Abstract
Disability weight for each disease plays a key role in combining years lived with disability and years of life lost in disability adjusted life year. For the Korean Burden of Disease 2012 study, we have conducted a re-estimation of disability weights for causes of disease by adapting the methodology of a recent Global Burden of Disease study. Our study was conducted through a self-administered web-based survey using a paired comparison (PC) as the main valuation method. A total of 496 physicians and medical college students who were attending in third or fourth grade of a regular course conducted the survey. We applied a probit regression on the PC data and computed the predicted probabilities of each cause of disease from the coefficient estimates of the probit regression. We used 'being dead (1)' and 'full health (0)' as anchor points to rescale the predicted probability of each cause of disease on a scale of 0 to 1. By this method, disability weights for a total of 228 causes of disease were estimated. There was a fairly high correlation between the disability weights of overlapping causes of disease from this study and a previous South Korean study despite the differences in valuation methods and time periods. In conclusion, we have shown that disability weights can be estimated based on a PC by including 'full health' and 'being dead' as anchor points without resorting to a person trade-off. Through developments in the methodology of disability weights estimation from this study, disability weights can be easily estimated and continuously revised.
Keywords: Disability Weight, Burden of Disease, Republic of Korea, Paired Comparison
Graphical Abstract

INTRODUCTION
Disability adjusted life year (DALY) has become a standard summary measure reflecting disease burden (1). The DALY aggregates the total burden of diseases into a single index by combining years lived with disability (YLD) and years of life lost (YLL) (2). With this measure, we can estimate the magnitude of disease burden and compare these values between diseases, so that we can prioritize policies and interventions to reduce the burden of disease (3). In DALY, the disability weight for each disease plays a key role in combining YLDs and YLLs (4). The level of disability for each disease is converted into a disability weight that rates a disease's disability from 0 (equivalent to full health) to 1 (equivalent to being dead).
Since 1996, there have been several studies that have tried to estimate the disability weights and to develop the methodology in response to criticisms about the methods for measuring disability weights (4,5,6,7,8). In the global burden of disease (GBD) 1990 study, the judgments of ten healthcare professionals were used to establish disability weights using a visual analogue scale (VAS) and a person trade-off (PTO) (5). Most recently, a disability weights measurement study for the GBD 2010 was conducted involving the general public to elicit judgments about disability due to many causes of disease through two valuation techniques: a paired comparison (PC) and a population health equivalence (PHE, modified from a PTO) (4). The results from a PC were hybridized with that from a PHE in the GBD 2010 disability weights measurement study.
In the GBD 2013 study, the disability weights were revised from previous versions by adding the results from a European disability weight study (9). The European disability weight study adapted the approach of the GBD 2010 disability weights measurement study by using a web-based sample survey with same valuation techniques (PC and PHE) (10). Likewise, the studies have been revising disability weights and methodology to effectively determine a valid and timely outcome and to efficiently elicit preferences about causes of diseases. However, several issues still remain as follows: the appropriateness of the health state or disease classification and valuation method, selection of survey participants to elicit preferences, validation of disability weights, and cross-cultural variability of disability weights (11,12,13,14,15). In particular, the problem of using PTO or PHE was questioned in terms of the lack of theoretical basis (16,17).
In the case of South Korea, the results for a disability weight measurement study were published for the first time in 2000, in which disability weights of major cancers were estimated by using the Delphi method (18). An addition and amendments to these disability weights were performed in 2002 (published in 2003) (19,20,21). A total of 16 indicator diseases' disability weights were calculated with a PTO, and the disability weights for 123 health states of diseases were interpolated using the disability weights for the indicator diseases (20). Although disability weights for 24 major cancers were recently evaluated based on a VAS, disability weights for the Korean Burden of Disease (KBD) 2012 study needed to be amended to reflect the latest changes (22). The disability level of diseases can change as medical technology advances the treatment and management of disease. Furthermore, a more efficient recent method to estimate disability weights, such as in the GBD 2010 or GBD 2013 studies, is required as the number of diseases to be considered increases in burden of disease studies.
For the KBD 2010 study, we have conducted a re-estimation of disability weights adapting the methodology of the recent GBD study. Specifically, in this study we asked medical professionals to evaluate disability weights for 228 causes of diseases using a PC and a PHE. We also attempted to determine a simple method of modifying the method based on a PC, rather than depending on a PTO.
MATERIALS AND METHODS
Study design and participants
The study was conducted through two types of self-administered web-based surveys in South Korea, adapting the methodology of a preceding disability weights measurement study (4). The two types of surveys were designed differently for the valuation methods. The first type (type A survey) used a PC and a PTO, while the second type (type B survey) used only a PC as a valuation method. The type A survey was performed on November 13 2014 and February 3 2015, and the type B survey was performed between November 18 2014 and October 21 2015.
A purposive sampling technique was utilized to select the participants who had knowledge of the causes of diseases. Accordingly, the participants in the survey differed between the two types of surveys. The participants in the type A survey were all specialists, but those in the type B survey were physicians and medical college students who were attending in the third or fourth grade of a regular course. The participants in the type A survey were delegated to represent various clinical departments and those of the type B survey were recruited from promotion of the survey in medical colleges and hospitals and an announcement at medical conferences and meetings.
Valuation method and causes of disease
In both types of surveys, participants were asked about their age, gender, and specialty at the beginning of the survey. Next, each participant evaluated the causes of diseases using a PC and a PHE. In the PC, the participants were requested to choose the healthier option between two causes of disease, which were randomly selected among the 230 causes of diseases. Among the 230 causes of disease, 228 causes of disease were taken from the disease classification of the KBD 2010 study. The remaining two causes of disease were 'full health' and 'being dead', which were included to utilize them as anchor points. Each participant in the type A and B surveys carried out a total of 60 PCs.
