Abstract
Dysplasia is a histologic precursor of esophageal squamous cell carcinoma (SCC). We previously showed that dietary freeze-dried, or lyophilized, strawberry powder inhibits N-nitrosomethylbenzylamine-induced SCC in the rat esophagus. On the basis of this observation, we conducted a randomized (noncomparative) phase II trial in China to investigate the effects of two doses of freeze-dried strawberries in patients with esophageal dysplastic lesions in a high-risk area for esophageal cancer. We randomly assigned 75 patients identified by endoscopy to have dysplastic esophageal premalignant lesions to receive freeze-dried strawberry powder at either 30 g/d (37 patients) or 60 g/d (38 patients) for six months; the powder was mixed with water and drunk. After six months, we assessed the changes in histologic grade of these lesions (primary endpoint) in a blinded fashion. The dose of 30 g/d, did not significantly affect histology or any other measured parameter. The dose of 60 g/d, however, reduced the histologic grade of dysplastic premalignant lesions in 29 (80.6%) of the 36 patients at this dose who were evaluated for histology (P < 0.0001). The strawberry powder was well tolerated, with no toxic effects or serious adverse events. Strawberries (60 g/d) also reduced protein expression levels of inducible nitric oxide synthase (iNOS) by 79.5% (P < 0.001), cyclooxygenase-2 (COX-2) by 62.9% (P < 0.001), phospho-nuclear factor kappa B (NFκB)-p65 (pNFκB-p65) by 62.6% (P < 0.001), and phospho-S6 (pS6) by 73.2% (P < 0.001). Freeze-dried strawberries (60 g/d) also significantly inhibited the Ki-67 labeling index by 37.9% (P = 0.023). Our present results indicate the potential of freeze-dried strawberry powder for preventing human esophageal cancer, supporting further clinical testing of this natural agent in this setting.
Introduction
Esophageal cancer remains a major public health concern worldwide. The 2 main types of esophageal cancer, squamous cell carcinoma (SCC), and adenocarcinoma (AC), have distinct etiologic and pathologic characteristics. More than 90% of esophageal cancer cases worldwide are SCC, and about 5% are AC (1). Esophageal SCC is one of the most common malignant neoplasms worldwide. Most patients present with advanced, metastatic disease at the time of diagnosis (2). The prognosis for these cases is poor; only 1 in 5 survives more than 3 years after the initial diagnosis (3, 4). The overall 5-year survival rate of esophageal SCC in the United States is only 13%, which is close to the observed rates in high-risk countries including China (5).
There is substantial geographic variation in the occurrence of esophageal SCC, with China having the highest incidence in the world (6). Esophageal SCC is the 4th most common cause of cancer death in China (7). A number of prospective studies have identified areas of a high risk for esophageal SCC in the Taihang Mountains region of China; these areas include Linxian and Anyang in Henan province (8, 9), Chaoshan in Guangdong province (10), and Cixian in Hebei province (11).
Esophageal carcinogenesis is a multistage process characterized by morphologic changes from normal esophagus to basal cell hyperplasia, dysplasia (mild, moderate, and severe), carcinoma in situ and SCC. Dysplasia is a histologic precursor of esophageal SCC and can be accurately visualized and targeted for biopsy by mucosal iodine staining during esophageal endoscopy (12). Severe dysplasia is usually treated either by endoscopic mucosal resection or argon plasma coagulation, whereas mild or moderate dysplasia generally is not treated but monitored by routine endoscopy. About 25% of patients with mild dysplasia and 50% of patients with moderate dysplasia will develop esophageal SCC over the subsequent decades (13, 14). Therefore, mild and moderate dysplasia are excellent surrogate endpoint biomarkers for the assessment of candidate chemoprevention agents.
Nitrosamine carcinogens in tobacco smoke, in the diet, and produced in the acidic conditions of the stomach seem to be important causative agents of esophageal SCC (15). Our laboratory has used the model of N-nitrosomethylbenzylamine (NMBA)-induced esophageal cancer in rats to identify putative chemopreventive agents, including natural food products, to prevent this disease. In our previous investigations, we found that freeze-dried, or lyophilized, strawberries and black raspberries (BRB) have chemopreventive potential in this preclinical animal model. Dietary freeze-dried strawberries significantly inhibit tumor development in the rat esophagus by inhibiting the metabolism of NMBA to DNA-damaging species and by reducing the growth rate of premalignant cells (16). The inhibition of rat esophageal tumorigenesis by BRBs was associated with its downregulation of genes involved in inflammation, gene transcription, and angiogenesis, genes including inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), c-Jun, and VEGF (17, 18).
Epidemiologic studies have revealed the protective effects of raw fruit and vegetable consumption on the development of several types of cancer in humans, and several studies have shown the protective effects of berry components, including the Nutrition Intervention Trial (NIT) conducted in Linxian, China. NIT showed that a triple combination of micronutrients also present in berries-reduced cancer mortality (19). A study conducted by Nouraie and colleagues suggested that high intake of vitamin C and tocopherols were protective for gastric noncardia cancer (20). None of the relevant epidemiologic studies reported to date have provided convincing evidence of a protective effect of whole berry consumption on the occurrence of any cancer type in humans (21). Lyophilized BRBs have shown preventive effects in a small clinical study in 10 patients with Barrett’s esophagus, a precursor of esophageal AC (22). This study found that 6-month BRB treatment inhibited oxidative stress, an effect that was detected via reduced levels of 8-epi-prostaglandin F2α (8-Iso-PGF2) and 8-hydroxy-2-deoxyguanosine (8-OHdG) in the urine. Until now, neither strawberries nor BRBs had been evaluated for chemopreventive effects against precursor lesions for esophageal SCC in humans.
We now report a randomized phase II trial we conducted to investigate the effects of freeze-dried strawberries in a cohort of adult individuals with esophageal dysplastic lesions in the Henan and Shandong provinces of China where the population is at the highest risk for esophageal cancer in the world. We assessed the effects of strawberries, which are the major berry grown in China, on the histologic grade of precancerous lesions and on biomarkers of cell proliferation, inflammation, and gene transcription.
