Abstract
Background
Dose-related toxicity is the major restriction of cisplatin and cisplatin-combination chemotherapy, and is a challenge for advanced gastric cancer treatment. We explored the possibility of using Paris saponin I as an agent to sensitize gastric cancer cells to cisplatin, and examined the underlying mechanism.
Material/Methods
Growth inhibition was detected by MTT assay. The cell cycle and apoptosis were detected using flow cytometry and Annexin V/PI staining. The P21waf1/cip1, Bcl-2, Bax, and caspase-3 protein expression were detected using Western blot analysis.
Results
The results revealed that PSI sensitized gastric cancer cells to cisplatin, with low toxicity. The IC50 value of cisplatin in SGC-7901 cell lines was decreased when combined with PSI. PSI promoted cisplatin-induced G2/M phase arrest and apoptosis in a cisplatin concentration-dependent manner. Bcl-2 protein expression decreased, but Bax, caspase-3, and P21waf1/cip1 protein expression increased with PSI treatment.
Conclusions
The underlying mechanism of Paris saponin I may be related to targeting the apoptosis pathway and cell cycle blocking, which suggests that PSI is a potential therapeutic sensitizer for cisplatin in treating gastric cancer.
MeSH Keywords: Antineoplastic Agents, Apoptosis, Cell Cycle Checkpoints, Stomach Neoplasms
Background
Gastric cancer (GC) has a high mortality worldwide. Most patients have advanced disease when they are diagnosed, and most experience relapse and metastasis after treatment. The prognosis of advanced gastric cancer is very poor; therefore, systemic multidisciplinary treatment of gastric cancer is particularly important, and chemotherapy plays a critical role in advanced gastric cancer treatment. Cisplatin (CDDP) is a first-line chemotherapeutic agent for gastric cancer [1]. Some CDDP-based combination chemotherapies have been used to improve the treatment outcomes. However, the response rate of CDDP treatment against advanced gastric cancer and the survival of patients remain unsatisfactory. Also, chemotherapy is often limited by dose-related toxicity, which restricts advanced gastric cancer treatment. Thus, developing more effective and less toxic therapeutic regimens for new therapeutic agents is urgently required for sensitizing gastric cancer cells to chemotherapy to improve the prognosis. Natural products such as steroidal saponins represent a source of novel anticancer alternatives.
Steroidal saponins have significant bioactivities, including their antitumor, hemostatic, immunotropic, and analgesic properties. Paris saponins are the most important potential agents that can be developed for new antitumor medicines [2–13]. Among them, Paris saponin I (PSI) has been extensively studied. The major advantages of PSI as an anticancer agent are related to its ability to inhibit tumor growth in various cancer types [14–19] associated with a low level of toxicity. Recent studies have demonstrated that PSI possesses pharmacological activities concerning the cytotoxic activity against many cancers with mechanism of increasing levels of Bax, cytochrome c, activating caspases, and by decreasing both Bcl-2 expression and specific kinase-1/2 activity [14–19]. PSI may be a potential anticancer drug by inhibiting proliferation and a good radiosensitizer of gefitinib resistant NSCLC cells [20,21].
However, the relationship between PSI and the sensitivity of gastric cancer cells to cisplatin has not been reported. This study was conducted to explore the possibility of using PSI as an agent to sensitize cancer cells to cisplatin, with an attempt to provide new methods and insights for the management of gastric cancer.
Material and Methods
Drugs and reagents
Paris saponin I (PS I) (C44H70O16) was obtained from the ZheJiang Institute for Food and Drug Control (batch no. 111590, Hangzhou, China). The purity was greater than 99%, and it was dissolved in dimethyl sulfoxide (DMSO) and stored at −20°C. Cisplatin was obtained from Sigma-Aldrich (St. Louis, MO). The drugs were diluted in RPMI-1640 to achieve the final concentration used for following experiment with the final DMSO concentration less than 0.25% (v/v). The RPMI-1640 medium and fetal calf serum were obtained from Hyclone Co. (Logan, UT). The Cycle TEST™ PLUS DNA Reagent Kit and FITC Annexin V Apoptosis Detection kit were obtained from BD Biosciences (NJ, USA). Rabbit anti-rat B cell lymphoma 2 (Bcl-2; #3498), Bcl-2-associated X protein (Bax; #5023), caspase-3 (#9665), and p21/Waf1/Cip1 (#2947) monoclonal primary antibodies at 1: 1000 dilution (Cell Signaling Technology, Danvers, MA); anti-GAPDH (Santa Cruz Biotechnology, Inc., Dallas, TX).
