Abstract
Our brains are composed of two distinct cell types: neurons and glia. Emerging data from recent investigations show that glial cells, especially astrocytes and microglia, are able to regulate synaptic transmission and thus brain information processing. This suggests that, not only neuronal activity, but communication between neurons and glia also plays a key role in brain function. Thus, it is currently well known that the physiology and pathophysiology of brain function can only be completely understood by considering the interplay between neurons and glia. However, it has not yet been possible to dissect glial cell type-specific roles in higher brain functions in vivo. Meanwhile, the recent development of optogenetics techniques has allowed investigators to manipulate neural activity with unprecedented temporal and spatial precision. Recently, a series of studies suggested the possibility of applying this cutting-edge technique to manipulate glial cell activity. This review briefly discusses the feasibility of optogenetic glia manipulation, which may provide a technical innovation in elucidating the in vivo role of glial cells in complex higher brain functions.
Keywords: Optogenetics, Astrocyte, Microglia, Higher brain functions, Synapse
GLIAL CELLS
Cells in the nervous system can be divided into two types: neurons and non-neuronal cells called neuroglia or glia that include astrocytes and microglia. For a century, neuroscientists considered glial cells as merely structural and supporting cells for neurons, and studies on the function of glial cells have been mainly focused on their roles in the context of neurological diseases. Thus, the belief that physiological brain functions are mediated solely by neuronal activity has been unchallenged for the past century. However, accumulating data from studies over the last two decades indisputably demonstrate that glial cells are also critically involved in synaptic transmission and plasticity. Based on these data, the classically accepted view of physiological brain function that exclusively revolves around neuronal activity has been updated to be regulated by the networks among neurons and glial cells.
Role of astrocyte in brain function
Astrocytes make up approximately half of the volume of the adult mammalian brain and are considered mainly as supportive cells for neurons [1]. It has been known for a long time that astrocytes play a role in maintaining extracellular homeostasis in the brain. Astrocytes maintain extracellular K+ and pH levels by expressing transporters for these ions on their membranes [2,3]. Likewise, astrocytes express transporters of neurotransmitters such as glutamate, which allow them to uptake extra-synaptic glutamate spill-over [4,5]. In addition, astrocytes are involved in maintaining structural integrity of the blood-brain barrier (BBB) [6,7].
Besides such traditional role of astrocytes for structural integrity and brain homeostasis, during the last decade it was discovered that astrocytes are crucially involved in the regulation of synaptic transmission. Astrocytes usually project their fine cytoplasmic processes in vivo and contact synapses and blood vessels in their vicinity in the brain. According to a study by Allen and colleagues, a single astrocyte can contact up to approximately 140,000 synapses, and more than 99% of the cerebrovascular surface is ensheathed by astrocyte end-feet [8]. Therefore, astrocytes are in a strategic position to monitor the microenvironment of neuronal synapses as well as brain microvessels. It has found that astrocytes express various neurotransmitter receptors including metabotropic (mGluR) and ionotropic (AMPAR) glutamate receptors, ATP receptors, and GABA receptors [5,8,9,10]. This finding suggests that neurotransmitters released from the presynaptic terminal not only activate postsynaptic neurons, but may also transmit information to peri-synaptic astrocytes. Indeed, it has been shown that activation of synaptic transmission induces intracellular calcium increase in peri-synaptic astrocytes [11,12]. In addition to having neurotransmitter receptors, astrocytes are also able to produce and release neurotransmitters by themselves such as glutamate [13], D-serine [14], ATP [15], and GABA [16], which are collectively called "gliotransmitters". Therefore, astrocytes are not merely listening to neuronal synaptic activity but also appear to be capable of modulating neuronal activity. Studies have shown that such gliotransmitters released from astrocytes are able to regulate the excitability of pre- and post-synaptic neurons [1,8,17,18,19]. Based on the evidence of bidirectional communication between astrocytes and pre/post-synaptic neurons, a novel concept of the tripartite synapse has been proposed (Fig. 1) [1].