Materials and Methods
Chemicals and reagent kits
Ki-67 antibody was obtained from Signet Laboratories. iNOS antibody was purchased from Santa Cruz Biotechnology. COX-2 antibody was purchased from Cayman Chemical Company. The antibodies for nuclear factor kappa B-p65 (NFκB-p65), phospho-NFκB-p65 (pNFκB-p65), S6, and phospho-S6 (pS6) were obtained from Cell Signaling Technology. The NuPAGE LDS sample buffer, NuPAGE sample reducing agent and 7% NuPAGE Novex Tris-acetate gel electrophoresis were obtained from Invitrogen. The Immun-star WesterC Kit was purchased from Bio-Rad Laboratories.
Lyophilized strawberries
Freeze-dried strawberries (Fragaria ananassa) were obtained from the California Strawberry Commission. Berries were hand harvested into plastic trays, transported to the processing facility and sorted, washed and frozen within 24 hours of harvest. Berries were then shipped frozen to Van Drunen Farms for dehydration, grinding to powder, and packaging for shipment while frozen to the Ohio State University and then to Beijing, China, where they were kept frozen at −20°C until used in the trial. A single batch of lyophilized strawberry powder was used throughout this trial and was analyzed for content of certain vitamins, minerals, phenols, carotenoids, and phytosterols by Covance Laboratories (Table 1).
Table 1.
Report of analysis of freeze-dried strawberries
| Assay | Analysis | Units |
|---|---|---|
| Sterols | ||
| Cholesterol | <1.0 | mg/100 g |
| Campesterol | 1.9 | mg/100 g |
| Stigmasterol | <1.0 | mg/100 g |
| Beta sitosterol | 31.1 | mg/100 g |
| Vitamins | ||
| Vitamin A | 51.0 | IU/100 g |
| Vitamin E (natural) | 4.1 | IU/100 g |
| Vitamin C | 488.0 | mg/100 g |
| Folic acid | 0.438 | mg/100 g |
| Phenylpropanoids | ||
| P-coumeric acid | 39.8 | mg/100 g |
| Ferulic acid | <5.0 | mg/100 g |
| Minerals | ||
| Calcium | 180.0 | mg/100 g |
| Copper | 0.3 | mg/100 g |
| Iron | 4.1 | mg/100 g |
| Magnesium | 125.0 | mg/100 g |
| Manganese | 3.7 | mg/100 g |
| Phosphorus | 244.0 | mg/100 g |
| Potassium | 1,600.0 | mg/100 g |
| Sodium | 17.0 | mg/100 g |
| Zinc | 1.2 | mg/100 g |
| Selenium | 0.0131 | mg/100 g |
| Ginkgo biloba flavones | ||
| Isorphamnetin | <7.5 | mg/100 g |
| Kaempferol | 10.9 | mg/100 g |
| Quercetin | 5.12 | mg/100 g |
| Total ginkgo flavone glycosides | 41.0 | mg/100 g |
| Pesticide screen | Limit of detection | |
| Organophosphates, ppm | NDa | <0.050 |
| Organonitrogens, ppm | NDa | <0.500 |
| Organochlorinated, ppm | NDa | <0.200 |
| N-methylcarbamates, ppm | NDa | <0.100 |
ND = None detected.
Intervention procedures and sample collections
This study was approved by the Institutional Review Boards of the Peking Union Medical College Hospital, Chinese PLA General Hospital (Beijing, China) and the Ohio State University Cancer Center (Columbus, OH). Both males and females were included in the trial.
To be eligible for the trial, individuals were required to meet all the following inclusion criteria: (i) must be over the age of 40 and give informed consent; (ii) must be diagnosed with mild or moderate esophageal dysplasia; (iii) must be able to consume strawberry slurry in the span of 5 minutes; and (iv) have no prior history of intolerance or allergy to strawberry or strawberry-containing products.
Individuals were excluded from the trial if they met one of the following criteria: (i) with a current malignancy identified histologically (with a history of malignancy or with evidence of recurrent or persistent disease); (ii) with severe dysplasia; (iii) unable to give informed consent; (iv) undergoing chemotherapy or radiation therapy; (v) taking non-steroidal antiinflammatory drugs (NSAID) including aspirin and cannot abstain from it due to a medical condition; and (v) pregnant or lactating.
Following completion of a detailed questionnaire on smoking/drinking habits and medical history, individuals interested in the study and who had the qualifications outlined above were asked to participate and sign an informed consent form. For the phase II trial, participants first underwent a physical examination and then were randomly assigned into 2 groups: 30 g/d (15 g × 2) or 60 g/d (30 g × 2) of freeze-dried strawberries (mixed with 240 mL of water); patients and health care providers were not blinded to treatment assignment. The participants were asked to drink the strawberry slurry slowly over a period of time, and return the empty bags of strawberries after the berries were taken. Compliance was assessed every 2 weeks (self-reports and empty bag counts) by a study associate.
Esophageal biopsies of dysplastic lesions were obtained before and after 6-months of strawberry treatment. During the endoscopy, 20 to 30 mL of 1.2% Lugol’s iodine solution was sprayed onto the mucosal surface from the gastroesophageal junction to the upper pharyngoesophageal sphincter. The dysplastic lesions seemed unstained relative to the surrounding normal mucosa. Biopsies were taken from unstained (whitish) areas according to the following size in diameter: 5 to 19 mm, 1 biopsy sample; 20 to 39 mm, 2 biopsy samples; and 40 mm or more, 3 biopsy samples. One biopsy sample was fixed in 10% neutral-buffered formalin for overnight, transferred to PBS, embedded in paraffin, and sectioned in 4-μm thickness for histologic evaluation. The other biopsy samples were stored in liquid nitrogen and then transferred to a −80°C freezer for subsequent cellular/molecular analyses.
Evaluation of histologic grade
The biopsy slides were stained with hematoxylin and eosin. The slides were independently reviewed by 3 gastrointestinal pathologists in a blinded fashion who were unaware of the participant’s history, the intervention group assignments, the time the specimens were collected and visual endoscopic findings. When a discrepancy occurred among pathologists, the final diagnosis was determined by agreement of 2 of 3 pathologists. Esophageal tissues collected from study participants were classified as normal, hyperplasia, and dysplasia (mild, moderate, and severe). If multiple biopsies were taken, the worst histologic grade was recorded for data analysis.
