Abstract
ATP sensitive potassium (KATP) channels connect the metabolic and energetic state of cells due to their sensitivity to ATP and ADP concentrations. KATP channels have been identified in multiple tissues and organs of the body including heart, pancreas, vascular smooth muscles and skeletal muscles. These channels are obligatory hetero-octamers and contain four sulfonylurea (SUR) and four potassium inward rectifier (Kir) subunits. Based on the particular type of SUR and Kir present, there are several tissue specific subtypes of KATP channels, each with their own unique set of functions. Recently, KATP channels have been reported in human and mouse ocular tissues. In ex vivo and in vivo model systems, KATP channel openers showed significant ocular hypotensive properties with no appearance of toxic side effects. Additionally, when used in conjunction with known intraocular pressure lowering drugs, an additive effect on IOP reduction was observed. These KATP channel openers have also been reported to protect the retinal ganglion cells during ischemic stress and glutamate induced toxicity suggesting a neuroprotective property for this drug class. Medications that are currently used for treating ocular hypertensive diseases like glaucoma do not directly protect the affected retinal cells, are sometimes ineffective and may show significant side effects. In light of this, KATP channel openers with both ocular hypotensive and neuroprotective properties, have the potential to develop into a new class of glaucoma therapeutics.
Introduction
ATP sensitive potassium (KATP) channels are evolutionarily conserved membrane proteins that connect the metabolic and energetic state of cells by virtue of their sensitivity to micromolar concentrations of intracellular ATP and ADP.(Babenko et al., 1998) KATP channels have been reported in multiple organs and tissues of the body including heart, (Noma, 1983) pancreas, (Ashcroft et al., 1984; Cook and Hales, 1984) brain, (Ashcroft et al., 1984; Ashford et al., 1988) pituitary gland (Bernardi et al., 1993) and skeletal (Spruce et al., 1985) and smooth muscles. (Standen et al., 1989) KATP channels perform a multitude of functions but because of their sensitivity to ATP and ADP concentrations, these channels are often linked to changes in cellular metabolic states such as ischemia and hypoxia. (Rodrigo and Standen, 2005) One of the most well understood functions of KATP channels is in regulation of insulin secretion from the pancreatic beta cells. (Proks and Lippiat, 2006) In other organs such as heart and brain, the functions of KATP channels are mostly protective in nature. (Gross and Auchampach, 1992; Parratt and Kane, 1994; Rodrigo and Standen, 2005)
KATP channels have recently been identified in tissues of the anterior and posterior chambers of human and mouse eyes. (Chowdhury et al., 2013; Roy Chowdhury et al., 2015a) The presence of these channels and the effects of KATP channel openers and closers have suggested a role in intraocular pressure regulation. (Chowdhury et al., 2011; Chowdhury et al., 2013; Roy Chowdhury et al., 2015a) Additionally, KATP channels have been shown to be involved in protecting retinal cells in the presence of ischemic stress or glutamate induced toxicity. (Atlasz et al., 2007; Roth et al., 2006; Roth et al., 2003) In this review we summarize the salient features of KATP channels and highlight recent studies suggesting that pharmacologic openers of KATP channels such as diazoxide, nicorandil and cromakalim may be a new class of ocular hypotensive agents.
Molecular structure of KATP channels
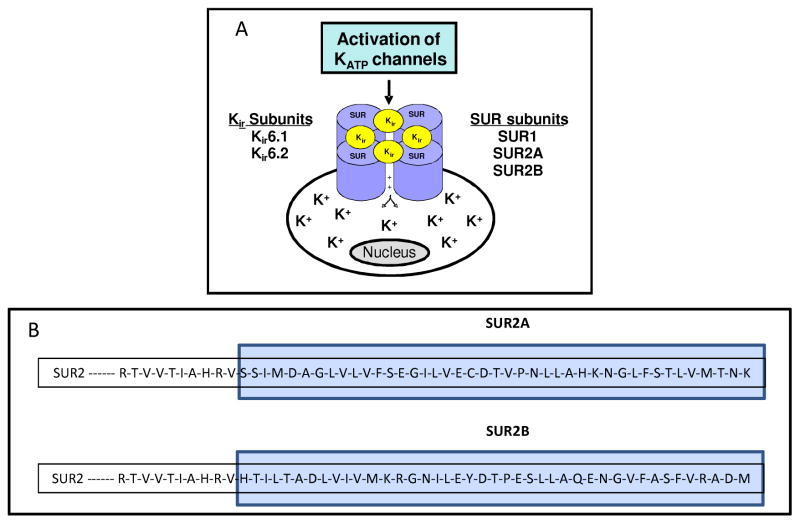
KATP channels are transmembrane octameric proteins formed from two separate subunits, the regulatory sulfonylurea (SUR) subunit and the pore forming potassium inward rectifier (Kir) subunit (Figure 1A). (Aguilar-Bryan et al., 1998; Aguilar-Bryan et al., 1995; Inagaki et al., 1995c) The SUR subunits are coded by two separate genes in humans – SUR1 and SUR2. (Chutkow et al., 1996; Inagaki et al., 1995a) The initial SUR gene (ABCC8) was mapped to chromosome band 11p15.1 and its protein product (SUR1) was identified based on studies utilizing glibenclamide, a sulfonylurea compound that inhibited KATP channel activity. (Aguilar-Bryan et al., 1995) A second SUR gene (ABCC9) was identified at chromosome locus 12p12.1. (Thomas et al., 1995) The ABCC9 gene gives rise to two separate subunits – SUR2A and SUR2B, through alternate splicing. (Chutkow et al., 1996) SUR2A and SUR2B are identical except for the terminal 42 amino acid residues (Figure 1B). (Aguilar-Bryan et al., 1998) Both SUR1 and SUR2 are members of the ATP binding cassette (ABC) transporter superfamily, a common membrane protein found in all species that use energy from ATP hydrolysis to selectively translocate specific molecules across the cell membrane. (Higgins and Linton, 2001; Tucker and Ashcroft, 1998) Similar to other ABC proteins, the SUR subunits contain two nuclear binding domains and two consensus nucleotide binding motifs. (Conti et al., 2001) Mutations in the ABCC8 gene are known to cause hyperinsulinemic hypoglycemia in infants and have been linked to type II diabetes, (Laukkanen et al., 2004; Lohmueller et al., 2003; Meirhaeghe et al., 2001) while mutations in the ABCC9 gene have been identified in sporadic cases of dilated cardiomyopathy, atrial fibrillation and hypertrichotic osteochondrodysplasia. (Bienengraeber et al., 2004; Harakalova et al., 2012; Olson et al., 2007; van Bon et al., 2012)
Figure 1. Molecular structure of KATP channels.
(A) Association of the sulfonylurea receptor (SUR) and the potassium inward rectifying (Kir) subunits in a functional KATP channel. The SURs form the regulatory subunit and are named due to their affinity for sulfonylureas which block the channel. The Kir subunits form the pore forming unit which allows selective translocation of K+ ions based on metabolic states of the cell. Depending on the particular SUR and Kir subunit combination, there can be six different sub-types of KATP channels. These subtypes are tissue specific and have varied specificity towards pharmacologic openers and closers of these channels. (B) SUR2A and SUR2B are both translated from the SUR2 gene and have 99% homology. The two subunits differ only in the last 42 amino acid residues (blue box) in the C-terminus. This is due to alternative splicing of the two 3′ terminal exons of the SUR2 gene.
