Abstract
Background
Animal models have demonstrated that allergen-specific IgG confers sensitivity to systemic anaphylaxis that relies on IgG receptors (FcγRs). Mouse IgG2a and IgG2b bind activating FcγRI, FcγRIII and FcγRIV, and inhibitory FcγRIIB; mouse IgG1 binds only FcγRIII and FcγRIIB. Although these interactions are of strikingly different affinities, these three IgG subclasses have been shown to enable induction of systemic anaphylaxis.
Objective
Determine which pathways control the induction of IgG1-, IgG2a- and IgG2b-passive systemic anaphylaxis.
Methods
Mice were sensitized with IgG1, IgG2a or IgG2b anti-TNP mAbs and challenged with TNP-BSA intravenously to induce systemic anaphylaxis that was monitored using rectal temperature. Anaphylaxis was evaluated in mice deficient for FcγRs, injected with mediator antagonists or in which basophils, monocyte/macrophages or neutrophils had been depleted. The expression of FcγRs was evaluated on these cells before and after anaphylaxis.
Results
Activating FcγRIII is the receptor primarily responsible for all three models of anaphylaxis, and subsequent down regulation of this receptor was observed. These models differentially relied on histamine release and on the contribution of mast cells, basophils, macrophages and neutrophils. Strikingly, basophil contribution and histamine predominance in IgG1- and IgG2b-mediated anaphylaxis correlated with the ability of inhibitory FcγRIIB to negatively regulate these models of anaphylaxis.
Conclusion
We propose that the differential expression of inhibitory FcγRIIB on myeloid cells and its differential binding of IgG subclasses controls the contributions of mast cells, basophils, neutrophils and macrophages to IgG subclass-dependent anaphylaxis. Collectively, our results unravel novel complexities in the involvement and regulation of cell populations in IgG-mediated reactions in vivo.
Keywords: Anaphylaxis, IgG, mouse model, basophil, neutrophil, monocyte, macrophage, FcγR, Platelet-activating Factor, Histamine
INTRODUCTION
Anaphylaxis is a hyperacute allergic reaction that occurs with increasing incidence in the population and can be of fatal consequence. Symptoms include skin rashes, hypotension, hypothermia, abdominal pain, bronchospasm and heart and lung failure that may lead to asphyxia and sometimes death1. The main treatment remains epinephrine (adrenaline) injection to restore heart and lung function. Since anaphylaxis represents an emergency situation, few clinical studies have been possible to address the mechanisms leading to anaphylaxis in patients. Experimental models of anaphylaxis identified mechanisms involving allergen-specific antibodies that trigger activating antibody receptors on myeloid cells, leading to the release of mediators. These mediators can, by themselves, recapitulate the symptoms of anaphylaxis as observed in humans2, 3.
The “classical” mechanism of anaphylaxis states that allergen-specific IgE binds the activating IgE receptor FcεRI on mast cells, which upon allergen encounter become activated and release histamine, among other mediators. Notably, histamine injection suffices to induce the signs of anaphylaxis in animal models4. In many cases, detectable allergen-specific IgE and elevated histamine levels do not accompany anaphylaxis in humans (discussed in 5), leading to the notion that “atypical” or “alternate” mechanisms of induction could explain these cases. One of these atypical/alternate models proposes a similar cascade of events, but instead based on allergen-specific IgG binding to allergen, forming IgG-allergen immune complexes that trigger activating IgG receptors (FcγRs) expressed on myeloid cells (i.e. macrophages, basophils and/or neutrophils), which in turn release Platelet-Activating Factor (PAF)2,3. Importantly, PAF injection suffices to induce the signs of anaphylaxis in animal models 6. IgG-induced anaphylaxis can be elicited by intravenous injection of allergen-specific IgG followed by allergen administration, and is termed IgG-induced passive systemic anaphylaxis (PSA).
IgG receptors in the mouse comprise four “classical“ IgG receptors termed FcγRs, but also the neonatal IgG receptor (FcRn) and the intracellular FcR tripartite motif-containing protein 21 (TRIM21)7, 8. Whereas FcRn and TRIM21 both participate in the intracellular routing of IgG, and FcRn in protection from catabolism and distribution to tissues9, FcγRs control cell activation in the presence of immune complexes. FcγRs in mice are subdivided into i) activating FcγRs, i.e. FcγRI, FcγRIII and FcγRIV, that lead to cell activation upon immune complex binding, and ii) an inhibitory FcγR, i.e. FcγRIIB, that inhibits cell activation when co-engaged by an immune complex with an activating FcγR co-expressed on the same cell10. Inhibition of cell activation by FcγRIIB thus requires that the immune complex contains IgG that are bound both by the activating and by the inhibitory FcγR.
Four IgG subclasses exist in mice, IgG1, IgG2a, IgG2b and IgG3. Among those, only IgG2a and IgG2b bind to all FcγRs, whereas IgG1 binds only to FcγRIIB and FcγRIII. It remains under debate whether IgG3 binds to FcγRs, particularly FcγRI11, 12. The affinities of these FcγRs towards IgG subclasses are strikingly different (Table 1) leading to the notion of “high-affinity” receptors that retain monomeric IgG and “low-affinity” receptors that do not8. The avidity of IgG-immune complexes, however, enables both types of receptors to retain IgG-immune complexes, leading to receptor clustering, intracellular signaling events and, eventually, to cell activation. FcγRI is a high-affinity receptor for IgG2a13, and FcγRIV is a high-affinity receptor for IgG2a and IgG2b14. All other FcγR-IgG interactions are of low affinity (reviewed in 7).
Table 1.
Affinities of mouse FcγR-IgG subclass interactions (KA values in M−1)
| IgG1 | IgG2a | IgG2b | IgG3 | |
|---|---|---|---|---|
| FcγRI | - | 1×108 | 1×105 | (+) |
| FcγRIIB | 3.3×106 | 4.2×105 | 2.2×106 | - |
| FcγRIII | 3.1×105 | 6.8×105 | 6.4×105 | - |
| FcγRIV | - | 2.9×107 | 1.7×107 | - |
Three out of the four IgG subclasses in the mouse, i.e. IgG1, IgG2a and IgG2b, have been reported to enable the induction of systemic anaphylaxis, inducing mild to severe hypothermia5, 15, 16. This is rather surprising for IgG1, considering that inhibitory FcγRIIB binds IgG1 with a 10-fold higher affinity (KA=3.3×106 M−1) than activating FcγRIII (KA=3.1×105 M−1)17 (Table 1), implying that inhibition should dominate over activation. WT mice, indeed, develop a very mild anaphylactic reaction during IgG1-PSA compared to FcγRIIB−/− mice18, indicating that inhibition by FcγRIIB occurs in WT mice during IgG1-PSA, reducing, but not protecting from, anaphylaxis. IgG1-PSA has been reported to rely on basophils19 that co-express FcγRIIB and FcγRIII20. In this apparently simple situation, only one activating receptor and one inhibitory receptor are engaged on a single cell type that, once activated, produces an anaphylactogenic mediator, like PAF19.
IgG2a and IgG2b, however, bind three activating FcγRs and inhibitory FcγRIIB with different affinities ranging over 2 logs. In particular, the affinity of FcγRIIB for IgG2a is significantly lower than for IgG2b, whereas activating IgG receptors FcγRIII and FcγRIV bind IgG2a and IgG2b with similar affinities, respectively (Table 1). Notably, FcγRIV is not expressed on basophils, but on monocytes/macrophages and neutrophils21 that have both been reported to contribute to experimental anaphylaxis16, 22-24. In addition, mice expressing only FcγRIV can develop IgG-PSA16. Together with expression and binding data, one would therefore hypothesize that FcγRIV contributes predominantly to IgG2a- and IgG2b-PSA. In this work, we present evidence contrary to this hypothesis, and reveal which activating FcγR on which cell type(s) releasing which mediator(s) are responsible for IgG2a-PSA and IgG2b-PSA, and the differential regulation of these models of anaphylaxis by FcγRIIB. Our results unravel a complex balance determined by FcγR expression patterns, inhibition potential by FcγRIIB and respective affinities of activating and inhibitory FcγRs for IgG subclasses that, altogether, regulate the contribution of cells and anaphylactogenic mediators to a given model of IgG-induced anaphylaxis.