Cell culture
The human gastric cancer cell line SGC-7901 was obtained from the ATCC Company (Manassas, VA, USA). Cells were grown in RPMI-1640 culture medium with 10% fetal bovine serum, 100 μg/ml penicillin and 100 μg/ml streptomycin at 37°C in a 5% CO2 humidified atmosphere.
Growth inhibition assay
The antiproliferative effects of PSI, Cisplatin-alone, and Cisplatin plus PSI were assessed using a MTT assay. SGC-7901 cells (100 μl/well, 1×104 cells/ml) were seeded and each group had triplicate treatments. Meanwhile, a nontreated group was established as the control (DMSO concentration was 0.25% (v/v)), and then treated with different concentrations of PSI (0.2, 0.4, 0.8, 1.6, 3.2, 6.4 μg/ml), or Cisplatin (0, 1, 2, 4, 8, 16, 32, 64 μM), or a combination of Cisplatin (at the concentrations shown above) plus PSI (0.3 μg/ml) for 48 h. Dose-dependent curves were generated. The cytotoxic effects of tested agents were expressed as the 50% inhibiting concentration (IC50).
Cell cycle assay
Four groups were divided as the control group, PSI group, Cisplatin group and PSI + Cisplatin group. The concentration of DMSO in the control group was 0.25% (v/v). PSI (0.3 μg/ml) and Cisplatin (0, 8,16, 32 μM) were used to treat the cells for 48 h, and then cells were harvested and fixed with 70% ethanol which were stored overnight at −20°C. Cells were incubated in RNase and then stained in propidium iodide (PI). Flow Cytometry with a Kaluza software, version 1.20 (Beckman Coulter, Inc., Brea, CA, USA) was used to analyze cell cycle.
Apoptosis analysis
Annexin V/PI method was used to analyze apoptosis with Annexin V FITC apoptosis detection kit (BD Biosciences). Four groups were divided as the control group, PSI group, Cisplatin group and PSI + Cisplatin group. The concentration of DMSO in the control group was 0.25% (v/v). Cells were treated with PSI (0.3 μg/ml) and Cisplatin (0, 8, 16, 32 μM), then harvested at 48 h, and stained following the kit instruction. Cells were incubated with the mixture of Annexin V FITC and PI in the dark. Apoptosis levels were detected by flow cytometry (Beckman Coulter, Inc., Brea, CA, USA).
Western blot analysis
Four groups were divided as the control group, Cisplatin group and PSI + Cisplatin group. The concentration of DMSO in the control group was 0.25% (v/v). Cells were treated with PSI (0.3 μg/ml) and Cisplatin (16 μM), Following treatment, cells were lysed with lysis buffer containing 50 mM Tris-HCl (pH 8.0) and 150 mM 1% TritonX-100 (Sigma-Aldrich). Equal amounts of protein were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, then transferred to nitrocellulose membranes (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The membranes were incubated overnight at 4°C with primary antibodies (rabbit anti-rat Bcl-2, Bax, caspase-3 and p21/Waf1/Cip1 monoclonal antibodies and mouse anti-rat GAPDH monoclonal antibody (1: 1,000)), and then washed three times with Tris-buffered saline supplemented with Tween-20, prior to being incubated for 2 h at room temperature with the HRP-conjugated goat anti-rabbit IgG secondary antibody (1: 10,000). The membranes were visualized using an enhanced chemiluminescence system and X-ray films (Santa Cruz Biotechnology Inc.).
Statistical analyses
Data were statistically analyzed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) and presented as means ±S.D. Groups were compared by one-way ANOVA test and SNK-q test considering P<0.05 as a significance level.
