Abstract
Lysosomes are the main catabolic organelles of a cell and play a pivotal role in a plethora of cellular processes, including responses to nutrient availability and composition, stress resistance, programmed cell death, plasma membrane repair, development, and cell differentiation. In line with this pleiotropic importance for cellular and organismal life and death, lysosomal dysfunction is associated with many age-related pathologies like Parkinson’s and Alzheimer’s disease, as well as with a decline in lifespan. Conversely, targeting lysosomal functional capacity is emerging as a means to promote longevity. Here, we analyze the current knowledge on the prominent influence of lysosomes on aging-related processes, such as their executory and regulatory roles during general and selective macroautophagy, or their storage capacity for amino acids and ions. In addition, we review and discuss the roles of lysosomes as active players in the mechanisms underlying known lifespan-extending interventions like, for example, spermidine or rapamycin administration. In conclusion, this review aims at critically examining the nature and pliability of the different layers, in which lysosomes are involved as a control hub for aging and longevity.
Keywords: Lysosome, aging, autophagy, lifespan, longevity, vacuole
1. Introduction
Lysosomes are found in all animal cell types (except erythrocytes) and represent the cell’s main catabolic organelles. The variety of substrates degraded in the lysosomes is wide, ranging from intracellular macromolecules and organelles to surface receptors and pathogens, among others. To exert their catabolic function, lysosomes contain an extensive set of hydrolases, including proteases, nucleases, lipases, sulfatases or phosphatases, whose pH optima are usually low (pH 4.5-5). Accordingly, their degradative capacity depends on a highly acidic milieu, which is maintained via the activity of a proton-pumping V-type ATPase that pumps protons from the cytoplasm into the lysosomal lumen. However, lysosomes are not mere sites for disposal and processing of cellular waste but also act as pivotal regulators of cell homeostasis at different levels. For instance, they are involved in the regulation of cellular responses to nutrient availability and composition, stress resistance, programmed cell death, plasma membrane repair, development, and cell differentiation, among many others (Braun et al., 2015a, 2015b; Boya, 2012; Settembre et al., 2013b). Thus, lysosomes play a determining role in processes that control cellular and organismal life and death.
Concurring with this pleiotropic importance, lysosomal dysfunction is associated to a plethora of disorders. Among them are those collectively known as lysosomal storage diseases (LSDs), which involve approximately 50 individual rare pathologies, whose combined incidence, however, is estimated to be about 1:5,000 live births (Fuller et al., 2006; Platt et al., 2012). LSDs are characterized by an anomalous accumulation of undigested intra-lysosomal material and each disease is caused by a specific monogenic deficiency, respectively. Mutations most commonly affect acidic hydrolases, but can also be found in non-enzymatic lysosomal proteins (soluble and membrane-bound) and non-lysosomal factors regulating lysosomal function. LSDs typically manifest in neurodegeneration during infancy/childhood and in some milder variants during adulthood (Nixon et al., 2008; Wraith, 2002). Accordingly, though tissue degeneration occurs in different organs (Beltroy et al., 2005), LSD progression most markedly triggers neuronal loss in patients and mouse models (Sleat et al., 2004; Tessitore et al., 2004; Walkley and Suzuki, 2004). In fact, the nervous system seems to be particularly susceptible to defects in lysosomal function (Hara et al., 2006; Komatsu et al., 2006). In addition to LSDs, age-related neurodegenerative disorders like Alzheimer’s or Parkinson’s disease are connected to impaired lysosomal activity through different but interweaved mechanisms that seem to include lysosomal enzyme malfunction, reduced intraluminal acidification or disrupted calcium regulation (Büttner et al., 2013; Jiang and Mizushima, 2014; McBrayer and Nixon, 2013; Menezes et al., 2015; Nixon, 2013; Wolfe et al., 2013). For instance, mutations in glucocerebrosidase (GBA), a lysosomal glucosylceramidase, are associated to an increase in the risk for Parkinson’s Disease (Westbroek et al., 2011). Reduced GBA activity has thereby been linked to increased levels of alpha-synuclein, a small protein, whose abnormal accumulation as aggregates is characteristic of synucleopathies like Parkinson’s Disease (Westbroek et al., 2011). Notably, lysosomal defects disturb the balance between damaged proteins and their proteolytic clearance, ultimately resulting in the accumulation of highly cross-linked aggregates. Accumulation of aggregates in post-mitotic cells appears to be particularly dramatic, since the material cannot be diluted via cell division. Many resulting aggregates of oxidized proteins may further react with cellular components like lipids and metals in different compositions, forming a fluorescent material termed lipofuscin (Jung et al., 2007). The exact cellular effects of lipofuscin are largely hypothetical and still under discussion, but may e.g. involve the production of oxidants, possibly via iron-mediated catalysis of free radicals (Höhn et al., 2010), or the inhibition of the proteasome (Höhn et al., 2011).
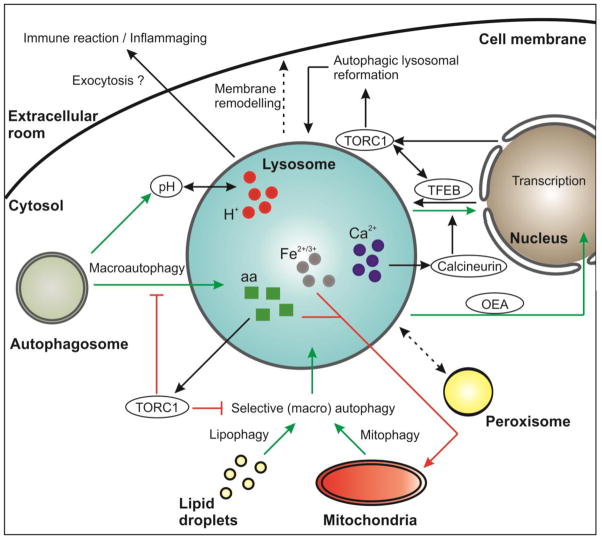
Of note, all the mentioned diseases exhibit a decline in healthspan and/or lifespan. Indeed, the aging process itself may be fueled by a decrease in lysosomal function. This may occur at several molecular levels, according to the multiple functions of the lysosomes and their broad intracellular impact. For instance, lysosomal degradation is tightly connected to the process of autophagy, the intracellular self-digestion machinery that orchestrates the elimination of unwanted or damaged material (e.g. macromolecules, organelles) that accumulates during aging (Klionsky, 2007; Madeo et al., 2015; Nakamura et al., 1997; Pierce et al., 2007; Sampaio-Marques et al., 2014). Thereby, lysosomes are not only the terminal degradation compartments, but are also connected to the autophagic process at the signaling level. For example, they represent a molecular hub controlling the activity of the mammalian target of rapamycin complex 1 (mTORC1) kinase complex, a negative master regulator of autophagy. Also, they are linked to the transcription factor EB (TFEB), which regulates both lysosomal biogenesis and autophagy activation (Settembre et al., 2013b). Besides their central implication in autophagy, lysosomes seem to crucially contribute to lifespan control via a diverse array of other signals as shown in different model organisms. For instance, deployment of active lipid molecules from the lysosomes to the nucleus results in altered gene expression that modulates lifespan in worms (Folick et al., 2015). Another aging-relevant signal might be the intralysosomal pH (Hughes and Gottschling, 2012), which impacts the degradative and storage functions of the lysosome in yeast. In fact, stored amino acids and metals like iron and calcium can significantly influence aging at several levels (Hughes and Gottschling, 2012; Klang et al., 2014; Kurz et al., 2007; Medina et al., 2015; Smaili et al., 2013). In this review, we will analyze the current evidence for these and other lysosome-lifespan connections and discuss the participation and pliability of lysosomal function in the frame of known anti-aging interventions (Figure 1).
Figure 1. Aging-relevant interactions of the lysosome with other cellular organelles.
Interaction of the lysosome with the general and selective (macro) autophagic machinery as well as the lysosomal-nuclear and lysosomal-mitochondrial axis are depicted. Some regulatory interactions of the lysosome with aging-relevant master regulators such as TORC1 and TFEB have feedback-loops that modulate lysosomal morphology and biogenesis. In addition, the lysosome may interact with the extracellular room and/or processes. Molecules are encircled. Longevity-mediating processes are depicted with green arrows whereas negative effects are depicted with red arrows. aa: amino acids; OEA: oleoylethanolamide.
2. Lysosomes and the regulation of autophagy during aging
An organism’s survival depends on its ability to maintain a balance between the production of new and the degradation of old and potentially harmful proteins and cellular structures. This balance is strongly dictated by the catabolic capacity of a cell, which is mainly executed via two distinct processes. On the one hand, the ubiquitin/proteasomal degradation pathway, which functions at the cytosolic level, primarily targets cytosolic proteins, ER proteins following their retrograde transport to the cytosol, and ubiquitinated mitochondrial proteins exposed on the outer membrane of mitochondria (Finley, 2009; Glickman and Ciechanover, 2002; Walter and Ron, 2011). On the other hand, the autophagic machinery degrades a wide array of cytosolic substrates, ranging from single proteins to whole organelles that are delivered to the lysosome for hydrolytic dismantling. Both degradation pathways have been associated to aging control (Carrard et al., 2002; Chondrogianni and Gonos, 2005; Cuervo and Dice, 2000; Kurz et al., 2008; Löw, 2011; Terman et al., 2010). Interestingly, they also seem to interact with each other (Ciechanover, 2005; Korolchuk et al., 2010), even though the regulatory extent of this crosstalk in the frame of lifespan control needs further investigation.