METHODS
Mice
Female C57Bl/6J mice (herein referred to as “WT”) were purchased from Charles River, female Balb/cJRj mice from Janvier Labs, FcγRIIB−/− (MGI:1857166), FcγRIII−/− mice (MGI: 3620982) and Rosa26-YFP mice from Jackson Laboratories. FcγRI−/− mice (MGI: 3664782) were provided by J. Leusen (University Medical Center, Utrecht, The Netherlands), FcγRIV−/− mice (MGI: 5428684) by J.V. Ravetch (The Rockefeller University, New York, NY, USA), Gfi1−/− mice by T. Moroy (Montreal University, Montreal, QC, Canada) and MRP8-cre mice by Clifford Lowell (University of California at San Francisco, CA, USA). MRP8-cre and Rosa26-YFP mice were intercrossed to generate MRP8-cre; Rosa26-YFP mice. Cpa3-Cre; Mcl-1fl/fl mice25 (backcrossed for at least 9 generations on a C57Bl/6J background) were kept in the Stanford University animal facility. All mouse protocols were approved by the Animal Ethics committee CETEA (Institut Pasteur, Paris, France) registered under #C2EA-89, and the Institutional Animal Care and Use Committee of Stanford University.
Antibodies and reagents
PBS- and clodronate-liposomes were prepared as previously described26. TNP(21-31)-BSA was obtained from Santa Cruz, ABT-491 from Sigma-Aldrich; cetirizine DiHCl from Selleck Chemicals; anti-mouse FcγRIII (275003) from R&D Systems; rat IgG2b isotype control (LTF-2) from Bio X Cell. Purified anti-CD200R3 (Ba103) was provided by H. Karasuyama (Tokyo Medical and Dental University Graduate School, Tokyo, Japan). The hybridoma producing mAbs anti-mouse FcγRIV (9E9) was provided by J.V. Ravetch (Rockefeller University, New York, New York, USA), anti-Ly6G (NIMP-R14) by C. Leclerc (Institut Pasteur, Paris, France), IgG1 anti-TNP (TIB-191) by D. Voehringer (Universitätsklinikum, Erlangen, Germany), IgG2a anti-TNP (Hy1.2) by Shozo Izui (University of Geneva, Geneva, Switzerland) and IgG2b anti-TNP (GORK) by B. Heyman (Uppsala Universitet, Uppsala, Sweden): corresponding antibodies were purified as described16. Purified mouse IgE anti-TNP was purchased from BD Pharmingen. MAb 9E9 was coupled to FITC using the Pierce™ FITC Antibody labeling kit (Life Technologies). The antibodies used for flow cytometry staining of c-Kit (clone 2B8), CD49b (clone DX5), IgE (clone R35-72), CD11b (clone M1/70), F4/80 (clone 6F12), CD115 (clone T38-320), Ly6G (clone 1A8) and Ly6C (clone AL-21) were purchased from BD Pharmingen; CD45 (clone 30F11) and Gr1 (clone RB6-8C5) were purchased from Miltenyi Biotec. FcγRIIB was detected using FITC-coupled mAb AT130-2 mIgG1 N297A27.
Passive Systemic Anaphylaxis
IgG-induced PSA: IgG1, IgG2a or IgG2b anti-TNP antibodies were administered intravenously at a dose of 500 μg, if not otherwise indicated, in 200 μL physiological saline, followed by an intravenous challenge with 200 μg of the antigen (TNPBSA) in physiological saline 16 hours later. IgE-induced PSA: IgE anti-TNP antibodies were administered intravenously at a dose of 50 μg in 200 μL physiological saline followed by an intravenous challenge with 500 μg of TNP-BSA in physiological saline 24 hours later. The body temperature of mice was monitored using a digital thermometer with rectal probe (YSI).
In vivo blocking and cellular depletion
300 μl/mouse of PBS- or clodronate-liposomes, 300 μl/mouse of rat IgG2b isotype control or anti-Ly6G, and 30 μg/mouse of anti-CD200R3 mAbs were injected i.v. 24 hours before challenge. Specificity of cell depletion was evaluated using flow cytometry on blood, bone marrow, spleen and peritoneal cells taken from naïve WT mice 24 hours after injection of the depleting antibody or clodronate-liposomes (Examples are shown in Supplemental Figures 1 & 2). 25 μg/mouse of ABT-491 or 300 μl/mouse of cetirizine were injected intravenously 20 minutes or intraperitoneally 30 minutes before challenge, respectively. 200 μg/mouse of anti-FcγRIV mAb were injected intravenously 30 minutes before challenge.
Flow cytometry analysis
Freshly isolated cells were stained with indicated fluorescently labeled mAbs for 30 minutes at 4°C. Cell populations were defined as follows: neutrophils (CD45+/CD11b+/Ly6Ghi/Ly6Cint), monocytes (CD45+/CD11b+/Ly6Glo/Ly6Clo or hi), basophils (CD45int/DX5+/IgE+); spleen macrophages (CD45+/CD11b+/Gr-1lo/CD115+/F4/80hi); peritoneal macrophages (CD45+/CD11b+/F4/80+); peritoneal mast cells (CD45+/c-Kit+/IgE+). Expression of FcγR on indicated cell population is represented as Δ Geomean between specific and isotype control staining. NB: In Figure 5: 1 or 0.5 mg IgG2b was injected to assess expression on neutrophils/monocytes or basophils, respectively.
Surface plasmon resonance analysis
Experiments were performed at 25°C using a ProteOn XPR36 real-time SPR biosensor (BioRad). Anti-TNP antibodies were immobilized covalently through amine coupling on the surface of a GLC chip. TNP-BSA was then injected on the chip at a flow rate of 25 μl.min-1, with contact and dissociation time of 8 minutes each. Binding responses were recorded in real time as resonance units (RU; 1 RU ≈ 1 pg/mm2). Background signals were subtracted, and binding rates (kon and koff) and equilibrium constants (Kd) were determined using the Biaevaluation software (GE Healthcare).
ELISAs
After the induction of IgG1-, IgG2a-, IgG2b- or IgE-induced PSA, plasma and serum were collected at 5 minutes and 3 hours later to determine the histamine and mMCP-1 content, respectively. Histamine and mMCP-1 concentration were determined using commercially available ELISA kits (Beckman Coulter; eBioscience) following the manufacturer's instructions. Relative binding affinity of IgG1, IgG2a and IgG2b anti-TNP antibodies to TNP-BSA was determined by ELISA. Briefly, TNP-BSA-coated plates were incubated with dilutions of IgG1, IgG2a or IgG2b anti-TNP antibodies. After washing, bound anti-TNP IgG were revealed using the same HRP-coupled anti-mouse IgG and SIGMAFAST OPD solution.
Mast cell histology
Mouse back skin biopsies were collected 24 hours after the induction of specific cell depletion and mouse ear skin biopsies were collected 30 minutes after IgE, IgG1, IgG2a or IgG2b-induced PSA, and embedded in paraffin prior to sectioning. Mast cells in toluidine blue-stained biopsies were counted visually in at least 15 FOV/mouse and > 6 mice per treatment (Supplemental Figure 1I).
Statistics
Data were analyzed using one-way or two-way ANOVA with Tukey's post-test. A p-value less than .05 was considered significant: (*p < .05; **p < .01; ***p < .001; ****p < .0001). If not stated otherwise, data are represented as mean +/− SEM.