Results
PSI inhibits cell growth and proliferation and promotes Cisplatin-induced cytotoxicity in human gastric cancer cell line by MTT assay
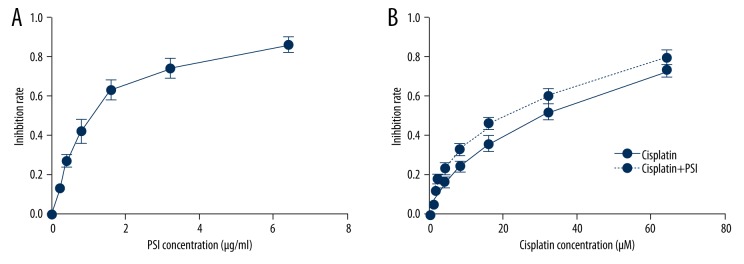
PSI concentrations of 0.2 to 6.4 μg/ml caused dose-dependent inhibition of the SGC-7901 cell growth at 48 h (Figure 1A). The IC50 value of PSI in SGC-7901 cell lines at 48 h was 1.12 μg/ml, indicating that PSI had a significant anticancer activity in gastric cancer cells in vitro. Then the cells were treated with different concentrations of Cisplatin, either alone or in combination with PSI (choose IC20, 0.3 μg/ml) at 48 h. Cisplatin at concentrations of 1 to 64 μM caused a dose-dependent inhibition at 48 h (Figure 1B). The IC50 value of Cisplatin in SGC-7901 cell lines was 30.4 μM at 48 h. When Cisplatin was combined with PSI (0.3 μg/ml), the IC50 value decreased significantly to 20.3 μM (Figure 1B). The results indicate that PSI significantly sensitizes SGC-7901 cell lines to Cisplatin-induced proliferation inhibition.
Figure 1.
PSI induced growth inhibition of gastric cancer cell line SGC-7901 following exposure at different concentrations at 48 h (A). Inhibition rates were significantly increased in the PSI+cisplatin treatment group compared with cisplatin only group at 48 h (P<0.05) and was dose-dependent (B).
PSI promotes Cisplatin-induced G2/M arrest in SGC-7901 cells
Cisplatin induced G2/M phase arrest in a concentration-dependent manner at 48 h. Furthermore, PSI promoted Cisplatin-induced G2/M phase arrest in a Cisplatin concentration-dependent manner (Figure 2). The results show that PSI promotes Cisplatin-induced inhibition of SGC-7901 cell proliferation according to the G2/M phase arrest.
Figure 2.
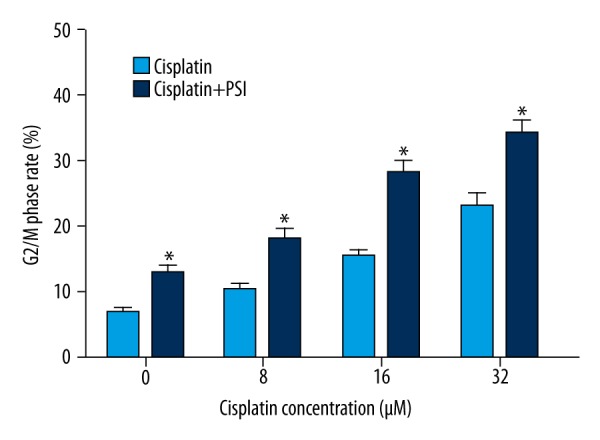
PSI promoted cisplatin-induced G2/M arrest in a cisplatin concentration-dependent manner. * Statistically significant difference (P<0.01) between the PSI+cisplatin treated groups and the cisplatin only group.
PSI promotes Cisplatin-induced apoptosis in SGC-7901 cells
Annexin V/PI double staining assay was used to evaluate the apoptosis induced by PSI and Cisplatin in SGC-7901 cells. Apoptosis was observed in Cisplatin treated group with different concentrations, and furthermore, significant apoptosis was observed in PSI plus Cisplatin group compare to Cisplatin treated group (Figure 3). The results reveal that PSI promotes Cisplatin-induced apoptosis in SGC-7901 cells at 48 h significantly in a Cisplatin concentration-dependent manner.
Figure 3.