2.1. Lysosomal execution during autophagy
Autophagy can mainly be divided into three subroutines: chaperone-mediated autophagy (CMA), microautophagy and macroautophagy (Singh and Cuervo, 2011). While all of them directly depend on functional lysosomes, the underlying mechanisms are distinct. During CMA, the cytosolic heat shock cognate 70 (hsc70) chaperone and its co-chaperones recognize the consensus motif (KFERQ) in target cytosolic proteins and are transported to the lysosomal membrane. There, lysosome-associated protein type 2A (LAMP-2A) is recognized by this substrate chaperone complex, allowing the target protein to unfold and cross the lysosomal membrane. CMA is thus characterized by selective protein targeting and direct substrate translocation to the lysosomes, which establishes a singular role of this process in diverse pathophysiological conditions, including aging (Cuervo, 2011; Dice, 2007; Martinez-Lopez et al., 2015)(see also this issue). Instead, microautophagy involves the direct invagination of cytosolic material (‘in bulk’ or selectively chosen by chaperones) at the lysosomal membrane (Li et al., 2012). Further studies will be needed to examine how microautophagy relates to aging (Martinez-Lopez et al., 2015; Sahu et al., 2011). Finally, during macroautophagy, the cellular target material is first engulfed in double-membrane-vesicles (autophagosomes) that are transported to the lysosome. There, the autophagosomal outer membrane fuses with the lysosome, and the resulting single-membrane vesicle containing the engulfed material is degraded in the lumen (Xie and Klionsky, 2007). Thereby, this machinery can either act as a selective or as a bulk degradation process. The macroautophagic machinery is highly conserved from the unicellular yeast Saccharomyces cerevisiae, over the nematode Caenorhabditis elegans and the fruitfly Drosophila melanogaster, up to mammals (Ohsumi, 2014). Of importance, rapidly accumulating evidence is revealing macroautophagy (hereafter referred to as autophagy) as a pivotal process in universal lifespan control (de Cabo et al., 2014; Eskelinen and Saftig, 2009; Madeo et al., 2015; Martinez-Lopez et al., 2015; Rajawat et al., 2009; Schroeder et al., 2014; Puleston et al. 2015). In fact, during aging, autophagic rates decline in most organisms examined (Rubinsztein et al., 2011). Conversely, genetic and pharmacological interventions that stimulate autophagy do promote longevity (de Cabo et al., 2014; Madeo et al., 2015, 2010).
Thereby, aging seems to be controlled at regulatory loci along all three different steps of the autophagic process: regulation/initiation, phagophore formation, and cargo loading/degradation (Madeo et al., 2015). Reduced levels or knock-downs in several of the so-called ATG genes, which comprise the core machinery of this multistep autophagic process (Xie and Klionsky, 2007), severely reduce lifespan, or accelerate aging-related pathologies. For example, the two main initiation complexes of autophagy are the ULK1 (also called Unc51 or ATG1) complex and the Beclin-1 (also known as ATG6) complex. ULK1 loss of function mutation in C. elegans as well as reduced expression in D. melanogaster results in decreased lifespan (Simonsen et al., 2008; Tóth et al., 2008). Similarly, Beclin-1 loss of function mutants in C. elegans as well as conditional mice models selectively deleted therein show decreased lifespan and early onset of aging and age-associated pathologies such as cancer (Liang et al., 1999; Tóth et al., 2008). Mice studies on conditional tissue-specific mutants in ATG7 and ATG5, both of which are involved in the second step of autophagy (phagophore assembly), revealed acceleration of age-associated phenomena (Hara et al., 2006; Komatsu et al., 2005). These included lysosomes filled with age-related pigment lipofuscin (Rajawat et al., 2009; Stroikin et al., 2004), accumulation of lipid droplets and defective mitochondria (Kim et al., 2007; Singh et al., 2009), increased protein oxidation and neurodegeneration (Komatsu et al., 2006; Nezis and Stenmark, 2012).
The third step of the autophagic process is intimately related with lysosomal function. It can be divided into three sub-stages: (i) Cargo recognition and loading, (ii) delivery to and fusion with the lysosome, and (iii) lysosomal degradation. A deficiency in these stages is characterized by increased autophagosome formation without increased autophagic flux (Füllgrabe et al., 2013) and tends to be severe and can contribute to neurodegenerative disorders like Huntington’s and Parkinson’s disease (Geisler et al., 2010; Martinez-Vicente et al., 2010). For instance, mutations in p62, which serves as a crucial adaptor involved in cargo recognition, are associated to multiple pathologies, including Paget’s disease of the bone or amyotrophic lateral sclerosis (Martinez-Vicente et al., 2010; Rea et al., 2014). Interestingly, p62 is also subject to phosphorylation events that regulate the antioxidant response pathway Keap1-Nrf2 (Ichimura et al., 2013). It is thus conceivable that the phosphorylation status of p62 might influence autophagy, as well, but this possibility remains to be explored (Martinez-Lopez et al., 2015).
2.2. Lysosomal regulation during autophagy
The effectiveness and general functionality of autophagy directly depends on the capacity of the lysosome to degrade the delivered cargo. Thus, lysosomal function determines the autophagic process as an executory player. However, it is becoming clear that lysosomes also control autophagy at the regulatory level. For instance, the mTORC1 kinase complex, a master regulator of cell and organism growth (Laplante and Sabatini, 2012), has a pivotal role in autophagy control. Upon nutrient availability, mTORC1 inhibits autophagosome biogenesis by directly phosphorylating and thus suppressing the activity of the initiator kinase complex ULK1–ATG13–FIP200 (Ganley et al., 2009; Hara et al., 2008). Accordingly, the starvation- or drug-induced inhibition of mTORC1 promotes autophagy and longevity (de Cabo et al., 2014). Interestingly, mTORC1 exerts its activity on the lysosomal surface (Sancak et al., 2010). Perhaps even more intriguingly, the docking of mTORC1 on its surface seems to be directly controlled by the lysosome itself, more precisely by the level of amino acids present in the lysosomal lumen. In other words: only upon accumulation of amino acids in the lysosomal lumen can mTORC1 dock to the lysosomal surface and become activated (Zoncu et al., 2011). The lysosome further interacts with mTORC1 via a membrane-located, ATP-sensitive Na+ channel (lysoNaATP). This channel is directly involved in nutrient sensing, since release of mTORC1 from the lysosome upon starvation, induces its constitutive activation to regulate amino acid homeostasis and pH stability in response to ATP levels (Cang et al., 2013). In fact, lysosomal pH control determines lifespan (Hughes and Gottschling, 2012; Ruckenstuhl et al., 2014a). In yeast, for instance, methionine restriction positively influences the pH of vacuoles (yeast lysosomes) and extends lifespan in dependence of functional autophagy (Ruckenstuhl et al., 2014a, 2014b). Intriguingly, a major activator of autophagy, the transcription factor TFEB (Lapierre et al., 2013), is also partly located on the lysosomal surface, when it is inactive (phosphorylated and non-nuclear). There, it interacts - among others - with mTORC1 (Settembre et al., 2012). In fact, mTORC1 is one of different kinases that has been shown to inhibit nuclear translocation of TFEB (Martina et al., 2012; Peña-Llopis et al., 2011). In addition, it is worth noting that TFEB underlies an autoregulatory feedback mechanism: it binds to its own promoter and can thus induce its own expression under nutrient-poor conditions (Settembre et al., 2013a).
This intimate connection between autophagy-regulating factors like mTORC1 or TFEB and the lysosome is further evinced by the fact that these same factors vice versa also regulate lysosomal function. For instance, the formation of nascent lysosomes from autolysosomal membranes (the so-called autophagic lysosomal reformation) requires the reactivation of mTORC1 upon prolonged starvation (Yu et al., 2010). TFEB is also involved in lysosomal biogenesis as well as in the expression of lysosomal genes and the control of lysosome numbers within the cell (Sardiello et al., 2009; Settembre et al., 2011). Altogether, the important signaling role of lysosomes in the frame of autophagy regulation is becoming increasingly clear and we expect that further layers of regulation will emerge in upcoming years. Recently, for example, it has been shown that lysosomal lipid composition influences vesicle fusion capacity. In a rodent high-fat-diet model, autophagy was partly inhibited by changes in the lipid composition of autophagosome and lysosomal membranes that interfered with efficient membrane fusion (Koga et al., 2010a, 2010b).