In the PTO, the participants were asked to make a trade-off between two different health programs. The first health program (program A) prevented 1,000 people from suffering a fatal disease causing rapid death. The second one (program B) prevented 10,000 people from being afflicted with a certain cause of disease, which was randomly selected from the 40 causes of disease. If program A was regarded as the worthier program, the number of people for program B was increased by 1,000. On the other hand, if program A was chosen as worthier program, the number of people for program B was decreased by 1,000. The questions were continued until the participants thought that the two health programs had the same or nearly the same worth. That is, the questions stopped at the point at which the participant did not have any differences in their preference for the two health programs. The number of people in program B ranged from 1,000 to 20,000, and the number of people changed by 500 or 1,000 depending on the participant's response. Each participant in the type A survey conducted a total of 40 iterations of PTO about 40 causes of disease, such as diabetes mellitus, ischemic stroke, and stomach cancer. The 40 causes of disease had a high disease burden in South Korea based on the scale from the GBD 2010 study (23).
Analysis
First, we conducted descriptive analyses for socio-demographic factors of the participants. Then, the disability weights for the cause of disease for each participant were computed in a PC and a PTO, respectively. In the PTO, the disability weights of causes of disease for each participant were estimated by the following formula: 1,000/final number of people in program B (19). The disability weights of causes of disease for all participants in the PTO were summarized using the mean, standard deviation, and median. In the case of the PC, we applied a probit regression that has been utilized in the analysis of discrete choice experimental data (24). We regarded the stated choice between the two causes of disease in the PC as the dependent variable. Furthermore, we treated the 230 causes of disease as independent variables and created them as dummy variables with 'being dead' as the reference. We computed the predicted probabilities for each cause of disease from the coefficient estimates of the probit regression. We took advantage of 'being dead (1)' and 'full health (0)' as anchor points to rescale the predicted probability of each cause of disease on a scale of 0 to 1. The 95% confidence interval of disability weight for each cause of disease in the PC was estimated using the 95% confidence interval of the predicted probabilities.
We compared the disability weights from this study to those estimated in a previous South Korean disability weight study (20). All statistical analyses were performed using Stata 13.1 software (StataCorp, College Station, TX, USA). P values less than 0.05 were considered statistically significant in this study.
Ethics statement
This study was approved by the institutional review board of the Asan Medical Center (S2014-1396-0002). Informed consent was waived by the board.
RESULTS
A total of 40 and 456 participants conducted type A and type B surveys, respectively. Table 1 lists the details of the participants' characteristics for each survey. Participants aged 30 to 39 were predominant in the type A survey; whereas, participants aged 19 to 29 were highest in the type B survey. The male respondents outnumbered female respondents in both surveys. In terms of the number of specialties involved in the surveys, 19 versus 22 specialties were involved in the type A and type B surveys, respectively.
Table 1. Characteristics of the study participants by type of survey.
| Demographic parameters | Type A survey | Type B survey | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, yr | 19-29 | 0 | 0.0 | 360 | 79.0 |
| 30-39 | 27 | 67.5 | 89 | 19.5 | |
| 40-49 | 13 | 32.5 | 5 | 1.1 | |
| 50-59 | 0 | 0.0 | 1 | 0.2 | |
| 60- | 0 | 0.0 | 1 | 0.2 | |
| Gender | Male | 30 | 75.0 | 284 | 62.3 |
| Female | 10 | 25.0 | 172 | 37.7 | |
| Specialty | Family medicine | 1 | 2.5 | 10 | 2.2 |
| Internal medicine | 10 | 25.0 | 23 | 5.0 | |
| Anesthesiology | 0 | 0.0 | 9 | 2.0 | |
| Radiation oncology | 1 | 2.5 | 6 | 1.3 | |
| Pathology | 1 | 2.5 | 10 | 2.2 | |
| Urology | 2 | 5.0 | 1 | 0.2 | |
| Obstetrics and gynecology | 0 | 0.0 | 2 | 0.4 | |
| Plastic surgery | 0 | 0.0 | 2 | 0.4 | |
| Pediatrics | 3 | 7.5 | 1 | 0.2 | |
| Neurology | 1 | 2.5 | 3 | 0.7 | |
| Neurosurgery | 2 | 5.0 | 1 | 0.2 | |
| Ophthalmology | 2 | 5.0 | 0 | 0.0 | |
| Radiology | 1 | 2.5 | 12 | 2.6 | |
| Preventive medicine | 6 | 15.0 | 11 | 2.4 | |
| General surgery | 2 | 5.0 | 4 | 0.9 | |
| Emergency medicine | 2 | 5.0 | 3 | 0.7 | |
| Otolaryngology | 1 | 2.5 | 13 | 2.9 | |
| Rehabilitation medicine | 1 | 2.5 | 4 | 0.9 | |
| Psychiatry | 1 | 2.5 | 6 | 1.3 | |
| Orthopedics | 1 | 2.5 | 8 | 1.8 | |
| Dermatology | 1 | 2.5 | 14 | 3.1 | |
| Nuclear medicine | 1 | 2.5 | 4 | 0.9 | |
| Cardiothoracic surgery | 0 | 0.0 | 1 | 0.2 | |
| General practitioner | 0 | 0.0 | 15 | 3.3 | |
| Medical student | 0 | 0.0 | 293 | 64.3 | |
| Total | 40.0 | 100.0 | 456 | 100.0 | |
Supplementary Fig. 1 shows the box plot of estimated disability weights for the 40 causes of disease from the PTO. On the basis of the mean, the majority of disability weights (27, 67.5%) from the PTO were located between 0.1 and 0.3. Furthermore, all disability weights from the PTO had a value less than 0.4. The cause of disease with the highest disability weight from the PTO was 'Pancreatic cancer (0.400)', followed by 'Gallbladder and biliary tract cancer (0.277)' and 'Hemorrhagic and other non-ischemic stroke (0.258)'. In contrast, the cause of disease with lowest disability weight from the PTO was 'Migraine (0.058)', followed by 'Neck pain (0.059)' and 'Dysthymia (0.061)'. An exact figure for the mean, standard deviation, and median of the disability weights from the PTO is presented in Supplementary Table 1.