RESULTS
FcγRIII dominates anaphylaxis induced by IgG subclasses
Passive systemic anaphylaxis was induced by an intravenous injection of one of the different anti-TNP IgG isotypes (IgG1, IgG2a, IgG2b) followed by an intravenous challenge with TNP-BSA 16 h later. This protocol induces a transient decrease in body temperature that is most pronounced between 30 and 40 minutes. As reported previously3, 16, 19, 22, 28, all three IgG isotypes were capable of inducing anaphylaxis in WT mice (Figure 1A-C). In these experimental conditions IgG1-PSA triggered a maximum temperature loss of ≈2°C, IgG2a-PSA of ≈4°C and IgG2b-PSA of ≈3°C in WT mice. Using single FcγR-knockout mice we evaluated the contribution of each of the four mouse FcγRs to these anaphylaxis models. The absence of either FcγRIV (with the exception of a single time point in IgG2b-PSA) or FcγRI had no significant impact on IgG-PSA-induced hypothermia, regardless of the subclass of IgG antibodies used to induce anaphylaxis (Figure 1A-C). The lack of FcγRIII, however, protected mice from anaphylaxis in all models. Mice lacking the inhibitory receptor FcγRIIB had a significantly more severe temperature drop than WT mice in both IgG1- and IgG2b-PSA, but showed no significant difference in the severity of IgG2a-PSA (Figure 1A-C). Even though the three anti-TNP IgG mAbs used are not switch variants of a unique anti-TNP antibody, they show comparable binding to TNP-BSA by ELISA, similar affinity (nanomolar range) and dissociation rates (koff) by surface plasmon resonance analysis, particularly the IgG2a and IgG2b anti-TNP antibodies (Supplemental Figures 3A, B & C). Of note, untreated FcγR-deficient mice presented modest variations in FcγR expression levels (Supplemental Figure 5) and leukocyte representation among blood cells compared to WT mice (Supplemental Figure 6). In particular, a mild lymphopenia in FcγRIV−/− mice and in FcγRIIB−/− mice (the latter also have a tendency to express higher levels of FcγRIII and FcγRIV); and a mild eosinophilia in FcγRIII−/− mice, that also express significantly more FcγRIIB on neutrophils and Ly6Chi monocytes. Together, we think that these variations do not explain the drastic phenotypes observed for PSA in FcγRIIB−/− and FcγRIII−/− mice compared to WT mice. Thus, these data demonstrate that FcγRIII predominates in the induction of IgG1-, IgG2a- and IgG2b-PSA, and that FcγRIIB specifically dampens anaphylaxis severity in IgG1- and IgG2b-PSA.
Figure 1. FcγRIII dominates in IgG-PSA models.
Mice injected with anti-TNP mAbs were challenged with TNP-BSA and body temperatures monitored. (A) IgG1-, (B) IgG2a- or (C) IgG2b-induced PSA in indicated mice (n≥3/group). Data are representative of at least two independent experiments (A: n=2; B: n=3; C: n=2). Significant differences compared to the WT group are indicated.
Basophils, mast cells, monocytes/macrophages and neutrophils contribute differentially to IgG isotype-dependent anaphylaxis models
FcγRIII is expressed by all myeloid cells7, 20 and to a lesser extent by NK cells29. One may therefore anticipate that IgG immune complexes formed in vivo as a consequence of TNP-BSA injection in anti-TNP sensitized mice would therefore engage FcγRIII on these cells, leading to cell activation and possibly contributing to anaphylaxis. Basophils, mast cells, neutrophils and monocyte/macrophages have indeed been reported to contribute to IgG-PSA16, 19, 22, 15, however the respective contribution of each of these different cell types remains debated2, 28. To investigate which cell types contribute to PSA induced by different IgG subclasses, we depleted basophils (anti-CD200R3 mAb), monocytes/macrophages (clodronate-filled liposomes) or neutrophils (anti-Ly6G) prior to anaphylaxis induction or evaluated anaphylaxis induction in transgenic mice deficient in certain cell populations.
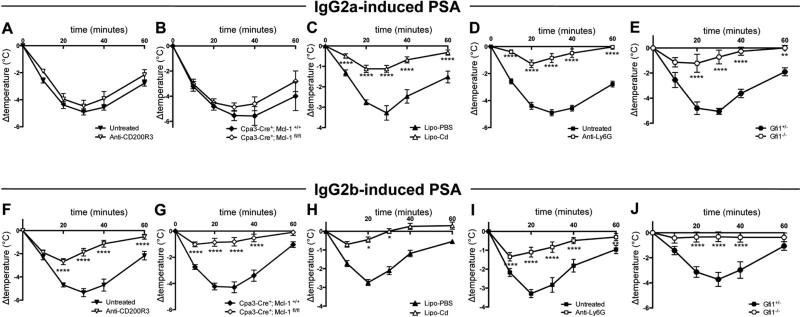
Of note, the relatively mild temperature loss in IgG1-PSA in WT mice (Supplemental Figure 4A), did not allow us to address reliably the contribution of either basophils or neutrophils to this model of anaphylaxis. We therefore restricted our analysis of the contribution of myeloid cell populations to IgG2a-PSA and IgG2b-PSA. Antibody-induced basophil depletion or genetically-induced mast cell and basophil deficiency (Supplemental Figure 2H, Cpa3-Cre; Mcl-1fl/fl mice25), did not affect IgG2a–PSA (Figure 2A&B), but significantly inhibited IgG2b-PSA (Figure 2F&G). Monocyte/macrophage depletion (Figure 2C&H) significantly inhibited both IgG2a- and IgG2b-PSA. The absence of neutrophils, either following antibody-mediated depletion (Figure 2D&I) or using neutropenic Gfi1−/− mice30 (Figure 2E&J), significantly inhibited both IgG2a- and IgG2b-PSA. Whereas monocytes/macrophages and neutrophils appear to contribute to both models of anaphylaxis, basophils and possibly mast cells therefore contribute specifically to IgG2b-PSA, but not to IgG2a-PSA.
Figure 2. Basophils, mast cells, monocytes/macrophages and neutrophils contribute differentially to IgG-PSA models.
Indicated mice (n≥8/group) were injected with IgG2a (A-E) or IgG2b (F-J) anti-TNP mAbs, challenged with TNP-BSA and body temperatures were monitored. WT mice (n=8/group) were pretreated as indicated (A, C-D, F, H-I). Lipo-PBS: PBS liposomes; Lipo-Cd: clodronate liposomes. Data are pooled from at least two independent experiments.
FcγRIII is down-regulated specifically on neutrophils following IgG2a PSA
Khodoun et al proposed to use the reduced expression level of FcγRIII on mouse neutrophils as a marker to distinguish IgE- from IgG1-induced PSA, both of which required priming with an antigen-specific antibody and challenge with the recognized antigen31. We therefore wondered if FcγRIII expression on neutrophils might also be a marker for IgG2a- and IgG2b-PSA. In addition, reduced expression of FcγR(s) following IgG-PSA may document that a particular cell population is activated following engagement of its FcγR(s) by IgG-immune complexes during anaphylaxis. This parameter may thus be used to discriminate cell populations contributing to anaphylaxis following direct activation by IgG-immune complexes from those contributing following activation by mediators liberated by IgG-immune complex-activated cells (e.g. histamine, PAF, leukotrienes and prostaglandins).
Among mouse IgG receptors, only FcγRIIB, FcγRIII and FcγRIV are significantly expressed on circulating myeloid cells, but not FcγRI7, 32, 33. Of circulating monocyte populations, “classical” Ly6Chi monocytes are FcγRIIBmed, FcγRIIImed FcγRIV−, whereas “non-classical” Ly6Clo monocytes are FcγRIIBlo, FcγRIIIlo FcγRIVhi 34. We therefore determined the expression of FcγRIIB, FcγRIII and FcγRIV before and after IgG2a-PSA induction on neutrophils and monocyte subsets. The expression of FcγRIII was down regulated on neutrophils, but not on Ly6Chi monocytes, during IgG2a-PSA (Figure 3A&D). The expression of FcγRIV was also down regulated on neutrophils, but not on Ly6Clo monocytes, during IgG2a-PSA (Figure 3B&D). This was unexpected considering that FcγRIV does not significantly contribute to this PSA model (Figure 1B). The expression of FcγRIIB, however, remained unchanged on Ly6Chi and Ly6Clo monocytes and neutrophils (Figure 3C&D), in agreement with the lack of contribution of this receptor to IgG2a-PSA (Figure 1B). Together these data suggest that neutrophils may directly be activated through FcγRIII by immune complexes formed during IgG2a-PSA. They also suggest that neutrophils, but not Ly6Clo monocytes, may be similarly activated through FcγRIV, even if no contribution of this receptor was identified in this model using FcγRIV−/− mice (Figure 1B).
Figure 3. Reduced expression of FcγRIII and FcγRIV, but not FcγRIIB, on neutrophils following IgG2a-PSA.
(A) FcγRIII, (B) FcγRIV and (C) FcγRIIB expression on blood cells from WT mice (A&B: n=11/group; C: n≥6/group) left untreated, injected with IgG2a anti-TNP mAbs, or injected with IgG2a anti-TNP mAbs and challenged with TNP-BSA. (D) Compilation of Geomean +/− SEM data from A-C.
