Abstract
Objective
To describe the methodology and report primary outcomes of an exploratory randomized clinical trial (RCT) of aerobic training for management of prolonged symptoms after mild traumatic brain injury (mTBI) in adolescents.
Setting
Outpatient research setting
Participants
Thirty adolescents between the ages of 12 and 17 years who sustained a mTBI and had between four and 16 weeks of persistent symptoms.
Design
Partially blinded, pilot RCT of sub-symptom exacerbation aerobic training compared to a full-body stretching program.
Main Measures
The primary outcome was post injury symptom improvement assessed by the adolescent’s self-reported Post Concussion Symptom Inventory (PCSI) repeated for at least six weeks of the intervention. Parent-reported PCSI and adherence are also described.
Results
Twenty-two percent of eligible participants enrolled in the trial. Repeated measures Analysis of Variance via mixed model analysis demonstrated a significant group by time interaction with self-reported PCSI ratings, indicating a greater rate of improvement in the sub-symptom exacerbation aerobic training compared to the full-body stretching group (F-value = 4.11, p-value = .044). Adherence to the home exercise programs was lower in the sub-symptom exacerbation aerobic training compared to the full-body stretching group (mean (SD) times per week = 4.42 (1.95) versus 5.85 (1.37), p < .0001) over the duration of the study.
Conclusion
Findings from this exploratory randomized clinical trial suggest sub-symptom exacerbation aerobic training is potentially beneficial for adolescents with persistent symptoms after mTBI. These findings and other recent research support the potential benefit of active rehabilitation programs for adolescents with persistent symptoms after mTBI. Larger replication studies are needed to verify findings and improve generalizability. Future work should focus on determining the optimal type, timing, and intensity of active rehabilitation programs and characteristics of individuals most likely to benefit.
Keywords: mild traumatic brain injury, concussion, adolescents, aerobic training, exercise, intervention
Introduction
Pediatric traumatic brain injury (TBI) is among the most common causes of acquired morbidity and mortality in children.1–3 There are an estimated 3.8 million sports/recreation-related mTBIs occurring each year in the United States.4 Approximately, 75–85% of these injuries are mild TBIs (mTBI) or concussions.1 Although most individuals recover within two to four weeks after mTBI, an estimated 10–33% of individuals have persistent symptoms beyond one to three months after injury.5–8 Because of the high incidence of mTBI and the unrecognized potential long-term consequences, mTBIs are recognized as a serious public health problem.8–10 There is a critical need to develop evidence-based interventions for children and adolescents after mTBI, especially for individuals with persistent symptoms.
Physiology of symptoms after mTBI is complex. Cortical, neurochemical, metabolic, cerebral blood flow, and mitochondrial dysfunction have been associated with the sequelae of mTBI;11–25 however, dysregulation in cerebral metabolism and cerebral blood flow may largely explain the sequelae. Therefore, interventions that improve or normalize cerebral metabolic function and blood flow would potentially be an effective treatment for persistent symptoms after mTBI.
Exercise is purported to improve cognition through improved cerebral blood flow, oxygen extraction, brain metabolism and neuroplasticity.26–37 These same positive biologic effects of exercise are thought to be beneficial and aid in recovery after TBI.38,39 However, studies have revealed conflicting results with regard to the benefits and potential detrimental effects of exercise after mTBI. In animal models of mTBI, exercise correlates with growth factor upregulation and improved performance on memory tasks.40 The timing of exercise after injury is also important.41,42 Exercise more than 14 days after injury is beneficial, while exercise introduced within six days after injury was detrimental in animal models.42 These findings highlight the potential for exercise to be beneficial for recovery after mTBI; however, the optimal intensity and timing for introduction of exercise following injury is still unknown.43
Based on the potential risk for repeat injury and the potential risk of physical or cognitive activities worsening symptoms and slowing recovery, physical and cognitive rest are commonly recommended after mTBI.44–46 On the contrary, prolonged rest may be detrimental to recovery47 and the introduction of activity at an optimal time after injury may accelerate recovery.39 Recent research suggests that recommendations of five versus one to two days of rest is associated with longer persistence of symptoms.48 Furthermore, aerobic exercise performed at an intensity and duration that does not exacerbate symptoms (i.e., sub-symptom exacerbation exercise) is potentially beneficial for adults with prolonged symptoms after mTBI.49 In a descriptive study of children and adolescent athletes (mean age 14.25 years, range 8–17 years) with symptoms for at least four weeks post injury, completion of a progressive rehabilitation program was associated with the return of all 16 participants to a normal lifestyle and full sports participation.50
The goal of this study was to describe the methodology and report primary outcomes of an exploratory randomized clinical trial (RCT) of aerobic training for management of prolonged symptoms after mTBI in adolescents. To our knowledge, this is the first randomized clinical trial of an aerobic intervention for adolescents with prolonged symptoms after mTBI. We predicted that it would be feasible to enroll participants with prolonged symptoms after mTBI into a randomized clinical trial and that sub-symptom exacerbation aerobic training would be associated with a more rapid resolution of symptoms compared to a full-body stretching intervention.
Methods
Design
A randomized clinical trial was performed to determine the potential benefits of a six week, sub-symptom exacerbation aerobic training intervention compared to a full-body stretching intervention. Randomization was performed within stratified age (12 – 14 and 15 – 17 years) and gender blocks to ensure these factors were balanced between groups. The study was single-blinded with the evaluator completing baseline and final outcome assessments unaware of group assignment. The study was institutional review board approved and the trial was registered on ClinicalTrials.gov, clinical trial registration: NCT02035579.