2.3. Different autophagic routes and aging control
The combination of major executors and crucial regulators of autophagy positions lysosomes as a central cellular hub for aging control. This involves a whole range of specific autophagy types that are intimately connected to longevity. Mitophagy, which refers to autophagy-dependent lysosomal degradation of damaged mitochondria, is probably one of the best described and most critical autophagy-variants during aging (Green et al., 2011; Terman et al., 2010; Weber and Reichert, 2010). It confers cytoprotection during senescense by inhibiting the cellular accumulation of deteriorated mitochondria that are prone to release pro-apoptotic factors and detrimental reactive oxygen species (Green et al., 2011). Also the regulated turnover of lipid droplets, lipid storage organelles, is at least partly regulated by a selective type of autophagy, lipophagy (Singh et al., 2009). Lipophagy modulates the energetic status of the cell and its dysfunction is consequently linked to metabolic disorders that may reflect processes of the metabolic syndrome upon aging (Singh and Cuervo, 2012, 2011). Besides selective autophagy pathways, also the autophagic activity in specific cells does impact aging. This is exemplified by liver cells or neuronal cells, where autophagy regulation is of great importance for the progress of aging and thus for longevity control (Carmona-Gutierrez et al., 2015; Schroeder et al., 2015; Singh et al., 2009). Other examples are cells involved in the innate immune response. Here, autophagy impacts aging at several levels, including the modulation of inflammasome activity, cytokine secretion, antigen presentation and lymphocyte function (Cuervo and Macian, 2014; Dengjel et al., 2005; Henault et al., 2012; Ireland and Unanue, 2011; Shi et al., 2012). The diversity in autophagic routes, including non-canonical types of autophagy, and autophagic specificities in single cell types are beginning to be deciphered and will need to be incorporated into the known network to understand the intricate connection between autophagy, lysosomal function and lifespan.
3. Lysosomes, nutrient storage and lifespan control
In addition to the lysosome’s function in cellular degradation pathways, it has become clear in recent years that this organelle also plays a central role in cellular nutrient homeostasis. As discussed above, the lysosome acts as sorting and recycling depot for nutrients such as amino acids during the process of autophagy. In addition to this function, the lysosome also acts as a storage organelle for many different metabolites, most prominently amino acids and ions (Li and Kane, 2009). Storage of nutrients occurs through the action of specific metabolite transporters and pumps localized to the lysosome membrane. Consistent with a prominent role in nutrient storage, the lysosome has recently emerged as a central regulator of nutrient sensing and signaling pathways (Efeyan et al., 2012).
3.1. Lysosomal nutrient storage
The storage function of the lysosome is best characterized in yeast, where it is well known that this organelle stores amino acids and ions at very high levels (Li and Kane, 2009). Storage of nutrients occurs through the action of specific metabolite transporters and pumps localized to the lysosome membrane. These transporters, which have been extensively reviewed elsewhere, exhibit specificity for various ions and amino acids, and predominantly promote uptake of nutrients into the lysosome in a proton-dependent manner (Miseta et al., 1999; Russnak et al., 2001; Sekito et al., 2008; Shimazu et al., 2005). The proton gradient for these transporters is set up by the evolutionarily conserved vacuolar H+-ATPase (V-ATPase), which utilizes ATP to drive protons into the lumen of the lysosome (Forgac, 2007). The nutrient carrier proteome of the yeast vacuole is almost completely catalogued (Blaby-Haas and Merchant, 2014; Miseta et al., 1999; Nass et al., 1997; Russnak et al., 2001; Shimazu et al., 2005), and we have an extensive view of the metabolites stored within the vacuole lumen through metabolite profiling studies (Kitamoto et al., 1988; Wiemken and Durr, 1974). These metabolites include ions and metals such as calcium, sodium, zinc, copper, iron, and manganese; as well as basic and neutral amino acids (Klionsky et al., 1990). Interestingly, acidic amino acids glutamate and aspartate are largely excluded from the lysosomal lumen in yeast. It is not entirely clear why amino acids are so distinctly compartmentalized, but the most likely explanation if that sequestration of certain types of amino acids in lysosomes may protect cells from toxicity (Braun et al., 2015a, 2015b; Klionsky et al., 1990).
Unlike the well-characterized yeast vacuole, our appreciation and understanding of the role of the lysosome in nutrient storage in mammals is only beginning to take shape. The mammalian lysosome also plays critical roles in regulation of metabolites such as calcium, iron, and amino acids (Harms et al., 1981; Kurz et al., 2011; Lloyd-Evans and Platt, 2011). However, the transporters that regulate influx and efflux of these metabolites are not well-characterized (Sagne and Gasnier, 2008). A recent proteome study of the lysosomal membrane identified many putative uncharacterized transporters (Chapel et al., 2013). The vast number of these proteins strongly implies that the storage function of this organelle may be as robust as its yeast counterpart. Because of the lysosome’s role in relaying nutrient signals to the mTOR pathway, the identification and characterization of orphaned lysosomal amino acid transporters is a very active area of research. Along these lines, three exciting studies recently identified an arginine transporter, SLC38A9, on the lysosome surface, and demonstrated a crucial role of this transporter in relaying amino acid status to the mTOR signaling machinery (Jung et al., 2015; Rebsamen et al., 2015; Wang et al., 2015). An exciting era of lysosome biology is upon us, and our understanding of the role of this organelle in cellular metabolism will no doubt significantly change over the next decade.
3.2. Physical and functional mitochondrial-lysosomal connection in lifespan regulation
As our knowledge on the role of the lysosome in cellular metabolism and nutrient storage continues to grow, so does our understanding of how alterations in the function of this organelle may impact cellular lifespan. In fact, lysosomal nutrient storage seems to be a critical regulator of cellular lifespan (Hughes and Gottschling, 2012). Aging yeast cells lose lysosomal acidity, and the resulting limited lifespan is mediated by inhibition of mitochondrial function. Loss of mitochondrial function in this context was attributed to an alteration in the storage of lysosome metabolites, which impair mitochondrial function through an unknown mechanism. The functional connection between lysosomes and mitochondrial was first recognized about 25 years ago (Ohya et al., 1991), and appears to be conserved in mammals as well (Kim et al., 2013). Consistent with this initial observation, it has now been shown that cells lacking subunits of the V-ATPase, or treated with inhibitors of this protein complex have a very short lifespan and exhibit a range of mitochondrial impairments, including alterations in mitochondrial structure, loss of the mitochondrial inner membrane potential, and a failure to import mitochondrial proteins (Dimmer et al., 2002; Hughes and Gottschling, 2012; Merz and Westermann, 2009; Schleit et al., 2013). Moreover, two recent studies identified a physical connection between lysosomes and mitochondria, mediated by a protein-tethering complex called vCLAMP (Elbaz-Alon et al., 2014; Honscher et al., 2014). This complex plays a role in lipid transport between these organelles, and may regulate the exchange of other nutrients as well. These studies highlight the importance of the mitochondria-lysosome connection, which has also been shown to play an important role in many age-related disorders, including neurodegenerative diseases and lysosomal-storage disorders (Nixon et al., 2008).
In the majority of studies examining the crosstalk of these organelles, mitochondrial dysfunction arising from lysosome impairment has been attributed to changes in degradation pathways such as autophagy (Terman et al., 2010). However, the emerging role of the lysosome in nutrient storage and metabolic regulation demands that future studies dissect the relationship between these organelles, and test whether changes in nutrient storage or signaling by lysosomes play a large role in regulating mitochondrial function and lifespan. A significant challenge in these studies is being able to separate distinct lysosome functions such as autophagy and metabolite storage from one another, in order to evaluate the contribution of each of these to lifespan control. Doing this will first require extensive knowledge of the metabolite transporter proteome, so that individual nutrient transporters can be modulated separately from one another. Given the complexity of metabolites stored within this organelle, many nutrients may ultimately contribute to mitochondrial deficits and lifespan regulation upon lysosomal impairment.
A few mechanisms by which impairment of lysosomal nutrient storage may alter mitochondrial function and lifespan are under discussion. The first possibility involves alterations in cytoplasmic amino acid pools. As mentioned above, basic and neutral amino acids are preferentially stored in the lysosome in yeast, likely to prevent toxicity that results from high cytoplasmic levels of these metabolites (Klionsky et al., 1990). Thus, impairment of lysosome function is expected to cause an elevation in cytoplasmic amino acid pools. It was previously shown that high levels of basic amino acids are toxic for cells, and cause generation of ROS as well as impairment of mitochondrial function (R J Braun et al., 2015; Vitiello et al., 2007). In addition, high levels of branched-chain amino acids lead to mitochondrial overload, potentially by import of these metabolites into the mitochondria at high rates, overwhelming mitochondrial catabolic pathways (Newgard et al., 2009; Wellen and Thompson, 2010). Thus, an elevation of cytoplasmic amino acid pools upon lysosome impairment may negatively impact mitochondrial function and lifespan through increased catabolism or production of toxic products by these amino acids. Consistent with this idea, the lysosomal neutral amino acid importer Avt1 was found to regulate mitochondrial function and lifespan in response to changes in lysosome function (Hughes and Gottschling, 2012). This suggests that neutral amino acids may be particularly important in lysosomal control of mitochondrial function, but the underlying mechanism of this connection is currently unclear.