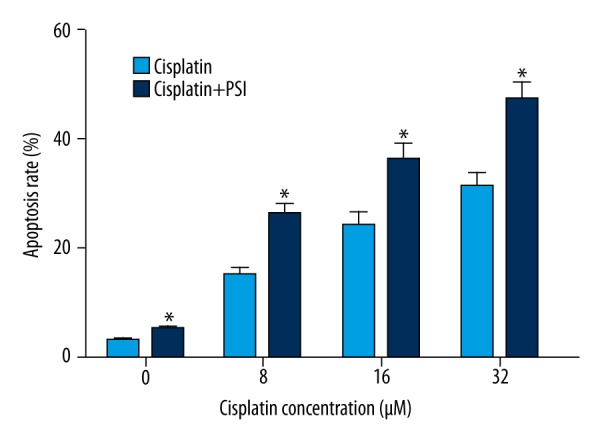
Apoptosis was increased which treated with PSI or cisplatin and was increased significantly when treated with PSI plus cisplatin in a cisplatin concentration-dependent manner. * Statistically significant difference (P<0.01) between the PSI+cisplatin-treated groups and the cisplatin only group.
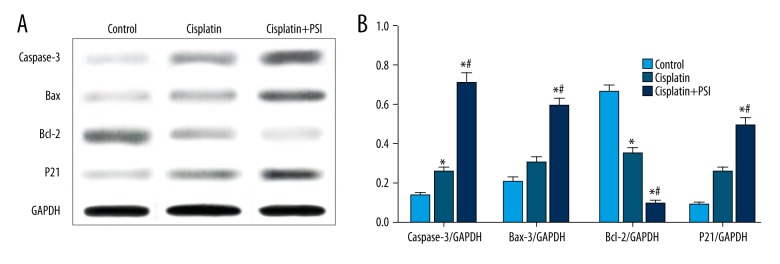
PSI increases Cisplatin-induced levels of apoptosis and G2/M regulatory proteins in SGC-7901 cells
To explore the mechanism by which PSI enhances the Cisplatin-induced apoptosis and cell cycle arrest in SGC-7901 cells, we examined the effects of PSI and/or Cisplatin on the expression of key regulators, including Caspase-3, Bax, Bcl-2 and P21waf1/cip1. Caspase-3, Bax and Bcl-2 were the most important apoptosis regulators and P21waf1/cip1 was the most important cell cycle checkpoint regulator. Cisplatin treatment decreased the level of Bcl-2 and increased the levels of Caspase-3, Bax, P21waf1/cip1, however, PSI enhanced these effects (Figure 4). The result suggested that the regulation of P21waf1/cip1, Caspase-3, Bax and Bcl-2 expressions contributed to G2/M phase arrest and apoptosis induced by PSI to Cisplatin in SGC-7901 cells.
Figure 4.
Effect of PSI to cisplatin treatment on levels of caspase-3, Bax, Bcl-2, and P21waf1/cip1 protein expression in SGC-7901 cells. Treatment with cisplatin decreased the level of Bcl-2 and increased the levels of caspase-3, Bax, Bcl-2, P21waf1/cip1, and the addition of PSI enhanced these effects of cisplatin (A). * Statistically significant difference (P<0.01) between the PSI+cisplatin-treated groups and the cisplatin only group; # Statistically significant difference (P<0.01) between the PSI+cisplatin-treated groups and the control group (B).
Discussion
Chemotherapy is the most important way to treat advanced gastric cancer for decades. Although new compounds have been developed to be active against transitional cell carcinoma, Cisplatin remains one of the most active ‘standard’ single-agents and the mainstay of typical combination regimens against gastric cancer [22–26]. However, Cisplatin is highly toxic, and its efficacy is limited owing to its adverse events. Furthermore, resistance to Cisplatin is developing gradually in the treatment [27–30]. Thus, developing effective agents to sensitize Cisplatin efficacy and overcome platinum resistance in gastric cancer makes a challenge. Paris saponin I, originally derived from Rhizoma paridis, which is the root of Chinese herbal, has been recently investigated as a new anticancer agent [14–19]. In the present study, we demonstrated that PSI sensitized gastric cancer cells to Cisplatin with low toxicity. We found that the IC50 value of Cisplatin in SGC-7901 cell lines was 30.4 μM at 48 h. When Cisplatin was combined with PSI (0.3 μg/ml), the IC50 value decreased significantly to 20.3 μM, revealing that PSI significantly sensitized SGC-7901 cell lines to Cisplatin-induced proliferation inhibition.
