Abstract
Objectives
Obesity and obesity-associated inflammation is central to a variety of end-organ sequelae including atherosclerosis, a leading cause of death worldwide. Although mouse models have provided important insights into the immunopathogenesis of various diseases, modeling atherosclerosis in mice has proven difficult. Specifically, wild-type (WT) mice are resistant to developing atherosclerosis, while commonly used genetically modified mouse models of atherosclerosis are poor mimics of human disease. The lack of a physiologically relevant experimental model of atherosclerosis has hindered the understanding of mechanisms regulating disease development and progression as well as the development of translational therapies. Recent evidence suggests that housing mice within their thermoneutral zone profoundly alters murine physiology, including both metabolic and immune processes. We hypothesized that thermoneutral housing would allow for augmentation of atherosclerosis induction and progression in mice.
Methods
ApoE−/− and WT mice were housed at either standard (TS) or thermoneutral (TN) temperatures and fed either a chow or obesogenic “Western” diet. Analysis included quantification of (i) obesity and obesity-associated downstream sequelae, (ii) the development and progression of atherosclerosis, and (iii) inflammatory gene expression pathways related to atherosclerosis.
Results
Housing mice at TN, in combination with an obesogenic “Western” diet, profoundly augmented obesity development, exacerbated atherosclerosis in ApoE−/− mice, and initiated atherosclerosis development in WT mice. This increased disease burden was associated with altered lipid profiles, including cholesterol levels and fractions, and increased aortic plaque size. In addition to the mild induction of atherosclerosis, we similarly observed increased levels of aortic and white adipose tissue inflammation and increased circulating immune cell expression of pathways related to adverse cardiovascular outcome.
Conclusions
In sum, our novel data in WT C57Bl/6 mice suggest that modulation of a single environmental variable, temperature, dramatically alters mouse physiology, metabolism, and inflammation, allowing for an improved mouse model of atherosclerosis. Thus, thermoneutral housing of mice shows promise in yielding a better understanding of the cellular and molecular pathways underlying the pathogenesis of diverse diseases.
Keywords: Atherosclerosis, Thermoneutrality, Inflammation
Highlights
-
•
Thermoneutral housing augments atherosclerosis in ApoE−/− and WT mice.
-
•
Thermoneutral housing increases serum LDL levels in obese WT mice.
-
•
Thermoneutral housing increases inflammatory potential in lean and obese mice.
1. Introduction
Obesity, a pandemic, is closely associated with the development and progression of atherosclerosis, the leading cause of mortality in the United States [1], [2]. In fact, along with age, increased body weight is a leading risk factor for atherosclerosis onset [3]. Atherosclerosis, or plaque buildup and immune cell infiltration into the arterial walls, is the most common pathological process leading to cardiovascular disease [2]. However, despite the public health and clinical significance, the molecular mechanisms that regulate atherosclerosis initiation and progression are not fully understood [2]. Consequently, a better understanding of the molecular and cellular pathways and mechanisms driving atherosclerosis pathogenesis could aid in the development of novel therapeutics.
Broad overall similarity in immune function between wild-type (WT) mice and humans has resulted in mice representing the most common experimental model for interrogation of cellular and molecular mechanisms underlying immunopathogenesis of various diseases. However, the same cannot be accomplished for atherosclerosis, as WT mice are highly resistant to atherosclerosis development, even when fed an obesogenic diet [4]. Thus, a major challenge in the mechanistic understanding of atherosclerosis pathogenesis is choosing an appropriate research model.
Given the lack of atherosclerosis development in WT mice, a number of knockout and transgenic mouse models, despite their distinct drawbacks, have been utilized to delineate the mechanisms underlying atherosclerosis [4]. The most widely used mouse models involve the genetic deletion of low-density lipoprotein receptor (Ldlr) or apolipoprotein E (ApoE) [4], [5], [6]. Ldlr−/− mice fed high fat, high cholesterol “Western” diets develop features of more advanced atherosclerotic lesions [5]. Fundamentally, however, Ldlr−/− mice exhibit levels of hypercholesterolemia that far exceed that seen in most patients with atherosclerosis and primarily provide a model of familial hypercholesterolemia [5]. Notably, familial hypercholesterolemia condition is rare in humans, despite the propensity of LDL-driven atherosclerosis in humans [5]. In contrast, although ApoE−/− mice develop early fatty streaks which evolve into complex lesions [6], ApoE−/− deficiency is rare in humans and results in a markedly different lipid profile (dramatically elevated cholesterol levels and a predominance of VLDL, as opposed to LDL) [5], [6]. Further, it has been reported that ApoE has anti-inflammatory potential, and ApoE deficiency drives an aberrant immune response associated with exacerbated levels of cytokine production and autoimmune disorders [7].
While the differences between animal and human atherosclerosis is typically attributed to fundamental biological differences between species, recent data suggest that the environmental conditions ubiquitously employed in mouse housing lead to profound physiological alterations [8], [9], [10], [11] – something that may impair our ability to model atherosclerosis in mice. Laboratory mice are standardly housed at 19–22 °C (TS), a temperature chosen for the comfort of clothed handlers [12]. However, the thermoneutral zone (TN), temperature at which metabolic homeostasis is maintained, for Mus musculus is 29–32 °C [13]. Thus, laboratory mice are subjected to constant cold stress, the extent of which can be measured by a dramatic decrease in heart rate (≈200 bpm) and basal metabolic rate (50–60%) in mice housed at TN as opposed to TS [13]. Specifically, among other physiological changes, housing mice at TN, as opposed to TS, (a) reduces sustained sympathetic nervous system activation—associated with decreased heart rate, (b) enhances the immune response to a variety of pathogens (typhus, rhinovirus, LPS, etc.) and tumors, and (c) augments obesity development [8], [10], [12], [13], [14], [15], [16], [17].
The emergence of ambient temperature as a potential variable in disease modeling has already gained interest in the atherosclerosis field. Specifically, recent, seminal reports have suggested both that severe cold exposure (5 °C) promotes atherosclerosis [18] and that TN housing, in combination with obesogenic Western diet (WD) exacerbates atherosclerosis [19]. However, these reports focused on atherosclerosis induction in ApoE−/− or Ldlr−/− mice [18], [19]. Thus, we hypothesized that modulation of ambient temperature would allow for exacerbated inflammation and the induction of atherosclerosis development in athero-resistant WT mice on a C57BL/background, resulting in a novel experimental model to study disease pathogenesis.