Fig. 1. A Schematic representation of a tripartite synapse. The tripartite synapse is composed of presynaptic and postsynaptic terminals with astrocytic processes enwrapping the synapses. The release of neurotransmitter from the presynaptic terminal acts on the postsynaptic terminal as well as with astrocytic receptors mediating intracellular Ca2+ elevation via Gq GPCR. Ca2+ elevation then triggers the release of gliotransmitters that react with the presynaptic and postsynaptic terminal receptors to modulate synaptic transmission.
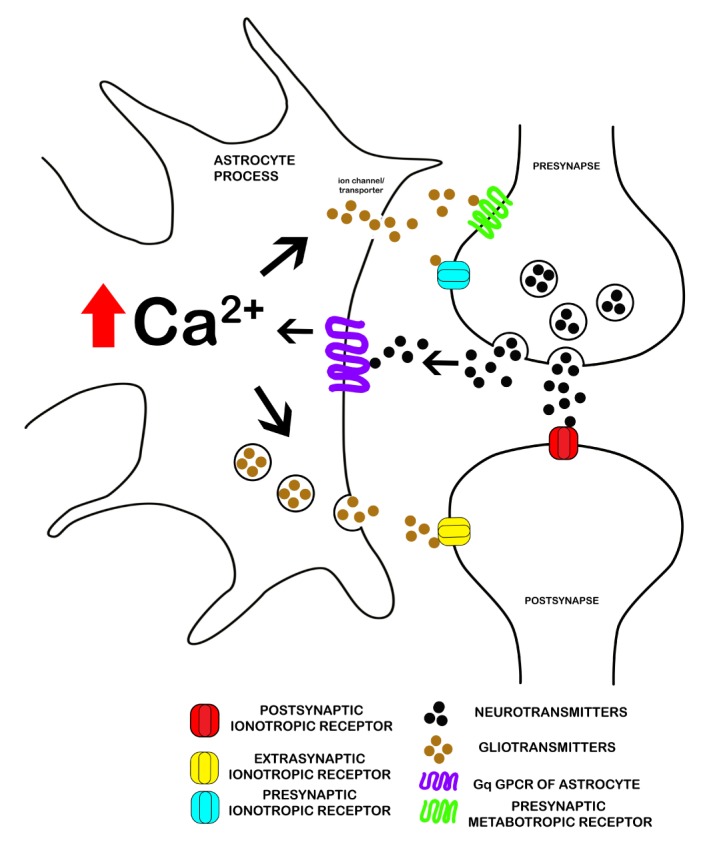
Moreover, astrocytes are connected with each other via gap junctions composed of connexin channels and form extensive communication networks on their own [20]. The pore size of the connexin channels are large enough to be permeable to intracellular second messenger molecules such as cAMP and calcium [21,22]. As a result, astrocytes can communicate with each other via these signaling molecules through open connexin channels. It is well documented that intracellular calcium increase in a single astrocyte can be propagated to other astrocytes resulting in Ca++ waves [20,23,24,25,26]. Thus far, how astrocytes release gliotransmitters has not been conclusively resolved. Data supporting vesicular as well as channel-mediated release have both been documented [10,17,26,27]. Intracellular calcium increase has been suggested as a common signal driving gliotransmitter release [1,8,11]. Assuming that calcium signals propagated via astrocyte gap junctions are capable of releasing gliotransmitters from other astrocytes, para-synaptic regulation and synchronized modulation of information processing by astrocytes are also conceivable.