In addition to impacting mitochondrial function through catabolism or toxicity, amino acid changes arising from lysosomal dysfunction may regulate mitochondria function and impact lifespan by affecting nutrient-sensing pathways with known connections to lifespan regulation such as mTORC1 or the Protein Kinase A (PKA) pathway (Hlavatá et al., 2008; Kaeberlein et al., 2005; Kapahi et al., 2010). As described earlier, mTORC1 is localized to the lysosome surface, and senses amino acids through several different mechanisms, some of which involve inside-out signaling through the V-ATPase (Zoncu et al., 2011) as well as the lysosomal arginine transporter SLC38A9 (Jung et al., 2015; Rebsamen et al., 2015; Wang et al., 2015). Inhibition of mTORC1 activity is well known to increase lifespan across a range of organisms, and changes in lysosomal function are known to impact mTORC1 regulation. Current data from mammalian systems suggests that inhibition of V-ATPase prevents activation of mTORC1 by amino acids (Zoncu et al., 2011). Based on this observation alone, one would predict that loss of V-ATPase function might be beneficial for lifespan because it causes mTOR inhibition. However, impairment of V-ATPase negatively impacts lifespan (Hughes and Gottschling, 2012; Schleit et al., 2013), suggesting that loss of V-ATPase activity with age impacts lifespan through mTORC1-independent mechanisms or that the relationship between these systems is more complex in the context of aging. In addition to the TOR pathway, lysosomal function is intricately connected to regulation of the PKA pathway (Bond and Forgac, 2008; Dechant et al., 2010; Hlavatá et al., 2008), which is also important for lifespan regulation. The crosstalk between V-ATPase function and PKA is complex, but is another great candidate for lifespan regulation through changes in lysosomal metabolites.
3.3. The impact of lysosomal ion storage on aging control
In addition to amino acids, two other prominent cellular metabolites widely connected to the lysosome are calcium and iron. It is likely that alteration in the homeostasis of these nutrients impacts lifespan and mitochondrial function upon lysosome impairment in aging organisms. Iron is stored in the lysosome at high levels in yeast, and lysosomal dysfunction leads to cellular iron deficits in both yeast and mammals (Diab and Kane, 2013; Kurz et al., 2011). Changes in cellular iron levels would have a significant impact on mitochondrial function, given the large number of iron requiring enzymes in the mitochondrial respiratory chain (Stehling and Lill, 2013). Furthermore, iron and other metals have been found to accumulate with age in C. elegans, and contribute to lifespan shortening through enhancement of aggregation of many proteins, several of which are mitochondrial proteins (Klang et al., 2014). In this model, addition of a metal chelator was sufficient to extend lifespan, suggesting that metal overload may be a significant regulator of lifespan in normal aging contexts. Like iron, calcium is also critically important for mitochondrial function (Finkel et al., 2015). Loss of lysosome function leads to rapid elevation in cytoplasmic calcium levels in yeast (Ohya et al., 1991), and may impact ER-localized calcium pools in mammals (Penny et al., 2015). Furthermore, elevated intracellular or extracellular levels of calcium shorten lifespan in yeast (Tsubakiyama et al., 2011). Thus, calcium and iron, as well as other ions and metals stored in the lysosome, are strong candidates to influence mitochondrial function and lifespan upon lysosome impairment in aged organisms. While we have focused here largely on the role of these metabolites in the mitochondria-lysosome relationship, it is also likely that they may impact lifespan through non-mitochondrial based pathways as well.
4. Lysosomes and anti-aging interventions
The idea that aging may be coupled to a progressive dysfunction of lysosomes raises the reciprocal question: is it possible to improve healthspan/lifespan by promoting lysosomal function? In fact, genetic overexpression data in diverse model organisms suggests that this is the case. In S. cerevisiae, for instance, overexpression of Pep4, the homolog of lysosomal cathepsin D, extends lifespan during chronological aging. This function is independent of its proteolytic activity and resides in the propeptide of the protein, which exerts anti-necrotic properties (Carmona-Gutiérrez et al., 2011). In C. elegans, the lysosomal acid lipase A LIPL-4 is highly expressed under conditions associated to extended lifespan (Lapierre et al., 2011). In line, overexpression of LIPL-4 promotes longevity in worms via the generation of the fatty acid oleoylethanolamide (OEA), which shuttles to the nucleus triggering the transcriptional activation of target genes (Folick et al., 2015). Also, overexpression of the TFEB homolog in C. elegans (HLH-30) extends lifespan, possibly via induction of autophagy (Lapierre et al., 2013). Interestingly, HLH-30 seems to regulate lysosomal lipolysis together with another transcription factor, MXL-3. While HLH-30 is crucial for lipolytic activity upon starvation, MXL-3 controls nutrient-triggered repression of lipolysis. Accordingly, mxl-3 mutant animals are long-lived (O’Rourke and Ruvkun, 2013). In D. melanogaster, overexpression of N-ethyl-maleimide sensitive fusion protein (NSF1) prevents neurodegeneration in a model of age-related Parkinson's disease, possibly by sustaining trafficking of lysosomal proteases and autophagy (Babcock et al., 2015). Collectively, these examples show the protective potential of increasing lysosomal performance under aging conditions.
Similarly, lysosomal function is connected to most of the proposed anti-aging interventions that can be considered reliable, i.e. such with healthspan and/or lifespan-extending effects that have been validated in several model organisms and have been confirmed by different laboratories (de Cabo et al., 2014). This connection mostly arises from the crucial role of lysosomes in autophagic processing. This is the case for caloric restriction (CR; the reduction in the intake of calories without malnutrition), fasting regimens, and most pharmacological interventions, including the use of spermidine, metformin, resveratrol and rapamycin (de Cabo et al., 2014). The longevity-promoting effects of these approaches are tightly associated to autophagy induction and thereof resulting control of aging-relevant processes like proteostasis and mitochondrial quality control. The mechanisms by which they impact autophagy are diverse, reaching from epigenetic control to sirtuin activation (de Cabo et al., 2014).
For example, spermidine, a naturally occurring polyamine that – with the remarkable exception of centenarians (Pucciarelli et al., 2012) - declines with ongoing age, triggers autophagy through control of nuclear and cytosolic acetylation. In yeast, for instance, spermidine treatment results in histone H3 hypoacetylation possibly via inhibition of acetyltransferases. Thereby, the promoter region of the autophagy-essential gene ATG7 is excluded, allowing its increased expression (Eisenberg et al., 2009). In mice, spermidine injections induce autophagy along with the inhibition of acetylation of cytosolic proteins such as ATG proteins (Morselli et al., 2011). Resveratrol, a polyphenolic compound found in grapes and red wine, for its part, affects a series of stress-related targets, among them the NAD+ dependent deacetylase SIRT1 (Baur and Sinclair, 2006; Lagouge et al., 2006). Intriguingly, SIRT1 is also stimulated by CR, which results in the deacetylation of autophagic proteins and their subsequent activation (Lee et al., 2008). Equally important, activation of autophagy is required for resveratrol-mediated longevity in C. elegans (Morselli et al., 2010).
Some anti-aging interventions also seem to converge in the direct or indirect repression of TOR signaling (Lamming et al., 2013). For instance, rapamycin is a direct mTORC1-inhibitor and thus a potent inducer of autophagy (Li et al., 2014), whereas CR reduces insulin signaling (and IGF-1 levels), which can inactivate protein kinase B (AKT/PKB) and its downstream target mTORC1 (de Cabo et al., 2014; Fontana et al., 2010). As a result, the ULK1 complex is activated via the adenosine monophosphate- activated protein kinase (AMPK), thus promoting autophagosome formation (Rubinsztein et al., 2011). In addition, acetyltransferase MEC-17 is activated, which stimulates the cellular microtubule transport machinery, a prerequisite for effective autophagy (Mackeh et al., 2014). The biguanide metformin, on the other hand, indirectly prevents TOR activity via inhibition of oxidative phosphorylation, which results in an increase of the AMP/ATP ratio and AMPK activation (Dowling et al., 2007). At the same time, metformin can also upregulate REDD1 (REgulated in Development and DNA damage responses 1) and inhibit the Ras-related GTP binding (Rag) GTPases, both of which promote TOR repression (Ben Sahra et al., 2011; Kalender et al., 2010).
The involvement of lysosomes in these interventions probably spans beyond their degradative potential. Through their luminal load of amino acids, lysosomes control mTORC1-docking on their surface, which is a prerequisite for its activity (Zoncu et al., 2011). Thus, this might be another layer of autophagic regulation for mTORC1-related interventions via the lysosomes. In the light of other, emerging lysosomal functions during senescence, new anti-aging interventions may come to the forefront. For instance, dietary supplementation of lysosomally generated OEA promotes longevity in worms (Folick et al., 2015) and may thus be a potential candidate for a dietary anti-aging approach.
A very effective behavioral strategy against aging is exercise, which does not extend lifespan (Mercken et al., 2012) but has multisystemic health benefits (Warburton et al., 2006). Exercise has been shown to induce autophagic flux in muscle (He et al., 2012; Tam and Siu, 2014). Interestingly, recent evidence suggests that exercise-induced autophagy requires the release of lysosomal Ca2+ through the lysosomal calcium channel mucolipin 1 and the subsequent activation of calcineurin, which in turn promotes nuclear translocation of TFEB (Medina et al., 2015). Altogether, it can be concluded that most anti-aging interventions converge in the lysosome at different levels, underlining lysosomal function as an essential and pliable molecular hub for health- and lifespan control.