Here, we confirmed previous reports [19] that housing mice at TN, in combination with WD, profoundly augmented obesity development and promoted atherosclerosis in not only ApoE−/− mice. Further, we show, for the first time, that TN housing in combination with WD promoted mild induction of atherosclerosis in WT mice as well. This increased disease burden was associated with altered cholesterol levels and fractions, augmented lipid profiles, and increased aortic plaque sizes. Further, the initiation of atherosclerosis in WT mice was accompanied with increased aortic and adipose tissue expression of inflammatory genes known to play a role in atherosclerosis, and increased circulating immune cell expression of pathways related to adverse cardiovascular outcome.
2. Methods
2.1. Mice
Male WT and ApoE−/− mice on a C57BL/6 background were purchased from Jackson Laboratories and bred at Cincinnati Children's Hospital Medical Center (CCHMC). All mice were housed in a specific pathogen-free (spf) facility, within the same barrier, with free access to irradiated food and water. Mice were housed either in a standard housing unit maintained at 22 °C or a thermoneutral room maintained at 30–33 °C. Mice were provided care in accordance with the Guide for the Care and Use of Laboratory Animals. All studies were approved by the CCHMC IACUC.
2.2. Atherosclerosis model
Diets: Mice were purchased at 6 weeks of age and housed in either a spf thermoneutral room (30–33 °C) or spf standard room (22 °C). After a 2-week acclimation period, mice were fed either an obesogenic “Western” diet (WD; 21% fat [wt/wt], 0.3% cholesterol; Dyets Inc. # 101977) or a chow diet (CD; LAB Diet #5010) ad libitum for 12 weeks (ApoE−/− mice) or 24 weeks (WT mice). Animal body weight and food consumption were quantified weekly. Fresh food was provided on a weekly basis. All mice were fasted overnight before sacrifice. At sacrifice, mice were anesthetized with isoflurane, exsanguinated by cardiac puncture, and perfused with phosphate buffered saline (PBS), followed by 10% sucrose in PBS. The heart was separated from the aorta at the root and embedded in OCT medium for sectioning.
2.3. Total body adiposity
Total body fat and lean and water mass were determined by nuclear magnetic resonance (Whole Body Composition Analyzer; Echo MRI) [20].
2.4. Atherosclerotic lesion size
Hearts were sectioned through the aortic root (8 μm) and stained with hematoxylin and eosin for lesion quantification or used for immunohistochemical analysis as previously described [21]. For morphometric analysis of lesions, 6 sections per mouse were imaged (Nikon Eclipse) with Image Pro Plus, spanning the entire aortic root, and lesions were quantified using iVision Software. Oil Red O and CD68 staining were performed to detect neutral lipid and lesional macrophages as described previously [21].
2.5. Serum cholesterol
Serum was initially diluted 1:10 in saline and 25 μL of diluted serum or standard was then added to a 96 well clear flat bottom plate (Costar) containing 100 μL of Infinity Cholesterol Liquid Stable Reagent (Thermo Scientific). Standards (Thermo Scientific) were prepared according to manufacturer's instructions. Serum cholesterol was quantified at 500–520 nm using Molecule Devices vmax and Kinetic Microplate Reader and SoftMax Pro v5 software.
2.6. Serum triglycerides
Ten micro litres of serum was added to a 96 well clear flat bottom plate (Costar) containing 200 μL of Triglyceride Reagent (Pointe Scientific). Standards (Pointe Scientific) were prepared according to manufacturer's instructions. Serum triglycerides were quantified at 500–520 nm using Molecule Devices vmax and Kinetic Microplate Reader and SoftMax Pro v5 software.
2.7. Lipoprotein separation
Pooled serum samples from three mice in the same group (minimum 0.3 mL) were subjected to fast-performance liqud chromatography (FPLC) gel filtration on two Superose 6 columns connected in series as previously described [22].
2.8. qRT-PCR
RNA was extracted from lysed tissue samples, and cDNA was transcribed according to previously described protocol [23], [24]. qPCR analysis was then completed utilizing a Light Cycler 480 II (Roche), Sybr Green I Master mix (Roche) and the following primers pairs: Abca1 For GCTGCAGGAATCCAGAGAAT Rev CATGCACAAGGTCCTGAGAA; Mmp2 For GATGGCATTCCAGGAGTCTG Rev CCAGCAAGTAGATGCTGCCT; Rag1 For CTGAAGCTCAGGGTAGACGG Rev CAACCAAGCTGCAGACATTC; Sox13 For ACTGCTCTCTGGTGAGGCAC Rev GTCTGAGGAGAAGAAGGAGCC; F4/80 For CTTTGGCTATGGGCTTCCAGTC Rev GCAAGGAGGACAGAGTTTATCGTG; Ccl2 For AGATGCAGTTAACGCCCCAC Rev TGTCTGGACCCATTCCTTCTTG; Ccr2 For CCACTGTCTTTGAGGCTTGTTG Rev GCTCTACATTCACTCCTTCCACTG; IFNγ For TGGCTGTTTCTGGCTGTTACTG Rev ACGCTTATGTTGTTGCTGATGG Arg1 For GTGGCAGAGGTCCAGAAGAATG Rev TTGTCAGGGGAGTGTTGATGTC; iNOS For GTTCTCAGCCCAACAATACAAGA Rev GTGGACGGGTCGATGTCAC; F4/80 For CCCCAGTGTCCTTACAGAGTG Rev GTGCCCAGAGTGGATGTCT; CD4 For TCCTTCCCACTCAACTTTGC Rev AAGCGAGACCTGGGGTATCT; Tbet For AGCAAGGACGGCGAATGTT Rev GGGTGGACATATAAGCGGTTC; IL6 For CCAAGAGGTGAGTGCTTCCC Rev CTGTTGTTCAGACTCTCTCCCT; TNF For ATGAGCACAGAAAGCATGATCCGC Rev CCAAAGTAGACCTGCCCGGACTC; Beta Actin For GGCCCAGAGCAAGAGAGGTA Rev GGTTGGCCTTAGGTTTCAGG.
2.9. Macrophage isolation
Murine thioglycollate elicited peritoneal macrophages (EPMs) were generated using standard protocol [25]. EPMs (1 × 106 cells/well) were cultured for 4 h and subsequently stimulated with LPS (100 ng/ml) for 18 h to determine cytokine production by ELISA.