The numerous studies showing astrocyte regulation of synaptic transmission suggested that astrocytes may also play a role in higher brain functions, which was substantiated by recent studies using astrocyte-specific genetically modified mice. For instance, Theis and colleagues reported that astrocyte-specific connexin-deficient mice showed enhanced locomotory activity [20]. These data indicated that the astrocyte network, possibly due to astrocyte calcium waves via gap junctions, is critical for such higher brain functions. In addition, conditional gene deletion of leptin receptors in hypothalamic astrocytes resulted in decreased feeding and altered synaptic inputs onto hypothalamic arcuate nucleus neurons [28]. This study suggests a putative role of hypothalamic astrocytes in synaptic transmission of hypothalamic neurons and subsequent feeding control. In addition, astrocytes were shown to modulate sleep behavior and the cognitive decline associated with sleep loss [29]. Likewise, astrocytes in the brainstem were shown to sense oxygen level in the blood via interstitial pH drop, and regulate respiration [30]. Based on these studies, it is nowadays indisputable that astrocytes play critical roles in physiological brain functions, although the underlying mechanisms still wait to be elucidated.
Microglia in synapse regulation
Microglia are another type of glia that have unique characteristics compared to other glial cells. Unlike other cell types in the brain, they are derived from the yolk sac during development and differentiate into brain-resident innate immune cells [31,32]. Like other innate immune cells in peripheral organs, they constantly monitor their microenvironment for infection or tissue damage with their fine processes [33,34]. Upon infection or tissue damage, they are immediately activated and express genes involved in inflammatory responses and wound healing. In addition, they obtain phagocytic functions and clear pathogens and cellular/tissue debris [35,36,37,38]. Due to their role in brain tissue inflammation, the function of microglia has been studied mainly in the context of neurological disorders for the past several decades.
However, a series of recent studies indicate that microglia are also actively involved in synapse remodeling. It was initially reported by Schafer and colleagues that microglia are involved in synapse pruning during brain development [35]. Later, it was found that microglia eliminate synapses in the adult brain as well [39,40,41]. Of interest, microglia utilize the same molecular machinery for synapse pruning as they utilize for phagocytic clearance of pathogens or cell/tissue debris; they utilize complement receptors to eliminate synapses [39] as well as for elimination of complement-coated pathogens. This discovery suggests that microglia may play an active role in regulating synaptic plasticity in the adult brain by regulating synapse remodeling. Synaptic plasticity is a key element of higher brain functions such as learning and memory. Therefore, it can be reasoned that microglia activity may play certain roles in such higher brain functions. This possibility was supported by the study by Parkhurst wherein microglia-depleted mice showed a significant reduction in learning and memory [42]. In this study, the authors showed that BDNF is released from activated microglia and affects synaptic plasticity by regulating synapse formation.
Taken together, these studies substantiated the active role of glia in physiological synaptic transmission, and support the putative role of glial cells in higher brain functions such as cognitive and affective brain functions. However, thus far, the in vivo function of glial cells in higher-order brain functions has not been clearly elucidated, mainly due to the lack of experimental techniques to manipulate glial cell activity in vivo in behaving animals. In this regard, recently developed optogenetic techniques may provide valuable tools to dissect in vivo glial cell functions.
OPTOGENTIC APPROACH IN GLIAL CELLS
Optogenetics is an innovative technique that utilizes genetically modified light-responsive ion channels allowing non-invasive and temporally precise manipulation of neural activity. This technique employs various proton-responsive ion channels such as channelrhodopsin-2 (ChR2), a non-selective cation channel, which induces membrane depolarization and thus neuronal excitation upon blue-light (470 nm) illumination. Halorhodopsin (NpHR), a light-responsive chloride channel, typically hyperpolarizes membrane potential upon illumination by chloride influx and thus suppresses neuronal excitability. Archaerhodopsin-3 (Arch) is a light-activated proton pump that can also hyperpolarize membrane potential. This technique allows depolarization or hyperpolarization of the opsin-expressing cells with high specificity and excellent temporal precision on a millisecond scale [43]. It is generally regarded that, neuronal, but not glial, activity is affected by membrane potential. Thus, optogenetics have been experimentally used mainly to manipulate neuronal activity and dissect the in vivo consequences in brain functions. However, recent studies showed that glial cell activity can be also optogenetically manipulated, which may serve as a new tool to elucidate the role of glial cells in higher brain functions(Fig. 2).