Participants
Participants were recruited from the community, brain/head injury clinics, and emergency departments across the greater Cincinnati, OH area between September, 1 2013 and February, 1 2015. Adolescents between ages 12 and 17 years who sustained a mTBI and had between four and 16 weeks of persistent symptoms were eligible for the study. Mild TBI was defined using the American Congress of Rehabilitation Definition.51 Persistent symptoms were defined according to the World Health Organization, ICD-10 criteria for post-concussion syndrome (PCS).52,53 Clinical diagnostic criteria for PCS require a prior history of TBI and the presence of at least three of eight symptoms (headache, dizziness, fatigue, irritability, insomnia, concentration problems, memory difficulty or intolerance of stress, emotion, or alcohol).52–54 Endorsement that symptoms were exacerbated with physical activity was an additional inclusion criteria because symptoms not exacerbated by physical activity may be related to other causes besides mTBI.55 Exclusion criteria included children and/or families who did not speak and/or read English, evidence of more severe brain injury defined as post-resuscitation Glasgow Coma Scale (GCS) score below 13, or evidence of more severe injury on clinically performed neuroimaging (e.g., subdural hematoma, epidural hematoma, and contusion), pre-existing neurologic impairment (e.g., stroke, cerebral spinal fluid shunt, brain tumor), cognitive disorders (seizure disorder, cognitive impairment), significant psychological problems or developmental delay, genetic disorders, metabolic disorders, hematologic disorders, cancer, pre-injury diagnosis of attention deficit hyperactivity disorder (ADHD) that requires two or more medications for control or recent (within past month) changes of medications, history of cardiovascular problem that would preclude participation in an aerobic training protocol, and evidence of neck pain as cervicogenic symptoms may be more consistent with a whiplash injury and/or confound the development of persistent mTBI symptoms. Participants actively on beta-blockers, anti-depressants, anti-anxiety, attention deficit related medications, other mood/behavioral medications, or prophylactic headache medications (e.g., amitriptyline, topiramate) were considered for enrollment only if they were on a stable medication dose, defined as on the medication for at least one month with no medication dose changes planned. Participants were allowed to take rescue pain medications (e.g., acetaminophen, ibuprofen, naproxen) as prescribed clinically by their treating provider. Participation in other therapy programs during the trial was an additional exclusion criteria. Information on history of prior concussion(s) was collected using the Ohio State University TBI identification method56, but was not used as an inclusion or exclusion criteria. There were 395 individuals assessed for eligibility, 259 did not meet eligibility criteria and of the 136 eligible participants, 102 declined participation (see Figure 1). Of the 102 that declined participation, 54 were “not interested”, 20 reported the time commitment was too much, 13 reported they were recovering, and 15 reported “other reasons”. Thirty-four individuals completed a baseline assessment; four were excluded at the baseline assessment and 30 participants were randomized (Figure 1, CONSORT flow diagram).
Figure 1.
Consort flow chart
Assessment and intervention procedures
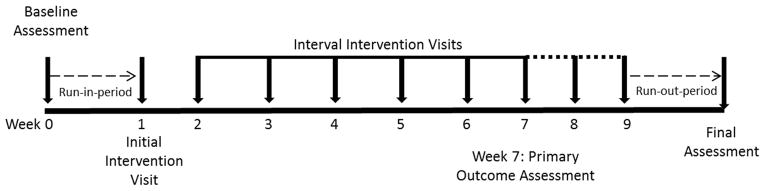
Figure 2 shows a schematic of the intervention timeline. Baseline (Week 0) visit: Participants were assessed for eligibility and consent and assent were completed at week 0. Participants were screened for significant neck symptoms. If neck range of motion was asymmetric or if there was significant tenderness to palpation of upper trapezius and/or cervical paraspinal muscles that reproduced post-concussion symptoms, participants were excluded. Participants also underwent an aerobic bike test using the Exerpeutic 400xl recumbent stationary bike. Participants started biking at a speed consistent with their Borg rate of perceived exertion (RPE) intensity level 11 (fairly light pace) with resistance fixed at level two for five minutes. Borg RPE ratings are valid and reliable in adolescents and may be used for development of exercise programs.57,58 At five minute intervals, participants were asked to increase their Borg intensity by one level until they either started to experience an increase in symptoms or until a maximum of 30 minutes (max intensity of 16). A Borg intensity range of 11–16 was chosen because it is correlated with aerobic training intensity.59 To limit heterogeneity and inclusion of children with potential other etiologies of their symptoms55,60, children unable to complete at least two minutes of cycling before exacerbation of symptoms or children able to complete 30 minutes without exacerbation of symptoms were excluded from further participation. Week 1 visit: Participants still meeting eligibility criteria after completion of the baseline visit moved to the pre-intervention/randomization or run-in period. The run-in period allowed an opportunity to monitor for change, specifically improvement, in symptoms that may occur as part of natural recovery. At the initial intervention visit (week 1, figure 2), participants were reassessed for eligibility and then randomized to the sub-symptom exacerbation aerobic training or full-body stretching intervention. Participants randomized to the sub-symptom exacerbation aerobic training group repeated the aerobic cycling test performed at the baseline assessment. Based on the cycling duration that was completed prior to symptom exacerbation, an individually tailored, sub-symptom exacerbation home exercise training program was developed. Individuals in the cycling group were given the same portable exercise bike used for study assessments to use at home. The bike was returned after study completion. Participants were asked to complete the cycling program five to six days per week at home at 80% of the duration that exacerbated symptoms during the interval and assessment visits. When participants in the cycling group returned for the weekly interval visits, the cycling test was repeated and the sub-symptom exacerbation home program was adjusted accordingly for the next week. Participants randomized to the stretching group were instructed on a full-body stretching program to be completed five to six days per week at home. The stretching program targeted the upper and lower extremity, as well as trunk-musculature. Each stretching program was rotated on a two week basis. The stretching group reviewed the full-body stretching program at weekly interval visits and every other week received a new group of stretches. All participants were asked to complete at least six weeks (week 7 in figure 2) of their respective training program. Participants in either group who returned to baseline at rest and were able to perform their exercise program without exacerbation of symptoms were considered to have recovered and moved to the post intervention or run-out period. This period was a time when participants completed their standard daily activities and allowed monitoring for change or maintenance of symptoms post intervention. Participants in either group who had not returned to their preinjury symptom level continued in their program for up to two additional weeks (week 8 and 9) before moving to the run-out period. The week seven visit was considered the primary outcome visit assessment. Additional visits were completed to determine if longer duration of the study would improve symptoms for individuals that did not return to baseline at week seven.
Figure 2.
Intervention overview
Outcome Measures
The Post-Concussion Symptom Inventory (PCSI) was used to assess self- and parent/guardian-rated symptoms.61,62 Self-ratings were considered as the primary outcome. The PCSI was used to obtain symptom ratings pre-injury, pre-intervention (week 0 and 1, figure 2), at interval visits (weeks 2 – 9, figure 2), and after the run-out period at the final assessment (figure 2). The adolescent version of the PCSI is a seven point Likert scale (0–6) rating 21 items on post-concussive symptoms in physical, cognitive, emotional, and sleep domains. Total scores range from 0 to 126 on the PCSI. The parent version of the PCSI uses the same seven point Likert scale to rate 20 items in physical, cognitive, emotional, and sleep domains. Total scores range from 0–120 on the parent version of the PCSI.