2.10. RNA sequencing and gene expression quantification
Circulating immune cells were isolated by terminal blood collection and subjection to ficoll gradient separation from mice fed an obesogenic diet (Research Diets #D12492) for 8 weeks. To eliminate contaminating cells, CD45+ cells were positively selected by magnetic separation (Miltenyi Biotec). Gene expression was determined by running 50 base pair single-end reads (∼20 million reads per sample). All analyses were performed in Strand NGS v2.5.1. Following the removal of primers and barcodes, raw reads were aligned to the mouse reference genome (mm10). Annotations were produced by RefSeq (NCBI Reference Sequence Database). Aligned reads were filtered on base quality (quality threshold ≥30, zero ‘Ns’ allowed). Reads per kilobase per million reads (RPKMs) were computed for each sample from aligned gene read counts (N = 36,186 transcripts). Raw counts were normalized (DESeq algorithm), thresholded to 1, baselined to the median of all samples, and further filtered to require at least three reads per transcript in all samples in at least one experimental condition (N = 14332 transcripts). Differential expression was defined as a fold change ≥2, with a P-value < 0.05, between experimental conditions. Raw data can be accessed at GSE80976.
2.11. Cytokine levels
For quantification of in vivo cytokine production, in vivo cytokine capture assays (IVCCA) were completed as previously described [24], [26], [27]. Briefly, biotinylated capture antibodies (all eBioscience) were injected intravenously, and terminal serum collection was performed 24 h later. For in vitro production, ELISAs for murine IL-6 and IFNγ (BD) were employed as previously described [24], [26].
2.12. Statistical analysis
Based on preliminary data, we assumed a normal distribution and employed parametric tests for statistical analysis. Student's t-test was used when the comparison was two groups, while a one-way ANOVA was employed for three or more groups, with Tukey's post hoc test to assess differences between specific groups. Statistical analysis was completed using Prism 5a (GraphPad Software, Inc.). All values are represented as means ± standard error mean (SEM).
3. Results
3.1. Thermoneutral housing exacerbates obesity and atherosclerosis in ApoE−/− mice
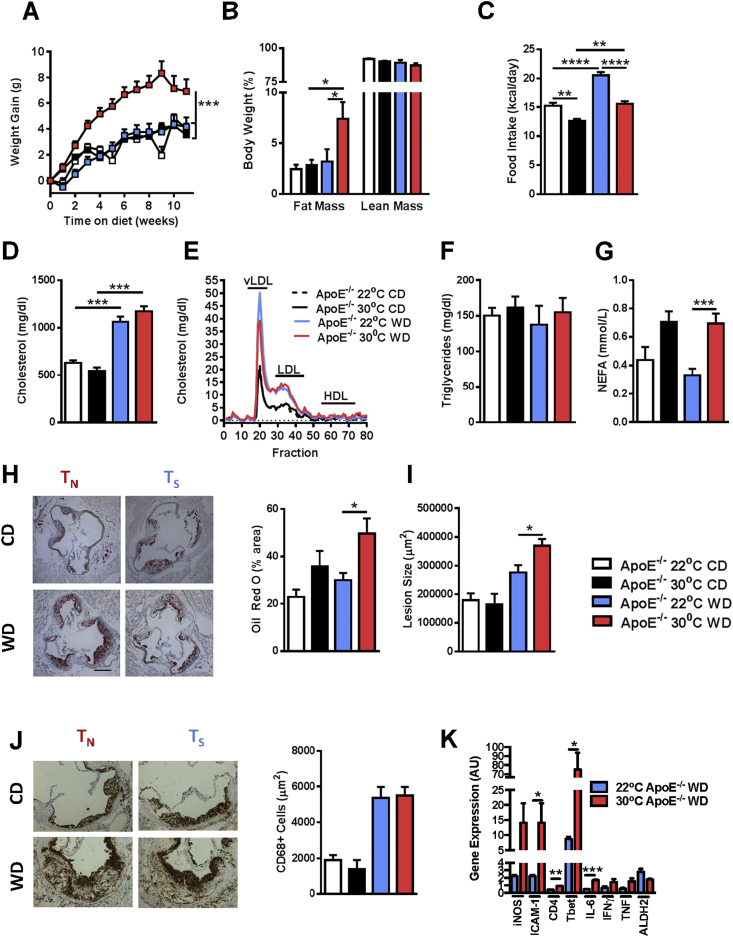
Thermoneutral (TN) housing allows for the development of obesity in nude mice–mice normally resistant to diet-induced obesity [14]. However, the role of TN housing in the development of atherosclerosis is less clear. Literature conflicts as to whether temperature-driven metabolic neutrality [19] or severe cold-driven stress [18] modulates atherosclerosis pathogenesis. Given that humans widely live in metabolic comfort [28], and TN housing has been demonstrated to alter metabolism and inflammation [12], [14], we hypothesized that TN housing in combination with WD would exacerbate obesity and atherosclerosis. ApoE−/− mice were housed in a standard (TS) or TN room and fed either WD or CD. Mice housed at TN gained significantly more weight than counterparts housed at TS (Figure 1A). Of note, this weight gain correlated with increased adiposity (Figure 1B), but was uncoupled from increased food/caloric intake (Figure 1C). Thus, these data confirm a previous report [19] and highlight the impact of TN housing on metabolic homeostasis and obesity development and suggest that thermoneutrality, in combination with WD, exacerbates obesity in ApoE−/− mice.
Figure 1.
Thermoneutral housing augments obesity and atherosclerosis pathogenesis in ApoE−/−mice. Eight week old male ApoE−/− mice (n = 10–13/condition) were housed at standard (23 °C; TS) or thermoneutral (30 °C; TN) temperatures and fed obesogenic “Western” diet (WD) or chow diet (CD) for 12 weeks. (A) Body weight gain over 12 weeks. (B) Percent of body weight composed of either fat mass or lean mass. (C) Average daily calorie intake per mouse. (D) Total serum cholesterol levels. (E) Serum cholesterol fraction profile. (F) Serum triglyceride levels. (G) Serum non-esterified fatty acids (NEFAs) levels. (H) Oil red O staining of aortic root and percent area positive for staining (Scale bar = 250 μm). (I) Quantification of average lesion size in aortic root. (J) Infiltration of CD68 + macrophages as determined by immunohistochemistry. (K) Aortic gene expression of iNOS, ICAM-1, CD4, Tbet, IL-6, IFNγ, TNF and ALDH2 in WD fed animals. Data represent means + SEM from a single experiment. (A) AUC ANOVA ***P < 0.001. (A–D, and G–I). ANOVA with Tukey's correction *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (K) Student's t-test *P < 0.05, **P < 0.01, ***P < 0.001.