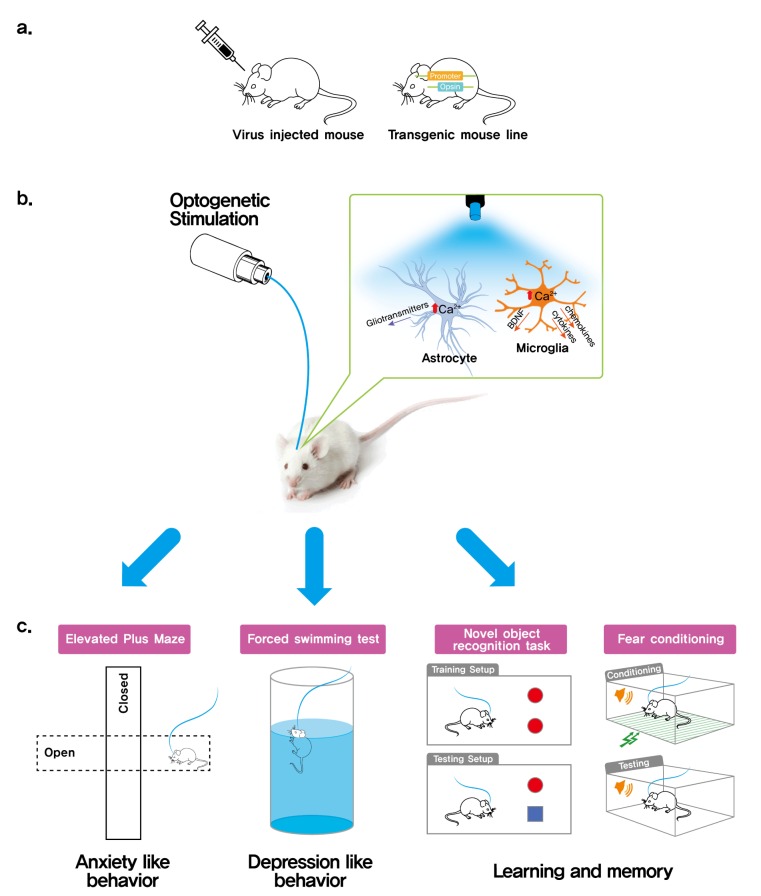
Fig. 2. A diagrammatic research scheme to dissect in vivo function of glia in higher brain function using optogenetics. This scheme shows glial cell type-specific opsin gene expression by using viral vectors or cell type-specific transgenic mice (a). Upon optogenetic glia activation/inhibition (b), the in vivo glia function can be assessed by subjecting the mice in a series of behavioral tests (c). The function of glia in anxiety can be measured by elevated plus maze that is based on the aversion of mice to open spaces. The forced swimming test is the most frequently used behavioral test for assessing depression-related behaviors. To assess the recognition and aversive memory, behavioral tests such as novel object recognition and fear conditioning task can be used.
Optogenetic astrocyte manipulation
Optogenetic manipulation of astrocytes was first reported by Gourine and colleagues [30]. In the study, they showed that illumination of ChR2-expressing astrocytes leads to a pH decrease followed by ATP release [30]. Using this optogenetic astrocyte manipulation system, they found that ATP released from activated astrocytes in the brainstem activated chemoreceptor neurons and regulated respiration. Optogenetic astrocyte manipulation was later shown to modulate astrocyte activity in the visual cortex and regulate response selectivity of visual cortex neurons [3]. In this study, light-stimulation of ChR2-expressing visual cortex astrocytes enhanced both excitatory and inhibitory synaptic transmission in neurons nearby. This regulatory effect of astrocytes was dependent on the metabotropic glutamate receptor (mGluR1) implicating glutamate as a gliotransmitter in the response upon optogenetic stimulation. Glutamate release by astrocyte ChR2 stimulation was directly demonstrated by Sasaki and colleagues [44]. In this study, optogenetic ChR2 stimulation of cerebellar astrocytes, including Bergmann glia, activated AMPA receptors on Purkinje cells and induced long-term depression of parallel fiber-to-Purkinje cell synapses. More recently, Yamashita and colleagues [45] reported that optogenetic astrocyte Ch2R activation in the anterior cingulate cortex during neuropathic pain can modulate wakefulness and sleep. Similarly, ChR2 stimulation of astrocytes in the posterior hypothalamus dramatically induced sleep during the active phase of the sleep-wake cycle [46] affirming the involvement of astrocytes in sleep-wake regulation. These studies demonstrate that astrocyte ChR2 stimulation successfully regulates astrocyte activity. Of note, the final outcome of optogenetic astrocyte activation is brain region-specific. Optogenetic Ch2R stimulation of brainstem astrocytes results in ATP release and respiratory regulation, while Ch2R activation of cerebellar astrocytes results in glutamate release and perturbation of cerebellar modulated motor behaviour [44].