Adherence
Participants were provided with a log to record whether they completed the prescribed home exercise program and the number of times per week they completed the exercise program. Participants were also asked to report the number of days per week that they participated in activity outside of the prescribed exercise program. Both logs were reviewed weekly at the interval visits.
Sample Size
Based on previous work in adults with persistent symptoms after mTBI,49,60 a large Cohen’s d effect size of 2.24 – 2.50 for an exercise intervention to reduce symptoms was reported. Using G*power 3.1.363 and assuming an effect size of 1.25 (approximately half of effect size reported in adults), alpha of 0.05, power of 0.9, and drop-out rate of 10%, we determined a priori that we would require 15 participants per group to provide an adequate sample size to begin to assess the potential benefits of the intervention and inform larger more confirmatory studies.
Analysis
Descriptive statistics were used to summarize data. T-tests and Chi-square comparisons were used to compare demographic and other covariates or potential confounders when appropriate. Repeated measures Analysis of Variance, via mixed models was used to compare trajectory of recovery between the sub-symptom exacerbation aerobic training and full-body stretching groups. In the mixed models, the primary dependent variable of interest was the self-reported PCSI. A group by time since randomization (week 1) interaction variable was used as the primary variable of interest to compare recovery trajectory between groups. A p-value of .05 was used to define significance. Consistent with intention to treat principles, mixed models allows for use of all available data from participants. Data available from all 30 participants that were randomized were included in the mixed models analysis. Participants’ data were included in the models until they discontinued or became ineligible for the study. The primary analyses focused on the PCSI assessed repeatedly through week seven. As a secondary analysis to better understand if extension of the program may be beneficial for individuals who did not fully recover after six weeks of intervention, descriptive analyses were performed on the data from individuals who completed seven and eight weeks (week 8 or 9) of their intervention. Additionally, post-hoc mixed model analyses were done that included data from visits one through nine and the final assessment after the run-out period to understand if effects were maintained after completion of the study.
Results
Participants
There were no differences between participants in the sub-symptom exacerbation aerobic training and full-body stretching groups in regards to age, sex, race, primary caregiver education, household income, prior history of concussion, and time since injury (Table 1). Participants in the full-body stretching group were more likely to have a non-sports related mechanism of injury; however, equal numbers between groups reported participating in an organized sport (Table 1). The sub-symptom exacerbation aerobic training and full-body stretching groups did not differ in self- and primary caregiver PCSI ratings preinjury, at initial screening visit (week 0), or after the run-in period (week 1) (see Table 1).
Table 1.
Comparison of baseline data between intervention and comparison groups
| Cycling (n=15) | Stretching (n=15) | p-value | |
|---|---|---|---|
| Age at enrollment, mean (stdv) in years | 15.22 (1.37) | 15.50 (1.80) | .64 |
| Time since injury, mean (stdv) in days | 52.30 (19.93) | 55.95 (22.16) | .64 |
| Sex, number males | 5 | 8 | .27 |
| Race, number non-white | 2 | 2 | 1.00 |
| Primary caregiver education, number with bachelor degree or higher | 9 | 7 | .46 |
| Income, number $70,000 and above annual income | 9 | 9* | .81 |
| Mechanism of injury, number sport-related | 6 | 12 | .03 |
| Number reporting typical participation in an organized sport prior to injury | 13 | 13 | 1.00 |
| History of 2 or more concussions, number including injury related to study | 10 | 6 | .14 |
| Pre-injury Self-PCSI ratings, mean (stdv) | 8.33 (8.36) | 8.20 (10.10) | .97 |
| Pre-injury Parent-PCSI ratings, mean (stdv) | 7.07 (6.71) | 3.6 (1.17) | .30 |
| Initial Baseline self-PCSI, mean (stdv) | 37.40 (25.01) | 40.27 (27.25) | .75 |
| Initial Baseline parent-PCSI, mean (stdv) | 38.93 (15.13) | 46.93 (25.22) | .30 |
| Pre-randomization self-PCSI, mean (stdv) | 23.93 (15.10) | 27.53 (18.94) | .57 |
| Pre-randomization parent-PCSI, mean (stdv) | 25.86 (19.11)* | 24.79 (17.54)* | .88 |
indicates value is based on n=14 for this variable as primary giver was unavailable or declined to complete questions or data was missing.