Elevated IgG2 antibody doses reveal FcγRIV contribution to IgG2a-PSA and IgG2b-PSA
In mice, FcγRIV binds monomeric IgG2a and IgG2b. At physiological concentrations of IgG2a (≈ 2.5 mg/mL) and IgG2b (≈ 1.5 mg/mL) in the serum, FcγRIV may therefore be occupied in vivo, particularly on circulating neutrophils and monocytes. Nevertheless, the short binding half-lives of monomeric IgG2a (t1/2 ≈ 3 min) and monomeric IgG2b (t1/2 ≈ 10 min) by FcγRIV, and their ability to be displaced from this receptor by immune complexes,14 may enable IgG2-immune complexes to interact with FcγRIV during anaphylaxis and therefore contribute to its induction and/or severity.
To explore this possibility, we primed FcγRIII−/− mice with various doses of anti-TNP IgG2a before challenge with TNP-BSA, in order to induce a range of in vivo concentrations of immune complexes. As expected, the low doses did not trigger FcγRIII−/− mice to develop anaphylaxis after challenge. Elevated doses (1 or 2 mg), however, enabled significant temperature drops in FcγRIII−/− mice, comparable to those observed in WT mice primed with 500 g IgG2, particularly at the highest dose of IgG2a (2 mg) (Figure 4A). Already at a dose of 1mg of IgG2, FcγRIII−/− mice developed mild hypothermia in IgG2a-PSA but not in IgG2b-PSA (Figure 4B&C). Unexpectedly in the same conditions, FcγRIV contributed to IgG2b-PSA that was not anymore dampened by inhibitory FcγRIIB (Figure 4C). At a dose of 2 mg of IgG, FcγRIII−/− mice developed hypothermia in both IgG2a-PSA and IgG2b-PSA that was abolished when FcγRIII−/− mice were pre-treated with a blocking antibody against FcγRIV (Figure 4D&E). FcγRI did not contribute to either model of IgG2-PSA at an elevated dose (Figure 4B&C). Furthermore, the expression of FcγRIII was down regulated on neutrophils and basophils, but not on Ly6Chi monocytes, following IgG2b-PSA (Figure 5A&D). The expression of FcγRIV was also down regulated on neutrophils, but not on Ly6Clo monocytes (Figure 5B&D). The expression of FcγRIIB, however, did not change on either neutrophils or Ly6Chi and Ly6Clo monocytes even though this inhibitory receptor regulates IgG2b-PSA (Figures 1C and 5C&D). This observation is in agreement with the report by Khodoun et al, reporting that FcγRIIB expression did not change on neutrophils following IgG1-PSA31. Altogether high doses of antigen-specific IgG2 reveal the contribution of FcγRIV to IgG2a-PSA and to IgG2b-PSA, and suggest the direct activation of neutrophils and basophils by IgG2b-immune complexes.
Figure 4. High doses of IgG2 antibodies reveal FcγRIV contribution to IgG2-PSA.
(A) PSA in indicated mice injected with various doses of IgG2a anti-TNP mAbs (n=2/group). (B-E) PSA in indicated mice (B&C: n=8/group; D&E: n≥3/group) injected with indicated doses of anti-TNP mAbs. Data are pooled from two independent experiments. Significant differences compared to the untreated WT group are indicated.
Figure 5. Expression of FcγRs on myeloid cells following IgG2b-PSA.
(A) FcγRIII (left: n=8/group, right: n=3/group), (B) FcγRIV (n=8/group) and (C) FcγRIIB expression (n≥6/group) on cells from WT mice (n=8/group) left untreated, injected with IgG2b anti-TNP mAbs, or injected with IgG2b anti-TNP mAbs and challenged with TNP-BSA. (D) Compilation of Geomean +/− SEM data from A-C.
IgG1 PSA in the absence of inhibitory FcγRIIB
The unexpected differences observed between IgG2a- and IgG2b-PSA induction pathways prompted us to find a mouse model more sensitive to IgG1-PSA than WT mice, to be able to evaluate the contribution of cell types and mediators also in this model. Indeed, as mentioned earlier, WT mice respond poorly in a model of IgG1-induced (Figure 1A; Supplemental Figure 4A)18. FcγRIIB−/− mice, however, develop a temperature drop of ≈4°C during IgG1-PSA, comparable to temperature losses observed in WT mice during IgG2a- or IgG2b-PSA (Figure 1B&C). We therefore analyzed the contribution of cell types to IgG1-PSA in FcγRIIB−/− mice. Basophil depletion mildly - but significantly - inhibited IgG1-PSA (Figure 6A), in agreement with previous data19. The depletion of neutrophils had the same effect, although not consistently as strongly as basophil depletion (Figure 6B and data not shown). Monocyte/macrophage depletion had only a tendency to ameliorate anaphylaxis that was reproducible but not significant (Figure 6C). These results suggest that IgG1-PSA relies on basophils and neutrophils, and possibly also on monocytes.
Figure 6. Cell contributions to IgG1-PSA in the absence of inhibitory FcγRIIB.
FcγRIIB−/− mice were pretreated as indicated, then injected with IgG1 anti-TNP mAbs, challenged with TNP-BSA and central temperatures were monitored (A: n=8/group; B: n=7/group; C: n=10/group). Data are represented as mean +/− SEM. Data are pooled from two independent experiments.
PAF and histamine contribute differentially to IgG2a- and IgG2b-PSA
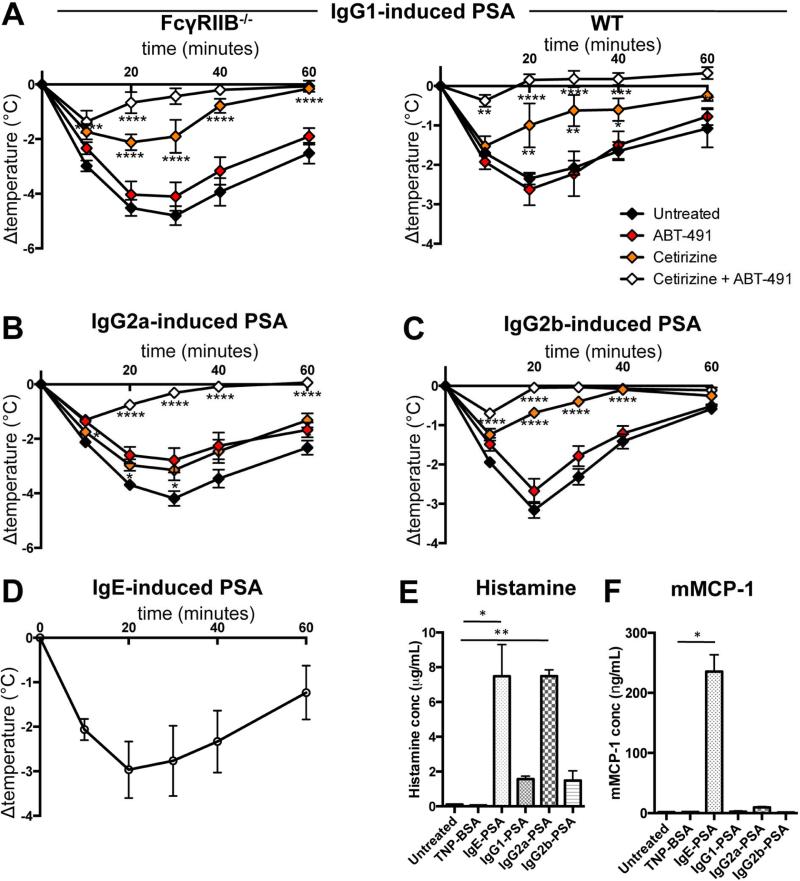
Because cell types contribute differently to IgG2-PSA models (i.e. IgG2a-PSA, neutrophils and monocytes; IgG2b-PSA, basophils, neutrophils and monocytes), one can expect that the mediators responsible for clinical signs also may differ between them. Platelet activating factor (PAF) has been shown to be responsible for anaphylactic reactions that required basophil19, neutrophil16, 24 and/or monocyte/macrophage22 activation, whereas histamine has been shown to be responsible for mast cell- and basophil-dependent anaphylaxis35, 36. Neutrophils are the main producers of PAF37, whereas mast cells and basophils are the main producers of histamine38, 39. We therefore analyzed the relative contribution of these two mediators to the three models of PSA using the histamine-receptor 1 antagonist cetirizine and the PAF-R antagonist ABT-491. Surprisingly, histamine-receptor 1 antagonist cetirizine significantly inhibited IgG1-PSA whereas PAF-R antagonist ABT-491 had no significant effect, in opposition with previous data19. The combination of both antagonists had an additive effect, and almost abolished IgG1-PSA (Figure 7A). These results obtained in FcγRIIB−/− mice were confirmed in WT mice (Figure 7A). Whereas cetirizine mildly reduced hypothermia in IgG2a-PSA, it significantly inhibited IgG2b-PSA. ABT-491 mildly reduced hypothermia in IgG2a-PSA, but had no significant effect on IgG2b-PSA (Figure 7B&C). The combination of cetirizine and ABT-491, however, almost abolished both IgG2a- and IgG2b-PSA. Elevated plasma histamine levels were detected 5 minutes post challenge in all three IgG-PSA models, and particularly high levels were observed in mice undergoing IgE-PSA (as a positive control) or undergoing IgG2a-PSA (Figures 7D&E). This latter finding is surprising as IgG2a-PSA is unaffected by the absence of both mast cells and basophils that are considered major sources of histamine. Mast cell protease-1 (mMCP-1), which is released upon activation of mucosal mast cells, could be detected in the serum of mice undergoing IgE-PSA, but not in those undergoing any one of the three models of IgGPSA, 3 hours post-PSA induction (Figure 7F). Collectively these results suggest that histamine predominantly contributes to IgG1- and IgG2b-PSA, whereas histamine and PAF, together, are necessary for IgG2a-PSA.