To drive astrocyte-specific opsin expression in vivo, two main approaches have been employed. First, a recombinant adeno-associated virus was used for expression of opsin constructs in the central nervous system [3,30,45,46]. Viral vectors encoding opsin genes under the astrocyte-specific promoter (e.g. GFAP) have been utilized to drive astrocyte-specific ChR2 expression in the mouse brain [3,30,46]. On the other hand, viral vectors encoding opsin genes preceded by a floxed-stop codon sequence can be transduced into astrocyte-specific Cre transgenic mice [45]. Alternatively, astrocyte-specific opsin transgenic or knock-in mice can also be used [44,47]. Both Cre/loxP and tetO-tTA systems can be used for astrocyte-specific opsin-transgenic mice, which can be generated by mating astrocyte-specific Cre or tTA mice with floxed-stop-opsin knock-in mice or tetO-opsin transgenic mice. Using this tetO-tTA system, Tanaka and colleagues [47] generated astrocyte specific ChR2 transgenic mice that express sufficient amounts of ChR2 in astrocytes for optogenetic astrocyte manipulation. In these bigenic mice (Mlc1-tTA::tetO-ChR2), they showed that optogenetic astrocyte ChR2 stimulation triggers glutamate release in vivo [44]. These prior studies support that astrocyte gliotransmitter release and subsequent synaptic transmission can be regulated by optogenetic manipulation, which can be a valuable tool to uncover the physiological role of astrocytes in higher-order brain functions.
Although optogenetics is a highly valuable tool, it should be mentioned that it has several limitations applying it to astrocyte manipulation. For instance, the intracellular calcium increase in astrocytes after optogenetic ChR2 activation does not exactly recapitulate physiological astrocyte calcium elevation that is mostly due to GPCRs activation. While optogenetic ChR2 stimulation supposedly increase calcium level by calcium influx [30], physiological calcium signals by GPCR activation triggers Ca2+ release from ER [48,49,50,51]. Furthermore, recent studies indicate that astrocytic Ca2+ responses are distinct depending on the subcellular compartments due to the heterogeneous expression of receptors and Ca2+ sources. Therefore, it is possible that astrocytic Ca2+ signaling induced by ChR2 may not represent the physiological astrocyte activation regulating synaptic functions. In this regard, development and utilization of other optogenetic tools such as optoXR [52] or OptoSTIM1 [53] might be required to dissect and manipulate physiological astrocyte function.