Initial studies with SUR1 and several known inward rectifiers (e.g. ROMK1, IRK1 etc.) (Ho et al., 1993; Makhina et al., 1994) failed to produce channel activity, (Aguilar-Bryan et al., 1995) suggesting that additional subunits were necessary to form functional KATP channels. This led to the cloning of KCNJ8 (12p11.23) and KCNJ11 (11p15.1), which code for Kir6.1 and Kir6.2. (Inagaki et al., 1996; Inagaki et al., 1995a; Inagaki et al., 1995b; Isomoto et al., 1996; Yamada et al., 1997) Mutations in the KCNJ8 or KCNJ11 genes can lead to impaired cardiac adaptation to stress, heart disease (Gumina et al., 2007; Hodgson et al., 2003; Yamada et al., 2006) or an increased susceptibility to endotoxemia. (Kane et al., 2006) This has been found to be true in both mice and humans. (Hodgson et al., 2003; Kane et al., 2005)
The amino acid sequences of Kir6.1 and Kir6.2 have approximately 70% homology and share around 40–50% similarity with other Kir channels. Like the SUR subunits, Kir6.1 and Kir6.2 failed to yield K+ currents on their own. (Aguilar-Bryan et al., 1995; Inagaki et al., 1995a) However, combination of SUR and Kir subunits forms active KATP channels. (Inagaki et al., 1996) SUR subunits contain nuclear binding domains and possess intrinsic ATPase activity making them the chief regulatory subunits that respond to intracellular metabolic changes. (Olson and Terzic, 2010) The SUR’s nuclear binding domains can also attach to Mg-ADP, subsequently activating the subunits. Therefore, the hydrolysis of Mg-ATP by one of the nuclear binding domains can provide ADP for a separate nuclear binding domain to initiate channel activation, (Ueda et al., 1997; Ueda et al., 1999; Zingman et al., 2001) increasing the probability of maximum channel activation in the absence of ATP. (Babenko et al., 1999) The Kir subunits can also bind with ATP but unlike SUR, they do so in the absence of Mg2+ and with much less affinity than that of a complete channel. (Tanabe et al., 2000; Tanabe et al., 1999) The SUR and Kir subunits interact through various transmembrane domains. (Doyle et al., 1998; Kuo et al., 2003; Nishida and MacKinnon, 2002) Functionally, the Kir subunit is involved in forming the channel pore while the SUR subunit is the main regulatory site, responsible for pharmacologic characteristics of the channels. (Aguilar-Bryan et al., 1995; Inagaki et al., 1995a; Sakura et al., 1995)
Localization and function
Depending on the particular SUR and Kir subunits, there can be a total of 6 different subtypes of KATP channels. These subtypes are often tissue specific and can have characteristic functionalities of their own. (Rodrigo and Standen, 2005) In this section, we briefly describe the subunit composition and associated function of KATP channels in heart, muscles and pancreas where they have been studied in detail. For more information regarding KATP function in other tissues, readers may refer to the reviews by Rodrigo and Standen and Seino and Miki. (Rodrigo and Standen, 2005; Seino and Miki, 2003)
Cardiac tissue
In cardiac tissue, KATP channels are comprised of SUR2A and Kir6.2 subunits. (Ashcroft and Ashcroft, 1990; Inagaki et al., 1996) These channels protect cardiac cells from Ca2+ overload, control cellular relaxation and are vital during ischemic episodes. (Noma, 1983; Ganote, 1983; Nichols and Lederer, 1991)
Vascular smooth muscle
In vascular smooth muscles, KATP channels are composed of SUR2B and Kir6.1 subunits. These channels have less affinity to inhibition by intracellular ATP but respond strongly to nucleoside diphosphates, (Beech et al., 1993; Quayle et al., 1997) likely due to rather static ATP levels in vascular smooth muscle tissues except during extreme metabolic stress. (Rodrigo and Standen, 2005) As a result, ATP inhibition occurs at much lower levels in SUR2B and Kir6.1 containing vascular KATP channels. The vascular KATP channels help in regulating blood flow by sensing various metabolic parameters like pH, adenosine levels and oxygen tension. Therefore it is not surprising that SUR2B and Kir6.1 subunit containing channels play an important role in body homeostasis during periods of high metabolic activity like physical exercise. (Miki et al., 2002) A similar protective function during physical stress is also exhibited by Kir6.2 subunit containing KATP channels of the heart. (Olson and Terzic, 2010; Zingman et al., 2002)
Skeletal muscle
One of the predominant roles of KATP channels in skeletal muscles is to increase vasodilation and formation of nitric oxide, (Marshall, 2000) particularly during fatigue. (Light et al., 1994) These channels are composed of SUR2A and Kir6.2 (Allard and Lazdunski, 1993; Forestier et al., 1996) and their degree of inhibition by ATP is strongly regulated by changes in pH. (Davies et al., 1992) Similar to KATP channels present in other tissues, activation of these channels in skeletal muscles protects cells from excess Ca2+ accumulation, subsequently regulating contractile properties. (Burton and Smith, 1997; Matar et al., 2000; Seino and Miki, 2003) Additionally, studies in Kir6.2−-/−) mice indicate a regulatory role for KATP channels in cellular glucose absorption in skeletal muscles. (Miki et al., 1998)
Pancreas
KATP channels of the pancreas are composed of SUR1 and Kir6.2 subunits (Inagaki et al., 1995a) and play a major role in regulating insulin secretion and subsequently glucose metabolism in the whole body. In pancreatic β cells, inhibition of KATP channels by ATP causes membrane depolarization that generates an associated voltage gated Ca2+ influx. Once the depolarization exceeds a cell specific threshold, (Ashcroft and Rorsman, 2013; Rorsman et al., 2011) insulin is released through exocytosis of intracellular granules. (Schulla et al., 2003) KATP channels have also been reported to affect glucagon secretion by α cells of the pancreas. (Gromada et al., 2004; Shiota et al., 2005) Both gain and loss of function mutations have been identified in the KATP channels of the pancreatic islets. Gain of function mutations in SUR1 or Kir6.2 cause neonatal diabetes mellitus and loss of function mutations in these subunits cause congenital hyperinsulinism where insulin is constitutively secreted despite low levels of blood glucose. (Huopio et al., 2003; Ocal et al., 2011) KATP channel mutations have also been reported as causal for type 2 diabetes mellitus. (Gloyn et al., 2003; Sakura et al., 1995)
Pharmacologic openers and closers of KATP channels
Pharmacologic agents that affect the activation (opening) and inhibition (closing) of KATP channels have played an important role in understanding the functional significance of KATP channels. As such, many of these agents are used clinically to treat a multitude of disorders. KATP channel openers such as diazoxide, nicorandil and cromakalim have been used to treat hypertension, myocardial ischemia, bronchial asthma, urinary incontinence, hyperinsulinism, angina pectoris and some forms of skeletal muscle myopathies.(Hibino et al., 2010) In contrast, KATP channel closers stimulate insulin secretion and therefore pharmacologic closers like glibenclamide are one of the only oral medicines to treat diabetes. More recently, development of KATP channel closers called PNU compounds (PNU-37883A, a morpholinoguanidine and PNU-99963, a cyanoguanidine) have been shown to inhibit the vasodilation and hypotension caused by traditional KATP channel openers. (Khan et al., 1997; Meisheri et al., 1993)
In general, activation of KATP channels leads to membrane hyperpolarization whereas KATP channel closers cause depolarization of the cell membrane. However, KATP channel openers and closers are often specific for the subunit combination and hence have diverse roles based on the involved tissue. This specificity appears to be due to allosteric interactions involving the binding domains of the openers and closers with the nuclear binding domains of the SUR subunits. Diazoxide acts on SUR1 and SUR2B containing channels and can activate SUR2A channels only in the presence of MgADP. (Ashcroft and Gribble, 2000; Hibino et al., 2010; Isomoto et al., 1996; Yamada et al., 1997) Because of its specificity for SUR1 and SUR2B subunits, diazoxide is used to treat uncontrolled insulin secretion in conditions like persistent hyperinsulinemic hypoglycemia in infants. (Hibino et al., 2010) Nicorandil specifically activates SUR2B containing KATP channels and by virtue of its vasodilating actions through vascular smooth muscles, is used to treat angina without affecting the SUR1 containing pancreatic β cells. KATP channel closers glibenclamide and meglitinide block both SUR1 and SUR2 channels with equal efficacy whereas SUR1 selective blockers like tolbutamide have low affinity for SUR2 containing channels. (Gribble and Reimann, 2003; Hibino et al., 2010; Rodrigo and Standen, 2005)
Distribution of KATP channels and their electrophysiological properties in ocular cells and tissues
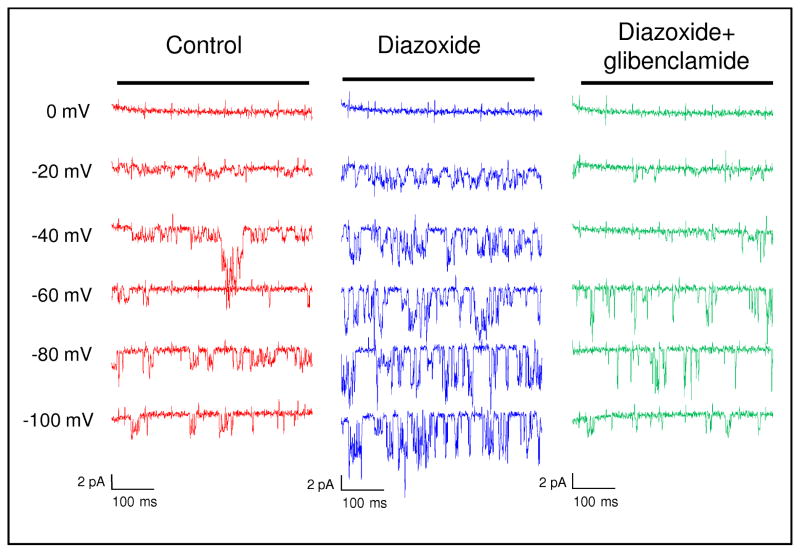
To evaluate the ocular hypotensive properties of KATP channel openers, it was essential to demonstrate the presence of functional KATP channels in the relevant ocular tissues. For this purpose we restricted our search to the trabecular meshwork and tissues of the anterior chamber as the primary regulatory units of the conventional outflow pathway. Patch clamp studies performed on isolated primary normal trabecular meshwork cells from human donors showed that channel opening probability at −60 mV increased from 0.29 ± 0.05 to 0.62 ± 0.05 in the presence of the KATP channel opener diazoxide (Figure 2). Additionally, the current magnitude increased from 1.68 ± 0.29 pA to 3.34 ± 0.35 pA. (Chowdhury et al., 2011) KATP channel activity was attenuated when glibenclamide (KATP channel closer) was added to the cells, confirming the presence of functional KATP channels in normal trabecular meshwork cells (Figure 2).