5. Conclusion
Mounting evidence suggests that a cell’s lifespan is partly determined by lysosomal function (Figure 1). This implies that processes in which lysosomes are generally involved, but which have not been clearly associated to aging yet, might also directly or indirectly modulate longevity. Lysosomal exocytosis, for example, in which lysosomes dock to the cell surface, fuse with the plasma membrane and release their content into the extracellular space, has an important role in membrane repair (Reddy et al., 2001) and may contribute to intracellular regeneration upon cellular senescence. At the same time, lysosomal exocytosis is involved in secretion processes that could interact with aging-related intercellular signals at the tissue and organismal level and/or help alleviate intracellular stress conditions, possibly in cooperation with selective secretion through exosomes (Baixauli et al., 2014; Lehmann et al., 2008). Interestingly, lysosomal exocytosis is modulated by Ca2+ and TFEB (Medina et al., 2011), both of which have regulatory functions during aging.
On the other hand, molecular processes known to impact aging may at least partly do so because they affect lysosomal function. Such processes may engage single components of the cellular network that are involved in lifespan control, including mitochondria, the nucleus, or peroxisomes (Fransen et al., 2013; Green et al., 2011; Terlecky et al., 2006). Intriguingly, lysosomes not only communicate with other organelles in the frame of their autophagic removal. For example, the peroxisome-lysosome interaction does not seem to be restricted to pexophagy. The membranes of both organelles can come in close apposition (without fusion), creating lysosomal-peroxisome membrane contacts (LPMC), which are essential for the cellular trafficking of cholesterol (Chu et al., 2015). Interestingly, cholesterol oxide derivatives (oxysterols) are involved in different aging-relevant processes like redox equilibrium and inflammation. In addition, they have been associated to major age-related pathologies like neurodegenerative and cardiovascular diseases (Poli et al., 2013; Zarrouk et al., 2014). Thus, organelles associated with the generation, transformation and transport of such molecules may strongly influence their impact on aging. The occurrence of membrane tethering sites (microdomains) like LPMCs allows an efficient interplay between organelles (Schrader et al., 2015). Thus, the establishment of microdomains between lysosomes and other organelles may allow signal exchanges that contribute to a dynamic and orchestrated control of aging. Though some remain speculative in their causality, these lysosome-aging connections exemplify the multilayered mechanisms through which lysosomal function may crucially contribute to aging control. Recognizing this potential opens doors not only to further understand the process of aging but also to improve the ravages of time via lysosomal avenues.
Highlights.
Lysosomal processes associated with aging and longevity
Executory and regulatory role of lysosomes for general and selective autophagy
Lifespan control via lysosomal storage functions
Lysosomal role in known anti-aging interventions
Acknowledgments
We are grateful to Andreas Zimmermann for help with figure illustration. We thank BioTechMed-Graz and NAWI Graz for their support. F. M. is grateful to the Austrian Science Fund FWF (Austria) for grants P23490-B12, P24381-B20, P 27893, I1000 and grant ‘SFB Lipotox’ and to BMWFW and the Karl-Franzens University for grant ‘Unkonventionelle Forschung’. A.L.H. is supported by the Searle Scholar Foundation, a Junior Research Grant from the American Federation for Aging Research, and NIH grant AG043095.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babcock DT, Shen W, Ganetzky B. A neuroprotective function of NSF1 sustains autophagy and lysosomal trafficking in Drosophila. Genetics. 2015;199:511–522. doi: 10.1534/genetics.114.172403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baixauli F, López-Otín C, Mittelbrunn M. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol. 2014;5:403. doi: 10.3389/fimmu.2014.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Beltroy EP, Richardson JA, Horton JD, Turley SD, Dietschy JM. Cholesterol accumulation and liver cell death in mice with Niemann-Pick type C disease. Hepatology. 2005;42:886–893. doi: 10.1002/hep.20868. [DOI] [PubMed] [Google Scholar]
- Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, Tanti J-F, Giorgetti-Peraldi S, Bost F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71:4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- Blaby-Haas CE, Merchant SS. Lysosome-related organelles as mediators of metal homeostasis. J Biol Chem. 2014;289:28129–28136. doi: 10.1074/jbc.R114.592618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond S, Forgac M. The Ras/cAMP/protein kinase A pathway regulates glucose-dependent assembly of the vacuolar (H+)-ATPase in yeast. J Biol Chem. 2008;283:36513–36521. doi: 10.1074/jbc.M805232200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya P. Lysosomal function and dysfunction: mechanism and disease. Antioxid Redox Signal. 2012;17:766–774. doi: 10.1089/ars.2011.4405. [DOI] [PubMed] [Google Scholar]
- Braun RJ, Sommer C, Leibiger C, Gentier RJ, Dumit VI, Paduch K, Eisenberg T, Habernig L, Trausinger G, Magnes C, Pieber T, Sinner F, Dengjel J, van Leeuwen FW, Kroemer G, Madeo F. Accumulation of Basic Amino Acids at Mitochondria Dictates the Cytotoxicity of Aberrant Ubiquitin. Cell Rep. 2015a doi: 10.1016/j.celrep.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun RJ, Sommer C, Leibiger C, Gentier RJG, Dumit VI, Paduch K, Eisenberg T, Habernig L, Trausinger G. Modeling non-hereditary mechanisms of Alzheimer disease during apoptosis in yeast. Microb Cell. 2015b;2:136–138. doi: 10.15698/mic2015.04.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner S, Faes L, Reichelt WN, Broeskamp F, Habernig L, Benke S, Kourtis N, Ruli D, Carmona-Gutierrez D, Eisenberg T, D’hooge P, Ghillebert R, Franssens V, Harger A, Pieber TR, Freudenberger P, Kroemer G, Sigrist SJ, Winderickx J, Callewaert G, Tavernarakis N, Madeo F. The Ca2+/Mn2+ ion-pump PMR1 links elevation of cytosolic Ca(2+) levels to α-synuclein toxicity in Parkinson’s disease models. Cell Death Differ. 2013;20:465–477. doi: 10.1038/cdd.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang C, Zhou Y, Navarro B, Seo Y-J, Aranda K, Shi L, Battaglia-Hsu S, Nissim I, Clapham DE, Ren D. mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell. 2013;152:778–90. doi: 10.1016/j.cell.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Gutiérrez D, Bauer MA, Ring J, Knauer H, Eisenberg T, Büttner S, Ruckenstuhl C, Reisenbichler A, Magnes C, Rechberger GN, Birner-Gruenberger R, Jungwirth H, Fröhlich K-U, Sinner F, Kroemer G, Madeo F. The propeptide of yeast cathepsin D inhibits programmed necrosis. Cell Death Dis. 2011;2:e161. doi: 10.1038/cddis.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Gutierrez D, Zimmermann A, Madeo F. A molecular mechanism for lipophagy regulation in the liver. Hepatology. 2015;61:1781–3. doi: 10.1002/hep.27738. [DOI] [PubMed] [Google Scholar]
- Carrard G, Bulteau A-L, Petropoulos I, Friguet B. Impairment of proteasome structure and function in aging. Int J Biochem Cell Biol. 2002;34:1461–74. doi: 10.1016/s1357-2725(02)00085-7. [DOI] [PubMed] [Google Scholar]
- Chapel A, Kieffer-Jaquinod S, Sagne C, Verdon Q, Ivaldi C, Mellal M, Thirion J, Jadot M, Bruley C, Garin J, Gasnier B, Journet A. An extended proteome map of the lysosomal membrane reveals novel potential transporters. Mol Cell Proteomics. 2013;12:1572–1588. doi: 10.1074/mcp.M112.021980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondrogianni N, Gonos ES. Proteasome dysfunction in mammalian aging: steps and factors involved. Exp Gerontol. 2005;40:931–8. doi: 10.1016/j.exger.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Chu B-B, Liao Y-C, Qi W, Xie C, Du X, Wang J, Yang H, Miao H-H, Li B-L, Song B-L. Cholesterol transport through lysosome-peroxisome membrane contacts. Cell. 2015;161:291–306. doi: 10.1016/j.cell.2015.02.019. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. Timeline: Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- Cuervo AM. Chaperone-mediated autophagy: Dice’s “wild” idea about lysosomal selectivity. Nat Rev Mol Cell Biol. 2011;12:535–41. doi: 10.1038/nrm3150. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. When lysosomes get old. Exp Gerontol. 2000;35:119–31. doi: 10.1016/s0531-5565(00)00075-9. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Macian F. Autophagy and the immune function in aging. Curr Opin Immunol. 2014;29:97–104. doi: 10.1016/j.coi.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN, Madeo F. The search for antiaging interventions: from elixirs to fasting regimens. Cell. 2014;157:1515–1526. doi: 10.1016/j.cell.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechant R, Binda M, Lee SS, Pelet S, Winderickx J, Peter M. Cytosolic pH is a second messenger for glucose and regulates the PKA pathway through V-ATPase. EMBO J. 2010;29:2515–2526. doi: 10.1038/emboj.2010.138. emboj2010138 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Müller M, Kreymborg K, Altenberend F, Brandenburg J, Kalbacher H, Brock R, Driessen C, Rammensee H-G, Stevanovic S. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci U S A. 2005;102:7922–7. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diab HI, Kane PM. Loss of vacuolar H+-ATPase (V-ATPase) activity in yeast generates an iron deprivation signal that is moderated by induction of the peroxiredoxin TSA2. J Biol Chem. 2013;288:11366–11377. doi: 10.1074/jbc.M112.419259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice JF. Chaperone-mediated autophagy. Autophagy. 2007;3:295–299. doi: 10.4161/auto.4144. [DOI] [PubMed] [Google Scholar]