The most important effect of chemotherapy for treating human cancers is cell proliferative inhibition according to the induction of cell apoptosis. Cisplatin is one of the most commonly used drug in treating various types of cancer. It has shown that Cisplatin can bind to DNA and inhibit DNA synthesis, suppress cell division and induce apoptosis [31]. It has been increasingly recognized that the apoptosis of tumor cells is a key indicator for measuring the effectiveness of chemotherapy, and the apoptosis is associated with the chemotherapy drugs in a time- and dose-dependent manner [32]. There are two major apoptosis pathways: an extrinsic pathway, which involves signal transduction through cell surface death receptors, and an intrinsic pathway, which is triggered by radiation or chemical agents. But most malignancies have defects in initiating or executing the apoptotic pathways. Therefore, targeting the regulation of apoptosis represents an important pharmacological strategy for the development of chemotherapeutic agents. Caspase-3 is the most important death protease in the caspase family, which play vital roles in mediating interactions through the apoptotic process, catalyzing the specific cleavage of many key proteins in the apoptosis pathway. The Bcl-2 family is composed of a series of anti-apoptotic and proapoptotic members. Bcl-2 and Bax are the crucial mediators of cell apoptosis between anti-apoptosis and proapoptosis. Bcl-2/Bax ratio is regarded as an indicator of apoptosis level, and a high Bcl-2/Bax value indicates cell resistance to apoptosis [20]. Therefore, pharmacological manipulation of caspases and Bcl-2 family is a potential therapeutic strategy. In the present study, apoptosis was increased by cisplatin treatment and was increased significantly by PSI plus cisplatin treatment. The results revealed that PSI increased cisplatin-induced apoptosis in SGC-7901 cells. Further, cisplatin treatment decreased the level of Bcl-2 and increased the levels of caspase-3, Bax, and PSI enhanced these effects of cisplatin. Thus, PSI induces apoptosis, especially together with the Cisplatin treatment, leading to enhanced efficacy. The mechanism may be by the regulation of Bcl-2, Bax, and caspase-3.
Cell cycle blocking is known to be a main process of cell proliferative inhibition. P21waf1/cip1 is the most significant cell cycle checkpoint protein. In the present study, PSI enhanced the effect of cisplatin to alter the cycle distribution with G2/M phase arrest in gastric cancer cells. Further study showed that PSI significantly increased the expression of P21waf1/cip1, finally resulting in cell cycle blocking of G2/M phase, which indicates that P21waf1/cip1 is an important mediator in cell cycle progression. PSI with cisplatin can induce significant G2/M phase arrest through P21waf1/cip1 regulation in gastric cancer cells.
Conclusions
PSI significantly sensitizes SGC-7901 cell lines to cisplatin-induced proliferation inhibition in a cisplatin concentration-dependent manner in vitro. The mechanism of this chemosensitivity is related to the G2/M phase arrest and apoptosis by increased caspase-3, Bax, P21waf1/cip1 and decreased Bcl-2 expression. Therefore, PSI shows promise as a chemosensitizer, but this needs further investigation in vivo.