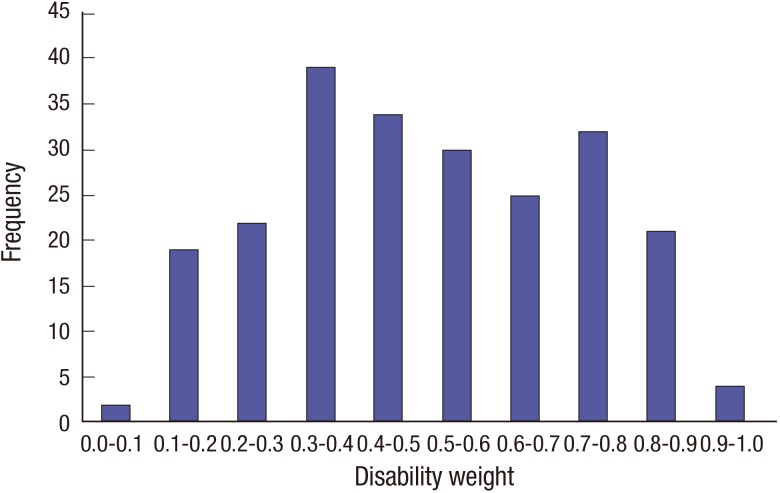
Table 2 presents the disability weights and their 95% confidence intervals for 228 causes of disease from the PC. Fig. 1 shows the frequency distribution of disability weights for causes of disease from the PC. Approximately half of the causes of disease (50.9%) had a disability weight less than 0.5. Furthermore, disability weights for approximately one third of causes of disease (65.8%) were located between 0.2 and 0.7. The cause of disease with the highest disability weight from the PC was 'Pancreatic cancer (0.938)', followed by 'Trachea, bronchus, and lung cancers (0.917)' and 'Neonatal encephalopathy (birth asphyxia and birth trauma) (0.911)'. On the other hand, the cause of disease with the lowest disability weight from the PC was 'Urticaria (0.084)', followed by 'Upper respiratory infections (0.088)' and 'Acne vulgaris (0.106)'.
Table 2. Disability weights for 228 causes of disease from a paired comparison.
| Causes of disease | Disability weight | 95% confidence interval |
|---|---|---|
| Tuberculosis | 0.464 | 0.367-0.563 |
| HIV disease resulting in mycobacterial infection | 0.837 | 0.770-0.891 |
| HIV disease resulting in other specified or unspecified diseases | 0.794 | 0.717-0.859 |
| Cholera | 0.324 | 0.237-0.421 |
| Other Salmonella infections | 0.283 | 0.199-0.381 |
| Shigellosis | 0.372 | 0.278-0.475 |
| Enteropathogenic E. coli infection | 0.257 | 0.174-0.357 |
| Enterotoxigenic E. coli infection | 0.347 | 0.255-0.449 |
| Campylobacter enteritis | 0.206 | 0.133-0.298 |
| Amoebiasis | 0.461 | 0.364-0.561 |
| Cryptosporidiosis | 0.583 | 0.486-0.676 |
| Rotaviral enteritis | 0.152 | 0.091-0.233 |
| Intestinal infection | 0.155 | 0.091-0.243 |
| Typhoid and paratyphoid fevers | 0.347 | 0.252-0.452 |
| Influenza | 0.131 | 0.075-0.211 |
| Pneumococcal pneumonia | 0.385 | 0.288-0.490 |
| H. influenza type B pneumonia | 0.309 | 0.215-0.418 |
| Respiratory syncytial virus pneumonia | 0.260 | 0.177-0.360 |
| Upper respiratory infections | 0.088 | 0.045-0.154 |
| Otitis media | 0.138 | 0.078-0.222 |
| Pneumococcal meningitis | 0.551 | 0.458-0.642 |
| H. influenza type B meningitis | 0.523 | 0.427-0.620 |
| Meningococcal infection | 0.598 | 0.500-0.691 |
| Encephalitis | 0.721 | 0.636-0.798 |
| Diphtheria | 0.389 | 0.291-0.495 |
| Whooping cough | 0.256 | 0.176-0.351 |
| Tetanus | 0.524 | 0.425-0.623 |
| Measles | 0.279 | 0.192-0.383 |
| Varicella | 0.223 | 0.147-0.316 |
| Malaria | 0.471 | 0.373-0.573 |
| Chagas disease | 0.647 | 0.550-0.739 |
| Leishmaniasis | 0.411 | 0.317-0.512 |
| African trypanosomiasis | 0.515 | 0.416-0.616 |
| Schistosomiasis | 0.340 | 0.253-0.435 |
| Cysticercosis | 0.471 | 0.370-0.577 |
| Echinococcosis | 0.354 | 0.264-0.454 |
| Lymphatic filariasis | 0.564 | 0.465-0.661 |
| Onchocerciasis | 0.357 | 0.268-0.455 |
| Trachoma | 0.431 | 0.333-0.535 |
| Dengue | 0.428 | 0.331-0.530 |
| Yellow fever | 0.496 | 0.392-0.602 |
| Rabies | 0.797 | 0.718-0.864 |
| Ascariasis | 0.256 | 0.175-0.352 |
| Trichuriasis | 0.320 | 0.229-0.422 |
| Hookworm disease | 0.302 | 0.211-0.407 |
| Food-borne trematodiases | 0.329 | 0.238-0.430 |
| Tsutsugamushi fever | 0.317 | 0.230-0.417 |
| Typhus fever | 0.435 | 0.341-0.533 |
| Hantaan virus disease | 0.479 | 0.382-0.580 |
| Intestinal helminth | 0.341 | 0.250-0.443 |
| Maternal hemorrhage | 0.514 | 0.415-0.613 |
| Maternal sepsis | 0.825 | 0.755-0.881 |
| Hypertensive disorders of pregnancy | 0.508 | 0.405-0.610 |
| Obstructed labor | 0.471 | 0.374-0.572 |
| Abortion | 0.455 | 0.358-0.556 |
| Preterm birth complications | 0.678 | 0.584-0.763 |
| Neonatal encephalopathy (birth asphyxia and birth trauma) | 0.911 | 0.858-0.949 |
| Sepsis and other infectious disorders of the newborn baby | 0.758 | 0.677-0.829 |
| Protein-energy malnutrition | 0.362 | 0.270-0.465 |
| Iodine deficiency | 0.166 | 0.101-0.253 |
| Vitamin A deficiency | 0.223 | 0.146-0.317 |
| Iron-deficiency anemia | 0.122 | 0.067-0.199 |
| Syphilis | 0.519 | 0.422-0.617 |
| Sexually transmitted chlamydial diseases | 0.413 | 0.317-0.516 |
