Abstract
Protein tyrosine phosphatases (PTPs) are key regulatory factors in inflammatory signaling pathways. Although PTPs have been extensively studied, little is known about their role in neuroinflammation. In the present study, we examined the expression of 6 different PTPs (PTP1B, TC-PTP, SHP2, MEG2, LYP, and RPTPβ) and their role in glial activation and neuroinflammation. All PTPs were expressed in brain and glia. The expression of PTP1B, SHP2, and LYP was enhanced in the inflamed brain. The expression of PTP1B, TC-PTP, and LYP was increased after treating microglia cells with lipopolysaccharide (LPS). To examine the role of PTPs in microglial activation and neuroinflammation, we used specific pharmacological inhibitors of PTPs. Inhibition of PTP1B, TC-PTP, SHP2, LYP, and RPTPβ suppressed nitric oxide production in LPS-treated microglial cells in a dose-dependent manner. Furthermore, intracerebroventricular injection of PTP1B, TC-PTP, SHP2, and RPTPβ inhibitors downregulated microglial activation in an LPS-induced neuroinflammation model. Our results indicate that multiple PTPs are involved in regulating microglial activation and neuroinflammation, with different expression patterns and specific functions. Thus, PTP inhibitors can be exploited for therapeutic modulation of microglial activation in neuroinflammatory diseases.
Keywords: neuroinflammation, protein tyrosine phosphatase, microglia
INTRODUCTION
Microglia are the innate immune cells in central nervous system (CNS). These cells contribute to the homeostasis of the CNS by participating in immune responses as the first-line defense [1]. Microglia also play an important role in the pathophysiology of neuroinflammatory and neurodegenerative diseases. Hypertrophic microglia are one of the hallmarks of brain tissue from patients with Alzheimer's disease, Parkinson's disease, ischemic injury, or multiple sclerosis [2]. These reactive microglia can produce a variety of proinflammatory molecules such as nitric oxide (NO) and TNF-α, which amplify the inflammatory response and have neurotoxic effects. Therefore, inhibition of microglial hyperactivation might be a good strategy to develop therapeutics for neurodegenerative diseases [3].
Recently, we reported a novel role for protein tyrosine phosphatase 1B (PTP1B), which is a member of the protein tyrosine phosphatases (PTPs) family, as a positive regulator of neuroinflammation [4]. We showed that the level of PTP1B expression was increased by inflammatory stimuli, and microglial cells overexpressing PTP1B exhibited an enhanced production of NO and proinflammatory cytokines. In addition, a small-molecule inhibitor of PTP1B significantly suppressed the production of proinflammatory cytokines in the brain.
PTPs regulate the tyrosine phosphorylation of many important signaling molecules that are involved in key cellular processes such as cell growth and inflammation. Although more than 100 members of the PTP superfamily have been identified [5], the functional significance of PTPs in neuroinflammation remains largely unknown. Here, we examined the expression of 6 different PTPs (PTP1B, TC-PTP, SHP2, MEG2, LYP2, and RPTPβ) and their role in glial activation and neuroinflammation.
MATERIALS AND METHODS
Cell culture
The immortalized murine microglial BV-2 cell line [6], was maintained in Dulbecco's modified Eagle's medium (DMEM) containing 5% heat-inactivated fetal bovine serum (FBS) and 50 µg/ml gentamicin at 37℃. Mouse primary microglial cells were maintained in DMEM containing 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin (Gibco) at 37℃ in a humidified atmosphere with 5% CO2. All animals and experimental procedures were approved by the Institutional Review Board of Kyungpook National University School of Medicine, and were carried out in accordance with the guidelines in the NIH Guide for the Care and Use of Laboratory Animals. The animals were maintained under temperature- and humidity-controlled conditions with a 12-h light/12-h dark cycle. The mouse primary microglial cultures were prepared using mild trypsinization as previously described, but with minor modifications [7]. In brief, the forebrains of 3~5 day-old C57BL/6 mice were chopped and dissociated through mechanical disruption using a nylon mesh. The cells were seeded into poly-L-lysine-coated flasks. After in vitro culture for 10~14 days, the microglial cells were isolated from the mixed glial cultures using mild trypsinization. The mixed glial cultures were then incubated with a trypsin solution (0.25% trypsin, 1 mM EDTA in Hank's balanced salt solution) diluted 1:4 in phosphate-buffered saline (PBS; 150 mM NaCl, 5 mM phosphate, pH 7.4) containing 1 mM CaCl2 for 30~60 min. This procedure resulted in the detachment of an upper layer of astrocytes; the microglia remained attached to the bottom of the culture. The detached layer of astrocytes and the remaining microglia were treated with 0.25% trypsin separately and used for subsequent experiments. The purity of the cultures was greater than 95%, as determined by immunocytochemistry using a rabbit polyclonal anti-Iba-1 antibody (1:1000 dilution; Wako) or anti-GFAP antibody (1:1000 dilution).