Footnotes
Source of support: The present study was supported by grants from the National Natural Science Foundation of China (grants no. 81303274 and 81202947)
References
- 1.Cunningham D, Allum WH, Stenning SP, et al. MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 2.Ma DD, Lu HX, Xu LS, Xiao W. Polyphyllin D exerts potent anti-tumour effects on Lewis cancer cells under hypoxic conditions. J Int Med Res. 2009;37:631–40. doi: 10.1177/147323000903700305. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Y, Kang LP, Liu YX, et al. Steroidal saponins from the rhizome of Paris polyphylla and their cytotoxic activities. Planta Med. 2009;75:356–63. doi: 10.1055/s-0028-1088380. [DOI] [PubMed] [Google Scholar]
- 4.Shuli M, Wenyuan G, Yanjun Z, et al. Paridis saponins inhibiting carcinoma growth and metastasis in vitro and in vivo. Arch Pharm Res. 2011;34:43–50. doi: 10.1007/s12272-011-0105-4. [DOI] [PubMed] [Google Scholar]
- 5.Wen F, Yin H, Chen C, et al. Chemical characteristics of saponins from Paris fargesii var. brevipetala and cytotoxic activity of its main ingredient, paris saponin H. Fitoterapia. 2012;83:627–35. doi: 10.1016/j.fitote.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 6.GuangLie C, WeiShi G, GaiLing H, JianPing C. Effect of Paris saponin on antitumor and immune function in U14 tumor-bearing mice. Afr J Tradit Complement Altern Med. 2013;10:503–7. doi: 10.4314/ajtcam.v10i3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L, Tan J, Wang B, et al. In-vitro antitumor activity and antifungal activity of pennogenin steroidal saponins from Paris polyphylla var. yunnanensis. Iran J Pharm Res. 2011;10:279–86. [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Gu JF, Zou X, et al. The anti-lung cancer activities of steroidal saponins of P. polyphylla Smith var. chinensis (Franch.) Hara through enhanced immunostimulation in experimental Lewis tumor-bearing C57BL/6 mice and induction of apoptosis in the A549 cell line. Molecules. 2013;18:12916–36. doi: 10.3390/molecules181012916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He H, Zheng L, Sun YP, et al. Steroidal saponins from Paris polyphylla suppress adhesion, migration and invasion of human lung cancer A549 cells via down-regulating MMP-2 and MMP-9. Asian Pac J Cancer Prev. 2014;15:10911–16. doi: 10.7314/apjcp.2014.15.24.10911. [DOI] [PubMed] [Google Scholar]
- 10.Ling Y, Fu Z, Zhang Q, et al. Identification and structural elucidation of steroidal saponins from the root of Paris polyphylla by HPLC-ESI-QTOF-MS/MS. Nat Prod Res. 2015;4:1–6. doi: 10.1080/14786419.2015.1007137. [DOI] [PubMed] [Google Scholar]
- 11.He H, Sun YP, Zheng L, Yue ZG. Steroidal saponins from Paris polyphylla induce apoptotic cell death and autophagy in A549 human lung cancer cells. Asian Pac J Cancer Prev. 2015;16:1169–73. doi: 10.7314/apjcp.2015.16.3.1169. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Yang Y, Lei L, Tian M. Rhizoma paridis saponins induces cell cycle arrest and apoptosis in non-small cell lung carcinoma a549 cells. Med Sci Monit. 2015;21:2535–41. doi: 10.12659/MSM.895084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao PJ, Song SC, Du LW, et al. Paris Saponins enhance radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell line by inducing apoptosis and G2/M cell cycle phase arrest. Mol Med Rep. 2016;13:2878–84. doi: 10.3892/mmr.2016.4865. [DOI] [PubMed] [Google Scholar]
- 14.Xiao X, Bai P, Bui Nguyen TM, et al. The antitumoral effect of Paris Saponin I associated with the induction of apoptosis through the mitochondrial pathway. Mol Cancer Ther. 2009;8:1179–88. doi: 10.1158/1535-7163.MCT-08-0939. [DOI] [PubMed] [Google Scholar]
- 15.Xiao M, Dai X, He X, et al. Paris saponin I induces G2/M cell cycle arrest and apoptosis in human gastric carcinoma SGC7901 cells. J Huazhong Univ Sci Technolog Med Sci. 2011;31:768–72. doi: 10.1007/s11596-011-0674-y. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H, Su D, Ma SL. [The effect of Chonglou Saponin I on proliferation and apoptosis in lung adenocarcinoma cell line PC9]. J Chin Oncol. 2012;18:166–69. [in Chinese] [Google Scholar]