| Gonococcal infection | 0.345 | 0.252-0.448 |
| Trichomoniasis | 0.346 | 0.253-0.448 |
| Herpes genitalia | 0.442 | 0.344-0.545 |
| Acute hepatitis A | 0.346 | 0.254-0.448 |
| Acute hepatitis B | 0.483 | 0.384-0.583 |
| Acute hepatitis C | 0.646 | 0.548-0.738 |
| Acute hepatitis E | 0.462 | 0.369-0.558 |
| Leprosy | 0.815 | 0.740-0.878 |
| Legionnaires' disease | 0.447 | 0.348-0.550 |
| Leptospirosis | 0.362 | 0.270-0.464 |
| Rubella | 0.366 | 0.270-0.470 |
| Mumps | 0.193 | 0.123-0.282 |
| Esophageal cancer | 0.875 | 0.809-0.924 |
| Stomach cancer | 0.724 | 0.640-0.799 |
| Liver cancer secondary to hepatitis B | 0.857 | 0.791-0.909 |
| Liver cancer secondary to hepatitis C | 0.819 | 0.745-0.881 |
| Liver cancer secondary to alcohol use | 0.824 | 0.748-0.885 |
| Larynx cancer | 0.872 | 0.812-0.919 |
| Trachea, bronchus, and lung cancers | 0.917 | 0.864-0.953 |
| Breast cancer | 0.704 | 0.614-0.787 |
| Cervical cancer | 0.744 | 0.659-0.819 |
| Uterine cancer | 0.745 | 0.659-0.820 |
| Prostate cancer | 0.701 | 0.609-0.783 |
| Colon and rectum cancers | 0.759 | 0.673-0.834 |
| Mouth cancer | 0.883 | 0.824-0.929 |
| Nasopharynx cancer | 0.816 | 0.739-0.879 |
| Cancer of other parts of pharynx and oropharynx | 0.890 | 0.837-0.931 |
| Gallbladder and biliary tract cancer | 0.827 | 0.757-0.886 |
| Pancreatic cancer | 0.938 | 0.891-0.968 |
| Malignant melanoma of skin | 0.790 | 0.706-0.860 |
| Non-melanoma skin cancer | 0.634 | 0.534-0.728 |
| Ovarian cancer | 0.791 | 0.713-0.856 |
| Testicular cancer | 0.799 | 0.721-0.865 |
| Kidney cancer | 0.777 | 0.698-0.846 |
| Other urinary organ cancers | 0.754 | 0.668-0.828 |
| Bladder cancer | 0.792 | 0.714-0.857 |
| Brain and nervous system cancers | 0.874 | 0.809-0.924 |
| Thyroid cancer | 0.466 | 0.369-0.564 |
| Hodgkin's disease | 0.718 | 0.631-0.795 |
| Non-Hodgkin's lymphoma | 0.754 | 0.672-0.827 |
| Multiple myeloma | 0.768 | 0.690-0.836 |
| Leukemia | 0.845 | 0.770-0.904 |
| Bone and connective tissue cancer | 0.816 | 0.744-0.876 |
| Benign neoplasm of brain and other parts of central nervous system | 0.571 | 0.472-0.667 |
| Rheumatic heart disease | 0.685 | 0.592-0.769 |
| Ischemic heart disease | 0.790 | 0.714-0.856 |
| Ischemic stroke | 0.809 | 0.734-0.872 |
| Hemorrhagic and other non-ischemic stroke | 0.864 | 0.796-0.917 |
| Hypertensive heart disease | 0.575 | 0.472-0.675 |
| Cardiomyopathy and myocarditis | 0.753 | 0.667-0.828 |
| Atrial fibrillation and flutter | 0.551 | 0.451-0.650 |
| Aortic aneurysm | 0.727 | 0.644-0.803 |
| Peripheral vascular disease | 0.530 | 0.432-0.627 |
| Endocarditis | 0.668 | 0.576-0.752 |
| Hemorrhoids | 0.171 | 0.103-0.260 |
| Varicose veins of lower extremities | 0.232 | 0.154-0.327 |
| Chronic obstructive pulmonary disease | 0.690 | 0.593-0.779 |
| Pneumoconiosis | 0.733 | 0.647-0.809 |
| Asthma | 0.367 | 0.269-0.477 |
| Interstitial lung disease and pulmonary sarcoidosis | 0.701 | 0.617-0.778 |
| Cirrhosis of the liver secondary to hepatitis B | 0.716 | 0.630-0.792 |
| Cirrhosis of the liver secondary to hepatitis C | 0.748 | 0.660-0.826 |
| Cirrhosis of the liver secondary to alcohol use | 0.614 | 0.517-0.706 |
| Peptic ulcer disease | 0.375 | 0.282-0.477 |
| Gastritis and duodenitis | 0.181 | 0.111-0.272 |
| Appendicitis | 0.133 | 0.077-0.212 |
| Paralytic ileus and intestinal obstruction without hernia | 0.491 | 0.390-0.594 |
| Inguinal or femoral hernia | 0.208 | 0.133-0.302 |
| Crohn's disease | 0.720 | 0.631-0.800 |
| Ulcerative colitis | 0.664 | 0.568-0.751 |
| Vascular disorders of intestine | 0.542 | 0.443-0.638 |
| Gall bladder and bile duct disease | 0.389 | 0.299-0.487 |
| Pancreatitis | 0.513 | 0.411-0.615 |
| Gastroesophageal reflux disease | 0.214 | 0.139-0.307 |
| Alzheimer's disease and other dementias | 0.854 | 0.784-0.911 |
| Parkinson's disease | 0.780 | 0.703-0.847 |
| Epilepsy | 0.778 | 0.698-0.846 |
| Multiple sclerosis | 0.778 | 0.696-0.848 |
| Migraine | 0.314 | 0.227-0.413 |
| Tension-type headache | 0.165 | 0.099-0.253 |