Optogenetic microglia manipulation
Unlike astrocytes, the possibility of optogenetic microglia manipulation has not yet been reported. As previously mentioned, microglia can regulate synapse formation/elimination by releasing BDNF. In addition, microglia-derived BDNF was shown to regulate synaptic transmission by disinhibiting inhibitory interneurons [54,55]. BDNF release can be evoked by an intracellular calcium increase in microglia. In this regard, it is conceivable that optogenetic stimulation that accompanies intracellular calcium increase may lead to BDNF release from the activated microglia, and thereby regulate synaptic transmission and plasticity [55,56]. Meanwhile, it has also been documented that microglia activation is accompanied by inward chloride conductance, which contributes to microglial phagocytic activity and NO production [57]. Chloride influx can be recapitulated by the introduction and activation of NpHR, a chloride transporter. In this regard, it will be interesting to test if optogenetic NpHR activation of microglia under yellow light (540 nm) illumination can regulate microglial phagocytic activity that is an underlying mechanism of microglial synaptic pruning. Taken together, it is theoretically possible that optogenetic ChR2 or NpHR stimulation of microglia may affect their ability to physiologically regulate synaptic activity, which should be tested in future studies.
Besides BDNF release, microglia activation is often accompanied by the expression and release of other proinflammatory genes including cytokines and chemokines. Some of these genes, namely TNF-α and ROS, are also implicated in the regulation of neuronal excitability [58,59], and thus have a potential to affect synaptic transmission. Intracellular calcium increase is a common signaling pathway leading to expression of these proinflammatory genes, which can potentially be regulated by optogenetic microglia stimulation. Although intracellular calcium increase after optogenetic ChR2 stimulation is detected in neurons and astrocytes, it has not been confirmed in microglia. Therefore, it is possible that ChR2 activation may not be sufficient to induce microglia activation (e.g. BDNF release). If this is the case, the optically regulated G-protein coupled receptor (GPCR) gene (opto-XR) [52] can be utilized instead. Since most microglial intracellular calcium signaling is dependent on the Gq-PLC pathway, optic stimulation of optoXR will be most likely to induce microglia activation. In addition, Heo and colleagues recently invented an ingenious light-responsive intracellular calcium signal activator called optoSTIM1 that activates the endogenous store-operated calcium channel CRAC by light-induced oligomerization of STIM1 [53]. Applying this novel genetically modified intracellular calcium signal activator to microglia will be a promising technique to provide more efficient tools to manipulate microglia activation and dissect their in vivo roles in brain functions in the future.
CONCLUSIONS
In summary, recent studies have established that neuroglia have a critical role in synaptic transmission, remodelling, and plasticity. In addition, accumulating evidence supports a putative role of neuroglia in higher-order brain functions such as cognitive and affective brain functions, which has yet to be conclusively demonstrated. This is partly due to technical limitations in the ability to manipulate glial cell activity in freely moving animals, by which researchers measure high order brain functions. Meanwhile, the recent advent of optogenetic techniques has allowed neuroscientists to control the activity of neurons in freely moving animals with excellent spatial and temporal precision. A series of pilot studies applying this technique to astrocytes has shown the potential of this technique in regulating gliotransmitter release from astrocytes and thereby regulating synaptic transmission. In addition, the increasing list of light-responsive channels and signaling molecules allows more efficient tools to manipulate microglia activity in vivo. Therefore, optogenetics will offer an exceptional opportunity to elucidate the physiological role of glial cells in higher-order brain functions in the near future.
ACKNOWLEDGEMENTS
This work was supported by the Samsung Science & Technology Foundation (SSTF-BA1502-13).