Figure 2. KATP channel activity in primary trabecular meshwork cells.
Patch clamp recordings show increased K+ conductance across the cell membranes of normal trabecular meshwork primary cells following diazoxide treatment. Channel opening probability in diazoxide treated cells was also increased at −60 mV as indicated by downward deflections. Effect of diazoxide was inhibited by the KATP channel closer glibenclamide. Figure shows representative sweep steps from 0 mV to −100 mV. Figure was previously published in Chowdhury et al., 2011.
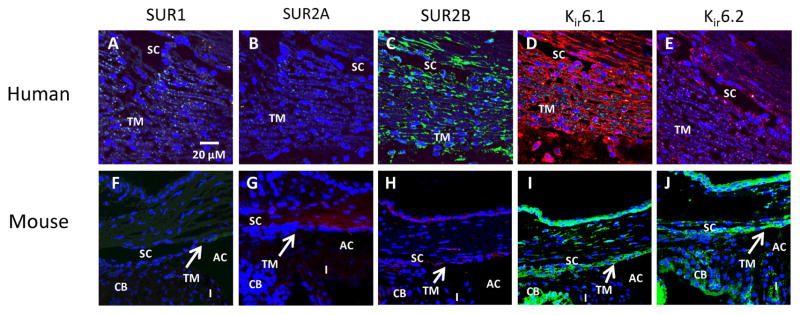
Among the various subunits, SUR2A, SUR2B, Kir6.1 and Kir6.2 mRNAs were identified in human trabecular meshwork tissue as well as primary cultures of normal human trabecular meshwork cells but SUR1 was not detected. (Chowdhury et al., 2011) At the protein level, antibodies for the specific subunits identified SUR2B, Kir6.1 and Kir6.2 in human trabecular meshwork and Schlemm’s canal tissue (Figure 3A–E). In mouse, tissues in the anterior chamber including the cornea, iris, ciliary body, trabecular meshwork and Schlemm’s canal were all positive for SUR2B, Kir6.1 and Kir6.2 (Figure 3F–J). (Chowdhury et al., 2011; Chowdhury et al., 2013) Although SUR2A mRNA was detected, this subunit stained negative in immunohistochemistry of human and mouse ocular tissues (Figure 3B and G). The reason behind this discrepancy is unclear, but could be reasonably explained by the inability of the SUR2A commercial antibody to recognize the subunit or alternatively, SUR2A could be minimally expressed on cell membranes. Nevertheless, it is clear that multiple KATP channel subunits are present in various tissues in human and mouse eyes.
Figure 3. Immunohistochemical localization of KATP channel subunits in tissues of the conventional outflow pathway.
(A–E) In normal human trabecular meshwork tissue, SUR2B, Kir6.1 and Kir6.2 were present while SUR1 and SUR2A were absent. (F–J) In mouse eyes, results similar to human studies were obtained – SUR2B, Kir6.1 and Kir6.2 were present while SUR1 and SUR2A were absent. Some auto-fluorescence was observed with SUR1 and SUR2A antibodies but the overall intensity was much lower than the subunits that stained positive. SUR2B, Kir6.1 and Kir6.2 were evenly distributed in the trabecular meshwork (TM), ciliary body (CB), iris (I) and inner and outer wall of Schlemm’s canal (SC). AC, anterior chamber. Figure reproduced from images published in Chowdhury et al., 2011 and Chowdhury et al., 2013.
KATP channel openers as ocular hypotensive agents
Glaucoma is a progressive neurodegenerative disorder and the leading cause of irreversible blindness worldwide. (Quigley and Broman, 2006; Weinreb et al., 2014) By the year 2040 an estimated 110 million people will be affected by the disease. (Tham et al., 2014) Among various risk factors of glaucoma, elevated IOP is the only one that can be therapeutically modified. As a result, all treatment options for the disease – both pharmacological and surgical – are aimed at lowering IOP. (Heijl et al., 2002) Unfortunately, all current drugs used to treat glaucoma (e.g. prostaglandin analogs, carbonic anhydrase inhibitors, β blockers, etc.) are associated with side effects. (Roy Chowdhury et al., 2015b) Contraindicative symptoms include hypertrichosis, allergic conjunctivitis, hyperemia, blurred vision and even severe systemic cardiovascular episodes, particularly with β-blockers. (Aydin Kurna et al., 2014; Diggory and Franks, 1996; Johnstone, 1997; Mandell et al., 1988; Nguyen, 2014; Roy Chowdhury et al., 2015b; Wistrand et al., 1997) Additionally, no current therapeutics to treat glaucoma specifically targets the trabecular meshwork which is in part due to the incomplete understanding of the disease pathogenesis. (Weinreb et al., 2014; Weinreb and Khaw, 2004) Therefore, searching for novel drugs that can help treat the disease by addressing underlying physiologic events is of particular importance. (Crooke et al., 2012) Over the past five years, our laboratory has published several studies showing a novel ocular hypotensive property of KATP channel openers. (Chowdhury et al., 2011; Chowdhury et al., 2013; Roy Chowdhury et al., 2015a) In the following paragraphs, we review the experimental evidence and discuss the potentials and pitfalls of developing the KATP channel openers into a future therapeutic drug for treating glaucoma.