As obesity is a primary risk factor for development of atherosclerosis, we next evaluated whether TN housing of ApoE−/− mice affects atherosclerosis pathogenesis. As shown in Figure 1D, although WD feeding increased total cholesterol levels, TN housing did not synergistically augment total serum cholesterol levels in ApoE−/− mice or alter the proportion of vLDL, LDL, and HDL cholesterol fractions in ApoE−/− mice fed WD (Figure 1E). Quantification of serum triglycerides similarly revealed no differences between TN and TS housing (Figure 1F). Notably, however, TN-housed ApoE−/− mice displayed significantly augmented levels of serum non-esterified fatty acids (NEFAs), a known marker and risk factor for atherosclerosis induction (Figure 1G) [29]. Quantification of lipid deposition in the aorta, via Oil Red O staining, revealed that WD fed, TN-housed ApoE−/− mice had significantly higher levels of aortic lipid accumulation (Figure 1H) and significantly larger atherosclerotic plaques (Figure 1I). Despite increased lipid deposition and lesion size, TS and TN housed ApoE−/− mice fed WD had similar levels of aortic CD68+ macrophage infiltration (Figure 1J). However, expression of genes related to increased macrophage activation, Th1 cell infiltration, and production of proinflammatory cytokines were upregulated in the aortic roots of mice housed at TN (Figure 1K). Notably, increased macrophage activation, characterized here by increased ICAM-1 expression and decreased ALDH2, as well as Th1 cell infiltration, and production of IL-6, IFNγ, and TNF previously have been associated with atherosclerosis pathogenesis (Figure 1K) [2], [30], [31]. These data confirm a previous report which demonstrated increased atherosclerosis in ApoE−/− mice housed at TN [19]. Therefore, TN housing augments inflammation, immune cell activation, and the propensity of ApoE−/− mice to develop atherosclerosis, despite similar levels of serum cholesterol and aortic macrophage infiltration.
3.2. Thermoneutrality exacerbates obesity development in WT mice
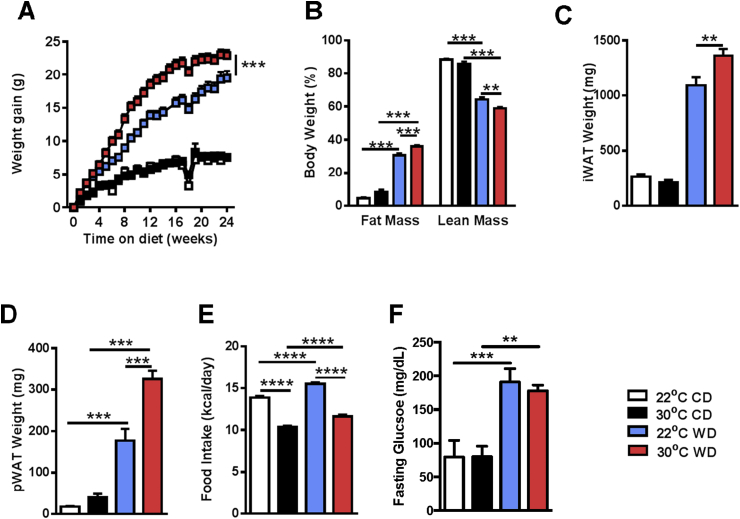
Although TN housing augments weight gain and atherosclerosis in ApoE−/− mice, ApoE−/− mice are suboptimal mimics of human physiology [5], [6], [7]. Thus, we asked whether TN housing would allow for the development of aortic lesions in WT mice, allowing for a novel, more “human-like” model of atherosclerosis. When fed WD, TN-housed WT mice gained significantly more weight than their TS-housed counterparts (Figure 2A). Notably, increased obesity was associated with an increased proportion of total body fat mass compared to TS housed mice (Figure 2B) and increased inguinal and perirenal adipose tissue deposition, both of which have been shown to increase risk for heart disease (Figure 3C,D) [32]. However, increased weight gain was independent from increased food intake (Figure 2E) and likely associated with altered energy expenditure at TN [14]. Additionally, in agreement with a previously published report [19], despite an increase in fasting glucose levels in WD fed animals, TN housing did not further alter glucose homeostasis compared to TS housing (Figure 2F). In sum, these data suggest that TN housing accelerates and exacerbates obesity and augments adipose tissue distribution in WT mice fed WD.
Figure 2.
Thermoneutral housing augments obesity development and progression in WT mice. Eight week old male WT C57BL/6 mice (n = 9–16/condition) were housed at standard (23 °C; TS) or thermoneutral (30 °C; TN) temperatures and fed obesogenic “western” diet (WD) or chow diet (CD) for 24 weeks. (A) Body weight gain over 24 weeks. (B) Percent of body weight composed of either fat mass or lean mass. (C) Inguinal white adipose tissue (iWAT) weight. (D) Perirenal white adipose tissue (pWAT) weight. (E) Average daily calorie intake per mouse. (F) Fasting glucose levels in serum after 18 weeks of chow or WD. Data represent means + SEM; a representative of two separate experiments. (A) AUC ANOVA ***P < 0.001. (A–F) ANOVA with Tukey's correction **P < 0.01, ***P < 0.001, ****P < 0.0001.
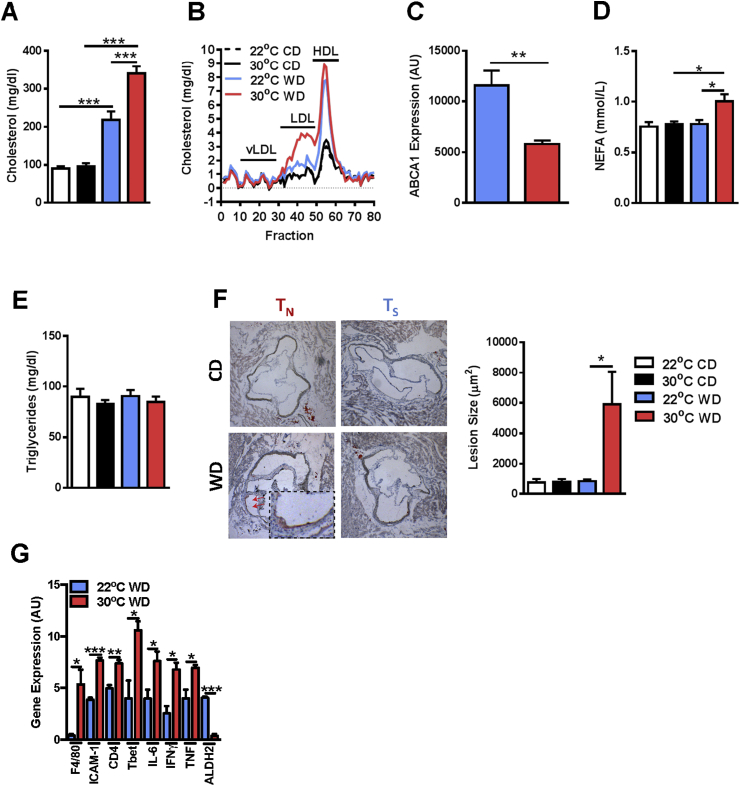
Figure 3.