References
- 1.Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59:932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walz W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem Int. 2000;36:291–300. doi: 10.1016/s0197-0186(99)00137-0. [DOI] [PubMed] [Google Scholar]
- 3.Perea G, Yang A, Boyden ES, Sur M. Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nat Commun. 2014;5:3262. doi: 10.1038/ncomms4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schousboe A, Svenneby G, Hertz L. Uptake and metabolism of glutamate in astrocytes cultured from dissociated mouse brain hemispheres. J Neurochem. 1977;29:999–1005. doi: 10.1111/j.1471-4159.1977.tb06503.x. [DOI] [PubMed] [Google Scholar]
- 5.Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol. 1997;51:439–455. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 6.Cabezas R, Ávila M, Gonzalez J, El-Bachá RS, Báez E, García-Segura LM, Jurado Coronel JC, Capani F, Cardona-Gomez GP, Barreto GE. Astrocytic modulation of blood brain barrier: perspectives on Parkinson's disease. Front Cell Neurosci. 2014;8:211. doi: 10.3389/fncel.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen NJ. Astrocyte regulation of synaptic behavior. Annu Rev Cell Dev Biol. 2014;30:439–463. doi: 10.1146/annurev-cellbio-100913-013053. [DOI] [PubMed] [Google Scholar]
- 9.Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7:338–353. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11:227–238. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- 11.Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci. 2016;19:182–189. doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Takano T, Nedergaard M. Astrocytic calcium signaling: mechanism and implications for functional brain imaging. Methods Mol Biol. 2009;489:93–109. doi: 10.1007/978-1-59745-543-5_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 14.Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- 16.Yoon BE, Lee CJ. GABA as a rising gliotransmitter. Front Neural Circuits. 2014;8:141. doi: 10.3389/fncir.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahlender DA, Savtchouk I, Volterra A. What do we know about gliotransmitter release from astrocytes? Philos Trans R Soc Lond B Biol Sci. 2014;369:20130592. doi: 10.1098/rstb.2013.0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Araque A, Navarrete M. Glial cells in neuronal network function. Philos Trans R Soc Lond B Biol Sci. 2010;365:2375–2381. doi: 10.1098/rstb.2009.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theis M, Jauch R, Zhuo L, Speidel D, Wallraff A, Döring B, Frisch C, Söhl G, Teubner B, Euwens C, Huston J, Steinhäuser C, Messing A, Heinemann U, Willecke K. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J Neurosci. 2003;23:766–776. doi: 10.1523/JNEUROSCI.23-03-00766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orthmann-Murphy JL, Abrams CK, Scherer SS. Gap junctions couple astrocytes and oligodendrocytes. J Mol Neurosci. 2008;35:101–116. doi: 10.1007/s12031-007-9027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94:120–143. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54:716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Höfer T, Venance L, Giaume C. Control and plasticity of intercellular calcium waves in astrocytes: a modeling approach. J Neurosci. 2002;22:4850–4859. doi: 10.1523/JNEUROSCI.22-12-04850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giaume C, Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia. 1998;24:50–64. [PubMed] [Google Scholar]
- 26.Theis M, Giaume C. Connexin-based intercellular communication and astrocyte heterogeneity. Brain Res. 2012;1487:88–98. doi: 10.1016/j.brainres.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 27.Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia. 2006;54:700–715. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- 28.Kim JG, Suyama S, Koch M, Jin S, Argente-Arizon P, Argente J, Liu ZW, Zimmer MR, Jeong JK, Szigeti-Buck K, Gao Y, Garcia-Caceres C, Yi CX, Salmaso N, Vaccarino FM, Chowen J, Diano S, Dietrich MO, Tschöp MH, Horvath TL. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci. 2014;17:908–910. doi: 10.1038/nn.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Town T, Nikolic V, Tan J. The microglial "activation" continuum: from innate to adaptive responses. J Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 33.Kofler J, Wiley CA. Microglia: key innate immune cells of the brain. Toxicol Pathol. 2011;39:103–114. doi: 10.1177/0192623310387619. [DOI] [PubMed] [Google Scholar]