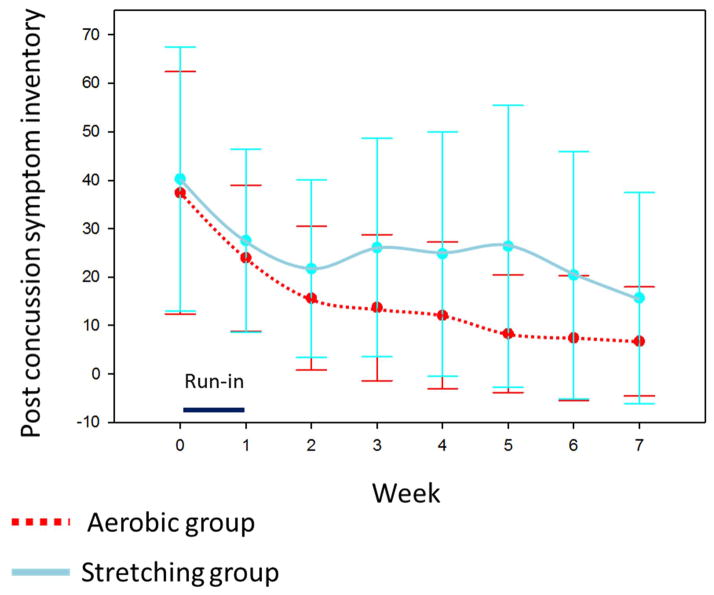
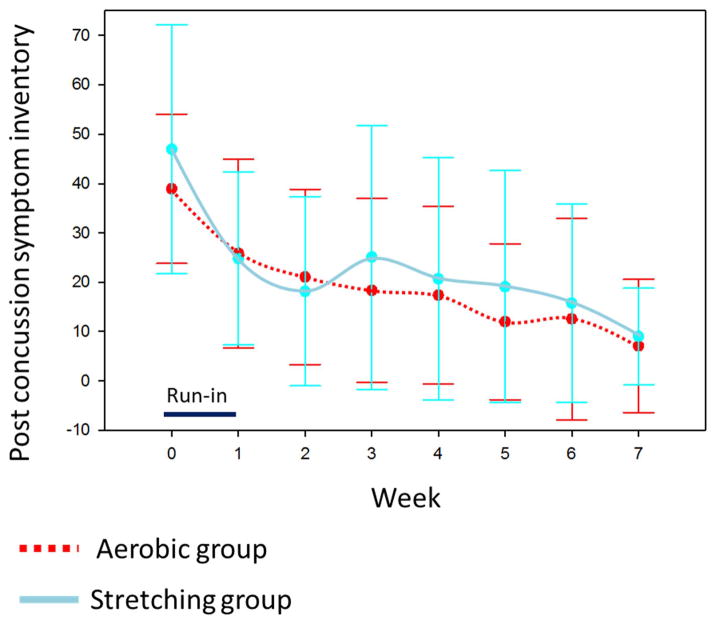
After randomization at week one, repeated measures mixed model analysis of self-PCSI ratings from week one through seven demonstrated a significant group by time interaction, indicating a greater rate of improvement in the sub-symptom aerobic training compared to the full-body stretching group (F-value = 4.11, p-value = .044, see figure 3). Based on repeated measures mixed model analysis, the effect size for the trend across time between groups is equivalent to a Cohen’s d effect size of ~.81 as measured by the F-value.64,65 The effect size at week seven between groups is equivalent to a Cohen’s d effect size of ~.51.64,65 Effect sizes of .5 to .8 are considered medium to large in magnitude and in this study correlate to approximately an eight to ten point greater improvement on the PCSI in the aerobic training compared to the full-body stretching group. Qualitative review of figure three indicates that the magnitude of difference between groups is largest after approximately four weeks (visit 5) of the intervention. The magnitude of the difference decreases after visit five. There was a non-significant group by time interaction for the primary caregiver PCSI ratings (F-value = .17, p-value = .68, see figure 4).
Figure 3.
Trajectory of self-reported post-concussion symptom inventory (PCSI) ratings
Means and standard deviations (represented by error bars) are reported for each weekly visit.
Figure 4.
Trajectory of parent-reported post-concussion symptom inventory (PCSI) ratings
Means and standard deviations (represented by error bars) are reported for each weekly visit.
After six weeks of the intervention, six of the possible 12 participants in the sub-symptom exacerbation aerobic training group (three participants dropped out or became ineligible) and 13 of a possible 14 in the full-body stretching group (one participant was lost to follow-up) continued in their respective program because they had not returned to baseline at rest and/or symptoms were still exacerbated by their training program. Individuals in the aerobic group that returned to baseline at week seven were similar to individuals that did not return to baseline with respect to age, sex, race, time since injury, and PCSI ratings preinjury, at initial screening visit (week 0), and after the run-in period (week 1). At week eight, the mean (SD) self- and parent-PCSI ratings for the sub-symptom exacerbation aerobic training group (n=6) were 6.00 (8.22) and 17.33 (23.01), respectively, and full-body stretching group (n=13) were 16.31 (24.15) and 9.15 (12.57), respectively. At week 9=nine, two and nine participants remained in the sub-symptom exacerbation and full-body stretching groups, respectively. The mean (SD) self- and parent-PCSI ratings for the sub-symptom exacerbation aerobic training group (n=2) were 13.50 (19.09) and 3.50 (4.95), respectively, and full-body stretching group (n=9) were 18.11 (26.16) and 9.22 (11.51), respectively.
At the final assessment, the mean (SD) self- and parent-ratings for the sub-symptom exacerbation aerobic training group (n=12) were 4.17 (7.36) and 9.50 (19.11), respectively, and full-body stretching group (n=14) were 15.93 (20.18) and 10.79 (13.33), respectively. Post-hoc repeated measures, mixed model analysis of self-PCSI ratings that included data from visits one through nine and the final assessment demonstrated a significant group by time interaction, indicating an improved rate of recovery for the sub-symptom exacerbation aerobic training compared to the full-body stretching group (F-value = 4.45, p-value = .036). There was not a significant group by time interaction for the primary caregiver PCSI ratings (F-value = .45, p-value = .50).
Adherence
Reported number of days per week that home exercise programs were completed was lower in the sub-symptom exacerbation aerobic training compared to the full-body stretching group (mean (SD) times per week = 4.42 (1.95) versus 5.85 (1.37), p < .0001) over the duration of the study. Days per week that participants reported physical activities in addition to their study related program was similar between groups (mean (SD) time per week = 1.72 (2.04) versus 1.92 (2.28), p = .5).
Adverse events
One participant in the sub-symptom exacerbation aerobic training group had a repeat head injury and associated neck injury between visits four and five that was unrelated to study procedures, thus she/he was ineligible after visit four due to the new injury and neck pain. Another participant broke his/her foot after a fall unrelated to study procedures between visits seven and eight of the sub-symptom exacerbation aerobic training intervention and dropped out of the study as she/he was unable to continue participation in the protocol. One participant in the full-body stretching group dropped out of the study due to worsening of symptoms after visit six to enroll in a formal therapy program.
Discussion
Our exploratory, randomized clinical trial demonstrates the benefits of sub-symptom exacerbation aerobic training in adolescents with an average of approximately two months of persistent symptoms after mTBI. Findings indicate sub-symptom exacerbation aerobic training program is potentially beneficial compared to a full-body stretching program. Additionally, both groups demonstrated improvement from baseline, indicating that even minimal activity may potentially be beneficial. The stretching comparison provided good control of visit procedures, contact with study staff, and practice or homework between visits, but may not represent true natural recovery. Because the comparison group in this study received an active intervention, albeit minimal activity, the magnitude of the effect of aerobic training from this study is likely a more conservative estimate than if compared to a truly non-active intervention. Furthermore, by engaging in more regular aerobic activity, cycling may be minimizing further effects of inactivity, and in theory, individuals in this group would be more fit and thus have greater benefit compared to the stretching group.66 A priori, six weeks was selected as the primary outcome time point; however, the largest benefits appear to occur after approximately four weeks of the intervention, indicating that four weeks may be adequate to assess benefits of the intervention. Continuation of the intervention program longer resulted in continued improvement, but not at the same rate or magnitude as the initial four weeks. This study also demonstrates the feasibility for adolescents to perform this program at home with intermittent visits. Larger, confirmatory active intervention trials are needed in the future.