Ki-67 immunohistochemistry staining
Ki-67 staining was carried out on paraffin-embedded biopsy specimens taken before and after berry treatment among all patients who have completed the 6 months of berry treatment. In brief, sections were incubated with antibody for Ki-67 (1:50, 1 hour, Signet Laboratories) after deparaffinized and blocked for nonspecific binding. Incubation with primary antibody was followed by 20 minutes incubation with secondary antibody (biotinylated immunoglobulin) and strepavidin-horseradish peroxidase label. The sections were developed with diaminobenzidine chromogen, and then counterstained with hematoxylin, dehydrated, and mounted. Stained slides were viewed and photographed with a Nikon Eclipse 80i microscope mounted with a high-resolution spot camera, which is interfaced with a computer loaded with image analysis software (NIS Element Research image analysis, version 3.1).
Western blot analysis
Proteins were extracted from frozen biopsy specimens from all patients who have completed the 6 months of berry treatment. Protein concentration was measured using a DC Protein Assay Kit (Bio-Rad Laboratories) according to the manufacturer’s recommendations. Fifteen microgram of protein with NuPAGE LDS Sample Buffer and NuPAGE sample reducing agent (Invitrogen) were heated at 100°C for 5 minute. After cooling at room temperature for 5 minutes, proteins were fractioned by 7% NuPAGE Novex Tris-acetate gel electrophoresis (Invitrogen). Proteins were then transferred to Invitrolon polyvinylidene difluoride (PVDF) membrane. The PVDF membrane was blocked with 5% (w/v) dry milk in TTBS for 1 hour, and then incubated overnight at 4°C. The blot was probed with iNOS (1:200, Santa Cruz Biotechnology), COX-2 (1:500, Cayman Chemical Co.), NFκB-p65 (1:1,000, Rabbit monoclonal, Cell Signaling Technology), pNFκB-p65 (1:1,000; Rabbit monoclonal, Cell Signaling Technology), S6 ribosomal protein (1:1,000; Rabbit monoclonal, Cell Signaling Technology), or pS6 ribosomal protein (1:1,000; Rabbit monoclonal, Cell Signaling Technology). Membranes were stripped by Restore Western Blot Stripping Buffer (Thermo Scientific) and reprobed β-actin antibody was used as a positive control. Membranes were washed with TTBS 4 times for 5 minutes each, incubated with anti-rabbit or mouse horseradish peroxidase antibody (1:1,000; Cell Signaling Technology) for 1 hour. During the end of incubation, the membrane was washed extensively with TTBS. The immunoreactive bands detected by Immun-star WesterC Kit (Bio-Rad Laboratories) were developed by Molecular Imager ChemiDoc XRS (Bio-Rad Laboratories) and bands quantity analyzed by Image Lab Software (version 2.0, Bio-Rad Laboratories).
Statistical analysis
The primary endpoint of the phase II trial was histologic grade of esophageal lesions (which was dysplasia at baseline). We assessed the primary endpoint in each study arm, and we compared histologic grade before and after treatment; the study was not designed to compare the 2 arms. We estimated a treatment effect of an improvement in histologic grade (between baseline and 6 months) in 50% of the patients in each study arm. A sample size of 30 patients in each arm would be needed to detect this treatment effect on the primary endpoint with an 80% power for a 2-sided test with a 0.05 level of significance. We estimated a patient withdrawal rate of 17% over the 6-month clinical trial, based on data reported by Limburg and colleagues on their clinical chemoprevention trial of selenomethionine and celecoxib for 10 months in China (15). Therefore, our overall accrual goal was 72 patients, 36 per arm, which would leave 30 evaluable patients per arm (36 − 17% × 36) at 6 months after the estimated withdrawals. We recognized that the limited sample size of each arm could make it impossible to characterize the dose–response relationship if the biological endpoints exhibit substantial variability (however, the resulting information may be useful when developing recruitment and retention strategies in subsequent phase II and III trials).
The histologic grade of esophageal tissues, Ki-67 labeling index, and the protein expression levels of iNOS, COX-2, NFκB-p65, pNFκB-p65, S6, and pS6 were determined for biopsy samples that were collected before and after strawberry treatment. The histologic grade was assessed by McNemar test. Ki-67 labeling index of pre- and posttreatment biopsies and differences in protein expression levels of the above genes were compared by Student t test. All statistical analysis was carried out using GraphPad Prism 5.0. Differences were considered statistically significant at P < 0.05. All P values were 2-sided.
Results
Participant characteristics
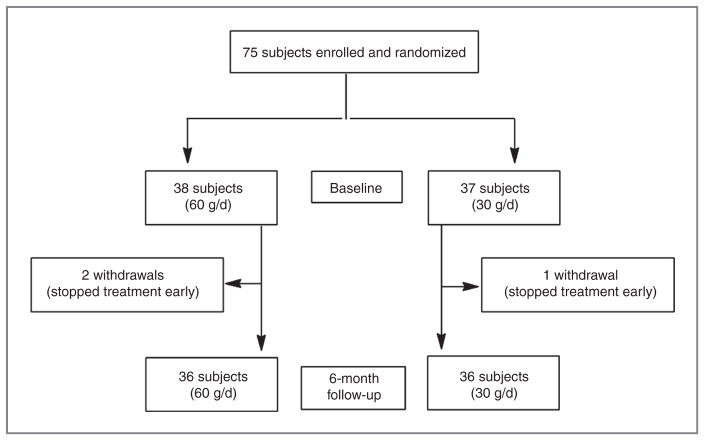
Seventy-five participants were recruited to this trial and randomly assigned to receive freeze-dried strawberries at either 60 g (30 g × 2/d; 38 participants) or 30 g (15 g × 2/d; 37 participants) for 6 months (Fig. 1). Patient characteristics of the 2 arms at baseline were similar (Table 2), including smoking status [12 (60 g) vs. 13 (30 g) smokers], drinking status (15 vs. 16 drinkers), and family history of esophageal SCC (7 vs. 5 participants). All 75 participants had esophageal dysplasia, with a similar distribution of mild (33 vs. 31) or moderate (5 vs. 6) dysplasia between the arms. There were 37 males and 38 females, and the average age of all participants was 57 years (range, 41–72 years). All patients signed written, informed consent to participate in the study.
Figure 1.
Trial flow diagram.
Table 2.