Thermoneutral housing augments serum lipid profile and initiates atherosclerosis induction in WT mice. Eight week old male WT C57BL/6 mice (n = 9–16/condition) were housed at standard (23 °C; TS) or thermoneutral (30 °C; TN) temperatures and fed obesogenic “Western” diet (WD) or chow diet (CD) for 24 weeks. (A) Total serum cholesterol levels. (B) Serum cholesterol fraction profile. (C) ABCA1 hepatic mRNA expression. (D) Serum NEFA levels. (E) Serum triglyceride levels. (F) Quantification of average lesion size in aortic root. (G) Aortic gene expression of F4/80, ICAM-1, CD4, Tbet, IL-6, IFNγ, TNF and ALDH2 in WD fed animals. Data represent means + SEM from a single experiment. (A, D and F) ANOVA with Tukey's correction *P < 0.05, ***P < 0.001. (C and G) Student's t-test *P < 0.05, **P < 0.01, ***P < 0.001.
3.3. Thermoneutrality allows for induction of atherosclerosis in WT mice
As TN housing exacerbates obesity development in WT mice, we next evaluated whether TN housing promoted initiation of atherosclerosis development in WT mice. As shown in Figure 3A, TN housing in combination with WD feeding, compared to TS housing, significantly increased total serum cholesterol levels, specifically serum LDL levels (Figure 3B), the predominate cholesterol fraction which drives human disease [2]. This increase in LDL vs HDL was supported by a reduced expression of ABCA1 in the liver of TN-housed, WD fed animals (Figure 3C). ABCA1 is a critical transporter for the formation of HDL, and its expression correlates with HDL levels and protects from atherosclerosis [33]. Further, TN housing in combination with WD feeding also increased serum NEFA levels without impacting serum triglyceride levels, as compared to TS-housed WT mice (Figure 3D,E). Quantification of atherosclerotic lesion size revealed that TN-housed WT mice fed WD had a significant induction of atherosclerotic lesions (Figure 3F). Additionally, TN-housed, WD-fed mice exhibited increased aortic root F4/80 expression compared to TS-housed counterparts, something suggestive of increased macrophage infiltration (Figure 3G). Additionally, there was also an observed decrease in ALDH2 gene expression, which is known to inhibit macrophage driven atherosclerosis [31]. Further similar to ApoE−/− mice, WT mice also exhibited increased aortic root expression of genes related to macrophage activation, Th1 cell infiltration, and IL-6, IFNγ, and TNF production (Figure 3G). In sum, these data suggest that TN housing, in combination with WD-stress, significantly alters serum lipid profiles and promotes induction of mild atherosclerosis in WT mice.
3.4. Increased inflammation and expression of pathways associated with atherosclerosis
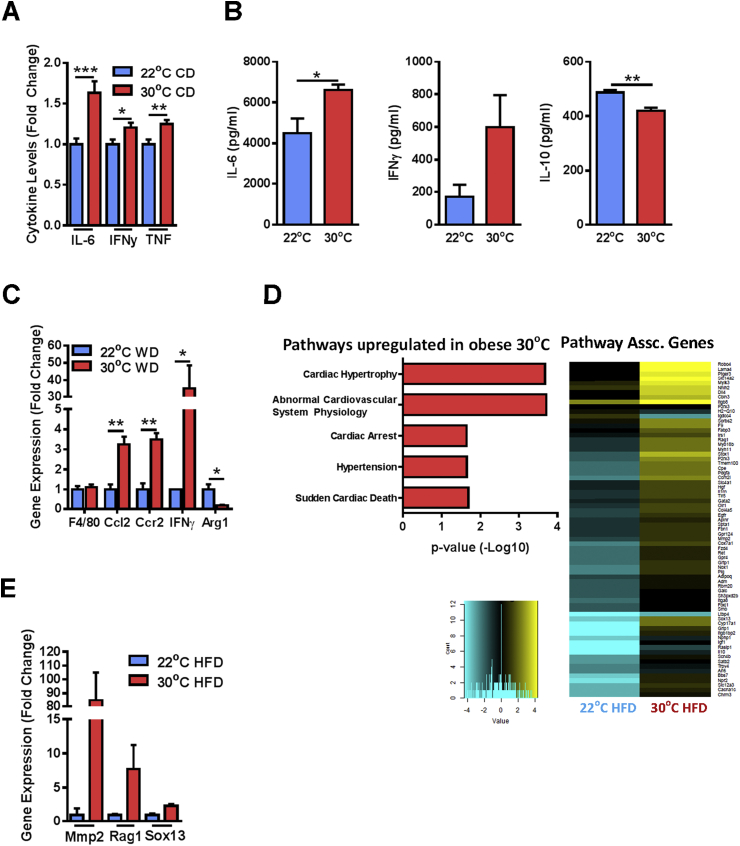
To begin to elucidate the mechanisms underlying TN-driven atherosclerosis induction, we evaluated the impact of TN housing on the inflammatory profile in WT mice. Notably, inflammation is central to atherosclerosis pathogenesis [2]. Analysis of circulating cytokine levels in TN housed WT mice revealed increased serum levels of proinflammatory cytokines (Figure 4A) [2]. Macrophage-driven inflammation, systemically and in the adipose tissue, contributes to atherosclerosis pathogenesis [34] and pathogenesis of other metabolic diseases [35]. Thus, we next evaluated macrophage activation and function at TN. Macrophages from TN, as compared to TS-housed mice, produced increased levels of proinflammatory cytokines IL-6 and IFNγ, and decreased levels of the anti-inflammatory cytokine IL-10 following bacterial ligand challenge (Figure 4B). Further, despite similar macrophage infiltration, as quantified by F4/80 (Figure 4C), into the adipose tissue [36], TN-housed mice displayed increased expression of CCL2, CCR2, and IFNγ, critical regulators of adipose tissue inflammation [19], and reduced Arg1 expression (Figure 4C). These data suggest a predilection towards an inflammatory M1 polarization phenotype at TN [34]. Notably, an inflammatory M1 skewing is associated with increased atherosclerosis, while an M2 promotion seems to be protective [34]. Given that obesity and TN housing alters adipose inflammation, we next examined whether there was a systemic effect by evaluating the gene expression profiles in primary blood mononuclear cells (PBMCs) of TN or TS housed mice fed obesogenic diet. Notably, TN housing significantly upregulated gene expression pathways associated with cardiovascular disease (Figure 4D and Supplementary Table 1). Expression of select genes associated with abnormal cardiovascular system physiology was further verified via qPCR (Figure 4E). These data suggest that increased inflammation observed at TN, may contribute induction of mild atherosclerosis in WT mice.