- Dimmer KS, Fritz S, Fuchs F, Messerschmitt M, Weinbach N, Neupert W, Westermann B. Genetic basis of mitochondrial function and morphology in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:847–853. doi: 10.1091/mbc.01-12-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling RJO, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med. 2012;18:524–533. doi: 10.1016/j.molmed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Fröhlich K-U, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–14. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- Elbaz-Alon Y, Rosenfeld-Gur E, Shinder V, Futerman AH, Geiger T, Schuldiner M. A dynamic interface between vacuoles and mitochondria in yeast. Dev Cell. 2014;30:95–102. doi: 10.1016/j.devcel.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Eskelinen E-L, Saftig P. Autophagy: A lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta - Mol Cell Res. 2009;1793:664–673. doi: 10.1016/j.bbamcr.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Finkel T, Menazza S, Holmstrom KM, Parks RJ, Liu J, Sun J, Liu J, Pan X, Murphy E. The ins and outs of mitochondrial calcium. Circ Res. 2015;116:1810–1819. doi: 10.1161/CIRCRESAHA.116.305484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D. Recognition and Processing of Ubiquitin-Protein Conjugates by the Proteasome. Annu Rev Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folick A, Oakley HD, Yu Y, Armstrong EH, Kumari M, Sanor L, Moore DD, Ortlund EA, Zechner R, Wang MC. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans. Science (80- ) 2015;347:83–86. doi: 10.1126/science.1258857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- Fransen M, Nordgren M, Wang B, Apanasets O, Van Veldhoven PP. Aging, age-related diseases and peroxisomes. Subcell Biochem. 2013;69:45–65. doi: 10.1007/978-94-007-6889-5_3. [DOI] [PubMed] [Google Scholar]
- Fuller M, Meikle PJ, Hopwood JJ. Epidemiology of lysosomal storage diseases: an overview. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry Disease: Perspectives from 5 Years of FOS. Oxford PharmaGenesis; Oxford: 2006. [PubMed] [Google Scholar]
- Füllgrabe J, Klionsky DJ, Joseph B. Histone post-translational modifications regulate autophagy flux and outcome. Autophagy. 2013;9:1621–3. doi: 10.4161/auto.25803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganley IG, Lam DH, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–31. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Hara T, Takamura A, Kishi C, Iemura S-i, Natsume T, Guan J-L, Mizushima N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol. 2008;181:497–510. doi: 10.1083/jcb.200712064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms E, Gochman N, Schneider JA. Lysosomal pool of free-amino acids. Biochem Biophys Res Commun. 1981;99:830–836. doi: 10.1016/0006-291x(81)91239-0. [DOI] [PubMed] [Google Scholar]
- He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henault J, Martinez J, Riggs JM, Tian J, Mehta P, Clarke L, Sasai M, Latz E, Brinkmann MM, Iwasaki A, Coyle AJ, Kolbeck R, Green DR, Sanjuan MA. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity. 2012;37:986–97. doi: 10.1016/j.immuni.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlavatá L, Nachin L, Jezek P, Nyström T. Elevated Ras/protein kinase A activity in Saccharomyces cerevisiae reduces proliferation rate and lifespan by two different reactive oxygen species-dependent routes. Aging Cell. 2008;7:148–157. doi: 10.1111/j.1474-9726.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- Höhn A, Jung T, Grimm S, Catalgol B, Weber D, Grune T. Lipofuscin inhibits the proteasome by binding to surface motifs. Free Radic Biol Med. 2011;50:585–91. doi: 10.1016/j.freeradbiomed.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Höhn A, Jung T, Grimm S, Grune T. Lipofuscin-bound iron is a major intracellular source of oxidants: role in senescent cells. Free Radic Biol Med. 2010;48:1100–8. doi: 10.1016/j.freeradbiomed.2010.01.030. [DOI] [PubMed] [Google Scholar]
- Honscher C, Mari M, Auffarth K, Bohnert M, Griffith J, Geerts W, van der Laan M, Cabrera M, Reggiori F, Ungermann C. Cellular metabolism regulates contact sites between vacuoles and mitochondria. Dev Cell. 2014;30:86–94. doi: 10.1016/j.devcel.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Hughes AL, Gottschling DE. An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature. 2012;492:261–5. doi: 10.1038/nature11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y, Waguri S, Sou Y-S, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, Hoshii T, Hirao A, Takagi K, Mizushima T, Motohashi H, Lee M-S, Yoshimori T, Tanaka K, Yamamoto M, Komatsu M. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51:618–31. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Ireland JM, Unanue ER. Autophagy in antigen-presenting cells results in presentation of citrullinated peptides to CD4 T cells. J Exp Med. 2011;208:2625–32. doi: 10.1084/jem.20110640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Mizushima N. Autophagy and human diseases. Cell Res. 2014;24:69–79. doi: 10.1038/cr.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Genau HM, Behrends C. Amino Acid-Dependent mTORC1 Regulation by the Lysosomal Membrane Protein SLC38A9. Mol Cell Biol. 2015;35:2479–2494. doi: 10.1128/MCB.00125-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Bader N, Grune T. Lipofuscin: formation, distribution, and metabolic consequences. Ann N Y Acad Sci. 2007;1119:97–111. doi: 10.1196/annals.1404.008. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science (80- ) 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kalender A, Selvaraj A, Kim SY, Gulati P, lé SB, Viollet B, Kemp B, Bardeesy N, Dennis P, Schlager JJ, Marette A, Kozma SC, Thomas G. Metformin, Independent of AMPK, Inhibits mTORC1 In a Rag GTPase-Dependent Manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW-L, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Mendiratta S, Kim J, Pecot CV, Larsen JE, Zubovych I, Seo BY, Kim J, Eskiocak B, Chung H, McMillan E, Wu S, De Brabander J, Komurov K, Toombs JE, Wei S, Peyton M, Williams N, Gazdar AF, Posner BA, Brekken RA, Sood AK, Deberardinis RJ, Roth MG, Minna JD, White MA. Systematic identification of molecular subtype-selective vulnerabilities in non-small-cell lung cancer. Cell. 2013;155:552–566. doi: 10.1016/j.cell.2013.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–53. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto K, Yoshizawa K, Ohsumi Y, Anraku Y. Dynamic aspects of vacuolar and cytosolic amino acid pools of Saccharomyces cerevisiae. J Bacteriol. 1988;170:2683–2686. doi: 10.1128/jb.170.6.2683-2686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klang IM, Schilling B, Sorensen DJ, Sahu AK, Kapahi P, Andersen JK, Swoboda P, Killilea DW, Gibson BW, Lithgow GJ. Iron promotes protein insolubility and aging in C. elegans. Aging (Albany NY) 2014;6:975–991. doi: 10.18632/aging.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. nrm2245 [pii] [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Herman PK, Emr SD. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990;54:266–292. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H, Kaushik S, Cuervo AM. Inhibitory effect of intracellular lipid load on macroautophagy. Autophagy. 2010a;6:825–7. doi: 10.1096/fj.09-144519. [DOI] [PubMed] [Google Scholar]
- Koga H, Kaushik S, Cuervo AM. Altered lipid content inhibits autophagic vesicular fusion. FASEB J. 2010b;24:3052–3065. doi: 10.1096/fj.09-144519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–34. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolchuk VI, Menzies FM, Rubinsztein DC. Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett. 2010;584:1393–8. doi: 10.1016/j.febslet.2009.12.047. [DOI] [PubMed] [Google Scholar]