Baseline characteristics of participants in this study
| No. of participants (%)
|
||
|---|---|---|
| Characteristic | 60 g/d | 30 g/d |
| No. of participants | 38 | 37 |
| Gender | ||
| Female | 21 (55.3) | 17 (45.9) |
| Male | 17 (44.7) | 20 (54.1) |
| Age | ||
| <45 | 5 (13.2) | 1 (2.7) |
| 45–60 | 24 (63.2) | 25 (67.6) |
| >60 | 9 (23.6) | 11 (29.7) |
| Range 41–72 | ||
| Smoking | ||
| Smoker | 12 (31.6) | 13 (35.1) |
| Nonsmoker | 26 (68.4) | 24 (64.9) |
| Alcohol drinking | ||
| Drinker | 15 (39.5) | 16 (43.2) |
| Nondrinker | 23 (60.5) | 21 (56.8) |
| Family history of esophageal SCC | ||
| Yes | 7 (18.4) | 5 (13.5) |
| No | 31 (81.6) | 32 (86.5) |
| Histologic grade of dysplasia | ||
| Mild dysplasia | 33 (86.8) | 31 (83.8) |
| Moderate dysplasia | 5 (13.2) | 6 (16.2) |
| Compliance | 36 (94.7) | 36 (97.3) |
| Toxicities reported | ||
| Diarrhea | 0 | 1 (0.03) |
| None | 38 (100) | 36 (97.3) |
Histologic evaluation
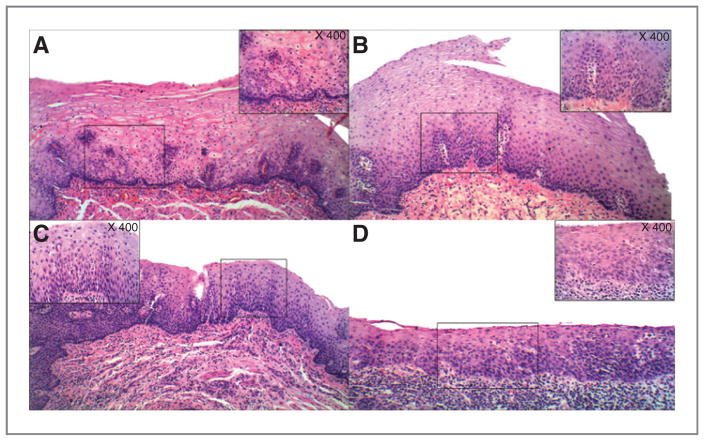
Histologically, esophageal epithelium of participants in this study was characterized into 3 categories: normal, hyperplasia and dysplasia (mild and moderate). As shown in Fig. 2, the normal esophageal epithelium is a nonkeratinizing, stratified, squamous epithelium. In hyperplasic epithelium, there is an increase in the number of basal cells, while the size and shape of the cells remains relatively unchanged. Squamous dysplasia is characterized by both architectural and cytologic abnormalities. The architectural abnormalities include epithelial disorganization, loss of cell polarity, and overlapping nuclei. The cytologic abnormalities include nuclear enlargement, hyperchromasia, and pleomorphism, increased nuclear/cytoplasmic ratio, and increased mitotic activity. Esophageal dysplasia was categorized into 3 grades: mild dysplasia (less than 25% of the thickness of epithelium), moderate dysplasia (less than 50% of the thickness of epithelium), and severe dysplasia (more than 50% of the thickness of epithelium). Because the patients with severe dysplasia are usually scheduled for surgery to have the lesion(s) removed, the participants who enrolled in this study had either mild or moderate dysplasia.
Figure 2.
Histologic grades of esophageal tissues collected from study participants. A, normal: the epithelium is nonkeratinizing and stratified. B, hyperplasia: there is an increase in the number of basal cells, but no atypia. C and D, dysplasia: there is an increase in the number of basal cells and some of them show nuclear overlapping and hyperchromasia. Mild dysplasia (C)—basal cells showing loss of polarity, composing less than 25% of the total thickness of the epithelium, and covered by thick squamous cell layer, while moderate dysplasia (D)—basal cells showing loss of polarity, composing closer to 50% of the total thickness of the epithelium, and with a reduced squamous layer (magnification ×200).
Among the participants, an average of 3.68 and 3.63 biopsy samples (range 2–5) were obtained from each participant at baseline and at the end of trial, respectively. Thirty-three (86.8%) and 31 (83.8%) patients who were diagnosed with esophageal mild dysplasia, and 5 (13.2%) and 6 (16.2%) patients who were diagnosed with esophageal moderate dysplasia, were assigned to 60 or 30 g per day of strawberry treatment, respectively. The strawberries were well tolerated. Only 1 participant who consumed 30 g/d of strawberry powder had diarrhea for only 1 day. The compliance was outstanding with 94.7% and 97.3% for 60 g/d and 30 g/d of strawberries, respectively.
Strawberries slow progression of precancerous growth
Of our 75 enrolled participants, 72 were evaluable for histology at the end of study—36 in each arm (Fig. 1; Table 3). Among participants who consumed 30 g of strawberries a day for 6 months, we did not observe any significant regression or progression of precancerous growth in the esophagus when compared with pretreatment. Only 5 patients (13.9%) improved histologically, and only 1 patient had an increase in histologic grade–all 6 had mild dysplasia at baseline. There was no change in 30 patients. Among participants who consumed 60 g total of strawberries daily for 6 months, 31 patients were diagnosed with mild dysplasia (86.1%) and 5 patients with moderate dysplasia (13.9%) as indicated in Table 3. After 6 months of strawberry treatment, in patients with mild dysplasia, there was no change in 4 patients, decrease in 26 patients (P < 0.0001) and an increase in 1 patient in histologic grade. In patients with moderate dysplasia, there was no change in 2 patients and a decrease in 3 patients in histologic grade. Overall, 29 of 36 participants (80.6%) treated with 60 g of strawberry powder daily experienced a decrease in histologic grade of the precancerous lesions during the study (P < 0.0001). The endoscopic appearance of normal esophagus and precancerous lesions before and after strawberry treatment is shown in Fig. 3.
Table 3.
Effect of 60 g (N = 36) or 30 g (N = 36) freeze-dried strawberries on histologic grade of esophageal precancerous lesions
| Normal | Hyperplasia | Mild dysplasia | Moderate dysplasia | |
|---|---|---|---|---|
| Treatmenta | Before/after (%) | Before/after (%) | Before/after (%) | Before/after (%) |
| 30 g | 0/2 (5.5) | 0/3 (8.3) | 30 (83.3)/24 (66.7) | 6 (16.7)/7 (19.4) |
| 60 g | 0/19 (52.7) | 0/9 (25) | 31 (86.1)/5 (13.9)b | 5 (13.9)/3 (8.3) |
Daily for 6 months.