| Schizophrenia | 0.885 | 0.824-0.931 |
| Alcohol use disorders | 0.436 | 0.338-0.539 |
| Opioid use disorders | 0.453 | 0.354-0.555 |
| Cocaine use disorders | 0.441 | 0.343-0.544 |
| Amphetamine use disorders | 0.461 | 0.364-0.562 |
| Cannabis use disorders | 0.318 | 0.231-0.415 |
| Major depressive disorders | 0.606 | 0.508-0.700 |
| Dysthymia | 0.194 | 0.122-0.286 |
| Bipolar affective disorder | 0.610 | 0.515-0.700 |
| Panic disorder | 0.526 | 0.424-0.628 |
| Obsessive-compulsive disorder | 0.391 | 0.294-0.495 |
| Post-traumatic stress disorder | 0.435 | 0.338-0.538 |
| Anorexia nervosa | 0.488 | 0.392-0.586 |
| Bulimia nervosa | 0.411 | 0.316-0.513 |
| Autism | 0.758 | 0.672-0.832 |
| Asperger's syndrome | 0.685 | 0.591-0.771 |
| Attention-deficit hyperactivity disorder | 0.379 | 0.288-0.480 |
| Conduct disorder | 0.502 | 0.399-0.606 |
| Idiopathic intellectual disability | 0.676 | 0.586-0.760 |
| Borderline personality disorder | 0.603 | 0.504-0.698 |
| Diabetes mellitus | 0.593 | 0.496-0.686 |
| Acute glomerulonephritis | 0.445 | 0.345-0.550 |
| Chronic kidney disease due to diabetes mellitus | 0.837 | 0.769-0.893 |
| Chronic kidney disease due to hypertension | 0.748 | 0.662-0.823 |
| Tubulointerstitial nephritis, pyelonephritis, and urinary tract infections | 0.483 | 0.384-0.584 |
| Urolithiasis | 0.283 | 0.199-0.382 |
| Benign prostatic hyperplasia | 0.289 | 0.205-0.387 |
| Male infertility | 0.588 | 0.494-0.680 |
| Urinary incontinence | 0.343 | 0.250-0.445 |
| Uterine fibroids | 0.242 | 0.163-0.338 |
| Polycystic ovarian syndrome | 0.439 | 0.338-0.544 |
| Female infertility | 0.561 | 0.466-0.655 |
| Endometriosis | 0.413 | 0.318-0.514 |
| Genital prolapse | 0.458 | 0.364-0.555 |
| Premenstrual syndrome | 0.142 | 0.082-0.225 |
| Thalassemias | 0.501 | 0.403-0.600 |
| Sickle cell disorders | 0.509 | 0.415-0.604 |
| G6PD deficiency | 0.679 | 0.588-0.763 |
| Rheumatoid arthritis | 0.549 | 0.449-0.647 |
| Osteoarthritis | 0.370 | 0.276-0.474 |
| Low back pain | 0.315 | 0.225-0.417 |
| Neck pain | 0.213 | 0.137-0.308 |
| Gout | 0.395 | 0.299-0.499 |
| Systemic lupus erythematosus (SLE) | 0.634 | 0.538-0.726 |
| Neural tube defects | 0.897 | 0.837-0.940 |
| Congenital heart anomalies | 0.686 | 0.596-0.769 |
| Cleft lip and cleft palate | 0.424 | 0.332-0.522 |
| Down's syndrome | 0.908 | 0.845-0.951 |
| Eczema | 0.162 | 0.098-0.248 |
| Psoriasis | 0.280 | 0.196-0.377 |
| Cellulitis | 0.302 | 0.214-0.404 |
| Abscess, impetigo, and other bacterial skin diseases | 0.205 | 0.133-0.295 |
| Scabies | 0.197 | 0.128-0.285 |
| Fungal skin diseases | 0.283 | 0.198-0.382 |
| Viral skin diseases | 0.240 | 0.159-0.337 |
| Acne vulgaris | 0.106 | 0.057-0.180 |
| Alopecia areata | 0.237 | 0.157-0.335 |
| Pruritus | 0.174 | 0.109-0.258 |
| Urticaria | 0.084 | 0.042-0.151 |
| Decubitus ulcer | 0.431 | 0.334-0.535 |
| Glaucoma | 0.640 | 0.545-0.727 |
| Cataracts | 0.384 | 0.294-0.483 |
| Macular degeneration | 0.576 | 0.480-0.669 |
| Refraction and accommodation disorders | 0.428 | 0.332-0.528 |
| Dental caries | 0.137 | 0.078-0.219 |
| Periodontal disease | 0.209 | 0.137-0.299 |
| Edentulism | 0.651 | 0.556-0.738 |
| Pedestrian injury by road vehicle | 0.534 | 0.437-0.629 |
| Pedal cycle vehicle | 0.357 | 0.266-0.458 |
| Motorized vehicle with two wheels | 0.529 | 0.428-0.630 |
| Motorized vehicle with three or more wheels | 0.588 | 0.487-0.685 |
| Falls | 0.613 | 0.513-0.708 |
| Drowning | 0.660 | 0.568-0.745 |
| Fire, heat, and hot substances | 0.545 | 0.443-0.645 |
| Poisonings | 0.546 | 0.447-0.644 |
| Mechanical forces (firearm) | 0.634 | 0.534-0.728 |
| Adverse effects of medical treatment | 0.385 | 0.286-0.494 |
| Animal contact (venomous) | 0.398 | 0.300-0.505 |
| Animal contact (non-venomous) | 0.107 | 0.059-0.176 |
| Self-harm | 0.614 | 0.517-0.706 |
| Assault by firearm | 0.611 | 0.516-0.701 |
| Assault by sharp object | 0.300 | 0.215-0.399 |
| Exposure to forces of nature | 0.389 | 0.292-0.494 |
| Collective violence and legal intervention | 0.613 | 0.520-0.702 |
Fig. 1.