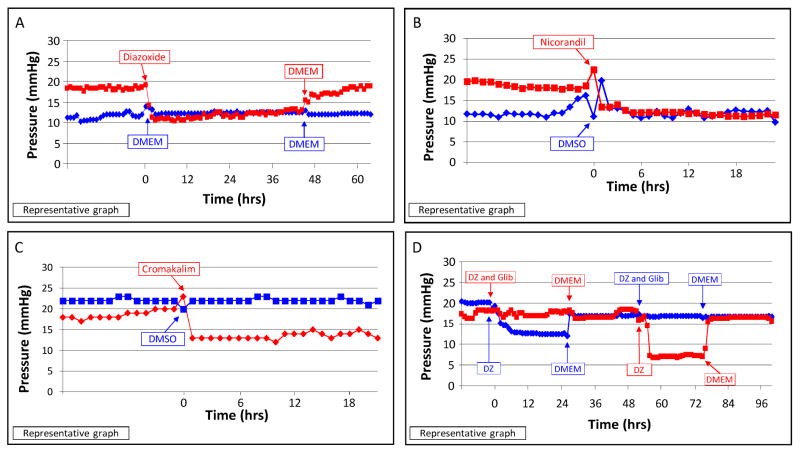
In non-ocular cells, the opening and closing of KATP channels modulates cellular contractility, cell adhesion, gap and tight junction regulation, adaptation to stress (shear, stretch, pressure, and oxidation), and improves overall cell well-being. (Brayden, 2002; Chatterjee et al., 2003; Dal-Secco et al., 2008; Gao et al., 2009; Kane et al., 2004; Kane et al., 2005; Kawamura et al., 2005; Nichols and Lederer, 1991; O’Donnell et al., 1995; Ozcan et al., 2007; Seino and Miki, 2003; Standen et al., 1989; Zingman et al., 2003; Zingman et al., 2002) Interestingly, all these processes have been implicated in altering outflow facility in the eye and therefore in the pathophysiology of glaucoma. However, the role of KATP channels in ocular tissues has never been studied. We reasoned that in light of this functional relevance of KATP channels to the underlying events leading to glaucoma, these channels may also have a role in IOP regulation. To determine whether the opening of KATP channels had an effect on the trabecular meshwork and intraocular pressure regulation, we added KATP channel openers diazoxide, nicorandil and cromakalim to human anterior segment perfusion organ cultures. Diazoxide, nicorandil and cromakalim all showed reduction in pressure (diazoxide, 41%; nicorandil, 35%; cromakalim, 31%; pressure reduction compared to baseline) and outflow facility (80% with diazoxide; 50% for nicorandil; 50% with cromakalim) (Figure 4A–C). (Chowdhury et al., 2011; Chowdhury et al., 2013; Roy Chowdhury et al., 2015a). In comparison, latanoprost free acid, which is the active component of the glaucoma drug xalatan, increased outflow facility by 67% in this model. (Bahler et al., 2008) Pressure reduction with KATP channel openers was reversible and was completely inhibited by the KATP channel closer glibenclamide (Figure 4D).
Figure 4. Effect of KATP channel openers on pressure in human anterior segment perfusion culture.
(A) Diazoxide, (B) nicorandil, and (C) cromakalim significantly lowered pressure in human anterior segment perfusion cultures. (D) Addition of glibenclamide (Glib) inhibited the pressure lowering effect of diazoxide (DZ). DMSO, dimethyl sulfoxide; DMEM, Dulbecco’s Modified Eagle’s Media. Images reproduced from figures published in Chowdhury et al., 2011 and Roy Chowdhury et al, 2015a.
For in vivo assessment, eye drops containing diazoxide or cromakalim were applied topically to one eye of wild type C57BL/6 mice once daily, while the contralateral eye received vehicle. Over a 14-day treatment period, diazoxide lowered IOP by 22% (maximal reduction of 25% on day 7) while cromakalim lowered IOP by 19% following a 5 day treatment period (maximal reduction of 23% on day 5) (Figure 5A and B), (Chowdhury et al., 2011; Chowdhury et al., 2013; Roy Chowdhury et al., 2015a) all slightly better than normotensive mice eyes treated with latanoprost, which produced an 18% IOP reduction when compared to control eyes. (Akaishi et al., 2009; Ota et al., 2005) Histologically, tissues of the conventional outflow pathway appeared normal in both human anterior segment cultures and in mouse eyes following treatment showing intact trabecular beams with viable trabecular meshwork cells and intact Schlemm’s canal inner and outer walls with healthy appearing cells. This suggests that treatment with pharmacologic KATP channel openers appears to be safe since no toxic side effects were observed (Figure 5C and D). (Chowdhury et al., 2011; Chowdhury et al., 2013; Roy Chowdhury et al., 2015a)
Figure 5. KATP channel openers significantly lower IOP in wild type C57BL/6 mice.
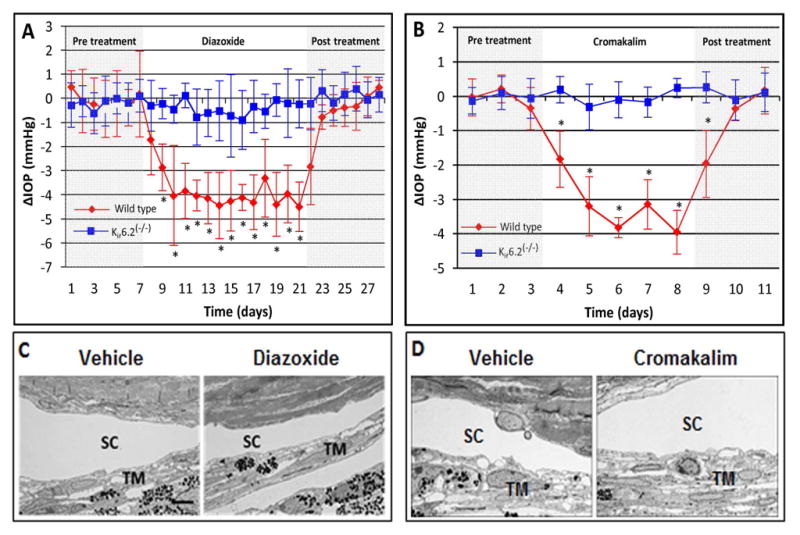
(A) Diazoxide lowered IOP in wild type C57BL/6 mice (red diamonds). However, diazoxide did not lower IOP in Kir6.2(−/−) mice (blue squares). (B) Cromakalim lowered IOP in wild type mice and similar to diazoxide (red diamonds), failed to lower IOP in Kir6.2(−/−) mice (blue squares). (C) Histology of cells and tissues of the conventional outflow pathway in wild type C57BL/6 mice following diazoxide treatment. (D) Histology of cells and tissues of the conventional outflow pathway in wild type C57BL/6 mice following cromakalim treatment. Neither diazoxide nor cromakalim showed toxic side effects in conventional outflow tissues following treatment. SC, Schlemm’s canal; TM, trabecular meshwork. Scale bar in (C) represents 5 μm for images (C) and (D). Average baseline IOP for wild type and Kir6.2(−/−) mice was 15.9 ± 0.6 and 16.5 ± 1.1 mm Hg respectively. *p<0.05. Figures reproduced from images published in Chowdhury et al., 2013 and Roy Chowdhury et al., 2015a.
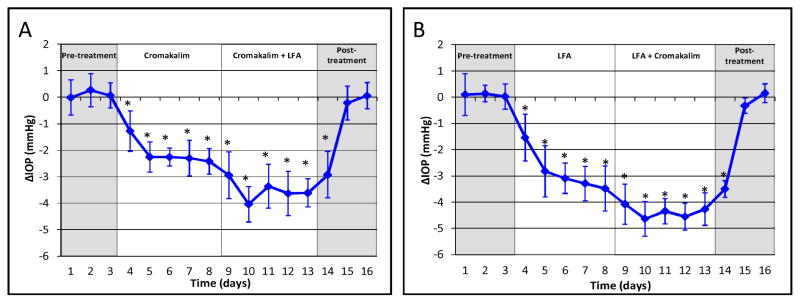
Besides ocular hypotensive activity, the ability of a novel drug to work with existing glaucoma therapeutics to additively lower IOP would be a significant benefit in treating glaucoma. We have shown that cromakalim produces an additive IOP reduction when used in combination with the free acid form of latanoprost. For example, when C57BL/6 wild type mice were treated with both cromakalim and latanoprost, IOP was lowered by an additional 79% compared to cromakalim alone (Figure 6A) and 56% compared to latanoprost alone (Figure 6B). (Roy Chowdhury et al., 2015a) Ongoing studies are defining a novel signaling pathway distinct from latanoprost that is used by KATP channels to lower IOP (manuscript under preparation).