Findings are consistent with other recent studies demonstrating that introduction of activity after concussion is potentially beneficial.48,67–69 Pilot studies in adults have demonstrated the potential benefit of sub-symptom threshold aerobic training.49,55 Additionally, non-randomized clinical trials have demonstrated potential benefits for active progressive rehabilitation in children.50 Furthermore, cervical and vestibular focused therapies have demonstrated benefit for individuals with primarily neck and vestibular symptoms after mTBI.70–72 Taken together, the findings from this study and other recent research indicate that active rehabilitation programs should be considered for management of mTBI. Likely, however, because symptom presentation is not uniform, programs targeted to each individual and their presenting symptoms need to be considered. Multidimensional (aerobic, cervical, vestibular-ocular, postural control) therapy programs that are targeted to each individual will need to be evaluated in the future.
There appeared to be large improvement in symptom scores during the run-in period and over the duration of the study in both groups. This may be related to either a placebo effect, natural recovery, or both. It is also possible that the cycling test given to all participants at week 0, to see if aerobic activity exacerbated symptoms, provided a psychological benefit or inadvertent “permission” to partake in more physical activities. Improvement seen in the stretching group could be attributed to partially active effects attributed to stretching.73 Additionally, improvements were seen in the self, but not the parent ratings of symptoms. Reasons for this discrepancy are unclear; however, previous research demonstrated only low to moderate agreement between self and parent ratings of mTBI symptoms in children62,74 and children and parent ratings may be differentially sensitive to recovery of mTBI symptoms.75 Furthermore, there is large variability in symptom scores throughout the study, indicating large variation of symptom burden among individuals.
Because prior research has shown dysregulation of cerebral blood flow and metabolism are likely key mechanism of persistent symptoms after mTBI16–23,76, it is plausible that aerobic activity may act through a mechanism that improves cerebral blood flow and reduces metabolism dysregulation to improve persistent symptoms after mTBI.39,77,78 A better understanding of the mechanism of action of active interventions for mTBI would allow for better targeting of interventions and allow more precise implementation of active therapy programs.
After the initial screening of participants, 136 participants met full eligibility criteria and approximately 22% enrolled in the study. Most commonly, families were not interested in participation or reported that the time commitment was too much. Individuals who refused participation did not always provide a clear reason, but 13% of this group reported sufficient recovery as to not justify the need for participation in a study. There may be other participants or families who did not specify a reason for nonparticipation with similar reasons for non-participation. Consideration of recovery trajectory prior to enrollment in future studies will be important. Having a more restrictive symptom threshold (i.e., higher symptom burden) as criteria for enrollment may identify individuals more likely to enroll and potentially more likely to benefit. After enrollment, only two participants were lost to follow-up or dropped, indicating that the program was feasible to complete for most families enrolled. The other two participants that did not complete the study had adverse events unrelated to study procedures. Qualitative feedback collected from families at the end of the study indicated that overall, they liked participating in the study; however, some reported that less frequent in-person visits and a program that was tailored to their symptoms would improve the program. Recruitment and dropout rates from this pilot study will inform sample size considerations for larger studies.
To develop a better evidence base for management of mTBI, there is a critical need to perform rigorous, controlled, clinical investigations in the mTBI population.79 Experience with this trial, demonstrates some of the challenges with performing trials in this population. With increasing time since injury, individuals typically continue to improve; therefore, controlled studies may have difficulty detecting a difference in trajectory of recovery between groups, especially due to variability in symptom reporting. Also, the longer the time since injury, the more likely other factors may confound recovery. Future studies evaluating the safety and tolerability of introducing active programs earlier are needed. Determining if earlier introduction is at least safe (i.e., does not worsen trajectory of recovery), may limit deconditioning that occurs with prolonged rest from activities. Recent studies suggest that recommendations for prolonged rest may not improve recovery and that abstaining from physical activity may not be associated with improved symptom recovery.48,80,81 Due to the wide variety of treatments that are utilized in clinical care, true control groups may be difficult to enroll; therefore, alternatives to RCT designs may need to be considered. Additionally, multimodal interventions need to be considered that may cross treatment domains, for example, combined medical, behavioral, cognitive, and active interventions should be considered or detailed information on management strategies used outside of controlled trials needs to be collected. Furthermore, multicenter trials are needed to improve generalizability and to account for potential differences based on geographic location.
Limitations
This was an exploratory randomized study, so the sample size limits the generalizability of findings to definitively inform clinical care, but demonstrates potential feasibility and benefits of this program for this population. This study targeted individuals with at least four weeks of persistent symptoms that were exacerbated by moderate aerobic activity. Therefore, individuals in better condition before injury may not have qualified for the study. Due to the relatively high non-participation rate, generalizability is limited. There may also be a bias towards increased benefits among participants compared to those who refused participation. Additionally, individuals with significant cervicogenic and neck symptoms or complaints were excluded, thus limiting the generalizability of this intervention to individuals without this constellation of symptoms after injury. Individuals with primarily vestibular or oculomotor symptoms may be less likely to benefit from this intervention as focused vestibular therapy was not delivered as part of this intervention. There were a higher proportion of individuals with non-sports related injuries in the stretching group compared to the control that may have introduced a bias as previous work suggests athletes may have a faster response to aerobic interventions;49 however, an equal number in each group reported typical participation in organized sports prior to injury. There were also three participants that did not complete the sub-symptom exacerbation aerobic training protocol compared to one in the full-body stretching group. Information on characteristics of non-participants is not available, so direct comparisons between participants and non-participants are not possible. Conclusions regarding characteristics associated with responders and non-responders are outside the scope of this initial report of the methodology and the primary outcome. The current study is undersized for sub-group analyses and the implication of analyses, regardless of findings, would be unclear. Larger studies are needed to better understand if there are individual, injury-related, and other contributory factors that predict response or non-response to active interventions. Due to the nature of the intervention, double blinding was not feasible and may have biased outcome reporting. There may have been unknown variation in clinical care provided to individuals that may have biased the study. Additionally, although participants reported equal amounts of activity outside of study procedures in both groups, we are unable to characterize the exact nature and intensity of these activities. Adherence was assessed by self-report logs at weekly visits, which may be associated with reporting bias. There were also a limited number of participants who were non-white and from lower socioeconomic status groups that limits generalizability.