Distribution of disability weights for cause of disease from a paired comparison.
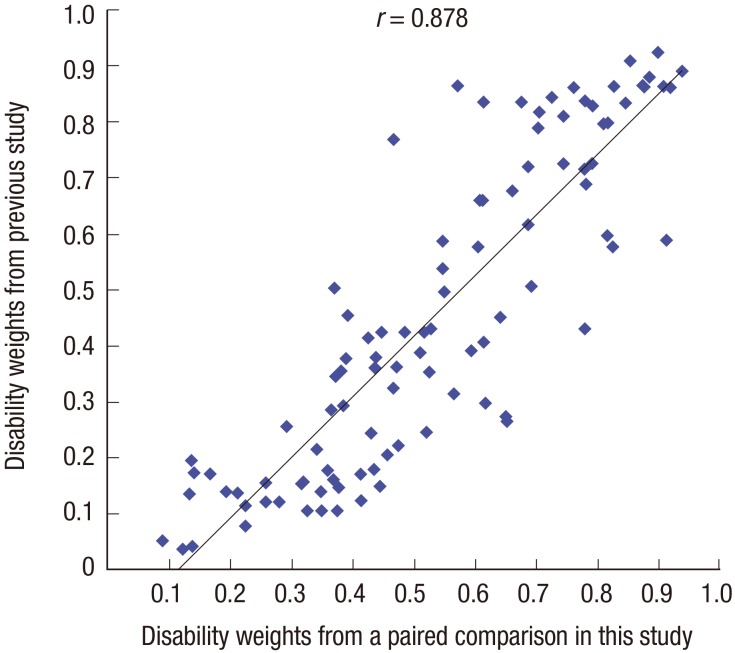
The Pearson correlation coefficient between the disability weights for the overlapping causes of disease from this study and a previous South Korean study was 0.878 (Fig. 2) (20). Among 100 overlapping causes of disease, the disability weights for 26 causes of disease from this study, such as 'Thyroid cancer' and 'Stomach cancer', were estimated to be lower than that from the previous study; whereas, the disability weights for 74 causes of disease from this study, such as 'Self-harm' and 'Low back pain', were determined to be higher than that from the previous study. The difference in disability weight between the two studies was largest for 'Edentulism (0.384)', followed by 'Chagas disease (0.371)' and 'Epilepsy (0.346)'. However, disability weights of some causes of disease (e.g., 'Schizophrenia' and 'Influenza') showed little change. Supplementary Table 2 shows detailed comparisons between the disability weights for causes of disease from this study and the previous study (20).
Fig. 2.
Comparison of disability weights between this study and a previous study.
DISCUSSION
In our present study, we re-estimated 228 disability weights for causes of disease by adapting the methodology of the recent GBD study. The disability weights were estimated by a survey with a PC and a PTO, involving physicians and medical college students. However, we suggest that the results from the PTO were not suitable to apply to estimation of disability weights, considering the span of values from the PTO. Furthermore, we showed that disability weights can be estimated from PC data alone by adding 'full health' and 'being dead' as anchor points. By using this simplified method in estimating disability weights, the problems on PTO or PHE could be overcome.
The PTO, including PHE, has been used to estimate disability weights in previous studies (4,5,6,9,19). The original purpose of a PTO was to anchor the results from a PTO and other valuation methods, such as a VAS or a PC. That is, the results from a VAS or a PC were interpolated into the values from a PTO. However, values from the PTO displayed little variation in the known severity of causes of disease, especially considering the result that majority of disability weights (67.5%) from the PTO in this study ranged between 0.1 and 0.3. Such a phenomenon also occurred in the European disability weight study (10). In that study, the responsiveness to the variation in the severity of health states was low, so the authors used the PHE data from the GBD 2010 disability weights measurement study to anchor the results from the PC on a scale from 0 to 1. We suspect that the poorer discrimination of values between causes of disease by the PTO might be due to the number of cause of disease applied to the PTO. In general, a PTO has been applied to only a few causes of disease or health states (5,6,19), but in this study we asked participants to evaluate 40 causes of disease using a PTO. To evaluate the disability weights for causes of disease might be hard for participants, considering the disability level between the causes of diseases. If the number of causes of disease or health states increases, a PTO may not be suitable to ascertain the differences of disability level between causes or health states.
As the number of causes of disease or health states compared increases, ordinal methods, such as a PC or a ranking method, would be more appropriate than cardinal methods, such as a PTO or a time trade-off (TTO). Understanding the methodology of cardinal methods is easier for a participant than ordinal methods (25). Consequently, in ordinal methods, participants can elicit preferences for causes of disease or health states more easily than cardinal methods, by comparing preferences between causes of diseases and health states. However, rescaling the values from ordinal methods on a certain scale is crucial to use the results from cardinal methods as continuous data. We overcame this issue by adding 'full health' and 'being dead' to the list of causes of disease used in the PC. Generally, 'being dead' is used as reference for eliciting preferences in other valuation methods, such as the TTO and the standard gamble (26). Furthermore, the 'best imaginable health state' and the 'worst imaginable health state' are defined as the top and bottom anchors in the VAS (27). By including 'full health' and 'being dead' as in these other valuation methods, disability weights can be estimated based on a PC without resorting to a PTO.