Figure 6. Combination treatment with KATP channel openers and latanoprost free acid (LFA).
(A) Cromakalim and LFA when used in combination therapy in C57BL/6 wild type mice reduced IOP by 79% more than cromakalim alone. (B) Combination treatment of cromakalim and LFA lowered IOP by 56% more than treatment with latanoprost alone. Average baseline IOP for wild-type mice was 16.2 ± 0.3 mm Hg. Figures reproduced from Roy Chowdhury et al., 2015a.
Subunit composition of KATP channels involved in lowering IOP
Since heterogeneity of KATP channels imparts selective specificity to the various pharmacologic openers, it is important to understand the particular subunit composition of the KATP channels involved in lowering IOP. To determine subunit specificity, we obtained Kir6.2(−/−) mice from Dr. Andre Terzic (Mayo Clinic). These mice are phenotypically normal, reproduce and live long lives similarly to wild-type controls. Other than a defective insulin secretion, these mice do not exhibit any major health issues under normal conditions. (Miki et al., 1998; Seino et al., 2000; Zingman et al., 2002) Only during cardiac adaptation in the event of physiologic stress (e.g. excessive physical exercise) do the animals encounter premature cardiac problems. (Zingman et al., 2003; Zingman et al., 2002) In the eye, Kir6.2(−/−) mice looked normal at the levels of morphology and histology. The IOP in Kir6.2(−/−) mice tends to be normal when compared to littermate controls indicating that the Kir6.2 subunit is not required for pressure regulation under homeostatic conditions. (Chowdhury et al., 2013) Treatment of Kir6.2(−/−) mice with diazoxide or cromakalim failed to lower IOP when compared to wild-type mice (Figure 5A and B). This indicates that Kir6.2 subunit containing channels are essential for KATP channel mediated reduction of IOP. Since diazoxide and nicorandil act mainly on SUR2B containing KATP channels and have little or no effect on SUR2A channels, (Lawson, 2000; Yokoshiki et al., 1998; Vivaudou et al, 2010) we concluded that KATP channels containing both Kir6.2 and SUR2B were the essential subunits required for IOP regulation.
KATP channels and neuroprotection of the retina
In addition to the ocular hypotensive effects of diazoxide, nicorandil and cromakalim, several reports have also indicated that KATP channel openers have a neuroprotective function in the eye. Opening of retinal KATP channels by diazoxide mimics ischemic preconditioning by activating downstream transduction of nitric oxide, particularly in the retinal ganglion cells. (Roth et al., 2006) Retinal ischemic preconditioning as seen by diazoxide treatment, can significantly decrease damage to the retina by inducing tolerance to ischemic events. (Roth et al., 1998) Diazoxide has also been shown to protect retinal degeneration caused by glutamate toxicity. (Atlasz et al., 2007) The neuroprotective effects of the KATP channel opener diazoxide is particularly interesting due to the fact that none of the current ocular hypotensive agents used for treating glaucoma has a direct cell protective effect on the retina.
Future direction with KATP channel openers
With excellent ocular hypotensive activity identified for KATP channel openers, the next logical step is to proceed to clinical trials. However, a significant roadblock to using KATP channel openers as a therapeutic for ocular hypertension is the insolubility of commercially available KATP channel openers in therapy friendly aqueous buffers. At present, diazoxide and cromakalim are soluble in dimethyl sulfoxide or other organic solvents which are not appropriate for topical application to human eyes. In addition, nicorandil has vasodilator activity attributed to a nitrate group in its chemical structure, (Nakae et al, 2000) making it a problematic compound to test the hypothesis that KATP channel openers lower IOP clinically in humans. In light of this, our laboratory has developed prodrugs based on the structure of the levo optical isomer of cromakalim. These novel prodrugs are aqueous soluble and on application to mouse eyes, are cleaved to generate the parent compound levcromakalim (unpublished data). The cromakalim prodrugs retain the IOP lowering ability of the parent compound and results are encouraging, with several prodrugs showing similar IOP reduction as the parent compound in mouse and rabbit eyes (unpublished data). Additional studies are underway in our laboratory to understand the IOP lowering abilities of these prodrugs as well as their pharmacokinetic profiles. Once completed, we intend to move one of these compounds forward into phase 1 clinical trials.
Concluding remarks
KATP channels and their pharmacologic openers have important applications in a myriad of physiologic functions. KATP channel openers have been shown to lower IOP in several experimental models including ex vivo human anterior segment cultures and in vivo mouse models. Additionally, other laboratories have shown that KATP channel openers can protect retinal ganglion cells. Existing therapeutic strategies for treating glaucoma are often inadequate; come with unwanted side effects and none have direct neuroprotective properties, despite the fact that glaucoma is principally a disease of the retinal neurons. With the development of aqueous soluble prodrugs, KATP channel openers have a strong potential to become a new class of glaucoma therapeutics that can lower IOP and directly protect the retinal ganglion cells and the optic nerve from neuronal degeneration caused by elevated IOP.
Research Highlights.
ATP sensitive potassium channels are heteroctomeric containing sulfonylurea receptor (SUR) and potassium inward rectifying (Kir) subunits.
ATP sensitive potassium channels are found in many tissues of the body including the trabecular meshwork and retina.
ATP sensitive potassium channel openers diazoxide, nicorandil and cromakalim show ocular hypotensive activity in ex vivo human anterior segment perfusion organ cultures and in vivo in mice.
ATP sensitive potassium channels have direct neuroprotective properties on retinal ganglion cells.
Acknowledgments
The study was supported in part by National Eye Institutes research grant EY 21727; Minnesota Partnership for Biotechnology and Medical Genomics MNP #12.06; Translational Project Development Fund TPDF #15.01; Mayo Foundation, Rochester, MN; and Research to Prevent Blindness, Inc., New York, NY (Department of Ophthalmology, Mayo Clinic is the recipient of an unrestricted grant).