Significantly lower than before strawberry treatment as determined by the McNemar test (P < 0.0001).
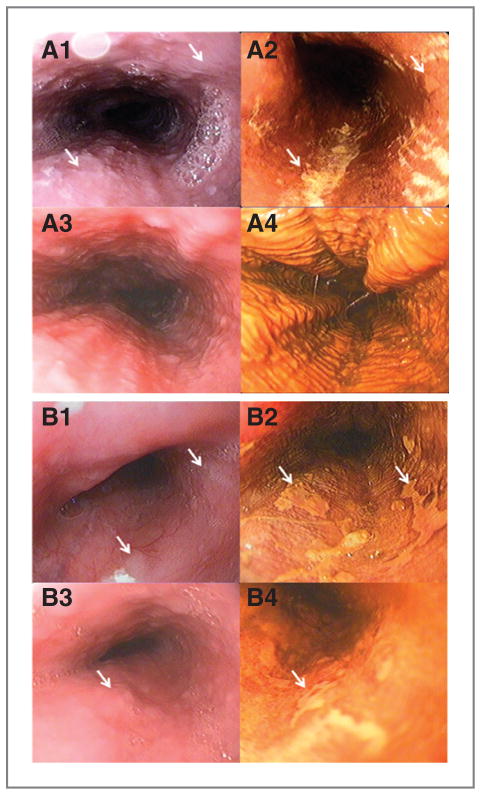
Figure 3.
Representative endoscopic images of esophagus before (A1, 2 and B1, 2) and after (A3, 4 and B3, 4) strawberry treatment. A1, 3 and B1, 3, endoscopic images of esophagus before iodine staining. A flat lesion with white patch (white arrows indicated) can be seen in A1, B1, and B3, although it is not clear. A2, B2, and B4, endoscopic images of the same lesion after iodine staining (white arrows indicated). Well-demarcated unstained mucosal areas are clearly observed. The histopathology reports showed that patient A had mild dysplasia (A1 and 2) before strawberry treatment and these lesions regressed to normal esophagus (A3 and 4) after strawberry treatment. Normal mucosa is smooth or mildly wrinkled and shows a diffuse change to brown with iodine staining (A3 and 4). Patient B had moderate dysplasia (B1 and 2) before treatment and mild dysplasia (B3 and 4) after treatment.
Strawberries inhibit protein expression of iNOS, COX-2, pNFκB-p65, and pS6
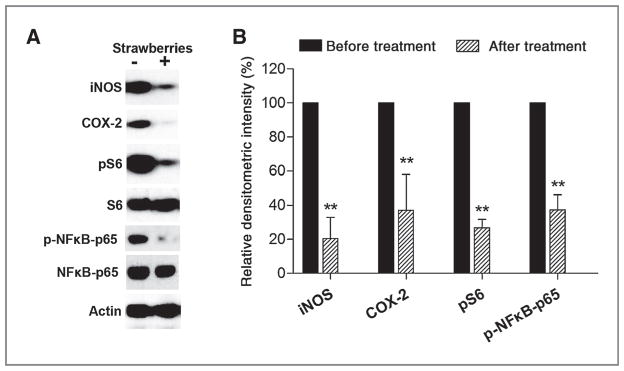
Western blot analysis was used to determine whether strawberries inhibited iNOS, COX-2, pNFκB-p65, and pS6 protein in esophageal mucosa after 6 months of berry treatment. Six months of a total 30 g/d of strawberry treatment statistically nonsignificantly decreased expression of the above proteins by 35.7% (iNOS), 25.5% (COX-2), 28.8% (pNFκB-p65), and 8.2% (pS6). As shown in Fig. 4, 6 months of a total of 60 g/d of strawberry treatment significantly inhibited protein expression of iNOS (79.50% reduction, P < 0.001), COX-2 (62.9% reduction, P < 0.001), pNFκB-p65 (62.6% reduction, P < 0.001), and pS6 (73.2% reduction, P < 0.001) in human esophageal mucosa.
Figure 4.
Effect of freeze-dried strawberries in the protein expression of iNOS, COX-2, p-NFκB-p65, and pS6. The indicated proteins were detected by Western blot analyses. Representative blots are shown; similar results were obtained from triplicate experiments. The values are relative densitometric intensity expressed as mean; bars, ± SE. **, P < 0.001 as determined by Student t test when compared with the expression level before strawberry treatment.
Strawberries inhibit cell proliferation
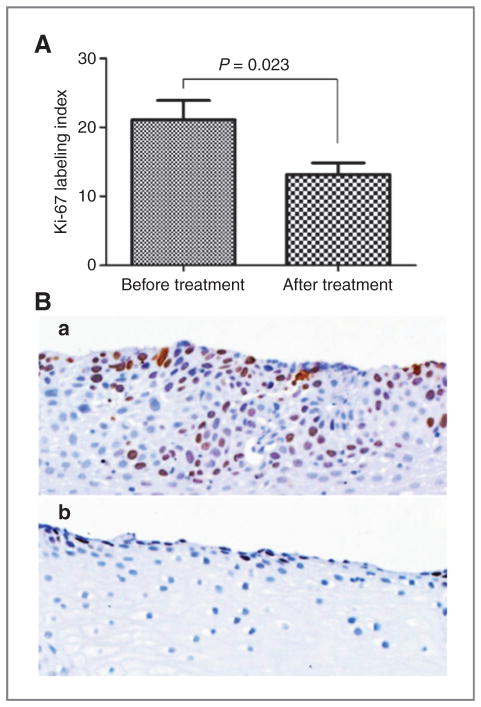
To evaluate the effect of strawberries on cell proliferation rate, all biopsy tissues of human esophagus collected before and after strawberry treatment were stained for Ki-67 protein by immunohistochemistry. As shown in Fig. 5, Ki-67 labeling index was significantly reduced by treatment with 60 g/d of strawberries from 21.1 ± 9.6% at baseline to 13.2 ± 5.9% at the end of the study (37.9% reduction, P < 0.05). Six months of a total 30 g/d of strawberry treatment did not reduce the labeling index of Ki-67 (17.7 ± 8.5% before and 18.0 ± 9.0% after berry treatment).
Figure 5.