Figure 4.
Thermoneutral housing augments systemic and aortic inflammation in WT mice. (A) 6 week old male WT C57BL/6 mice (n = 29–34/condition) were housed at standard (23 °C; TS) or thermoneutral (30 °C; TN) temperatures and fed chow diet (CD) for 3 weeks. Serum levels of IL-6, IFNγ, and TNF were quantified via IVCCA. (B) 6 week old male WT C57BL/6 mice (n = 4/condition) were housed at standard (23 °C; TS) or thermoneutral (30 °C; TN) temperatures and fed chow diet (CD) for 3 weeks. Thioglycollate elicited peritoneal macrophages were stimulated with lipopolysaccharide (100 ng/mL) and production of IL-6, IFNγ and IL-10 was quantified via ELISA. (C) Epididymal white adipose tissue expression of macrophage associated genes by RT-PCR from WT C57BL/6 mice fed obesogenic “Western” diet (WD) as described above (Figure 3). IFNγ levels were undetectable in TS-housed mice and assigned a value of 1. (D) 8 week old male WT C57BL/6 mice (n = 2/condition) were housed at standard (23 °C; TS) or thermoneutral (30 °C; TN) temperatures and fed obesogenic diet for 8 weeks. Pathways related to atherosclerosis were quantified in PBMCs by RNASeq analysis. (E) Relative immune cell expression of select genes identified by RNASeq as determined by qPCR. (A–C and E) Data represent means + SEM from a single experiment. Student's t-test *P < 0.05, **P < 0.01, ***P < 0.001.
4. Discussion
Obesity has emerged as a leading risk factor for induction of atherosclerosis [1], a primary cause of mortality in the US [37]. Despite the clinical and public health significance, effective therapies for atherosclerosis are lacking. Additionally, animal models of atherosclerosis fail to properly mimic human disease. Development of novel treatment strategies for atherosclerosis will likely depend on our ability to better experimentally model the disease development and progression. Of note, the temperature at which mice are housed affects their heart rate, weight gain, and inflammation [8], [9], [10], [11], [12], [13], [14] – physiological parameters all shown to play an important role in atherosclerosis. Our data confirm previous findings [19] and demonstrate that housing mice at TN, in combination with WD, led to increased atherosclerosis pathogenesis in ApoE−/− mice. Further, our data, for the first time, demonstrate induction of mild atherosclerosis in WT mice. Increased atherosclerosis induction and progression was associated with increased weight gain, adiposity, and altered serum cholesterol levels and profiles. Specifically, in addition to the mild induction of atherosclerosis, we similarly observed increased levels of systemic, aortic and white adipose tissue inflammation, macrophage activation and circulating immune cell expression of genes associated with adverse cardiac outcomes. This increased disease burden was associated with altered lipid profiles, including cholesterol levels and fractions, and increased aortic plaque size. Thus, our data suggest that housing mice at TN represents a novel, improved murine model of inflammatory atherosclerosis—a model in which the molecular mechanisms regulating disease progression potentially can be more fully interrogated. Of particular interest, a more thorough investigation of the role of the immune system in the induction of atherosclerosis may be possible now.
While the mechanisms underlying atherosclerosis pathogenesis remain incompletely defined, it is well accepted that inflammation plays a central role in disease pathogenesis [2]. Of note, housing mice within their thermoneutral zone drives increased immune function in obesity, infection, and tumor models [10], [14], [38], but whether specific immune pathways are upregulated by TN housing in either ApoE−/− or WT mice is unknown. Our data suggest that TN housing, in combination with obesogenic diet, drives a significant increase in the expression of genes associated with a variety of adverse cardiac outcomes. These data suggest that TN housing may allow for improved modeling of multiple cardiac diseases, although formal evaluation of kinetics and diets will need to be completed. Further, given that these gene expression pathways are increased specifically in immune cells, evaluation of differential inflammatory pathway expression in TN versus TS-housed mice fed WD could yield insight into novel, critical inflammatory pathways central to atherosclerosis progression.
Analysis of serum cholesterol in WT mice fed WD revealed that these mice exhibited increased levels of LDL. Notably, LDL drives cholesterol accumulation in the aortic wall and acts as a ligand for macrophage activation. In addition to increased LDL levels, it is not known whether housing mice at TN augments the inflammatory response to LDL. Thus, utility of TN housing could represent an initial step towards defining the underlying mechanisms of increased inflammatory response to LDL in atherosclerosis. Additional experiments evaluating the response of immune cells in TN housed mice to LDL may aid in understanding how environmental changes augment mouse physiology and identifying novel, or previously underappreciated immune mediators in atherosclerosis. In fact, such findings may be highly relevant for the development of new therapies for atherosclerosis.
A central site of inflammation during obesity is the adipose tissue [35]. Increased activity of inflammatory macrophages in the adipose tissue has been associated with the exacerbation of obesity's downstream sequelae, including insulin resistance [35]. While macrophage driven inflammation in the arterial wall is central to atherosclerosis [34], the impact of increased adipose tissue macrophage activation on atherosclerosis is less well-understood. A published report suggests that obesity induction at TN housing allows for increased macrophage infiltration to the adipose tissue in both WT and ApoE−/− mice [19]. This increased infiltration was also associated with an increased skewing of M1 over M2 macrophages and increased atherosclerosis induction in ApoE−/− mice [19]. However, whether altering the macrophage phenotype (M1 or M2) or the numbers infiltrating the adipose tissue affects atherosclerosis induction requires further examination. To this point, TN housing may represent a model for characterizing the mechanisms inducing and maintaining macrophage polarization. Further, TN housing may represent a novel model where the critical loci of inflammation and critical cell type(s) in atherosclerosis can be further explored.