Conclusion
Findings from this exploratory randomized clinical trial suggests sub-symptom exacerbation aerobic training is potentially beneficial for adolescents with persistent symptoms after mTBI. Findings will inform future larger multicenter, replication studies to verify findings and improve the generalizability of the results. Studies that evaluate multimodal active rehab interventions are needed to more robustly inform clinical practice. Overall, findings from this study and other recent research indicate that active rehabilitation programs are potentially beneficial for adolescents with persistent symptoms after mTBI. Future work should focus on determining the optimal type, timing, and intensity of active rehabilitation programs and characteristics of individuals most likely to benefit from these interventions. Larger randomized comparison or other controlled studies are warranted.
Acknowledgments
Funding for this study was supported in part by the Cincinnati Children’s Research Foundation Trustees Grant program, Ohio Department of Public Safety, National Institute for Child Health and Human Development K23HD074683-01A1, and Grant 8 UL1 TR000077 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
Abbreviations
- mTBI
mild traumatic brain injury
- TBI
traumatic brain injury
Footnotes
Trial Registration: NCT02035579
The authors have no financial relationships relevant to this article to disclose.
The authors have no conflicts of interest to disclose.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other supporting agencies.
References
- 1.Faul M, Xu L, Wald M, Coronado V. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Accessed September 9, 2013]. http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf. [Google Scholar]
- 2.Langlois J, Rutland-Brown W, Thomas K. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta, GA: US Department of Health and Human Servicies, CDC; 2004. [Google Scholar]
- 3.Moreno MA. Advice for patients. Children and organized sports. Arch Pediatr Adolesc Med. 2011;165(4):376. doi: 10.1001/archpediatrics.2011.31. [DOI] [PubMed] [Google Scholar]
- 4.Moreno MA. Youth sports and concussion risk. Arch Pediatr Adolesc Med. 2012;166(4):396. doi: 10.1001/archpediatrics.2012.79. [DOI] [PubMed] [Google Scholar]
- 5.Rimel RW, Giordani B, Barth JT, Boll TJ, Jane JA. Disability caused by minor head injury. Neurosurgery. 1981;9(3):221–228. [PubMed] [Google Scholar]
- 6.Willer B, Leddy JJ. Management of concussion and post-concussion syndrome. Curr Treat Options Neurol. 2006;8(5):415–426. doi: 10.1007/s11940-006-0031-9. [DOI] [PubMed] [Google Scholar]
- 7.Barlow KM, Crawford S, Stevenson A, Sandhu SS, Belanger F, Dewey D. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics. 2010;126(2):e374–381. doi: 10.1542/peds.2009-0925. [DOI] [PubMed] [Google Scholar]
- 8.Yeates KO. Mild traumatic brain injury and postconcussive symptoms in children and adolescents. Journal of the International Neuropsychological Society : JINS. 2010;16(6):953–960. doi: 10.1017/S1355617710000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Control NCfIPa. Report to Congress on Mild Traumatic brainInjury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta, GA: Center for Disease Control and Prevention; 2003. [Google Scholar]
- 10.Langlois JA, Marr A, Mitchko J, Johnson RL. Tracking the silent epidemic and educating the public: CDC’s traumatic brain injury-associated activities under the TBI Act of 1996 and the Children’s Health Act of 2000. J Head Trauma Rehabil. 2005;20(3):196–204. doi: 10.1097/00001199-200505000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Hovda DA, Lee SM, Smith ML, et al. The neurochemical and metabolic cascade following brain injury: moving from animal models to man. J Neurotrauma. 1995;12(5):903–906. doi: 10.1089/neu.1995.12.903. [DOI] [PubMed] [Google Scholar]
- 12.Hovda DA, Yoshino A, Kawamata T, Katayama Y, Becker DP. Diffuse prolonged depression of cerebral oxidative metabolism following concussive brain injury in the rat: a cytochrome oxidase histochemistry study. Brain Res. 1991;567(1):1–10. doi: 10.1016/0006-8993(91)91429-5. [DOI] [PubMed] [Google Scholar]
- 13.Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73(6):889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 14.Kawamata T, Katayama Y, Hovda DA, Yoshino A, Becker DP. Administration of excitatory amino acid antagonists via microdialysis attenuates the increase in glucose utilization seen following concussive brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1992;12(1):12–24. doi: 10.1038/jcbfm.1992.3. [DOI] [PubMed] [Google Scholar]
- 15.McCrory P, Johnston KM, Mohtadi NG, Meeuwisse W. Evidence-based review of sport-related concussion: basic science. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2001;11(3):160–165. doi: 10.1097/00042752-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Korn A, Golan H, Melamed I, Pascual-Marqui R, Friedman A. Focal cortical dysfunction and blood-brain barrier disruption in patients with Postconcussion syndrome. J Clin Neurophysiol. 2005;22(1):1–9. doi: 10.1097/01.wnp.0000150973.24324.a7. [DOI] [PubMed] [Google Scholar]
- 17.Vagnozzi R, Signoretti S, Cristofori L, et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain : a journal of neurology. 2010;133(11):3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- 18.Vagnozzi R, Signoretti S, Tavazzi B, et al. Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes--part III. Neurosurgery. 2008;62(6):1286–1295. doi: 10.1227/01.neu.0000333300.34189.74. discussion 1295–1286. [DOI] [PubMed] [Google Scholar]
- 19.Junger EC, Newell DW, Grant GA, et al. Cerebral autoregulation following minor head injury. J Neurosurg. 1997;86(3):425–432. doi: 10.3171/jns.1997.86.3.0425. [DOI] [PubMed] [Google Scholar]
- 20.Strebel S, Lam AM, Matta BF, Newell DW. Impaired cerebral autoregulation after mild brain injury. Surg Neurol. 1997;47(2):128–131. doi: 10.1016/s0090-3019(96)00459-4. [DOI] [PubMed] [Google Scholar]
- 21.Len TK, Neary JP. Cerebrovascular pathophysiology following mild traumatic brain injury. Clin Physiol Funct Imaging. 2011;31(2):85–93. doi: 10.1111/j.1475-097X.2010.00990.x. [DOI] [PubMed] [Google Scholar]
- 22.Len TK, Neary JP, Asmundson GJ, Goodman DG, Bjornson B, Bhambhani YN. Cerebrovascular reactivity impairment after sport-induced concussion. Med Sci Sports Exerc. 2011;43(12):2241–2248. doi: 10.1249/MSS.0b013e3182249539. [DOI] [PubMed] [Google Scholar]
- 23.Maugans TA, Farley C, Altaye M, Leach J, Cecil KM. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics. 2012;129(1):28–37. doi: 10.1542/peds.2011-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gowda NK, Agrawal D, Bal C, et al. Technetium Tc-99m ethyl cysteinate dimer brain single-photon emission CT in mild traumatic brain injury: a prospective study. AJNR Am J Neuroradiol. 2006;27(2):447–451. [PMC free article] [PubMed] [Google Scholar]
- 25.Lemka M, Brockhuis B, Lass P, Pilarska E. Headache occurrence and assessment of regional blood flow after brain concussion in children. Neurol Neurochir Pol. 2005;39(4 Suppl 1):S42–48. [PubMed] [Google Scholar]