We recruited physicians and medical college students, who were attending in third or fourth grade of a regular course, as study participants rather than the general public. Although the preferences in economic evaluation of the general public have more significance than those of healthcare professionals or patients (28), completely capturing the preferences of the general public is not an easy task. When evaluating the preferences of the general public, health states or diseases need to be explained in a manner that can be understood by all, especially people with a low level of education. Therefore, health states explaining various aspects of causes of disease should be carefully developed and repeatedly reviewed. The GBD 2010 disability weights measurement study conducted surveys of the general public (4), but the disability weights from that study were revised by adding additional results from the European disability weight study, in which the lay descriptions of 33 health states were modified to fully reflect the key manifestations of the causes of disease (10). The disability weights from the general public are sensitive to specific descriptions of health states (9). To deflect this issue in this study, we conducted surveys involving physicians and medical college students who are assumed to possess sufficient experience and knowledge in the causes of disease. Furthermore, conducting surveys of healthcare professionals ensured comparability between the results from this study and a previous South Korean study that targeted medical experts as survey participants. For the KBD 2010 study, the Korean disability weights study aimed at the South Korean general public was also performed and is described in detail elsewhere (29).
Because we estimated a large number of disability weights for causes of disease on a scale from 0 to 1, some disability weights might seem counterintuitive in terms of degree and ranking compared to others. However, evaluating the validity of disability weights is difficult due to the absence of a gold standard (11). Consequently, comparing the ranking of disability weights between similar studies and detecting inversion of disability weights in specific diseases with different severity levels have been suggested to confirm the validity of disability weights (11). Furthermore, increasing the sample size of survey participants and including diverse specialists among the survey participants are additional options that can enhance the validity of disability weights. Although a relatively large number of physicians and medical college students conducted the survey in this study, further studies will be required to ensure the validity of disability weights.
When comparing disability weights for overlapping causes of disease from this study and the previous South Korean study (20), there is a fairly high correlation between the two studies despite their differences in valuation methods and time periods. However, change in perception of healthcare professionals about particular causes of disease is predictable. For example, the disability weights of 'Thyroid cancer' and 'Stomach cancer' were lower than those of previous studies. On the other hand, the disability weights of 'Self-harm' and 'Low back pain' were higher than those of previous studies. The outcome of medical treatments, prognosis of causes of disease, and recent changes in epidemiologic data are expected to affect participants' evaluation of the disability level for causes of disease (30,31). Therefore, disability weights must periodically be revised to reflect the up-to-date advances in medical science and epidemiological changes.
One limitation of our current study design is that the severity levels of causes of disease were not considered. The disability weights of 'Trachea, bronchus, and lung cancers (0.917)' and 'Pancreatic cancer (0.938)' were estimated with high values in this study. If these cancers were divided up into different disease stages, the results might be more valid because they would reflect the severity levels of causes of disease. Further disability weight studies that consider the stages of causes of disease will be required in the near future.
In conclusion, we re-estimated 228 disability weights for causes of disease based on the responses of a large number of medical experts. We showed that disability weights can be estimated based on a PC without resorting to a PTO by including 'full health' and 'being dead' as anchor points. Disability weights can be easily estimated and continuously revised through new methodology developed from this study in the estimation of disability weights.
ACKNOWLEDGMENT
The authors would like to thank the Gallup Korea for help in conducting survey. The authors also are grateful to the survey participants.
Footnotes
Funding: This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (number of study: HI13C0729).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study conception and design: Jo MW, Ock M. Acquisition of data: all authors. Data analysis: Jo MW, Ock M, Lee JY. Manuscript preparation: Ock M, Jo MW. Revision and approval of manuscript: all authors.
Supplementary Materials
Means, standard deviations, and medians of disability weights for causes of disease from a person trade-off
Comparison of disability weights for causes of disease between this study and a previous study
Box plot of disability weights of 40 causes of disease from a person trade-off
References
- 1.Yoon J, Oh IH, Seo H, Kim EJ, Gong YH, Ock M, Lim D, Lee WK, Lee YR, Kim D, et al. Disability-adjusted Life Years for 313 Diseases and Injuries: the 2012 Korean Burden of Disease Study. J Korean Med Sci. 2016;31(Suppl 2):S146–S157. doi: 10.3346/jkms.2016.31.S2.S146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salomon JA, Mathers CD, Chatterji S, Sadana R, Ustun TB, Murray CJ. Quantifying individual levels of health: definitions, concepts and measurement issues. In: Murray CJ, Evans DB, editors. Health Systems Performance Assessment: Debate, Methods, and Empiricism. Geneva: World Health Organization; 2003. pp. 301–318. [Google Scholar]