Figure 7. Contributions of histamine and PAF to IgG-PSA.
Body temperatures of pretreated mice during (A) IgG1-PSA in FcγRIIB−/− (n=6/group) or WT mice (n=4/group), (B) IgG2a-PSA, (C) IgG2b-PSA or (D) IgE-PSA in WT mice (n≥7/group). (E) Histamine and (F) mMCP-1 concentrations post-PSA (n=3/group). Data are representative of at least two independent experiments, except for A&C (pooled from two independent experiments).
DISCUSSION
Our work suggests that the activating IgG receptor FcγRIII predominantly contributes to IgG-dependent passive systemic anaphylaxis, whether induced by IgG1, IgG2a or IgG2b antibodies. A contribution of the activating IgG receptor FcγRIV was only identified when using very high amounts of IgG2 antibodies, whereas the activating IgG receptor FcγRI played no detectable role. Remarkably, the inhibitory IgG receptor FcγRIIB controlled the severity of IgG1- and IgG2b-, but not IgG2a-induced anaphylaxis. The ability of FcγRIIB to inhibit a given model of IgG-induced anaphylaxis correlated with the contribution of basophils and histamine to that model. Indeed, basophils, and possibly mast cells, contributed with neutrophils to IgG1-PSA, and with neutrophils and monocytes to IgG2b-PSA, but not to IgG2a-PSA that appeared to depend entirely on neutrophils and monocytes/macrophages. Altogether our data propose that the three IgG subclasses IgG1, IgG2a and IgG2b induce three qualitatively different pathways of anaphylaxis that are nevertheless triggered primarily by a single IgG receptor, FcγRIII.
FcγRIII is a low-affinity receptor for IgG1, IgG2a and IgG2b, whereas FcγRI is a high-affinity receptor for IgG2a, and FcγRIV is a high affinity receptor for IgG2a and IgG2b. One would therefore assume that FcγRIII predominates in IgG1-PSA, FcγRI and FcγRIV in IgG2a-PSA, and FcγRIV in IgG2b-PSA. However, our data from FcγRIII−/− mice indicate that this receptor predominates in all three models. Notably, we found an increased expression of FcγRIIB on neutrophils and Ly6Chi monocytes in FcγRIII−/− mice, which could mask a potential contribution of FcγRIV in these conditions. In support of the notion that FcγRIII predominates IgG-PSA induction, an alternative model of PSA induced by sensitization and challenge with goat antibodies was found to be driven by FcγRIII22 and blocking antibodies against FcγRIII were protective in a model of PSA induced by IgG immune complexes16. In addition, IgG2a-PSA in FcγRIIB−/− mice was abolished following injection of anti-FcγRIIB/III blocking mAbs5. FcγRIII is the only activating IgG receptor in the mouse that does not bind an IgG subclass with high affinity, thus it remains unoccupied by monomeric IgG and accessible for binding of immune complexes. This is theoretically not the case for FcγRI and FcγRIV, which at physiological serum concentrations of IgG2a (≈ 2.5 mg/mL) and IgG2b (≈ 1.5 mg/mL), are likely occupied in vivo, particularly on circulating cells. Of note, C57Bl/6 mice produce IgG2c, but not IgG2a antibodies, whose amino acid sequence varies by about 15%. Experiments performed in Balb/c mice that express endogenous IgG2a (but no IgG2c) gave similar results regarding the contribution of basophils, neutrophils and monocytes to IgG2a (Supplemental Figure 4B), indicating that IgG2a and IgG2c sequence variations probably do not affect the mechanisms of anaphylaxis induction that we describe herein.
Adult female mice of 20 g, as used in this study, possess a circulating blood volume of 1.4-1.5 mL. Injection of 500 μg antibody thus corresponds to ≈330 μg/mL of circulating antibody, injection of 1 mg to ≈660 μg/mL, and injection of 2 mg to ≈1,3 mg/mL. In cases of anaphylaxis the circulating concentration of allergen-specific IgG has not been evaluated due to lack of testing and appropriate controls (i.e. monoclonal anti-allergen antibodies); although we have reported high circulating antigen-specific IgG levels in an autoimmune model of arthritis33. It seems rather unlikely that patients suffering from anaphylaxis possess such elevated circulating levels of IgG anti-allergen as in the mice receiving the high doses we used in this study. Nevertheless, our results in high-dose IgG2a- and IgG2b-PSA demonstrate that FcγRIV can by itself (i.e. in the absence of FcγRIII) trigger anaphylaxis. Similar results have been obtained in mice expressing only FcγRIV: “FcγRIV-only” mice developed IgG2b-PSA after injection of pre-formed IgG2b immune complexes and also upon injection of polyclonal anti-sera followed by a challenge with the antigen16. We reported previously that IgG2b-PSA triggered by the injection of preformed IgG2b-immune complexes in WT mice was abolished following injection of anti-FcγRIV blocking mAb 9E9. This contrasts with the findings of the current study, in which we show that FcγRIII is the major activating receptor in all models of IgG-PSA, and FcγRIV contributes only at high antibody concentrations. Two hypotheses may explain these discrepant results: i) the injection of preformed IgG2b-immune complexes leads to an immediate circulating bolus of immune complexes, which are similarly formed only after injection of high amounts of IgG2b and antigen, thus triggering FcγRIV; 2) as recently reported40 mAb 9E9 may not only block FcγRIV through its Fab portions, but also FcγRIII via its Fc portion once 9E9 is bound to FcγRIV. In our view, it is likely that a combination of these mechanisms reconcile our previous and herein described results, and suggest that IgG2b-PSA induced following injection of preformed IgG2b-immune complexes relies rather on both FcγRIII and FcγRIV than on FcγRIV alone as we reported previously16. Together this body of evidence supports the notion that FcγRIV is capable of triggering cell activation leading to anaphylaxis, yet in restricted conditions, i.e. in the absence/blockade of FcγRIII or in presence of large amounts of IgG2a and/or IgG2b antibodies.
The differential contribution of FcγRs to IgG-PSA may rely on their respective expression patterns on myeloid cells. Indeed, FcγRI is not32, 33 or only barely34 expressed on circulating monocytes, and its expression is largely restricted to tissue-resident macrophages. The level of its expression on cells reported to contribute to anaphylaxis (i.e. monocytes in this case) may therefore not suffice to induce their activation. This notion is supported by the absence of any detectable effect of FcγRI deficiency in IgG2-PSA that we report in this study, even at high doses of IgG2 antibodies. FcγRIII, however, is expressed on all myeloid cells7 and moreover at comparably high levels on all those cell types that have been reported to contribute to anaphylaxis; basophils, monocytes and neutrophils20. This pattern of cellular expression may explain its predominant contribution to all models of IgG-induced anaphylaxis. FcγRIV is expressed on neutrophils and Ly6Clo monocytes. It remains unclear, however, if Ly6Clo, Ly6Chi or both monocyte subsets contribute to anaphylaxis. FcγRIV could contribute to PSA induction in exceptional conditions (FcγRIII deficiency or high IgG2 antibody doses). The lack of FcγRIV contribution in classical conditions of PSA may suggest that its expression level is not sufficient in WT mice. Notably, it has been reported previously that particular FcγR deficiencies modify the expression levels of other FcγRs. In particular FcγRIII−/− mice, but not FcγRI−/− mice, presented a significant increase in FcγRIV expression levels on neutrophils16, 41, 42 and a tendency for increased expression on Ly6Clo monocytes (Supplemental Figure 5B). This could explain why the contribution of FcγRIV to IgG2-PSA becomes apparent in FcγRIII−/− mice. FcγRIV−/− mice did not, conversely, present alterations of FcγRIII expression on neutrophils or Ly6Chi monocytes compared to WT littermates (Supplemental Figure 5A). FcγRIIB−/− mice expressed significantly higher levels of FcγRIII and FcγRIV on neutrophils and increased FcγRIII on Ly6Chi monocytes that may, altogether, contribute to their higher susceptibility to anaphylaxis induction (Supplemental Figure 5A&B).