Given that TN housing alters the immune response, food intake, and weight gain, it is likely that it also alters the intestinal microbiome. As both a metabolic disorder and inflammatory disease, the pathogenesis of atherosclerosis has, in fact, been linked to the intestinal microbiome [39]. Intuitively, an altered microbiome may affect atherosclerosis initiation and/or progression through a variety of mechanisms including increased LPS production, cholesterol uptake, altered systemic metabolic function, or inflammation [39]. To date, a majority of studies implicating the microbiome in atherosclerosis pathogenesis remain correlative. In addition to the complexities underlying microbiome composition and atherosclerosis progression, the lack of a cohesive model has also impacted the relatively little mechanistic data concerning this link. As TN housing represents a novel model of atherosclerosis, largely devoid of need for genetic alterations, evaluating the microbiome differences of mice housed at varying temperatures may provide insight into whether specific bacteria are critical to atherosclerosis development and progression. Subsequent studies involving monocolonization of specific bacteria may allow for initial mechanistic studies in determining the link between the microbiome and atherosclerosis pathogenesis.
Finally, studying the pathogenesis of atherosclerosis in female mice has not been widely performed. This is likely due to the fact that female mice are protected from diet-induced obesity, as compared to male mice [40]. However, human obesity rates are similar in both men and women, and atherosclerosis is the leading cause of mortality in both sexes [41], [42]. As TN housing significantly augments weight gain in male mice fed WD, whether or not this is the case for female mice requires further exploration. In fact, if TN housing were to promote obesity and atherosclerosis in female mice, it may allow for the realization of various gender-related mechanisms involved in atherosclerosis development and progression.
In sum, this report describes a novel, inflammatory murine model for atherosclerosis in the absence of previously utilized genetic manipulations. While atherosclerosis induction in WT mice was mild (compared to ApoE−/− mice), and likely requires further optimization, this approach represents a significant step forward in atherosclerosis modeling. In fact, further manipulation of atherosclerosis induction in TN housing may improve our ability to interrogate the metabolic and immune mechanisms central for atherosclerosis initiation and progression—something that may lead to the development of novel therapeutic approaches. Given the growing obese population worldwide, it is unlikely that morbidity and mortality associated with atherosclerosis will diminish without development of novel therapies.
Author contribution
All authors have reviewed the manuscript and approve the final version.
Acknowledgments
This work was supported in part by National Institutes of Health R01DK099222 and R21HL113907 (to S.D.); National Institutes of Health T32AI118697 (to D.A.G.); National Institutes of Health K99HL125667 (to B.R.); National Institute of Environmental Health Sciences Grant P30 ES006096 University of Cincinnati Center for Environmental Genomics; and National Institutes of Health Grant P30 DK078392 Pathology of the Digestive Disease Research Core Center at CCHMC.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.09.008.
Conflict of interest
All authors declare no conflict of interest.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Table 1Thermoneutral housing increases expression of genes within identified atherosclerosis-related pathways. Eight week old male WT C57BL/6 mice (n = 2/condition) were housed at standard (23 °C; TS) or thermoneutral (30 °C; TN) temperatures and fed obesogenic diet for 8 weeks. Pathways related to atherosclerosis were quantified in PBMCs by RNASeq analysis. This table reports the fold increase of expression in mice housed at TN.
References
- 1.Finucane M.M., Stevens G.A., Cowan M.J., Danaei G., Lin J.K., Paciorek C.J. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galkina E., Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annual Review of Immunology. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmermund A., Mohlenkamp S. No paradox: relationship between obesity and coronary atherosclerosis. European Heart Journal – Cardiovascular Imaging. 2013;14(9):928–929. doi: 10.1093/ehjci/jet115. [DOI] [PubMed] [Google Scholar]
- 4.Leong X.F., Ng C.Y., Jaarin K. Animal models in cardiovascular research: hypertension and atherosclerosis. BioMed Research International. 2015;2015:528757. doi: 10.1155/2015/528757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith J.D., Breslow J.L. The emergence of mouse models of atherosclerosis and their relevance to clinical research. Journal of Internal Medicine. 1997;242(2):99–109. doi: 10.1046/j.1365-2796.1997.00197.x. [DOI] [PubMed] [Google Scholar]
- 6.Zadelaar S., Kleemann R., Verschuren L., de Vries-Van der Weij J., van der Hoorn J., Princen H.M. Mouse models for atherosclerosis and pharmaceutical modifiers. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(8):1706–1721. doi: 10.1161/ATVBAHA.107.142570. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H., Wu L.M., Wu J. Cross-talk between apolipoprotein E and cytokines. Mediators of Inflammation. 2011;2011:949072. doi: 10.1155/2011/949072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swoap S.J., Li C., Wess J., Parsons A.D., Williams T.D., Overton J.M. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. American Journal of Physiology, Heart and Circulatory Physiology. 2008;294(4):H1581–H1588. doi: 10.1152/ajpheart.01000.2007. [DOI] [PubMed] [Google Scholar]
- 9.Cannon B., Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. Journal of Experimental Biology. 2011;214(Pt 2):242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 10.Rudaya A.Y., Steiner A.A., Robbins J.R., Dragic A.S., Romanovsky A.A. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 2005;289(5):R1244–R1252. doi: 10.1152/ajpregu.00370.2005. [DOI] [PubMed] [Google Scholar]
- 11.Won W.D., Ross H. Relationship of low temperature to mouse resistance to infection with Klebsiella pneumoniae. Aerospace Medicine. 1971;42(6):642–645. [PubMed] [Google Scholar]
- 12.Karp C.L. Unstressing intemperate models: how cold stress undermines mouse modeling. Journal of Experimental Medicine. 2012;209(6):1069–1074. doi: 10.1084/jem.20120988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overton J.M. Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters. International Journal of Obesity (London) 2010;34(Suppl. 2):S53–S58. doi: 10.1038/ijo.2010.240. [DOI] [PubMed] [Google Scholar]