Effect of freeze-dried strawberries on cell proliferation (A). Strawberries significantly reduced Ki-67 expression after 6-month treatment (B, b) when compared with before treatment (B, a; magnification ×200).
Discussion
This study shows that lyophilized strawberries (60 g/d for 6 months) inhibit the progression of precancerous growth in patients who have been diagnosed with esophageal dysplasia, at least in part by reducing the protein expression of iNOS, COX-2, pNFκB-p65, and pS6 and the Ki-67 cell proliferation index. Patients who consumed lower-dose lyophilized strawberries (30 g/d for 6 months) did not experience a significant regression of esophageal dysplasia, cell proliferation, or expression of iNOS, COX-2, pNFκB-p65, and pS6, although there may have been a modest biological affect on certain of these biomarkers. These results suggest that the 30-g dose was either not high enough and/or required a longer intervention to have chemopreventive effects. Furthermore, it may be that the 30-g dose was too dilute to be effective—15 g of strawberries mixed with 240 mL of water twice a day, versus 30 g mixed with the same amount of water twice a day (60-g preparation).
Pathologic and epidemiologic studies have found that there is a positive correlation between histologic progression of premalignant lesions from hyperplasia to dysplasia and increasing risk for esophageal SCC (13, 14). Therefore, dysplasia is considered to be an important precursor of esophageal SCC and has been proposed as a surrogate endpoint biomarker that can serve as the primary endpoint of clinical trials of chemopreventive agents (14, 23, 24). Endoscopy with mucosal iodine staining combined with histopathology has proved useful in identifying high-risk populations and offers multiple opportunities for assessment and intervention. Blocking the progression of premalignant lesions, such as epithelial dysplasia, to malignant SCC is an important component of chemoprevention of human esophageal SCC. Therefore, candidate chemopreventive agents that may inhibit progression of dysplasia in the esophagus have been tested in clinical trials, some of which did not have very encouraging results (25–28). For example, Limburg and colleagues found that neither selenomethionine nor celecoxib for 10 months inhibited esophageal SCC (14). Selenomethionine had a protective effect in the subgroup with mild dysplasia, but the correlation was not significant (P = 0.08). On the other hand, an earlier study by Lin and colleagues in Henan province in China found that the progression of esophageal dysplasia was inhibited by antitumor-B (a mixture of Chinese herbs) by 52.2% (3-year treatment) and 47.3% (5-year treatment) and by retinamide (4-ethoxycarbophenylretinamide) by 37.3% (3-year treatment) and 43.2% (5-year treatment; ref. 28). The mechanism(s) of action for antitumor-B and retinamide in inhibition of esophageal dysplasia remain to be elucidated in future studies.
Strawberries are an abundant source of flavonoid compounds (e.g., ellagic acid, ferulic acid, coumaric acid, quercetin, and the anthocyanins), vitamins (e.g., vitamins A, C, and E and folic acid), and minerals (e.g., calcium, selenium, and zinc; ref. 29; Table 1). Many of the known compounds have antioxidant and antiinflammatory activities and protect against cancer including ellagic acid (30), anthocyanins (31, 32), ascorbic acid (vitamin C; ref. 33), calcium (34), and selenium (35). The effective chemical composition of strawberries has not been fully determined. By freeze-drying the berries, these components are concentrated approximately 10-fold because strawberries are approximately 90% water by weight (21). The obvious practical/clinical advantage of lyophilized strawberries is the 10-fold concentration of potentially chemopreventive components in a volume one-tenth that of fresh strawberries. For example, it would take consumption of 1 1/3 lb (600 g) of fresh strawberries to equal the 60-g dose of lyophilized strawberries in our present trial.
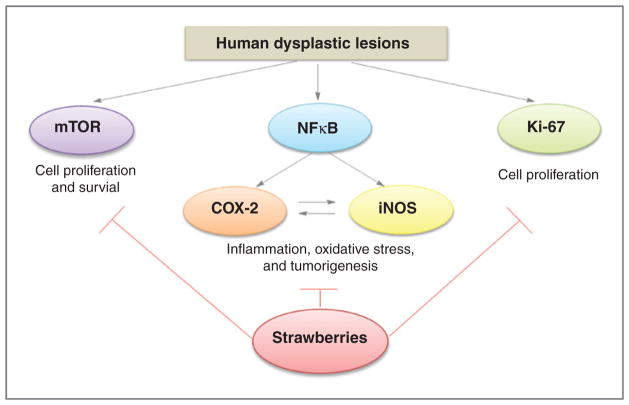
This study found that freeze-dried strawberries inhibited the progression of precancerous growth in the human esophagus. Our data suggest that strawberries reduced the expression levels of iNOS and COX-2, 2 enzymes previously shown in our laboratory to be upregulated in rat esophageal tumorigenesis (36, 37). Strawberries also downregulated the expression of pNFκB and pS6 ribosomal protein in dysplastic lesions of the human esophagus. NFκB is a pivotal transcription factor mediating the expression of multiple genes including COX-2 and iNOS (38). NFκB has 2 major subunits, p50 and p65. Activation of NFκB results in a degradation of inhibitor κB alpha (IκB-α) by phosphorylation and translocation of NFκB to the nucleus. pNFκB-p65 is commonly used as an index for the activation of NFκB (39). Strawberries may inhibit COX-2 and iNOS through the suppression of upstream mediators such as NFκB. mTOR plays central roles in the regulation of cell growth and proliferation (40). One of the downstream targets of mTOR is p70S6Kinase, whose subtract is pS6, a component of the S40 ribosome subunit (41). Measurement of pS6 by Western blot has been used to assess the activity of mTOR (42). The current study shows that strawberries inhibit mTOR signaling through downregulation of pS6 in human esophageal precancerous lesions. Because iNOS (43), COX-2 (44), NFκB (45), and mTOR (46) are overexpressed/activated in human esophageal SCC, agents like strawberries that inhibit these pathways may have chemopreventive potential in esophageal SCC. We depict the possible mechanisms of strawberries for inhibiting esophageal precancerous progression in Fig. 6.
Figure 6.
Possible mechanisms for the inhibition of esophageal precancerous progression by strawberries.