The contribution of a rather restricted subset of myeloid cells to these (and other) models of anaphylaxis2, 3 appears to be determined by at least two factors: their capacity to release anaphylactogenic mediators (e.g. histamine or PAF) and their expression of sufficient levels of activating IgG receptors. Mast cells and basophils release histamine, and neutrophils, monocytes/ macrophages and basophils release PAF, upon FcγR-triggering. Other mediators may induce anaphylaxis or contribute to its severity, among them lipid mediators like prostaglandins, thromboxanes and leukotrienes. Some of these have indeed been reported to trigger bronchoconstriction and an increase in vascular permeability43. The release of such mediators is sufficiently rapid to coincide with the celerity of hypothermia, which is detectable within minutes after allergen challenge. It is therefore surprising that eosinophils do not contribute to IgG-PSA, as they express high levels of activating FcγRIII and FcγRIIB20 (but no FcγRI or FcγRIV), and are capable of releasing Leukotriene C4, Prostaglandin E2, thromboxane and PAF upon activation43. Though eosinophils represent relatively low numbers among blood cells (≈2×105/mL), this is an unlikely explanation because basophils are significantly less numerous (≈5×104/mL) but do contribute to anaphylaxis models. Most revealingly, it has been reported that eosinophils do not release PAF following IgG-dependent activation44. Whether eosinophils produce other potentially anaphylactogenic mediators following IgG-immune complex activation has not been investigated, but the lack of such an effect appears the most reasonable hypothesis to explain why eosinophils have not been found to contribute to IgG-induced anaphylaxis.
We investigated the contribution of neutrophils and monocytes to IgG-PSA models using depletion approaches. Ly6G+ cell depletion using NIMP-R14 resulted in an efficient depletion of neutrophils in the blood and the spleen (Supplemental Figures 1B&2B). The same treatment resulted only in a partial depletion in the bone marrow, in which a proportion of Ly6G+ cells are masked from fluorescent anti-Ly6G staining, but not depleted by NIMPR14 treatment (refer to bone marrow panels in Supplemental Figures 1C,D & 2C,D,I). Importantly, we found that NIMP-R14 depletion has a significant impact on monocyte populations in the blood and to some extent in the spleen. This should be taken into consideration when interpreting the results of NIMP-R14 depletion experiments. All IgG-PSA models were ameliorated following NIMP-R14 depletion, but also when monocytes/macrophages were targeted using clodronate liposomes. Intravenous injection of clodronate liposomes resulted in a significant depletion of monocytes from the blood and monocytes/macrophages from the spleen and BM, but not from the skin (data not shown) and peritoneum (Supplemental Figures 1&2, as reported26), and to a significant increase in blood leukocyte counts and particularly of neutrophils (Supplemental Figures 1&2). Thus the anti-Ly6G and the clodronate liposome treatments alter also the monocytes and neutrophil compartment, respectively, but reduced hypothermia in the three models of IgG-PSA studied. Constitutive deficiency in neutrophils, studied using Gfi1−/− mice, confirmed the role of neutrophils in IgG2a- and IgG2b-PSA models. Both neutrophils and monocytes can therefore be considered to contribute to IgG-induced anaphylaxis in mice, whether dependent on IgG1, IgG2a or IgG2b. The role of macrophages in the different IgG-PSA models remains to be investigated more deeply, as clodronate liposomes injected intravenously efficiently targeted macrophages in the spleen, but not in other tissues like peritoneum or skin, and thus do not allow conclusions on their contribution.
The contribution of basophils to models of anaphylaxis has been a recent matter of debate. Tsujimura et al reported that depletion of basophils using anti-CD200R3 (clone Ba103) monoclonal antibodies strongly inhibited IgG1-PSA and rescued mast cell-deficient mice from active anaphylaxis19. Ohnmacht et al, however, found that basophil-deficient Mcpt8cre mice demonstrated slightly decreased but significant hypothermia in response to IgG1-PSA (induced with the same antibody clone) when compared to WT mice45. More recently, Reber et al. reported that peanut-induced anaphylaxis was reduced following Diphtheria toxin injection in Mcpt8DTR mice that selectively depletes basophils, and confirmed that basophil depletion using anti-CD200R3 mAbs inhibited anaphylaxis36. Moreover, Khodoun et al found a contribution of basophils to anaphylaxis mortality, but not to hypothermia, in a model of IgG2a-PSA following anti-CD200R3 mAb injection5. It therefore appears that differences between inducible basophil depletion using specific antibodies or toxin administration and a constitutive lack of basophils, possibly leading to compensatory mechanisms during development of these mice, may account for the divergent results observed. Intriguingly however, basophils have been reported to be resistant to IgG-immune complex triggering ex vivo due to dominant inhibition by FcγRIIB over activation by FcγRIII20. In this study, we report that both basophil depletion following anti-CD200R3 mAb (Ba103) injection or constitutive deficiency of basophils and mast cells in Cpa3-Cre; Mcl-1fl/fl mice inhibits IgG2b-PSA but not IgG2a-PSA, confirming a role for basophils (and potentially mast cells) to specific IgG-PSA models. Of note, Ba103 efficiently depleted basophils from the blood and partially from the spleen and the bone marrow, but had no significant effect on mast cells in the peritoneum or skin (Supplemental Figures 1A&1E and 2A&2E). The difference in the ability of basophils to respond to IgG-immune complex triggering in vitro and the various in vivo models may be explained by functional alterations during basophil purification or a requirement for co-stimulation by other cells or their products that are present in vivo, but not ex vivo, for basophils to respond to IgG-immune complexes. Our results using Cpa3-Cre; Mcl-1fl/fl mice indicate that mast cells were not necessary for IgG2a-PSA. We could not formally define their role in IgG2b-PSA as basophil depletion and deficiency in basophils and mast cells lead to similar reduction in IgG2b-PSA. Notably, increased plasma histamine levels, but no increase in mMCP-1 levels could be detected, suggesting that mucosal mast cells were not activated during IgG-PSA. Intriguingly, however, some dermal mast cells displayed a degranulated morphology 30 minutes after challenge in all IgG PSA models tested (examples are shown in Supplemental Figure 7). Whether their degranulation is a cause or a consequence of anaphylaxis remains however elusive.
The ability of cells expressing activating FcγRs to respond to IgG-immune complexes has been proposed to be regulated by co-expression of FcγRIIB46. FcγRIIB−/− mice develop increased hypersensitivity and anaphylactic reactions to IgG1-PSA (this report and16, 18). Our results further demonstrate that FcγRIIB inhibits IgG2b-, but not IgG2a-PSA. This latter finding is supported by results from Khodoun et al5: these authors proposed that the lack of this inhibitory receptor may lead to increased spontaneous formation of immune complexes in FcγRIIB−/− mice, that could compete with IgG2a-immune complexes. In light of our results comparing IgG1-, IgG2a- and IgG2b-PSA, we rather propose that the significantly lower affinity of inhibitory FcγRIIB for IgG2a (KA = 4.2 105 M−1) than for IgG1 (KA = 3.3 106 M−1) and IgG2b (KA = 2.2 106 M−1) is the determining factor (Table 1). Another factor may be the variance in expression of FcγRIIB on circulating myeloid cells: basophils > monocytes > eosinophils >> neutrophils20. Whereas the exact numbers of expressed activating FcγRIII and inhibitory FcγRIIB per cell remain unknown, flow cytometric analysis allowed the estimation of their relative expression: indeed, the ratio FcγRIII/FcγRIIB is higher on neutrophils than on monocytes and basophils. These differential expression levels may thus explain why neutrophils contribute to anaphylaxis, as the receptor balance is in favor of the activating receptor. Strikingly, FcγRIIB is co-expressed only with FcγRIII on basophils and Ly6Chi monocytes, whereas it is co-expressed with FcγRIII and FcγRIV on neutrophils and Ly6Clo monocytes34. Contribution of a given cell type to anaphylaxis may therefore be favored when inhibitory FcγRIIB is required to dampen the stimulatory potential of two activating IgG receptors instead of one. This concept extends to IgG1-immune complexes that only engage one activating receptor, FcγRIII.