- 14.Stemmer K., Kotzbeck P., Zani F., Bauer M., Neff C., Müller T.D. Thermoneutral housing is a critical factor for immune function and diet-induced obesity in C57BL/6 nude mice. International Journal of Obesity (London) 2014 doi: 10.1038/ijo.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eng J.W., Reed C.B., Kokolus K.M., Pitoniak R., Utley A., Bucsek M.J. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through beta2-adrenergic receptor activation. Nature Communication. 2015;6:6426. doi: 10.1038/ncomms7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foxman Ellen F., Storer James A., Fitzgerald Megan E., Wasik Bethany R., Hou Lin, Zhao Hongyu. Temperature-dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proceedings of the National Academy of Sciences of the United States of America. 2014:6. doi: 10.1073/pnas.1411030112. Early Edition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moragues V., Pinkerton H. Variation in morbidity and mortality of murine typhus infection in mice with changes in the environmental temperature. Journal of Experimental Medicine. 1944;79(1):41–43. doi: 10.1084/jem.79.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong M., Yang X., Lim S., Cao Z., Honek J., Lu H. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell Metabolism. 2013;18(1):118–129. doi: 10.1016/j.cmet.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian X.Y., Ganeshan K., Hong C., Nguyen K.D., Qiu Y., Kim J. Thermoneutral housing accelerates metabolic inflammation to potentiate atherosclerosis but not insulin resistance. Cell Metabolism. 2015 doi: 10.1016/j.cmet.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castaneda T.R., Abplanalp W., Um S.H., Pfluger P.T., Schrott B., Brown K. Metabolic control by S6 kinases depends on dietary lipids. PLoS One. 2012;7(3):e32631. doi: 10.1371/journal.pone.0032631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rayner K.J., Sheedy F.J., Esau C.C., Hussain F.N., Temel R.E., Parathath S. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. Journal of Clinical Investigation. 2011;121(7):2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann S.M., Perez-Tilve D., Greer T.M., Coburn B.A., Grant E., Basford J.E. Defective lipid delivery modulates glucose tolerance and metabolic response to diet in apolipoprotein E-deficient mice. Diabetes. 2008;57(1):5–12. doi: 10.2337/db07-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giles D.A., Moreno-Fernandez M.E., Stankiewicz T.E., Cappelletti M., Huppert S.S., Iwakura Y. Regulation of inflammation by IL-17A and IL-17F modulates non-alcoholic fatty liver disease pathogenesis. PLoS One. 2016;11(2):e0149783. doi: 10.1371/journal.pone.0149783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harley I.T., Stankiewicz T.E., Giles D.A., Softic S., Flick L.M., Cappelletti M. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology. 2014;59(5):1830–1839. doi: 10.1002/hep.26746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Divanovic S., Trompette A., Atabani S.F., Madan R., Golenbock D.T., Visintin A. Negative regulation of toll-like receptor 4 signaling by the toll-like receptor homolog RP105. Nature Immunology. 2005;6(6):571–578. doi: 10.1038/ni1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Divanovic S., Trompette A., Ashworth J.I., Rao M.B., Karp C.L. Therapeutic enhancement of protective immunity during experimental leishmaniasis. PLoS Neglected Tropical Diseases. 2011;5(9):e1316. doi: 10.1371/journal.pntd.0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkelman F., Morris S., Orekhova T., Sehy D. The in vivo cytokine capture assay for measurement of cytokine production in the mouse. Current Protocols in Immunology. 2003 doi: 10.1002/0471142735.im0628s54. Chapter 6: p. Unit 6 28. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenbelt W., Kingma B., van der Lans A., Schellen L. Cold exposure–an approach to increasing energy expenditure in humans. Trends in Endocrinology & Metabolism. 2014;25(4):165–167. doi: 10.1016/j.tem.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Frohnert B.I., Jacobs D.R., Jr., Steinberger J., Moran A., Steffen L.M., Sinaiko A.R. Relation between serum free fatty acids and adiposity, insulin resistance, and cardiovascular risk factors from adolescence to adulthood. Diabetes. 2013;62(9):3163–3169. doi: 10.2337/db12-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernatchez S.F., Atkinson M.R., Parks P.J. Expression of intercellular adhesion molecule-1 on macrophages in vitro as a marker of activation. Biomaterials. 1997;18(20):1371–1378. doi: 10.1016/s0142-9612(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 31.Ouimet M., Ediriweera H.N., Gundra U.M., Sheedy F.J., Ramkhelawon B., Hutchison S.B., Rinehold K. MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. Journal of Clinical Investigation. 2015;125(12):4334–4348. doi: 10.1172/JCI81676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim S., Meigs J.B. Links between ectopic fat and vascular disease in humans. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(9):1820–1826. doi: 10.1161/ATVBAHA.114.303035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singaraja R.R., Fievet C., Castro G., James E.R., Hennuyer N., Clee S.M. Increased ABCA1 activity protects against atherosclerosis. Journal of Clinical Investigation. 2002;110(1):35–42. doi: 10.1172/JCI15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore K.J., Sheedy F.J., Fisher E.A. Macrophages in atherosclerosis: a dynamic balance. Nature Reviews Immunology. 2013;13(10):709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metabolism. 2013;17(6):851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujisaka S., Usui I., Bukhari A., Ikutani M., Oya T., Kanatani Y. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58(11):2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anand S.S., Yusuf S. Stemming the global tsunami of cardiovascular disease. Lancet. 2011;377(9765):529–532. doi: 10.1016/S0140-6736(10)62346-X. [DOI] [PubMed] [Google Scholar]
- 38.Kokolus K.M., Capitano M.L., Lee C.T., Eng J.W., Waight J.D., Hylander B.L. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(50):20176–20181. doi: 10.1073/pnas.1304291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chistiakov D.A., Bobryshev Y.V., Kozarov E., Sobenin I.A., Orekhov A.N. Role of gut microbiota in the modulation of atherosclerosis-associated immune response. Frontiers in Microbiology. 2015;6:671. doi: 10.3389/fmicb.2015.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettersson U.S., Waldén T.B., Carlsson P.O., Jansson L., Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One. 2012;7(9):e46057. doi: 10.1371/journal.pone.0046057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heron M. Deaths: leading causes for 2004. National Vital Statistics Reports. 2007;56(5):1–95. [PubMed] [Google Scholar]
- 42.Wang Y., Beydoun M.A. The obesity epidemic in the United States–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiologic Reviews. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1Thermoneutral housing increases expression of genes within identified atherosclerosis-related pathways. Eight week old male WT C57BL/6 mice (n = 2/condition) were housed at standard (23 °C; TS) or thermoneutral (30 °C; TN) temperatures and fed obesogenic diet for 8 weeks. Pathways related to atherosclerosis were quantified in PBMCs by RNASeq analysis. This table reports the fold increase of expression in mice housed at TN.