In summary, our present study showed for the first time that dietary strawberries (freeze-dried, 60 g/d for 6 months) significantly decreased the histologic grade of patients’ precancerous esophageal lesions. Although the precise mechanisms of this promising effect remain to be elucidated, it seems to be associated, in part, with downregulation of genes involved in inflammation, cell proliferation, and gene transcription including COX-2, iNOS, NFκB, and mTOR. Biodirected fractionation studies are underway to identify the most active inhibitory components in strawberries. Because our results show that strawberries have inhibitory properties during the promotion/progression stages of esophageal carcinogenesis in humans, strawberries may be an alternative or work together with other chemopreventive drugs for the prevention of esophageal cancer. On the basis of the promising results of this study and our overall program of strawberry research, we propose to test strawberries alone and combined with other preventive agents in randomized placebo-controlled trials in the future.
Acknowledgments
Grant Support
This work was supported by the California Strawberry Commission and Faculty Startup Fund from the Division of Medical Oncology, Department of Internal Medicine, The Ohio State University.
Footnotes
Disclosure of Potential Conflicts of Interest
Dr. Gary Stoner is part owner of BerriProducts, Inc., a company in Corvallis, Oregon that sells freeze-dried berries including strawberries.
References
- 1.Souza RF. Molecular and biologic basis of upper gastrointestinal malignancy–esophageal carcinoma. Surg Oncol Clin N Am. 2002;11:257–72. doi: 10.1016/s1055-3207(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 2.Layke JC, Lopez PP. Esophageal cancer: a review and update. Am Fam Physician. 2006;73:2187–94. [PubMed] [Google Scholar]
- 3.Polednak AP. Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer. 2003;105:98–100. doi: 10.1002/ijc.11029. [DOI] [PubMed] [Google Scholar]
- 4.Younes M, Henson DE, Ertan A, Miller CC. Incidence and survival trends of esophageal carcinoma in the United States: racial and gender differences by histological type. Scand J Gastroenterol. 2002;37:1359–65. doi: 10.1080/003655202762671215. [DOI] [PubMed] [Google Scholar]
- 5.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11:235–56. doi: 10.1016/s1055-3207(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, Parkin DM, Li LD, Chen YD, Bray F. Estimation and projection of the national profile of cancer mortality in China: 1991–2005. Br J Cancer. 2004;90:2157–66. doi: 10.1038/sj.bjc.6601813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy G, Fan JH, Mark SD, Dawsey SM, Selhub J, Wang J, et al. Prospective study of serum cysteine levels and oesophageal and gastric cancers in China. Gut. 2011;60:618–23. doi: 10.1136/gut.2010.225854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Z, Zhao Y, Guo C, Liu Y, Sun M, Liu F, et al. Prevalence and risk factors for esophageal squamous cell cancer and precursor lesions in Anyang, China: a population-based endoscopic survey. Br J Cancer. 2010;103:1085–8. doi: 10.1038/sj.bjc.6605843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang GH, Su M, Tian DP, Huang HH, Wu XY, Zheng RM, et al. Analysis of basement membrane structure and inflammation during the development of esophageal squamous cell carcinoma in the Chinese Chaoshan high risk region. Cancer Invest. 2008;26:296–305. doi: 10.1080/07357900701683901. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Wang L, Chang A, Jin Y, Rao J. Immunohistochemical analysis of cyclooxygenase-2 expression in premalignant and malignant esophageal glandular and squamous lesions in Cixian, China. Cancer Detect Prev. 2003;27:243–9. doi: 10.1016/s0361-090x(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu M, Nagata K, Yamaguchi H, Kita H. Squamous intraepithelial neoplasia of the esophagus: past, present, and future. J Gastroenterol. 2009;44:103–12. doi: 10.1007/s00535-008-2298-y. [DOI] [PubMed] [Google Scholar]
- 13.Wang GQ, Abnet CC, Shen Q, Lewin KJ, Sun XD, Roth MJ, et al. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187–92. doi: 10.1136/gut.2004.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Limburg PJ, Wei W, Ahnen DJ, Qiao Y, Hawk ET, Wang G, et al. Randomized, placebo-controlled, esophageal squamous cell cancer chemoprevention trial of selenomethionine and celecoxib. Gastroenterology. 2005;129:863–73. doi: 10.1053/j.gastro.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001;22:1737–46. doi: 10.1093/carcin/22.11.1737. [DOI] [PubMed] [Google Scholar]
- 16.Carlton PS, Kresty LA, Siglin JC, Morse MA, Lu J, Morgan C, et al. Inhibition of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus by dietary freeze-dried strawberries. Carcinogenesis. 2001;22:441–6. doi: 10.1093/carcin/22.3.441. [DOI] [PubMed] [Google Scholar]
- 17.Chen T, Hwang H, Rose ME, Nines RG, Stoner GD. Chemopreventive properties of black raspberries in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis: down-regulation of cyclooxygenase-2, inducible nitric oxide synthase, and c-Jun. Cancer Res. 2006;66:2853–9. doi: 10.1158/0008-5472.CAN-05-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen T, Rose ME, Hwang H, Nines RG, Stoner GD. Black raspberries inhibit N-nitrosomethylbenzylamine (NMBA)-induced angiogenesis in rat esophagus parallel to the suppression of COX-2 and iNOS. Carcinogenesis. 2006;27:2301–7. doi: 10.1093/carcin/bgl109. [DOI] [PubMed] [Google Scholar]
- 19.Qiao YL, Dawsey SM, Kamangar F, Fan JH, Abnet CC, Sun XD, et al. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101:507–18. doi: 10.1093/jnci/djp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nouraie M, Pietinen P, Kamangar F, Dawsey SM, Abnet CC, Albanes D, et al. Fruits, vegetables, and antioxidants and risk of gastric cancer among male smokers. Cancer Epidemiol Biomarkers Prev. 2005;14:2087–92. doi: 10.1158/1055-9965.EPI-05-0038. [DOI] [PubMed] [Google Scholar]