Our results on the contribution of mouse IgG receptors, cells and mediators in IgG-induced anaphylaxis can potentially be translated to human IgG-mediated anaphylaxis, e.g. following intravenous IgG or therapeutic IgG antibody administration. Indeed, even though IgG receptors are different in the two species, we have already reported that human FcγRI (hFcγRI) and human FcγRIIA (hFcγRIIA) can induce anaphylaxis when expressed under the control of their own promoter in transgenic mice23, 24. hFcγRI (CD64) is the equivalent of mouse FcγRI whereas hFcγRIIA (CD32A) can be regarded as the equivalent of mouse FcγRIII, and hFcγRIIIA (CD16A) the equivalent of mouse FcγRIV7. hFcγRIIA, like mouse FcγRIII, is expressed on all myeloid cells and could therefore act as the principal IgG receptor responsible for anaphylaxis in humans. hFcγRIIB, the equivalent of mouse FcγRIIB, is scarcely expressed on most circulating myeloid cells47 except for its high expression on basophils20, suggesting that among myeloid cells only human basophils are highly sensitive to hFcγRIIB-mediated inhibition. In contrast to mouse FcγRI, hFcγRI is constitutively expressed on circulating monocytes and inducibly on neutrophils, allowing this receptor to induce anaphylaxis24. The binding of human IgG subclasses to hFcγRs differs strikingly from the binding of mouse IgG subclasses to mouse FcγRs. Noticeably, the affinity of hFcγRIIB for any human IgG subclass is the lowest among human IgG-hFcγR interactions. For example, human IgG1, the equivalent of mouse IgG2a, is bound by all activating hFcγRs (KA > 106 M−1) with at least a ten-fold higher affinity than by inhibitory hFcγRIIB (KA ≈ 105 M−1)48. If we consider the translation of our results obtained in the mouse to human IgG-induced anaphylaxis, one could anticipate that hFcγRIIB-mediated inhibition of IgG-induced anaphylaxis is inefficient in human neutrophils and monocytes, and efficient only in human basophils for which the elevated hFcγRIIB expression may compensate for the low-affinity of this receptor for human IgG subclasses. Certainly, FcγR-engagement by IgG immune complexes on human basophils could not trigger any detectable basophil activation in vitro20, similar to the results we reported for mouse basophil activation. Our data altogether propose that the differential expression of inhibitory FcγRIIB on myeloid cells and its differential binding of IgG subclasses control the contribution of basophils, neutrophils and monocytes to IgG-dependent anaphylaxis, thus revealing novel complexities in the mechanism of regulation of cell populations, and therefore their contribution to IgG-mediated reactions in vivo.
Supplementary Material
CLINICAL IMPLICATIONS.
Anaphylactic pathways induced by different IgG subclasses in mice vary in terms of contributions by different cell types, mediators and antibody receptors. These results may help in the design of efforts to understand and treat IgG-mediated anaphylaxis in humans, e.g., as seen following intravenous IgG or administration of therapeutic IgG antibodies.
CAPSULE SUMMARY.
Antibodies of the IgG class can contribute to anaphylaxis. This report reveals pathways induced by each IgG subclass in experimental anaphylaxis, demonstrating varying contributions of cells, mediators and antibody receptors.
ACKNOWLEDGMENTS
We are thankful to our colleagues at Institut Pasteur, Paris: D. Sinnaya for administrative help, Stéphane Petres for help with antibody purifications and Laurence Fiette for help with histological analyses. We are thankful to our colleagues for their generous gifts: T. Moroy (Montreal University, Montreal, QC, Canada), Clifford Lowell (University of California at San Francisco, CA, USA), J.V. Ravetch (Rockefeller University, New York, NY, USA) and J. Leusen (University Medical Center, Utrecht, The Netherlands) for mice; R. Coffman (DNAX, Palo Alto, CA, USA), R. Good (USFCM, Tampa, FL, USA), B. Heyman (Uppsala Universitet, Uppsala, Sweden), H. Karasuyama (Tokyo Medical and Dental University Graduate School, Tokyo, Japan) and D. Voehringer (Universitätsklinikum, Erlangen, Germany) for antibodies. Cl2MDP was a gift of Roche Diagnostics GmbH. This work was supported by the Institut Pasteur, the Institut National de la Santé et de la Recherche Médicale (INSERM), the European Research Council (ERC)–Seventh Framework Program (ERC-2013-CoG 616050), the Société Française d’Allergologie (SFA; Soutien de la Recherche en Allergologie) and the Balsan company. H.B. is supported by a fellowship from the University Pierre et Marie Curie. C.M.G. is a scholar of the Pasteur Paris University International Doctoral Program and supported by a stipend from the Institut Carnot Pasteur Maladies Infectieuses. F.J. is an employee of the Centre National de La Recherche Scientifique (CNRS). R.S. and S.J.G. are supported by NIH/NIAMS grant R01 AR067145 and the Department of Pathology at Stanford University.
Sources of funding: none of the sources of funding have an interest in the subject matter or materials discussed in the submitted manuscript
ABBREVIATIONS USED
- FcγR
IgG Fc receptor
- PAF
Platelet-activating factor
- KA
Affinity constant
- WT
C57Bl/6 Wild-type
- PSA
Passive systemic anaphylaxis
- TNP
Trinitrophenyl
- BSA
Bovine serum albumin
- mAb
Monoclonal antibody
- PBS
Phosphate Buffered Saline
- Gfi1
Growth Factor Independence-1
- GeoMean
Geometric Mean
- SEM
Standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORSHIP AND CONFLICT OF INTEREST STATEMENTS
H.B. performed all experiments at the Institut Pasteur with contributions from P.E, C.M.G, F.J.; R.S. and L.L.R contributed experiments using Cpa3-Cre; Mcl-1fl/fl mice; B.I. and O.G. genotyped mice and produced reagents; M.S.C., S.J.G. and N.v.R. provided reagents; H.B., P.B., P.E., C.M.G., S.J.G, F.J., D.A.M., L.L.R. and R.S. analyzed and discussed results; F.J., P.B. and D.A.M. supervised and designed the research; P.B. and F.J. wrote the manuscript. All authors read and had an opportunity to contribute to the editing of the manuscript, and declare no competing financial interests.