Abbreviations
- KATP channels
ATP sensitive potassium channels
- IOP
Intraocular pressure
- SUR
Sulfonylurea
- Kir
Potassium inward rectifier
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar-Bryan L, Clement JP, 4th, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78:227–245. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, 4th, Boyd AE, 3rd, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the β Cell High-Affinity Sulfonylurea Receptor: A Regulator of Insulin Secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Akaishi T, Odani-Kawabata N, Ishida N, Nakamura M. Ocular hypotensive effects of antiglaucoma agents in mice. J Ocul Pharmacol Ther. 2009;25:401–408. doi: 10.1089/jop.2009.0006. [DOI] [PubMed] [Google Scholar]
- Allard B, Lazdunski M. Pharmacological properties of ATP-sensitive K+ channels in mammalian skeletal muscle cells. Eur J Pharmacol. 1993;236:419–426. doi: 10.1016/0014-2999(93)90480-6. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Gribble FM. New windows on the mechanism of action of K(ATP) channel openers. Trends Pharmacol Sci. 2000;21:439–445. doi: 10.1016/s0165-6147(00)01563-7. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Rorsman P. K(ATP) channels and islet hormone secretion: new insights and controversies. Nat Rev Endocrinol. 2013;9:660–669. doi: 10.1038/nrendo.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft SJ, Ashcroft FM. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2:197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Ashford ML, Sturgess NC, Trout NJ, Gardner NJ, Hales CN. Adenosine-5′-triphosphate-sensitive ion channels in neonatal rat cultured central neurones. Pflugers Archiv. 1988;412:297–304. doi: 10.1007/BF00582512. [DOI] [PubMed] [Google Scholar]
- Atlasz T, Babai N, Reglodi D, Kiss P, Tamas A, Bari F, Domoki F, Gabriel R. Diazoxide is protective in the rat retina against ischemic injury induced by bilateral carotid occlusion and glutamate-induced degeneration. Neurotox Res. 2007;12:105–111. doi: 10.1007/BF03033919. [DOI] [PubMed] [Google Scholar]
- Aydin Kurna S, Acikgoz S, Altun A, Ozbay N, Sengor T, Olcaysu OO. The effects of topical antiglaucoma drugs as monotherapy on the ocular surface: a prospective study. J Ophthalmol. 2014;2014:460483. doi: 10.1155/2014/460483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko AP, Aguilar-Bryan L, Bryan J. A view of sur/KIR6 X, KATP channels. Annu Rev Physiol. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Sulfonylurea receptors set the maximal open probability, ATP sensitivity and plasma membrane density of KATP channels. FEBS Lett. 1999;445:131–136. doi: 10.1016/s0014-5793(99)00102-7. [DOI] [PubMed] [Google Scholar]
- Bahler CK, Howell KG, Hann CR, Fautsch MP, Johnson DH. Prostaglandins increase trabecular meshwork outflow facility in cultured human anterior segments. Am J Ophthalmol. 2008;145:114–119. doi: 10.1016/j.ajo.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ, Zhang H, Nakao K, Bolton TB. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol. 1993;110:573–582. doi: 10.1111/j.1476-5381.1993.tb13849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi H, De Weille JR, Epelbaum J, Mourre C, Amoroso S, Slama A, Fosset M, Lazdunski M. ATP-modulated K+ channels sensitive to antidiabetic sulfonylureas are present in adenohypophysis and are involved in growth hormone release. Proc Natl Acad Sci U S A. 1993;90:1340–1344. doi: 10.1073/pnas.90.4.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O’Cochlain F, Gao F, Karger AB, Ballew JD, Hodgson DM, Zingman LV, Pang YP, Alekseev AE, Terzic A. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet. 2004;36:382–387. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2002;29:312–316. doi: 10.1046/j.1440-1681.2002.03650.x. [DOI] [PubMed] [Google Scholar]
- Burton FL, Smith GL. The effect of cromakalim on intracellular [Ca2+] in isolated rat skeletal muscle during fatigue and metabolic blockade. Exp Physiol. 1997;82:469–483. doi: 10.1113/expphysiol.1997.sp004040. [DOI] [PubMed] [Google Scholar]
- Cecchetti V, Tabarrini O, Sabatini S. From cromakalim to different structural classes of K(ATP) channel openers. Curr Top Med Chem. 2006;6:1049–1068. doi: 10.2174/156802606777323683. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Al-Mehdi AB, Levitan I, Stevens T, Fisher AB. Shear stress increases expression of a KATP channel in rat and bovine pulmonary vascular endothelial cells. Am J Physiol Cell Physiol. 2003;285:C959–967. doi: 10.1152/ajpcell.00511.2002. [DOI] [PubMed] [Google Scholar]
- Chowdhury UR, Bahler CK, Hann CR, Chang M, Resch ZT, Romero MF, Fautsch MP. ATP-sensitive potassium (KATP) channel activation decreases intraocular pressure in the anterior chamber of the eye. Invest Ophthalmol Vis Sci. 2011;52:6435–6442. doi: 10.1167/iovs.11-7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury UR, Holman BH, Fautsch MP. ATP-Sensitive Potassium (KATP) Channel Openers Diazoxide and Nicorandil Lower Intraocular Pressure In Vivo. Invest Ophthalmol Vis Sci. 2013;54:4892–4899. doi: 10.1167/iovs.13-11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutkow WA, Simon MC, Le Beau MM, Burant CF. Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular KATP channels. Diabetes. 1996;45:1439–1445. doi: 10.2337/diab.45.10.1439. [DOI] [PubMed] [Google Scholar]
- Conti LR, Radeke CM, Shyng SL, Vandenberg CA. Transmembrane topology of the sulfonylurea receptor SUR1. J Biol Chem. 2001;276:41270–41278. doi: 10.1074/jbc.M106555200. [DOI] [PubMed] [Google Scholar]
- Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984;311:271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Crooke A, Colligris B, Pintor J. Update in glaucoma medicinal chemistry: emerging evidence for the importance of melatonin analogues. Curr Med Chem. 2012;19:3508–3522. doi: 10.2174/092986712801323234. [DOI] [PubMed] [Google Scholar]
- Dal-Secco D, Cunha TM, Freitas A, Alves-Filho JC, Souto FO, Fukada SY, Grespan R, Alencar NM, Neto AF, Rossi MA, Ferreira SH, Hothersall JS, Cunha FQ. Hydrogen sulfide augments neutrophil migration through enhancement of adhesion molecule expression and prevention of CXCR2 internalization: role of ATP-sensitive potassium channels. J Immunol. 2008;181:4287–4298. doi: 10.4049/jimmunol.181.6.4287. [DOI] [PubMed] [Google Scholar]
- Davies NW, Standen NB, Stanfield PR. The effect of intracellular pH on ATP-dependent potassium channels of frog skeletal muscle. J Physiol. 1992;445:549–568. doi: 10.1113/jphysiol.1992.sp018939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggory P, Franks W. Medical treatment of glaucoma--a reappraisal of the risks. Br J Ophthalmol. 1996;80:85–89. doi: 10.1136/bjo.80.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Forestier C, Pierrard J, Vivaudou M. Mechanism of action of K channel openers on skeletal muscle KATP channels. Interactions with nucleotides and protons. J Gen Physiol. 1996;107:489–502. doi: 10.1085/jgp.107.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganote CE. Contraction band necrosis and irreversible myocardial injury. J Mol Cell Cardiol. 1983;15:67–73. doi: 10.1016/0022-2828(83)90283-3. [DOI] [PubMed] [Google Scholar]
- Gao S, Long CL, Wang RH, Wang H. K(ATP) activation prevents progression of cardiac hypertrophy to failure induced by pressure overload via protecting endothelial function. Cardiovasc Res. 2009;83:444–456. doi: 10.1093/cvr/cvp099. [DOI] [PubMed] [Google Scholar]
- Gloyn AL, Weedon MN, Owen KR, Turner MJ, Knight BA, Hitman G, Walker M, Levy JC, Sampson M, Halford S, McCarthy MI, Hattersley AT, Frayling TM. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes. 2003;52:568–572. doi: 10.2337/diabetes.52.2.568. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875–891. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- Gromada J, Ma X, Hoy M, Bokvist K, Salehi A, Berggren PO, Rorsman P. ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1−/− mouse alpha-cells. Diabetes. 2004;53(Suppl 3):S181–189. doi: 10.2337/diabetes.53.suppl_3.s181. [DOI] [PubMed] [Google Scholar]