- 17.Jiang H, Zhao PJ, Ma SL. [The effect of Paris saponin I on apoptosis associating with PI3K/Akt pathway in pancreatic carcinoma cell line PANC-1]. J Chin Oncol. 2014;20:127–30. [in Chinese[ [Google Scholar]
- 18.Jiang H, Zhao PJ, Feng JG, et al. [Radiosensitivity of Paris saponin I on pancreatic carcinoma cell line PANC-1 in vitro]. J Chin Oncol. 2014;20:483–87. [in Chinese] [Google Scholar]
- 19.Zhao P, Jiang H, Su D, et al. Inhibition of cell proliferation by mild hyperthermia at 43°C with Paris Saponin I in the lung adenocarcinoma cell line PC-9. Mol Med Rep. 2015;11:327–32. doi: 10.3892/mmr.2014.2655. [DOI] [PubMed] [Google Scholar]
- 20.Jiang H, Zhao P, Feng J, et al. Effect of Paris saponin I on radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell line. Oncol Lett. 2014;7:2059–64. doi: 10.3892/ol.2014.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang H, Zhao PJ, Su D, et al. Paris saponin I induces apoptosis via increasing the Bax/Bcl-2 ratio and caspase-3 expression in gefitinib-resistant non-small cell lung cancer in vitro and in vivo. Mol Med Rep. 2014;9:2265–72. doi: 10.3892/mmr.2014.2108. [DOI] [PubMed] [Google Scholar]
- 22.Cavanna L, Bodini FC, Stroppa EM, et al. Advanced gastric cancer with liver and lymph node metastases successfully resected after induction chemotherapy with docetaxel, Cisplatin and 5-fluorouracil. Chemotherapy. 2014;60:224–27. doi: 10.1159/000375156. [DOI] [PubMed] [Google Scholar]
- 23.Wu D, Li X, Tong J, et al. S-1 combined with Cisplatin versus Cisplatin alone for the treatment of advanced gastric cancer: A pilot randomized-controlled trial. Anticancer Drugs. 2015;26:774–78. doi: 10.1097/CAD.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa K, Fujitani K, Inagaki H, et al. Randomised phase III trial of second-line irinotecan plus Cisplatin versus irinotecan alone in patients with advanced gastric cancer refractory to S-1 monotherapy: TRICS trial. Eur J Cancer. 2015;51:808–16. doi: 10.1016/j.ejca.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Turkeli M, Aldemir MN, Cayir K, et al. Efficacy and tolerability of weekly docetaxel, Cisplatin, and 5-fluorouracil for locally advanced or metastatic gastric cancer patients with ECOG performance scores of 1 and 2. Asian Pac J Cancer Prev. 2015;16:985–89. doi: 10.7314/apjcp.2015.16.3.985. [DOI] [PubMed] [Google Scholar]
- 26.Bodoky G, Scheulen ME, Rivera F, et al. Clinical benefit and health-related quality of life assessment in patients treated with Cisplatin/S-1 versus Cisplatin/5-FU: Secondary end point results from the First-Line Advanced Gastric Cancer Study (FLAGS) J Gastrointest Cancer. 2015;46:109–17. doi: 10.1007/s12029-014-9680-1. [DOI] [PubMed] [Google Scholar]
- 27.Zhi X, Tao J, Xiang G, et al. APRIL induces Cisplatin resistance in gastric cancer cells via activation of the NF-κB pathway. Cell Physiol Biochem. 2015;35:571–585. doi: 10.1159/000369720. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Jin W, Jia H, et al. MiR-223 promotes the Cisplatin resistance of human gastric cancer cells via regulating cell cycle by targeting FBXW7. J Exp Clin Cancer Res. 2015;34:28. doi: 10.1186/s13046-015-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou W, Wang J, Man WY, et al. siRNA silencing EZH2 reverses Cisplatin-resistance of human non-small cell lung and gastric cancer cells. Asian Pac J Cancer Prev. 2015;16:2425–30. doi: 10.7314/apjcp.2015.16.6.2425. [DOI] [PubMed] [Google Scholar]
- 30.Hang Q, Sun R, Jiang C, Li Y. Notch 1 promotes Cisplatin-resistant gastric cancer formation by upregulating lncRNA AK022798 expression. Anticancer Drugs. 2015;26:632–40. doi: 10.1097/CAD.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 31.Peres LA, Cunha AD., Junior Acute nephrotoxicity of Cisplatin: Molecular mechanisms. J Bras Nefrol Orgao Oficial Soc Bras Latino-Am Nefrol. 2013;35:332–40. doi: 10.5935/0101-2800.20130052. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Li P, Li B, et al. RKIP promotes Cisplatin-induced gastric cancer cell death through NF-κB/Snail pathway. Tumour Biol. 2015;36:1445–53. doi: 10.1007/s13277-014-2496-6. [DOI] [PubMed] [Google Scholar]