- 34.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 35.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu R, Shen Q, Xu P, Luo JJ, Tang Y. Phagocytosis of microglia in the central nervous system diseases. Mol Neurobiol. 2014;49:1422–1434. doi: 10.1007/s12035-013-8620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 39.Miyamoto A, Wake H, Moorhouse AJ, Nabekura J. Microglia and synapse interactions: fine tuning neural circuits and candidate molecules. Front Cell Neurosci. 2013;7:70. doi: 10.3389/fncel.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rice RA, Spangenberg EE, Yamate-Morgan H, Lee RJ, Arora RP, Hernandez MX, Tenner AJ, West BL, Green KN. Elimination of microglia improves functional outcomes following extensive neuronal loss in the hippocampus. J Neurosci. 2015;35:9977–9989. doi: 10.1523/JNEUROSCI.0336-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 42.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ, 3rd, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsáki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sasaki T, Beppu K, Tanaka KF, Fukazawa Y, Shigemoto R, Matsui K. Application of an optogenetic byway for perturbing neuronal activity via glial photostimulation. Proc Natl Acad Sci U S A. 2012;109:20720–20725. doi: 10.1073/pnas.1213458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamashita A, Hamada A, Suhara Y, Kawabe R, Yanase M, Kuzumaki N, Narita M, Matsui R, Okano H, Narita M. Astrocytic activation in the anterior cingulate cortex is critical for sleep disorder under neuropathic pain. Synapse. 2014;68:235–247. doi: 10.1002/syn.21733. [DOI] [PubMed] [Google Scholar]
- 46.Pelluru D, Konadhode RR, Bhat NR, Shiromani PJ. Optogenetic stimulation of astrocytes in the posterior hypothalamus increases sleep at night in C57BL/6J mice. Eur J Neurosci. 2016;43:1298–1306. doi: 10.1111/ejn.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka KF, Matsui K, Sasaki T, Sano H, Sugio S, Fan K, Hen R, Nakai J, Yanagawa Y, Hasuwa H, Okabe M, Deisseroth K, Ikenaka K, Yamanaka A. Expanding the repertoire of optogenetically targeted cells with an enhanced gene expression system. Cell Rep. 2012;2:397–406. doi: 10.1016/j.celrep.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 48.Waldo GL, Harden TK. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol Pharmacol. 2004;65:426–436. doi: 10.1124/mol.65.2.426. [DOI] [PubMed] [Google Scholar]
- 49.Pinto JC, Potié F, Rice KC, Boring D, Johnson MR, Evans DM, Wilken GH, Cantrell CH, Howlett AC. Cannabinoid receptor binding and agonist activity of amides and esters of arachidonic acid. Mol Pharmacol. 1994;46:516–522. [PubMed] [Google Scholar]
- 50.Bowery NG, Hill DR, Hudson AL. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br J Pharmacol. 1983;78:191–206. doi: 10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nash MS, Schell MJ, Atkinson PJ, Johnston NR, Nahorski SR, Challiss RA. Determinants of metabotropic glutamate receptor-5-mediated Ca2+ and inositol 1,4,5-trisphosphate oscillation frequency. Receptor density versus agonist concentration. J Biol Chem. 2002;277:35947–35960. doi: 10.1074/jbc.M205622200. [DOI] [PubMed] [Google Scholar]
- 52.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- 53.Kyung T, Lee S, Kim JE, Cho T, Park H, Jeong YM, Kim D, Shin A, Kim S, Baek J, Kim J, Kim NY, Woo D, Chae S, Kim CH, Shin HS, Han YM, Kim D, Heo WD. Optogenetic control of endogenous Ca(2+) channels in vivo. Nat Biotechnol. 2015;33:1092–1096. doi: 10.1038/nbt.3350. [DOI] [PubMed] [Google Scholar]
- 54.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 55.Ferrini F, De Koninck Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013;2013:429815. doi: 10.1155/2013/429815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gottmann K, Mittmann T, Lessmann V. BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp Brain Res. 2009;199:203–234. doi: 10.1007/s00221-009-1994-z. [DOI] [PubMed] [Google Scholar]
- 57.Averaimo S, Milton RH, Duchen MR, Mazzanti M. Chloride intracellular channel 1 (CLIC1): Sensor and effector during oxidative stress. FEBS Lett. 2010;584:2076–2084. doi: 10.1016/j.febslet.2010.02.073. [DOI] [PubMed] [Google Scholar]
- 58.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Malik A, Choi HB, Ko RW, Dissing-Olesen L, MacVicar BA. Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron. 2014;82:195–207. doi: 10.1016/j.neuron.2014.01.043. [DOI] [PubMed] [Google Scholar]