- 21.Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev Res. 2009;2:187–94. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kresty LA, Frankel WL, Hammond CD, Baird ME, Mele JM, Stoner GD, et al. Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: interim results show berries modulate markers of oxidative stress in Barrett's esophagus patients. Nutr Cancer. 2006;54:148–56. doi: 10.1207/s15327914nc5401_15. [DOI] [PubMed] [Google Scholar]
- 23.Posner M, Forastiere A, Minsky B. Cancer of esophagus. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer principals & practices of oncology. 7. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 861–909. [Google Scholar]
- 24.Kelloff GJ, Sigman CC, Johnson KM, Boone CW, Greenwald P, Crowell JA, et al. Perspective on surrogate end points in the development of drugs that reduce the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:127–37. [PubMed] [Google Scholar]
- 25.O'Shaughnessy JA, Knelloff GJ, Gordon GB, Dannengerg AJ, Hong WK, Fabian CJ, et al. Treatment and prevention for intraepithelial neoplasia: an important target for accelerated new agent development. Clin Cancer Res. 2002;8:314–46. [PubMed] [Google Scholar]
- 26.Wang LD, Qiu SL, Yang GR, Lipkin M, Newmark HL, Yang CS. A randomized double blind intervention study on the effect of calcium supplementation on esophageal precancerous lesions in a high-risk population in China. Cancer Epidemiol Biomarkers Prev. 1993;2:71–8. [PubMed] [Google Scholar]
- 27.Rao M, Liu FS, Dawsey SM, Yang K, Lipkin M, Li JY, et al. (1994) Effects of vitamin/mineral supplementation on the proliferation of esophageal squamous epithelium in Linxian, China. Cancer Epidemiol Biomarkers Prev. 1994;3:277–9. [PubMed] [Google Scholar]
- 28.Lin P, Zhang J, Rong Z, Han R, Xu S, Gao R, et al. Studies on medicamentous inhibitory therapy for esophageal precancerous lesions–3- and 5-year inhibitory effects of antitumor-B, retinamide and riboflavin. Proc Chin Acad Med Sci Peking Union Med Coll. 1990;5:121–9. [PubMed] [Google Scholar]
- 29.Olsson ME, Andersson CS, Oredsson S, Berglund RH, Gustavsson KE. Antioxidant levels and inhibition of cancer cell proliferation in vitro by extracts from organically and conventionally cultivated strawberries. J Agric Food Chem. 2006;54:1248–55. doi: 10.1021/jf0524776. [DOI] [PubMed] [Google Scholar]
- 30.Mandal S, Stoner GD. Inhibition of N-nitrosomethylbenzylamine-induced esophageal tumorigenesis in rats by ellagic acid. Carcinogenesis. 1990;11:55–61. doi: 10.1093/carcin/11.1.55. [DOI] [PubMed] [Google Scholar]
- 31.Wang LS, Hecht SS, Carmella SG, Yu N, Larue B, Henry C, et al. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev Res. 2009;2:84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bi X, Fang W, Wang LS, Stoner GD, Yang W. Black raspberries inhibit intestinal tumorigenesis in apc1638+/− and Muc2−/− mouse models of colorectal cancer. Cancer Prev Res. 2010;3:1443–50. doi: 10.1158/1940-6207.CAPR-10-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosetti C, La Vecchia C, Talamini R, Simonato L, Zambon P, Negri E, et al. Food groups and risk of squamous cell esophageal cancer in northern Italy. Int J Cancer. 2000;87:289–94. [PubMed] [Google Scholar]
- 34.Slattery ML, Neuhausen SL, Hoffman M, Caan B, Curtin K, Khe NM, et al. Dietary calcium, vitamin D, VDR genotypes and colorectal cancer. Int J Cancer. 2004;111:750–6. doi: 10.1002/ijc.20330. [DOI] [PubMed] [Google Scholar]
- 35.Yang H, Fang J, Jia X, Han C, Chen X, Yang CS, et al. Chemopreventive effects of early-stage and late-stage supplementation of vitamin E and selenium on esophageal carcinogenesis in rats maintained on a low vitamin E/selenium diet. Carcinogenesis. 2011;32:381–8. doi: 10.1093/carcin/bgq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen T, Stoner GD. Inducible nitric oxide synthase expression in N-Nitrosomethylbenzylamine (NMBA)-induced rat esophageal tumorigenesis. Mol Carcinog. 2004;40:232–40. doi: 10.1002/mc.20035. [DOI] [PubMed] [Google Scholar]
- 37.Carlton PS, Gopalakrishnan R, Gupta A, Liston BW, Habib S, Morse MA, et al. Piroxicam is an ineffective inhibitor of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus. Cancer Res. 2002;62:4376–82. [PubMed] [Google Scholar]
- 38.Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Hayden MS, Ghosh S. Shared principles in NF-kappa B signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 40.Wu P, Hu YZ. PI3K/Akt/mTOR pathway inhibitors in cancer: a perspective on clinical progress. Curr Med Chem. 2010;17:4326–41. doi: 10.2174/092986710793361234. [DOI] [PubMed] [Google Scholar]
- 41.Iwenofu OH, Lackman RD, Staddon AP, Goodwin DG, Haupt HM, Brooks JS. Phospho-S6 ribosomal protein: a potential new predictive sarcoma marker for targeted mTOR therapy. Mod Pathol. 2008;21:231–37. doi: 10.1038/modpathol.3800995. [DOI] [PubMed] [Google Scholar]
- 42.Sun Q, Chen X, Ma J, Peng H, Wang F, Zha X, et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci. 2011;108:4129–34. doi: 10.1073/pnas.1014769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin Y, Zhang W, Liu B, Wang H, Han Z, Wang L. Expression of inducible nitric oxide synthase in human esophageal biopsies from carcinoma and precancerous lesions. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2000;22:570–2. [PubMed] [Google Scholar]
- 44.Yu HP, Xu SQ, Liu L, Shi LY, Cai XK, Lu WH, et al. Cyclooxygenase-2 expression in squamous dysplasia and squamous cell carcinoma of the esophagus. Cancer Lett. 2003;198:193–201. doi: 10.1016/s0304-3835(03)00340-9. [DOI] [PubMed] [Google Scholar]
- 45.Izzo JG, Malhotra U, Wu TT, Ensor J, Luthra R, Lee JH, et al. Association of activated transcription factor nuclear factor kappa B with chemoradiation resistance and poor outcome in esophageal carcinoma. J Clin Oncol. 2006;24:748–54. doi: 10.1200/JCO.2005.03.8810. [DOI] [PubMed] [Google Scholar]
- 46.Boone J, Ten Kate FJ, Offerhaus GJ, van Diest PJ, Rinkes IH, van Hillegersberg R. mTOR in squamous cell carcinoma of the oesophagus: a potential target for molecular therapy? J Clin Pathol. 2008;61:909–13. doi: 10.1136/jcp.2008.055772. [DOI] [PubMed] [Google Scholar]