- Gross GJ, Auchampach JA. Role of ATP dependent potassium channels in myocardial ischaemia. Cardiovasc Res. 1992;26:1011–1016. doi: 10.1093/cvr/26.11.1011. [DOI] [PubMed] [Google Scholar]
- Gumina RJ, O’Cochlain DF, Kurtz CE, Bast P, Pucar D, Mishra P, Miki T, Seino S, Macura S, Terzic A. KATP channel knockout worsens myocardial calcium stress load in vivo and impairs recovery in stunned heart. Am J Physiol Heart Circ Physiol. 2007;292:H1706–1713. doi: 10.1152/ajpheart.01305.2006. [DOI] [PubMed] [Google Scholar]
- Harakalova M, van Harssel JJ, Terhal PA, van Lieshout S, Duran K, Renkens I, Amor DJ, Wilson LC, Kirk EP, Turner CL, Shears D, Garcia-Minaur S, Lees MM, Ross A, Venselaar H, Vriend G, Takanari H, Rook MB, van der Heyden MA, Asselbergs FW, Breur HM, Swinkels ME, Scurr IJ, Smithson SF, Knoers NV, van der Smagt JJ, Nijman IJ, Kloosterman WP, van Haelst MM, van Haaften G, Cuppen E. Dominant missense mutations in ABCC9 cause Cantu syndrome. Nat Genet. 2012;44:793–796. doi: 10.1038/ng.2324. [DOI] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Higgins CF, Linton KJ. Structural biology The xyz of ABC transporters. Science. 2001;293:1782–1784. doi: 10.1126/science.1065588. [DOI] [PubMed] [Google Scholar]
- Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Hodgson DM, Zingman LV, Kane GC, Perez-Terzic C, Bienengraeber M, Ozcan C, Gumina RJ, Pucar D, O’Coclain F, Mann DL, Alekseev AE, Terzic A. Cellular remodeling in heart failure disrupts K(ATP) channel-dependent stress tolerance. EMBO J. 2003;22:1732–1742. doi: 10.1093/emboj/cdg192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huopio H, Otonkoski T, Vauhkonen I, Reimann F, Ashcroft FM, Laakso M. A new subtype of autosomal dominant diabetes attributable to a mutation in the gene for sulfonylurea receptor 1. Lancet. 2003;361:301–307. doi: 10.1016/S0140-6736(03)12325-2. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JPt, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995a;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Inazawa J, Seino S. cDNA sequence, gene structure, and chromosomal localization of the human ATP-sensitive potassium channel, uKATP-1, gene (KCNJ8) Genomics. 1995b;30:102–104. doi: 10.1006/geno.1995.0018. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Tsuura Y, Namba N, Masuda K, Gonoi T, Horie M, Seino Y, Mizuta M, Seino S. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J Biol Chem. 1995c;270:5691–5694. doi: 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997;124:544–547. doi: 10.1016/s0002-9394(14)70870-0. [DOI] [PubMed] [Google Scholar]
- Kane GC, Behfar A, Yamada S, Perez-Terzic C, O’Cochlain F, Reyes S, Dzeja PP, Miki T, Seino S, Terzic A. ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes. 2004;53(Suppl 3):S169–175. doi: 10.2337/diabetes.53.suppl_3.s169. [DOI] [PubMed] [Google Scholar]
- Kane GC, Lam CF, O’Cochlain F, Hodgson DM, Reyes S, Liu XK, Miki T, Seino S, Katusic ZS, Terzic A. Gene knockout of the KCNJ8-encoded Kir6.1 K(ATP) channel imparts fatal susceptibility to endotoxemia. FASEB J. 2006;20:2271–2280. doi: 10.1096/fj.06-6349com. [DOI] [PubMed] [Google Scholar]
- Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005;38:937–943. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Kadosaki M, Nara N, Wei J, Endo S, Inada K. Nicorandil attenuates NF-kappaB activation, adhesion molecule expression, and cytokine production in patients with coronary artery bypass surgery. Shock. 2005;24:103–108. doi: 10.1097/01.shk.0000168874.83401.3f. [DOI] [PubMed] [Google Scholar]
- Khan SA, Higdon NR, Hester JB, Meisheri KD. Pharmacological characterization of novel cyanoguanidines as vascular KATP channel blockers. J Pharm Exp Therap. 1997;283:1207–1213. [PubMed] [Google Scholar]
- Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- Laukkanen O, Pihlajamaki J, Lindstrom J, Eriksson J, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Tuomilehto J, Uusitupa M, Laakso M. Polymorphisms of the SUR1 (ABCC8) and Kir6.2 (KCNJ11) genes predict the conversion from impaired glucose tolerance to type 2 diabetes. The Finnish Diabetes Prevention Study. J Clin Endo Met. 2004;89:6286–6290. doi: 10.1210/jc.2004-1204. [DOI] [PubMed] [Google Scholar]
- Lawson K. Potassium channel openers as potential therapeutic weapons in ion channel disease. Kidney Int. 2000;57:838–845. doi: 10.1046/j.1523-1755.2000.00923.x. [DOI] [PubMed] [Google Scholar]
- Light PE, Comtois AS, Renaud JM. The effect of glibenclamide on frog skeletal muscle: evidence for K+ATP channel activation during fatigue. J Physiol. 1994;475:495–507. doi: 10.1113/jphysiol.1994.sp020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33:177–182. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- Makhina EN, Kelly AJ, Lopatin AN, Mercer RW, Nichols CG. Cloning and expression of a novel human brain inward rectifier potassium channel. J Biol Chem. 1994;269:20468–20474. [PubMed] [Google Scholar]
- Mandell AI, Bruce LA, Khalifa MA. Reduced cyclic myopia with pilocarpine gel. Ann Ophthalmol. 1988;20:133–135. [PubMed] [Google Scholar]
- Marshall JM. Adenosine and muscle vasodilatation in acute systemic hypoxia. Acta Physiol Scand. 2000;168:561–573. doi: 10.1046/j.1365-201x.2000.00709.x. [DOI] [PubMed] [Google Scholar]
- Matar W, Nosek TM, Wong D, Renaud J. Pinacidil suppresses contractility and preserves energy but glibenclamide has no effect during muscle fatigue. Am J Physiol Cell Physiol. 2000;278:C404–416. doi: 10.1152/ajpcell.2000.278.2.C404. [DOI] [PubMed] [Google Scholar]
- Meirhaeghe A, Helbecque N, Cottel D, Arveiler D, Ruidavets JB, Haas B, Ferrieres J, Tauber JP, Bingham A, Amouyel P. Impact of sulfonylurea receptor 1 genetic variability on non-insulin-dependent diabetes mellitus prevalence and treatment: a population study. Am J Med Genet. 2001;101:4–8. doi: 10.1002/ajmg.1297. [DOI] [PubMed] [Google Scholar]
- Meisheri KD, Humphrey SJ, Khan SA, Cipkus-Dubray LA, Smith MP, Jones AW. 4-morpholinecarboximidine-N-1-adamantyl-N′-cyclohexylhydrochloride (U-37883A): pharmacological characterization of a novel antagonist of vascular ATP-sensitive K+ channel openers. J Pharm Exp Therap. 1993;266:655–665. [PubMed] [Google Scholar]
- Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- Nakae I, Matsumoto T, Horie H, Yokohama H, Omura T, Minai K, Matsui T, Nozawa M, Takahashi M, Sugimoto Y, Ito M, Izumi M, Nakamura Y, Mitsunami K, Kinoshita M. Effects of Intravenous Nicorandil on Coronary Circulation in Humans: Plasma Concentration and Action Mechanism. J Cardio Pharm. 2000;35:919–925. doi: 10.1097/00005344-200006000-00014. [DOI] [PubMed] [Google Scholar]
- Nguyen QH. Combination of brinzolamide and brimonidine for glaucoma and ocular hypertension: critical appraisal and patient focus. Patient Prefer Adherence. 2014;8:853–864. doi: 10.2147/PPA.S53162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991;261:H1675–1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- Nishida M, MacKinnon R. Structural basis of inward rectification: cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 A resolution. Cell. 2002;111:957–965. doi: 10.1016/s0092-8674(02)01227-8. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- O’Donnell ME, Brandt JD, Curry FR. Na-K-Cl cotransport regulates intracellular volume and monolayer permeability of trabecular meshwork cells. Am J Physiol. 1995;268:C1067–1074. doi: 10.1152/ajpcell.1995.268.4.C1067. [DOI] [PubMed] [Google Scholar]