REFERENCES
- 1.Brown SG, Stone SF, Fatovich DM, Burrows SA, Holdgate A, Celenza A, et al. Anaphylaxis: Clinical patterns, mediator release, and severity. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol. 2005;115:449–57. doi: 10.1016/j.jaci.2004.12.1125. quiz 58. [DOI] [PubMed] [Google Scholar]
- 3.Jonsson F, Mancardi DA, Albanesi M, Bruhns P. Neutrophils in local and systemic antibody-dependent inflammatory and anaphylactic reactions. J Leukoc Biol. 2013;94:643–56. doi: 10.1189/jlb.1212623. [DOI] [PubMed] [Google Scholar]
- 4.Iff ET, Vaz NM. Mechanisms of anaphylaxis in the mouse. Similarity of shock induced by anaphylaxis and by mixtures of histamine and serotonin. Int Arch Allergy Appl Immunol. 1966;30:313–22. doi: 10.1159/000229815. [DOI] [PubMed] [Google Scholar]
- 5.Khodoun MV, Kucuk ZY, Strait RT, Krishnamurthy D, Janek K, Clay CD, et al. Rapid desensitization of mice with anti-FcgammaRIIb/FcgammaRIII mAb safely prevents IgG-mediated anaphylaxis. J Allergy Clin Immunol. 2013;132:1375–87. doi: 10.1016/j.jaci.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Million M, Fioramonti J, Zajac JM, Bueno L. Effects of neuropeptide FF on intestinal motility and temperature changes induced by endotoxin and platelet-activating factor. Eur J Pharmacol. 1997;334:67–73. doi: 10.1016/s0014-2999(97)01142-4. [DOI] [PubMed] [Google Scholar]
- 7.Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–9. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- 8.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014;14:94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- 9.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 10.Bruhns P, Fremont S, Daëron M. Regulation of allergy by Fc receptors. Curr Opin Immunol. 2005;17:662–9. doi: 10.1016/j.coi.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Gavin AL, Barnes N, Dijstelbloem HM, Hogarth PM. Identification of the mouse IgG3 receptor: implications for antibody effector function at the interface between innate and adaptive immunity. J Immunol. 1998;160:20–3. [PubMed] [Google Scholar]
- 12.Saylor CA, Dadachova E, Casadevall A. Murine IgG1 and IgG3 isotype switch variants promote phagocytosis of Cryptococcus neoformans through different receptors. J Immunol. 2010;184:336–43. doi: 10.4049/jimmunol.0902752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unkeless JC, Eisen HN. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J Exp Med. 1975;142:1520–33. doi: 10.1084/jem.142.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancardi DA, Iannascoli B, Hoos S, England P, Daeron M, Bruhns P. FcgammaRIV is a mouse IgE receptor that resembles macrophage FcepsilonRI in humans and promotes IgE-induced lung inflammation. J Clin Invest. 2008;118:3738–50. doi: 10.1172/JCI36452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99:901–14. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jönsson F, Mancardi DA, Kita Y, Karasuyama H, Iannascoli B, Van Rooijen N, et al. Mouse and human neutrophils induce anaphylaxis. J Clin Invest. 2011;121:1484–96. doi: 10.1172/JCI45232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–2. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 18.Ujike A, Ishikawa Y, Ono M, Yuasa T, Yoshino T, Fukumoto M, et al. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J Exp Med. 1999;189:1573–9. doi: 10.1084/jem.189.10.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin- E-mediated systemic anaphylaxis. Immunity. 2008;28:581–9. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Cassard L, Jonsson F, Arnaud S, Daeron M. Fcgamma receptors inhibit mouse and human basophil activation. J Immunol. 2012;189:2995–3006. doi: 10.4049/jimmunol.1200968. [DOI] [PubMed] [Google Scholar]
- 21.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. Fc gamma RIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol. 2002;109:658–68. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 23.Jonsson F, Mancardi DA, Zhao W, Kita Y, Iannascoli B, Khun H, et al. Human FcgammaRIIA induces anaphylactic and allergic reactions. Blood. 2012;119:2533–44. doi: 10.1182/blood-2011-07-367334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancardi DA, Albanesi M, Jonsson F, Iannascoli B, Van Rooijen N, Kang X, et al. The high-affinity human IgG receptor FcgammaRI (CD64) promotes IgG-mediated inflammation, anaphylaxis, and antitumor immunotherapy. Blood. 2013;121:1563–73. doi: 10.1182/blood-2012-07-442541. [DOI] [PubMed] [Google Scholar]
- 25.Lilla JN, Chen CC, Mukai K, BenBarak MJ, Franco CB, Kalesnikoff J, et al. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930–8. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 27.Williams EL, Tutt AL, French RR, Chan HT, Lau B, Penfold CA, et al. Development and characterisation of monoclonal antibodies specific for the murine inhibitory FcgammaRIIB (CD32B). Eur J Immunol. 2012;42:2109–20. doi: 10.1002/eji.201142302. [DOI] [PubMed] [Google Scholar]
- 28.Jiao D, Liu Y, Lu X, Liu B, Pan Q, Liu Y, et al. Macrophages are the dominant effector cells responsible for IgG-mediated passive systemic anaphylaxis challenged by natural protein antigen in BALB/c and C57BL/6 mice. Cell Immunol. 2014;289:97–105. doi: 10.1016/j.cellimm.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Biburger M, Nimmerjahn F. Low level of FcgammaRIII expression on murine natural killer cells. Immunol Lett. 2012;143:53–9. doi: 10.1016/j.imlet.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Yucel R, Kosan C, Heyd F, Moroy T. Gfi1:green fluorescent protein knock-in mutant reveals differential expression and autoregulation of the growth factor independence 1 (Gfi1) gene during lymphocyte development. J Biol Chem. 2004;279:40906–17. doi: 10.1074/jbc.M400808200. [DOI] [PubMed] [Google Scholar]
- 31.Khodoun MV, Strait R, Armstrong L, Yanase N, Finkelman FD. Identification of markers that distinguish IgE- from IgG-mediated anaphylaxis. Proc Natl Acad Sci U S A. 2011;108:12413–8. doi: 10.1073/pnas.1105695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan PS, Gavin AL, Barnes N, Sears DW, Vremec D, Shortman K, et al. Unique monoclonal antibodies define expression of Fc gamma RI on macrophages and mast cell lines and demonstrate heterogeneity among subcutaneous and other dendritic cells. J Immunol. 2003;170:2549–56. doi: 10.4049/jimmunol.170.5.2549. [DOI] [PubMed] [Google Scholar]
- 33.Mancardi DA, Jonsson F, Iannascoli B, Khun H, Van Rooijen N, Huerre M, et al. The murine high-affinity IgG receptor Fc(gamma)RIV is sufficient for autoantibody- induced arthritis. J Immunol. 2011;186:1899–903. doi: 10.4049/jimmunol.1003642. [DOI] [PubMed] [Google Scholar]
- 34.Biburger M, Aschermann S, Schwab I, Lux A, Albert H, Danzer H, et al. Monocyte subsets responsible for immunoglobulin G-dependent effector functions in vivo. Immunity. 2011;35:932–44. doi: 10.1016/j.immuni.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Makabe-Kobayashi Y, Hori Y, Adachi T, Ishigaki-Suzuki S, Kikuchi Y, Kagaya Y, et al. The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. J Allergy Clin Immunol. 2002;110:298–303. doi: 10.1067/mai.2002.125977. [DOI] [PubMed] [Google Scholar]
- 36.Reber LL, Marichal T, Mukai K, Kita Y, Tokuoka SM, Roers A, et al. Selective ablation of mast cells or basophils reduces peanut-induced anaphylaxis in mice. J Allergy Clin Immunol. 2013;132:881–8. e11. doi: 10.1016/j.jaci.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camussi G, Aglietta M, Coda R, Bussolino F, Piacibello W, Tetta C. Release of platelet-activating factor (PAF) and histamine. II. The cellular origin of human PAF: monocytes, polymorphonuclear neutrophils and basophils. Immunology. 1981;42:191–9. [PMC free article] [PubMed] [Google Scholar]
- 38.Wedemeyer J, Tsai M, Galli SJ. Roles of mast cells and basophils in innate and acquired immunity. Curr Opin Immunol. 2000;12:624–31. doi: 10.1016/s0952-7915(00)00154-0. [DOI] [PubMed] [Google Scholar]
- 39.Jonsson F, Daeron M. Mast cells and company. Front Immunol. 2012;3:16. doi: 10.3389/fimmu.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tipton TR, Mockridge CI, French RR, Tutt AL, Cragg MS, Beers SA. Anti-mouse FcgammaRIV antibody 9E9 also blocks FcgammaRIII in vivo. Blood. 2015;126:2643–5. doi: 10.1182/blood-2015-09-671339. [DOI] [PubMed] [Google Scholar]
- 41.Syed SN, Konrad S, Wiege K, Nieswandt B, Nimmerjahn F, Schmidt RE, et al. Both FcgammaRIV and FcgammaRIII are essential receptors mediating type II and type III autoimmune responses via FcRgamma-LAT-dependent generation of C5a. Eur J Immunol. 2009;39:3343–56. doi: 10.1002/eji.200939884. [DOI] [PubMed] [Google Scholar]
- 42.Nimmerjahn F, Lux A, Albert H, Woigk M, Lehmann C, Dudziak D, et al. FcgammaRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc Natl Acad Sci U S A. 2010;107:19396–401. doi: 10.1073/pnas.1014515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capron M. Eosinophils: receptors and mediators in hypersensitivity. Clin Exp Allergy. 1989;19(Suppl 1):3–8. [PubMed] [Google Scholar]
- 45.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–74. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Smith KG, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol. 2010;10:328–43. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veri MC, Gorlatov S, Li H, Burke S, Johnson S, Stavenhagen J, et al. Monoclonal antibodies capable of discriminating the human inhibitory Fcgamma-receptor IIB (CD32B) from the activating Fcgamma-receptor IIA (CD32A): biochemical, biological and functional characterization. Immunology. 2007;121:392–404. doi: 10.1111/j.1365-2567.2007.02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human Fc{gamma} receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–25. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.