- 3.Lee YH, Yoon SJ, Kim A, Seo H, Ko S. Health Performance and Challenges in Korea: a Review of the Global Burden of Disease Study 2013. J Korean Med Sci. 2016;31(Suppl 2):S114–S120. doi: 10.3346/jkms.2016.31.S2.S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, Begum N, Shah R, Karyana M, Kosen S, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the global burden of disease study 2010. Lancet. 2012;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray CJ, Lopez AD. The Global Burden of Disease: a Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Cambridge, MA: Harvard University Press; 1996. [Google Scholar]
- 6.Stouthard ME, Essink-Bot ML, Bonsel GJ, Barendregt JJ, Kramers P, van de Water HP, Gunning-Schepers LJ, van der Maas PJ. Disability Weights for Diseases in the Netherlands. Rotterdam: Erasmus University; 1997. [Google Scholar]
- 7.Stouthard ME, Essink-Bot ML, Bonsel GJ. Disability weights for diseases. A modified protocol and results for a Western European region. Eur J Public Health. 2000;10:24–30. [Google Scholar]
- 8.Ustün TB, Rehm J, Chatterji S, Saxena S, Trotter R, Room R, Bickenbach J, WHO/NIH Joint Project CAR Study Group Multiple-informant ranking of the disabling effects of different health conditions in 14 countries. Lancet. 1999;354:111–115. doi: 10.1016/s0140-6736(98)07507-2. [DOI] [PubMed] [Google Scholar]
- 9.Salomon JA, Haagsma JA, Davis A, de Noordhout CM, Polinder S, Havelaar AH, Cassini A, Devleesschauwer B, Kretzschmar M, Speybroeck N, et al. Disability weights for the global burden of disease 2013 study. Lancet Glob Health. 2015;3:e712–23. doi: 10.1016/S2214-109X(15)00069-8. [DOI] [PubMed] [Google Scholar]
- 10.Haagsma JA, Maertens de Noordhout C, Polinder S, Vos T, Havelaar AH, Cassini A, Devleesschauwer B, Kretzschmar ME, Speybroeck N, Salomon JA. Assessing disability weights based on the responses of 30,660 people from four European countries. Popul Health Metr. 2015;13:10. doi: 10.1186/s12963-015-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haagsma JA, Polinder S, Cassini A, Colzani E, Havelaar AH. Review of disability weight studies: comparison of methodological choices and values. Popul Health Metr. 2014;12:20. doi: 10.1186/s12963-014-0020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polinder S, Haagsma JA, Stein C, Havelaar AH. Systematic review of general burden of disease studies using disability-adjusted life years. Popul Health Metr. 2012;10:21. doi: 10.1186/1478-7954-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnesen T, Nord E. The value of DALY life: problems with ethics and validity of disability adjusted life years. BMJ. 1999;319:1423–1425. doi: 10.1136/bmj.319.7222.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehm J, Frick U. Valuation of health states in the US study to establish disability weights: lessons from the literature. Int J Methods Psychiatr Res. 2010;19:18–33. doi: 10.1002/mpr.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groce NE. Disability in cross-cultural perspective: rethinking disability. Lancet. 1999;354:756–757. doi: 10.1016/s0140-6736(99)06140-1. [DOI] [PubMed] [Google Scholar]
- 16.Østerdal LP. The lack of theoretical support for using person trade-offs in QALY-type models. Eur J Health Econ. 2009;10:429–436. doi: 10.1007/s10198-009-0150-9. [DOI] [PubMed] [Google Scholar]
- 17.Doctor JN, Miyamoto J, Bleichrodt H. When are person tradeoffs valid? J Health Econ. 2009;28:1018–1027. doi: 10.1016/j.jhealeco.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon SJ, Kwon YD, Kim BY. Estimating the disability weight of major cancers in Korea using Delphi method. Korean J Prev Med. 2000;33:409–414. [Google Scholar]
- 19.Lee JK, Yoon SJ, Do YK, Kwon YH, Kim CY, Park K, Kim YI, Shin YS. Disability weights for diseases in Korea. Korean J Prev Med. 2003;36:163–170. [Google Scholar]
- 20.Do YK, Yoon SJ, Lee JK, Kwon YH, Lee SI, Kim C, Park K, Kim YI, Shin Y. Disability weights for the Korean burden of disease study: focused on comparison with disability weights in the Australian burden of disease study. J Prev Med Public Health. 2004;37:59–71. [PubMed] [Google Scholar]
- 21.Yoon SJ, Bae SC, Lee SI, Chang H, Jo HS, Sung JH, Park JH, Lee JY, Shin Y. Measuring the burden of disease in Korea. J Korean Med Sci. 2007;22:518–523. doi: 10.3346/jkms.2007.22.3.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi KS, Park JH, Lee KS. Disability weights for cancers in Korea. J Korean Med Sci. 2013;28:808–813. doi: 10.3346/jkms.2013.28.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Institute for Health Metrics and Evaluation (US) GBD compare (South Korea) [Internet] [accessed on 24 November 2015]. Available at http://vizhub.healthdata.org/gbd-compare/
- 24.de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21:145–172. doi: 10.1002/hec.1697. [DOI] [PubMed] [Google Scholar]
- 25.Ali S, Ronaldson S. Ordinal preference elicitation methods in health economics and health services research: using discrete choice experiments and ranking methods. Br Med Bull. 2012;103:21–44. doi: 10.1093/bmb/lds020. [DOI] [PubMed] [Google Scholar]
- 26.Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96:5–21. doi: 10.1093/bmb/ldq033. [DOI] [PubMed] [Google Scholar]
- 27.Torrance GW, Feeny D, Furlong W. Visual analog scales: do they have a role in the measurement of preferences for health states? Med Decis Making. 2001;21:329–334. doi: 10.1177/0272989X0102100408. [DOI] [PubMed] [Google Scholar]
- 28.Dolan P, Olsen JA, Menzel P, Richardson J. An inquiry into the different perspectives that can be used when eliciting preferences in health. Health Econ. 2003;12:545–551. doi: 10.1002/hec.760. [DOI] [PubMed] [Google Scholar]
- 29.Jo MW, Ock M. Estimation of disability weights in the general population of South Korea using a paired comparison; Proceedings of the 37th Annual Meeting of the Society for Medical Decision Making; 2015 Oct 18-21; St. Louis, MO. Hillsborough, NJ: Society for Medical Decision Making; 2015. [Google Scholar]
- 30.Lim D, Ha M, Song I. Trends in the leading causes of death in Korea, 1983-2012. J Korean Med Sci. 2014;29:1597–1603. doi: 10.3346/jkms.2014.29.12.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim D, Ha M, Song I. Trends in major cancer mortality in Korea, 1983-2012, with a joinpoint analysis. Cancer Epidemiol. 2015;39:939–946. doi: 10.1016/j.canep.2015.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Means, standard deviations, and medians of disability weights for causes of disease from a person trade-off
Comparison of disability weights for causes of disease between this study and a previous study
Box plot of disability weights of 40 causes of disease from a person trade-off