- Ocal G, Flanagan SE, Hacihamdioglu B, Berberoglu M, Siklar Z, Ellard S, Savas Erdeve S, Okulu E, Akin IM, Atasay B, Arsan S, Yagmurlu A. Clinical characteristics of recessive and dominant congenital hyperinsulinism due to mutation(s) in the ABCC8/KCNJ11 genes encoding the ATP-sensitive potasium channel in the pancreatic beta cell. J Ped Endo Met. 2011;24:1019–1023. doi: 10.1515/jpem.2011.347. [DOI] [PubMed] [Google Scholar]
- Olson TM, Alekseev AE, Moreau C, Liu XK, Zingman LV, Miki T, Seino S, Asirvatham SJ, Jahangir A, Terzic A. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2007;4:110–116. doi: 10.1038/ncpcardio0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson TM, Terzic A. Human K(ATP) channelopathies: diseases of metabolic homeostasis. Pflugers Archiv : Eur J Physiol. 2010;460:295–306. doi: 10.1007/s00424-009-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota T, Murata H, Sugimoto E, Aihara M, Araie M. Prostaglandin analogues and mouse intraocular pressure: effects of tafluprost, latanoprost, travoprost, and unoprostone, considering 24-hour variation. Invest Ophthalmol Vis Sci. 2005;46:2006–2011. doi: 10.1167/iovs.04-1527. [DOI] [PubMed] [Google Scholar]
- Ozcan C, Terzic A, Bienengraeber M. Effective pharmacotherapy against oxidative injury: alternative utility of an ATP-sensitive potassium channel opener. J Cardiovasc Pharmacol. 2007;50:411–418. doi: 10.1097/FJC.0b013e31812378df. [DOI] [PubMed] [Google Scholar]
- Parratt JR, Kane KA. KATP channels in ischaemic preconditioning. Cardiovasc Res. 1994;28:783–787. doi: 10.1093/cvr/28.6.783. [DOI] [PubMed] [Google Scholar]
- Proks P, Lippiat JD. Membrane ion channels and diabetes. Curr Pharm Des. 2006;12:485–501. doi: 10.2174/138161206775474431. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo GC, Standen NB. ATP-sensitive potassium channels. Curr Pharm Des. 2005;11:1915–1940. doi: 10.2174/1381612054021015. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Eliasson L, Kanno T, Zhang Q, Gopel S. Electrophysiology of pancreatic beta-cells in intact mouse islets of Langerhans. Prog Biophys Mol Biol. 2011;107:224–235. doi: 10.1016/j.pbiomolbio.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Roth S, Dreixler JC, Shaikh AR, Lee KH, Bindokas V. Mitochondrial potassium ATP channels and retinal ischemic preconditioning. Invest Ophthalmol Vis Sci. 2006;47:2114–2124. doi: 10.1167/iovs.05-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, Li B, Rosenbaum PS, Gupta H, Goldstein IM, Maxwell KM, Gidday JM. Preconditioning provides complete protection against retinal ischemic injury in rats. Invest Ophthalmol Vis Sci. 1998;39:777–785. [PubMed] [Google Scholar]
- Roth S, Shaikh AR, Hennelly MM, Li Q, Bindokas V, Graham CE. Mitogen-activated protein kinases and retinal ischemia. Invest Ophthalmol Vis Sci. 2003;44:5383–5395. doi: 10.1167/iovs.03-0451. [DOI] [PubMed] [Google Scholar]
- Roy Chowdhury U, Bahler CK, Holman BH, Dosa PI, Fautsch MP. Ocular Hypotensive Effects of the ATP-Sensitive Potassium Channel Opener Cromakalim in Human and Murine Experimental Model Systems. PLoS One. 2015a;10:e0141783. doi: 10.1371/journal.pone.0141783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Chowdhury U, Hann CR, Stamer WD, Fautsch MP. Aqueous humor outflow: dynamics and disease. Invest Ophthalmol Vis Sci. 2015b;56:2993–3003. doi: 10.1167/iovs.15-16744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakura H, Ammala C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- Schulla V, Renstrom E, Feil R, Feil S, Franklin I, Gjinovci A, Jing XJ, Laux D, Lundquist I, Magnuson MA, Obermuller S, Olofsson CS, Salehi A, Wendt A, Klugbauer N, Wollheim CB, Rorsman P, Hofmann F. Impaired insulin secretion and glucose tolerance in beta cell-selective Ca(v)1.2 Ca2+ channel null mice. EMBO J. 2003;22:3844–3854. doi: 10.1093/emboj/cdg389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebille S, De Tullio P, Boverie S, Antoine MH, Lebrun P, Pirotte B. Recent developments in the chemistry of potassium channel activators: the cromakalim analogs. Curr Med Chem. 2004;11:1213–1222. doi: 10.2174/0929867043365378. [DOI] [PubMed] [Google Scholar]
- Seino S, Iwanaga T, Nagashima K, Miki T. Diverse roles of K(ATP) channels learned from Kir6.2 genetically engineered mice. Diabetes. 2000;49:311–318. doi: 10.2337/diabetes.49.3.311. [DOI] [PubMed] [Google Scholar]
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- Shiota C, Rocheleau JV, Shiota M, Piston DW, Magnuson MA. Impaired glucagon secretory responses in mice lacking the type 1 sulfonylurea receptor. Am J Physiol Endocrinol Metab. 2005;289:E570–577. doi: 10.1152/ajpendo.00102.2005. [DOI] [PubMed] [Google Scholar]
- Spruce AE, Standen NB, Stanfield PR. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985;316:736–738. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Tucker SJ, Ashcroft FM, Proks P, Kioka N, Amachi T, Ueda K. Direct photoaffinity labeling of Kir6.2 by [gamma-(32)P]ATP-[gamma]4-azidoanilide. Biochem Biophys Res Commun. 2000;272:316–319. doi: 10.1006/bbrc.2000.2780. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Tucker SJ, Matsuo M, Proks P, Ashcroft FM, Seino S, Amachi T, Ueda K. Direct photoaffinity labeling of the Kir6.2 subunit of the ATP-sensitive K+ channel by 8-azido-ATP. J Biol Chem. 1999;274:3931–3933. doi: 10.1074/jbc.274.7.3931. [DOI] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmol. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Tucker SJ, Ashcroft FM. A touching case of channel regulation: the ATP-sensitive K+ channel. Curr Opin Neurobiol. 1998;8:316–320. doi: 10.1016/s0959-4388(98)80055-x. [DOI] [PubMed] [Google Scholar]
- Ueda K, Inagaki N, Seino S. MgADP antagonism to Mg2+-independent ATP binding of the sulfonylurea receptor SUR1. J Biol Chem. 1997;272:22983–22986. doi: 10.1074/jbc.272.37.22983. [DOI] [PubMed] [Google Scholar]
- Ueda K, Komine J, Matsuo M, Seino S, Amachi T. Cooperative binding of ATP and MgADP in the sulfonylurea receptor is modulated by glibenclamide. Proc Natl Acad Sci U S A. 1999;96:1268–1272. doi: 10.1073/pnas.96.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bon BW, Gilissen C, Grange DK, Hennekam RC, Kayserili H, Engels H, Reutter H, Ostergaard JR, Morava E, Tsiakas K, Isidor B, Le Merrer M, Eser M, Wieskamp N, de Vries P, Steehouwer M, Veltman JA, Robertson SP, Brunner HG, de Vries BB, Hoischen A. Cantu syndrome is caused by mutations in ABCC9. Am J Hum Genet. 2012;90:1094–1101. doi: 10.1016/j.ajhg.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivaudou M, Moreau C, Terzic A. ATP-sensitive K+ Channels. In: Kew JN, Davies CH, editors. Ion Channels: from Structure to Function. Oxford University Press; Oxford: 2010. pp. 454–473. [Google Scholar]
- Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol. 1997;41(Suppl 2):S129–138. doi: 10.1016/s0039-6257(97)80020-3. [DOI] [PubMed] [Google Scholar]
- Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol. 1997;499(Pt 3):715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Kane GC, Behfar A, Liu XK, Dyer RB, Faustino RS, Miki T, Seino S, Terzic A. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J Physiol. 2006;577:1053–1065. doi: 10.1113/jphysiol.2006.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. Am J Physiol. 1998;274:C25–37. doi: 10.1152/ajpcell.1998.274.1.C25. [DOI] [PubMed] [Google Scholar]
- Zingman LV, Alekseev AE, Bienengraeber M, Hodgson D, Karger AB, Dzeja PP, Terzic A. Signaling in channel/enzyme multimers: ATPase transitions in SUR module gate ATP-sensitive K+ conductance. Neuron. 2001;31:233–245. doi: 10.1016/s0896-6273(01)00356-7. [DOI] [PubMed] [Google Scholar]
- Zingman LV, Hodgson DM, Alekseev AE, Terzic A. Stress without distress: homeostatic role for K(ATP) channels. Mol Psychiatry. 2003;8:253–254. doi: 10.1038/sj.mp.4001323. [DOI] [PubMed] [Google Scholar]
- Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]