- 26.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 27.Cotman CW, Engesser-Cesar C. Exercise enhances and protects brain function. Exercise and sport sciences reviews. 2002;30(2):75–79. doi: 10.1097/00003677-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiol Aging. 2002;23(5):941–955. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 29.Davenport MH, Hogan DB, Eskes GA, Longman RS, Poulin MJ. Cerebrovascular reserve: the link between fitness and cognitive function? Exercise and sport sciences reviews. 2012;40(3):153–158. doi: 10.1097/JES.0b013e3182553430. [DOI] [PubMed] [Google Scholar]
- 30.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 32.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 33.Vaynman S, Ying Z, Gomez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. Journal of neuroscience research. 2004;76(3):356–362. doi: 10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- 34.Epp JR, Spritzer MD, Galea LA. Hippocampus-dependent learning promotes survival of new neurons in the dentate gyrus at a specific time during cell maturation. Neuroscience. 2007;149(2):273–285. doi: 10.1016/j.neuroscience.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 35.Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124(1):71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 36.Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16(6):1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- 37.Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nature Neuroscience. 2000;3(4):323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- 38.Devine JM, Zafonte RD. Physical exercise and cognitive recovery in acquired brain injury: a review of the literature. PM & R : the journal of injury, function, and rehabilitation. 2009;1(6):560–575. doi: 10.1016/j.pmrj.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Fogelman D, Zafonte R. Exercise to enhance neurocognitive function after traumatic brain injury. PM & R : the journal of injury, function, and rehabilitation. 2012;4(11):908–913. doi: 10.1016/j.pmrj.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 40.Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004;1016(2):154–162. doi: 10.1016/j.brainres.2004.04.079. [DOI] [PubMed] [Google Scholar]
- 42.Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125(1):129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 43.Griesbach GS. Exercise after traumatic brain injury: is it a double-edged sword? PM & R : the journal of injury, function, and rehabilitation. 2011;3(6 Suppl 1):S64–72. doi: 10.1016/j.pmrj.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 44.McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport - The 3rd international conference on concussion in sport held in Zurich, November 2008. PM & R : the journal of injury, function, and rehabilitation. 2009;1(5):406–420. doi: 10.1016/j.pmrj.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Halstead ME, Walter KD Council on Sports M Fitness, American Academy of Pediatrics. Clinical report--sport-related concussion in children and adolescents. Pediatrics. 2010;126(3):597–615. doi: 10.1542/peds.2010-2005. [DOI] [PubMed] [Google Scholar]
- 46.Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. British journal of sports medicine. 2013;47(1):15–26. doi: 10.1136/bjsports-2012-091941. [DOI] [PubMed] [Google Scholar]
- 47.Silverberg ND, Iverson GL. Is Rest After Concussion “The Best Medicine?”: Recommendations for Activity Resumption Following Concussion in Athletes, Civilians, and Military Service Members. J Head Trauma Rehabil. 2012 doi: 10.1097/HTR.0b013e31825ad658. [DOI] [PubMed] [Google Scholar]
- 48.Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of Strict Rest After Acute Concussion: A Randomized Controlled Trial. Pediatrics. 2015 doi: 10.1542/peds.2014-0966. [DOI] [PubMed] [Google Scholar]
- 49.Leddy JJ, Kozlowski K, Donnelly JP, Pendergast DR, Epstein LH, Willer B. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2010;20(1):21–27. doi: 10.1097/JSM.0b013e3181c6c22c. [DOI] [PubMed] [Google Scholar]
- 50.Gagnon I, Galli C, Friedman D, Grilli L, Iverson GL. Active rehabilitation for children who are slow to recover following sport-related concussion. Brain injury : [BI] 2009;23(12):956–964. doi: 10.3109/02699050903373477. [DOI] [PubMed] [Google Scholar]
- 51.Ruff RM, Iverson GL, Barth JT, et al. Recommendations for diagnosing a mild traumatic brain injury: a National Academy of Neuropsychology education paper. Arch Clin Neuropsychol. 2009;24(1):3–10. doi: 10.1093/arclin/acp006. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- 53.World Helath Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnositc Criteria for Research. Geneva: World Health Organization; 1993. [Google Scholar]
- 54.Boake C, McCauley SR, Levin HS, et al. Diagnostic criteria for postconcussional syndrome after mild to moderate traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2005;17(3):350–356. doi: 10.1176/jnp.17.3.350. [DOI] [PubMed] [Google Scholar]
- 55.Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of Concussion and Post-concussion Syndrome. Sports health. 2012;4(2):147–154. doi: 10.1177/1941738111433673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corrigan JD, Bogner J. Initial reliability and validity of the Ohio State University TBI Identification Method. J Head Trauma Rehabil. 2007;22(6):318–329. doi: 10.1097/01.HTR.0000300227.67748.77. [DOI] [PubMed] [Google Scholar]
- 57.Eston RG, Williams JG. Exercise intensity and perceived exertion in adolescent boys. British journal of sports medicine. 1986;20(1):27–30. doi: 10.1136/bjsm.20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eakin BL, Finta KM, Serwer GA, Beekman RH. Perceived exertion and exercise intensity in children with or without structural heart defects. The Journal of pediatrics. 1992;120(1):90–93. doi: 10.1016/s0022-3476(05)80608-0. [DOI] [PubMed] [Google Scholar]
- 59.GB . An introduction to Borg’s RPE-scale. Ithaca, NY: Mouvement Publications; 1985. [Google Scholar]
- 60.Leddy JJ, Cox JL, Baker JG, et al. Exercise Treatment for Postconcussion Syndrome: A Pilot Study of Changes in Functional Magnetic Resonance Imaging Activation, Physiology, and Symptoms. J Head Trauma Rehabil. 2012 doi: 10.1097/HTR.0b013e31826da964. [DOI] [PubMed] [Google Scholar]
- 61.McCauley SR, Wilde EA, Anderson VA, et al. Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. J Neurotrauma. 2012;29(4):678–705. doi: 10.1089/neu.2011.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gioia GA, Schneider JC, Vaughan CG, Isquith PK. Which symptom assessments and approaches are uniquely appropriate for paediatric concussion? British journal of sports medicine. 2009;43(Suppl 1):i13–i22. doi: 10.1136/bjsm.2009.058255. [DOI] [PubMed] [Google Scholar]
- 63.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 64.Wilson DB. [Accessed Decenber 14, 2015];Practical Meta-Analysis Effect Size Calculator. http://www.campbellcollaboration.org/escalc/html/EffectSizeCalculator-Home.php.