- Kurz T, Eaton JW, Brunk UT. The role of lysosomes in iron metabolism and recycling. Int J Biochem Cell Biol. 2011;43:1686–1697. doi: 10.1016/j.biocel.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Kurz T, Terman A, Brunk UT. Autophagy, ageing and apoptosis: the role of oxidative stress and lysosomal iron. Arch Biochem Biophys. 2007;462:220–230. doi: 10.1016/j.abb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Kurz T, Terman A, Gustafsson B, Brunk UT. Lysosomes and oxidative stress in aging and apoptosis. Biochim Biophys Acta. 2008;1780:1291–303. doi: 10.1016/j.bbagen.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Sabatini DM, Baur JA. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest. 2013;123:980–989. doi: 10.1172/JCI64099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre LR, De Magalhaes Filho CD, McQuary PR, Chu C-C, Visvikis O, Chang JT, Gelino S, Ong B, Davis AE, Irazoqui JE, Dillin A, Hansen M. The TFEB orthologue HLH-30 regulates autophagy and modulates longevity in Caenorhabditis elegans. Nat Commun. 2013;4:2267. doi: 10.1038/ncomms3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre LR, Gelino S, Meléndez A, Hansen M. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr Biol CB. 2011;21:1507–1514. doi: 10.1016/j.cub.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, Terrian DM. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008;68:7864–7871. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19:373–9. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SC, Kane PM. The yeast lysosome-like vacuole: endpoint and crossroads. Biochim Biophys Acta. 2009;1793:650–663. doi: 10.1016/j.bbamcr.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li J, Bao J. Microautophagy: lesser-known self-eating. Cell Mol Life Sci. 2012;69:1125–36. doi: 10.1007/s00018-011-0865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–6. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, Platt FM. Lysosomal Ca(2+) homeostasis: role in pathogenesis of lysosomal storage diseases. Cell Calcium. 2011;50:200–205. doi: 10.1016/j.ceca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Löw P. The role of ubiquitin-proteasome system in ageing. Gen Comp Endocrinol. 2011;172:39–43. doi: 10.1016/j.ygcen.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Mackeh R, Lorin S, Ratier A, Mejdoubi-Charef N, Baillet A, Bruneel A, Hamaï A, Codogno P, Poüs C, Perdiz D. Reactive oxygen species, AMP-activated protein kinase, and the transcription cofactor p300 regulate α-tubulin acetyltransferase-1 (αTAT-1/MEC-17)-dependent microtubule hyperacetylation during cell stress. J Biol Chem. 2014;289:11816–28. doi: 10.1074/jbc.M113.507400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol. 2010;12:842–846. doi: 10.1038/ncb0910-842. ncb0910-842 [pii] [DOI] [PubMed] [Google Scholar]
- Madeo F, Zimmermann A, Maiuri MC, Kroemer G. Essential role for autophagy in life span extension. J Clin Invest. 2015;125:85–93. doi: 10.1172/JCI73946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8:903–14. doi: 10.4161/auto.19653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lopez N, Athonvarangkul D, Singh R. Autophagy and aging. Adv Exp Med Biol. 2015;847:73–87. doi: 10.1007/978-1-4939-2404-2_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vicente M, Talloczy Z, Wong E, Tang G, Koga H, Kaushik S, de Vries R, Arias E, Harris S, Sulzer D, Cuervo AM. Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nat Neurosci. 2010;13:567–76. doi: 10.1038/nn.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBrayer M, Nixon RA. Lysosome and Calcium Dysregulation in Alzheimer’s Disease – Partners in Crime. Biochem Soc Trans. 2013;41:1495–1502. doi: 10.1042/BST20130201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. 2015;17:288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Fraldi A, Bouche V, Annunziata F, Mansueto G, Spampanato C, Puri C, Pignata A, Martina JA, Sardiello M, Palmieri M, Polishchuk R, Puertollano R, Ballabio A. Transcriptional Activation of Lysosomal Exocytosis Promotes Cellular Clearance. Dev Cell. 2011;21:421–430. doi: 10.1016/j.devcel.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes R, Tenreiro S, Macedo D, Santos CN, Outeiro TF. From the baker to the bedside: yeast models of Parkinson’s disease. Microb Cell. 2015;2:262–279. doi: 10.15698/mic2015.08.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz S, Westermann B. Genome-wide deletion mutant analysis reveals genes required for respiratory growth, mitochondrial genome maintenance and mitochondrial protein synthesis in Saccharomyces cerevisiae. Genome Biol. 2009;10:R95. doi: 10.1186/gb-2009-10-9-r95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miseta A, Kellermayer R, Aiello DP, Fu L, Bedwell DM. The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 1999;451:132–136. doi: 10.1016/s0014-5793(99)00519-0. S0014-5793(99)00519-0 [pii] [DOI] [PubMed] [Google Scholar]
- Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G. The life span-prolonging effect of sirtuin-1 is mediated by autophagy. Autophagy. 2010;6:186–188. doi: 10.4161/auto.6.1.10817. 10817 [pii] [DOI] [PubMed] [Google Scholar]
- Morselli E, Mariño G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, Cabrera S, Bénit P, Rustin P, Criollo A, Kepp O, Galluzzi L, Shen S, Malik SA, Maiuri MC, Horio Y, López-Otín C, Andersen JS, Tavernarakis N, Madeo F, Kroemer G. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol. 2011;192:615–29. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Matsuura A, Wada Y, Ohsumi Y. Acidification of Vacuoles Is Required for Autophagic Degradation in the Yeast, Saccharomyces cerevisiae. J Biochem. 1997;121:338–344. doi: 10.1093/oxfordjournals.jbchem.a021592. [DOI] [PubMed] [Google Scholar]
- Nass R, Cunningham KW, Rao R. Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+-ATPase. Insights into mechanisms of sodium tolerance. J Biol Chem. 1997;272:26145–26152. doi: 10.1074/jbc.272.42.26145. [DOI] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezis IP, Stenmark H. p62 at the interface of autophagy, oxidative stress signaling, and cancer. Antioxid Redox Signal. 2012;17:786–93. doi: 10.1089/ars.2011.4394. [DOI] [PubMed] [Google Scholar]
- Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19:983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Yang D-S, Lee J-H. Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy. 2008;4:590–599. doi: 10.4161/auto.6259. [DOI] [PubMed] [Google Scholar]
- O’Rourke EJ, Ruvkun G. MXL-3 and HLH-30 transcriptionally link lipolysis and autophagy to nutrient availability. Nat Cell Biol. 2013;15:668–676. doi: 10.1038/ncb2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24:9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y, Umemoto N, Tanida I, Ohta A, Iida H, Anraku Y. Calcium-sensitive cls mutants of Saccharomyces cerevisiae showing a Pet- phenotype are ascribable to defects of vacuolar membrane H(+)-ATPase activity. J Biol Chem. 1991;266:13971–13977. [PubMed] [Google Scholar]
- Peña-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC, Wolff NC, Tran TAT, Zou L, Xie X-J, Corey DR, Brugarolas J. Regulation of TFEB and V-ATPases by mTORC1. EMBO J. 2011;30:3242–58. doi: 10.1038/emboj.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny CJ, Kilpatrick BS, Eden ER, Patel S. Coupling acidic organelles with the ER through Ca(2+) microdomains at membrane contact sites. Cell Calcium. 2015;58:387–396. doi: 10.1016/j.ceca.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Pierce SE, Davis RW, Nislow C, Giaever G. Genome-wide analysis of barcoded Saccharomyces cerevisiae gene-deletion mutants in pooled cultures. Nat Protoc. 2007;2:2958–2974. doi: 10.1038/nprot.2007.427. nprot.2007.427 [pii] [DOI] [PubMed] [Google Scholar]
- Platt FM, Boland B, van der Spoel AC. Lysosomal storage disorders: The cellular impact of lysosomal dysfunction. J Cell Biol. 2012;199:723–734. doi: 10.1083/jcb.201208152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G, Biasi F, Leonarduzzi G. Oxysterols in the pathogenesis of major chronic diseases. Redox Biol. 2013;1:125–130. doi: 10.1016/j.redox.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucciarelli S, Moreschini B, Micozzi D, De Fronzo GS, Carpi FM, Polzonetti V, Vincenzetti S, Mignini F, Napolioni V. Spermidine and spermine are enriched in whole blood of nona/centenarians. Rejuvenation Res. 2012;15:590–595. doi: 10.1089/rej.2012.1349. [DOI] [PubMed] [Google Scholar]
- Rajawat YS, Hilioti Z, Bossis I. Aging: central role for autophagy and the lysosomal degradative system. Ageing Res Rev. 2009;8:199–213. doi: 10.1016/j.arr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Rea SL, Majcher V, Searle MS, Layfield R. SQSTM1 mutations--bridging Paget disease of bone and ALS/FTLD. Exp Cell Res. 2014;325:27–37. doi: 10.1016/j.yexcr.2014.01.020. [DOI] [PubMed] [Google Scholar]
- Rebsamen M, Pochini L, Stasyk T, de Araujo ME, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M, Scorzoni S, Filipek PA, Huber KV, Bigenzahn JW, Heinz LX, Kraft C, Bennett KL, Indiveri C, Huber LA, Superti-Furga G. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature. 2015;519:477–481. doi: 10.1038/nature14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. S0092-8674(11)00828-2 [pii] [DOI] [PubMed] [Google Scholar]
- Ruckenstuhl C, Netzberger C, Entfellner I, Carmona-Gutierrez D, Kickenweiz T, Stekovic S, Gleixner C, Schmid C, Klug L, Sorgo AG, Eisenberg T, Büttner S, Mariño G, Koziel R, Jansen-Dürr P, Fröhlich K-U, Kroemer G, Madeo F. Lifespan Extension by Methionine Restriction Requires Autophagy-Dependent Vacuolar Acidification. PLoS Genet. 2014a;10:e1004347. doi: 10.1371/journal.pgen.1004347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckenstuhl C, Netzberger C, Iryna Entfellner I, Didac Carmona-Gutierrez D, Thomas Kickenweiz T, Slaven Stekovic S, Christina Gleixner C, Schmid C, Klug L, Hajnal I, Sorgo AG, Eisenberg T, Büttner S, Mariño G, Koziel R, Magnes C, Sinner F, Pieber TR, Jansen-Dürr P, Fröhlich K-U, Kroemer G, Madeo F. Autophagy extends lifespan via vacuolar acidification. Microb Cell. 2014b;1:160–162. doi: 10.15698/mic2014.05.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russnak R, Konczal D, McIntire SL. A family of yeast proteins mediating bidirectional vacuolar amino acid transport. J Biol Chem. 2001;276:23849–23857. doi: 10.1074/jbc.M008028200. M008028200 [pii] [DOI] [PubMed] [Google Scholar]