- 65.Lipsey MW, Wilson DB. Practical Meta-Analysis (Applied Social Research Methods) 1. Thousand Oaks, California: SAGE Publications, Inc; 2001. [Google Scholar]
- 66.Chin LM, Chan L, Woolstenhulme JG, Christensen EJ, Shenouda CN, Keyser RE. Improved Cardiorespiratory Fitness With Aerobic Exercise Training in Individuals With Traumatic Brain Injury. J Head Trauma Rehabil. 2014 doi: 10.1097/HTR.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schneider KJ, Iverson GL, Emery CA, McCrory P, Herring SA, Meeuwisse WH. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. British journal of sports medicine. 2013;47(5):304–307. doi: 10.1136/bjsports-2013-092190. [DOI] [PubMed] [Google Scholar]
- 68.Vidal PG, Goodman AM, Colin A, Leddy JJ, Grady MF. Rehabilitation strategies for prolonged recovery in pediatric and adolescent concussion. Pediatric annals. 2012;41(9):1–7. doi: 10.3928/00904481-20120827-10. [DOI] [PubMed] [Google Scholar]
- 69.Majerske CW, Mihalik JP, Ren D, et al. Concussion in sports: postconcussive activity levels, symptoms, and neurocognitive performance. J Athl Train. 2008;43(3):265–274. doi: 10.4085/1062-6050-43.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alsalaheen BA, Mucha A, Morris LO, et al. Vestibular rehabilitation for dizziness and balance disorders after concussion. J Neurol Phys Ther. 2010;34(2):87–93. doi: 10.1097/NPT.0b013e3181dde568. [DOI] [PubMed] [Google Scholar]
- 71.Alsalaheen BA, Whitney SL, Mucha A, Morris LO, Furman JM, Sparto PJ. Exercise prescription patterns in patients treated with vestibular rehabilitation after concussion. Physiother Res Int. 2013;18(2):100–108. doi: 10.1002/pri.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schneider KJ, Meeuwisse WH, Nettel-Aguirre A, et al. Cervicovestibular rehabilitation in sport-related concussion: a randomised controlled trial. British journal of sports medicine. 2014 doi: 10.1136/bjsports-2013-093267. [DOI] [PubMed] [Google Scholar]
- 73.Ylinen J, Nikander R, Nykanen M, Kautiainen H, Hakkinen A. Effect of neck exercises on cervicogenic headache: a randomized controlled trial. Journal of rehabilitation medicine. 2010;42(4):344–349. doi: 10.2340/16501977-0527. [DOI] [PubMed] [Google Scholar]
- 74.Hajek CA, Yeates KO, Taylor HG, et al. Agreement between Parents and Children on Ratings of Post-Concussive Symptoms Following Mild Traumatic Brain Injury. Child Neuropsychol. 2011;17(1):17–33. doi: 10.1080/09297049.2010.495058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor HG, Dietrich A, Nuss K, et al. Post-concussive symptoms in children with mild traumatic brain injury. Neuropsychology. 2010;24(2):148–159. doi: 10.1037/a0018112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clausen M, Pendergast DR, Willer B, Leddy J. Cerebral Blood Flow During Treadmill Exercise Is a Marker of Physiological Postconcussion Syndrome in Female Athletes. J Head Trauma Rehabil. 2015 doi: 10.1097/HTR.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 77.Brooks GA, Martin NA. Cerebral metabolism following traumatic brain injury: new discoveries with implications for treatment. Front Neurosci. 2014;8:408. doi: 10.3389/fnins.2014.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rooks CR, Thom NJ, McCully KK, Dishman RK. Effects of incremental exercise on cerebral oxygenation measured by near-infrared spectroscopy: a systematic review. Prog Neurobiol. 2010;92(2):134–150. doi: 10.1016/j.pneurobio.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 79.Burke MJ, Fralick M, Nejatbakhsh N, Tartaglia MC, Tator CH. In search of evidence-based treatment for concussion: characteristics of current clinical trials. Brain injury : [BI] 2015;29(3):300–305. doi: 10.3109/02699052.2014.974673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Howell DR, Mannix RC, Quinn B, Taylor JA, Tan CO, Meehan WP., 3rd Physical Activity Level and Symptom Duration Are Not Associated After Concussion. Am J Sports Med. 2016 doi: 10.1177/0363546515625045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maerlender A, Rieman W, Lichtenstein J, Condiracci C. Programmed Physical Exertion in Recovery From Sports-Related Concussion: A Randomized Pilot Study. Dev Neuropsychol. 2015;40(5):273–278. doi: 10.1080/87565641.2015.1067706. [DOI] [PubMed] [Google Scholar]