- Sagne C, Gasnier B. Molecular physiology and pathophysiology of lysosomal membrane transporters. J Inherit Metab Dis. 2008;31:258–266. doi: 10.1007/s10545-008-0879-9. [DOI] [PubMed] [Google Scholar]
- Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, Potolicchio I, Nieves E, Cuervo AM, Santambrogio L. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20:131–9. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Marques B, Burhans WC, Ludovico P. Longevity pathways and maintenance of the proteome: the role of autophagy and mitophagy during yeast ageing. Microb Cell. 2014;1:118–127. doi: 10.15698/mic2014.04.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–7. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- Schleit J, Johnson SC, Bennett CF, Simko M, Trongtham N, Castanza A, Hsieh EJ, Moller RM, Wasko BM, Delaney JR, Sutphin GL, Carr D, Murakami CJ, Tocchi A, Xian B, Chen W, Yu T, Goswami S, Higgins S, Holmberg M, Jeong KS, Kim JR, Klum S, Liao E, Lin MS, Lo W, Miller H, Olsen B, Peng ZJ, Pollard T, Pradeep P, Pruett D, Rai D, Ros V, Singh M, Spector BL, Vander Wende H, An EH, Fletcher M, Jelic M, Rabinovitch PS, MacCoss MJ, Han JD, Kennedy BK, Kaeberlein M. Molecular mechanisms underlying genotype-dependent responses to dietary restriction. Aging Cell. 2013;12:1050–1061. doi: 10.1111/acel.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M, Godinho LF, Costello JL, Islinger M. The different facets of organelle interplay-an overview of organelle interactions. Front Cell Dev Biol. 2015;3:56. doi: 10.3389/fcell.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder B, Schulze RJ, Weller SG, Sletten AC, Casey Ca, McNiven Ma. The small GTPase Rab7 as a central regulator of hepatocellular lipophagy. Hepatology. 2015;61:1896–907. doi: 10.1002/hep.27667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder S, Zimmermann A, Carmona-Gutierrez D, Eisenberg T, Ruckenstuhl C, Andryushkova A, Pendl T, Harger A, Madeo F. Metabolites in aging and autophagy. Microb Cell. 2014;1:110–114. doi: 10.15698/mic2014.04.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekito T, Fujiki Y, Ohsumi Y, Kakinuma Y. Novel families of vacuolar amino acid transporters. IUBMB Life. 2008;60:519–525. doi: 10.1002/iub.92. [DOI] [PubMed] [Google Scholar]
- Settembre C, De Cegli R, Mansueto G, Saha PK, Vetrini F, Visvikis O, Huynh T, Carissimo A, Palmer D, Jürgen Klisch T, Wollenberg AC, Di Bernardo D, Chan L, Irazoqui JE, Ballabio A. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. 2013a;15:647–658. doi: 10.1038/ncb2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–33. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Fraldi A, Medina DL, Ballabio A. Signals for the lysosome: a control center for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013b;14:283–296. doi: 10.1038/nrm3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, Ballabio A. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012;31:1095–108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C-S, Shenderov K, Huang N-N, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, Kehrl JH. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu M, Sekito T, Akiyama K, Ohsumi Y, Kakinuma Y. A family of basic amino acid transporters of the vacuolar membrane from Saccharomyces cerevisiae. J Biol Chem. 2005;280:4851–4857. doi: 10.1074/jbc.M412617200. M412617200 [pii] [DOI] [PubMed] [Google Scholar]
- Puleston DJ, Simon AK. New roles for autophagy and spermidine in T cells. Microb Cell. 2015;2:91–93. doi: 10.15698/mic2015.03.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13:495–504. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Cuervo AM. Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol. 2012;2012:282041. doi: 10.1155/2012/282041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat DE, Wiseman JA, El-Banna M, Kim K-H, Mao Q, Price S, Macauley SL, Sidman RL, Shen MM, Zhao Q, Passini MA, Davidson BL, Stewart GR, Lobel P. A mouse model of classical late-infantile neuronal ceroid lipofuscinosis based on targeted disruption of the CLN2 gene results in a loss of tripeptidyl-peptidase I activity and progressive neurodegeneration. J Neurosci Off J Soc Neurosci. 2004;24:9117–9126. doi: 10.1523/JNEUROSCI.2729-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaili SS, Pereira GJS, Costa MM, Rocha KK, Rodrigues L, do Carmo LG, Hirata H, Hsu Y-T. The role of calcium stores in apoptosis and autophagy. Curr Mol Med. 2013;13:252–265. doi: 10.2174/156652413804810772. [DOI] [PubMed] [Google Scholar]
- Stehling O, Lill R. The role of mitochondria in cellular iron-sulfur protein biogenesis: mechanisms, connected processes, and diseases. Cold Spring Harb Perspect Biol. 2013;5:a011312. doi: 10.1101/cshperspect.a011312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroikin Y, Dalen H, Lööf S, Terman A. Inhibition of autophagy with 3-methyladenine results in impaired turnover of lysosomes and accumulation of lipofuscin-like material. Eur J Cell Biol. 2004;83:583–90. doi: 10.1078/0171-9335-00433. [DOI] [PubMed] [Google Scholar]
- Tam BT, Siu PM. Autophagic Cellular Responses to Physical Exercise in Skeletal Muscle. Sport Med. 2014;44:625–640. doi: 10.1007/s40279-013-0140-z. [DOI] [PubMed] [Google Scholar]
- Terlecky SR, Koepke JI, Walton PA. Peroxisomes and aging. Biochim Biophys Acta. 2006;1763:1749–1754. doi: 10.1016/j.bbamcr.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid Redox Signal. 2010;12:503–35. doi: 10.1089/ars.2009.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, del Martin PM, Sano R, Ma Y, Mann L, Ingrassia A, Laywell ED, Steindler DA, Hendershot LM, d’Azzo A. GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis. Mol Cell. 2004;15:753–766. doi: 10.1016/j.molcel.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Tóth ML, Sigmond T, Borsos E, Barna J, Erdélyi P, Takács-Vellai K, Orosz L, Kovács AL, Csikós G, Sass M, Vellai T. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy. 2008;4:330–8. doi: 10.4161/auto.5618. [DOI] [PubMed] [Google Scholar]
- Tsubakiyama R, Mizunuma M, Gengyo A, Yamamoto J, Kume K, Miyakawa T, Hirata D. Implication of Ca2+ in the regulation of replicative life span of budding yeast. J Biol Chem. 2011;286:28681–28687. doi: 10.1074/jbc.M111.231415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello SP, Wolfe DM, Pearce DA. Absence of Btn1p in the yeast model for juvenile Batten disease may cause arginine to become toxic to yeast cells. Hum Mol Genet. 2007;16:1007–1016. doi: 10.1093/hmg/ddm046. [DOI] [PubMed] [Google Scholar]
- Walkley SU, Suzuki K. Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim Biophys Acta. 2004;1685:48–62. doi: 10.1016/j.bbalip.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W, Wang T, Bar-Peled L, Zoncu R, Straub C, Kim C, Park J, Sabatini BL, Sabatini DM. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science (80- ) 2015;347:188–194. doi: 10.1126/science.1257132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. C Can Med Assoc J = J l’Association medicale Can. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber TA, Reichert AS. Impaired quality control of mitochondria: aging from a new perspective. Exp Gerontol. 2010;45:503–511. doi: 10.1016/j.exger.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Thompson CB. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell. 2010;40:323–332. doi: 10.1016/j.molcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbroek W, Gustafson AM, Sidransky E. Exploring the link between glucocerebrosidase mutations and parkinsonism. Trends Mol Med. 2011;17:485–93. doi: 10.1016/j.molmed.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemken A, Durr M. Characterization of amino acid pools in the vacuolar compartment of Saccharomyces cerevisiae. Arch Microbiol. 1974;101:45–57. doi: 10.1007/BF00455924. [DOI] [PubMed] [Google Scholar]
- Wolfe DM, Lee J-H, Kumar A, Lee S, Orenstein SJ, Nixon RA. Autophagy failure in Alzheimer’s disease and the role of defective lysosomal acidification. Eur J Neurosci. 2013;37:1949–1961. doi: 10.1111/ejn.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith JE. Lysosomal disorders. Semin Neonatol SN. 2002;7:75–83. doi: 10.1053/siny.2001.0088. [DOI] [PubMed] [Google Scholar]
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–9. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, Hailey DW, Oorschot V, Klumperman J, Baehrecke EH, Lenardo MJ. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–6. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrouk A, Vejux A, Mackrill J, O’Callaghan Y, Hammami M, O’Brien N, Lizard G. Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res Rev. 2014;18:148–162. doi: 10.1016/j.arr.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]