Measurement of nitric oxide production
The production of nitric oxide (NO) was estimated by measuring the amount of nitrite, a stable metabolite of NO. The cells were treated with lipopolysaccharide (LPS from E. coli 055: B5; Sigma) in the presence or absence of the inhibitors for PTP1B [8,9], TC-PTP, SHP2, MEG2, LYP, and RPTPβ. At the end of a 24 h incubation period, 50 µl of the cell culture media was mixed with an equal volume of a Griess reagent (0.1% naphthylethylenediamine dihydrochloride and 1% sulfanilamide in 5% phosphoric acid) in a 96-well microtiter plate. The light absorbance was read at 540 nm and sodium nitrite was used for a standard curve.
Assessment of cell viability
Cel l viabi lity was assessed using a modified 3-(4,5 dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay, as previously described [10]. After LPS treatment for 24 h, either in the presence or absence of pharmacological inhibitors, the culture media was aspirated. MTT (0.5 mg/ml in PBS) was added to cells, which were then incubated at 37℃ for 4 h. The resulting formazan crystals were dissolved in DMSO. The absorbance was determined at 570 nm using a microplate reader.
RT-PCR
Total RNA was extracted from the brain tissues or treated cells using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's protocol. The reverse transcription (RT) was conducted using the Superscript II reverse transcriptase (Invitrogen) and an oligo (dT) primer. Traditional polymerase chain reaction (PCR) amplification was performed using specific primer sets at an annealing temperature of 55~60℃ for 20~30 cycles. PCR was performed using a C1000 Touch Thermal Cycler (Bio-Rad, Richmond, CA, USA). For the PCR product analysis, 10 µl of each PCR reaction was electrophoresed on a 1% agarose gel and detected under ultraviolet light following ethidium bromide staining. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. The primer sequences were designed based on published cDNA sequences (Table 1).
Table 1. Primers used for RT-PCR.
| Target genes | Accession number | Forward primer (5’-->3’) | Reverse primer (5’-->3’) |
|---|---|---|---|
| PTP1B | NM_011201.3 | AAGACCCATCTTCCGTGGAC | ACAGACGCCTGAGCACTTTG |
| TC-PTP | NM_008977.3 | GCTGGCAGCCGTTATACTTG | TGGCCAGGTGGTATAATGGA |
| SHP2 | NM_011202.3 | TGGTTTCACCCCAACATC | CGTGGGTCACTTTGGACTTG |
| MEG2 | NM_019651.2 | CCTGGAATGTGGCTGTCAAG | ATGCTCCCTTCAGCAGGTTT |
| LYP | NM_008979.2 | TTCCTGAACAAAGCCTCACG | GGGAGTTGATTTGGTCCGTT |
| RPTPβ | NM_001311064.1 | AGATCAAGGGTGGGCATT | ATGGGACTATCCGGATTTGG |
| GAPDH | NM_008084 | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA |
Immunostaining
For histochemical analysis, mice were transcardially perfused with saline and whole brains were fixed in 4% paraformaldehyde for 72 h. The fixed brains were incubated in 30% sucrose for 72 h, embedded in Optimal Cutting Temperature (O.C.T.) compound (Tissue-Tek), and then cut into 12-µm-thick sagittal sections. The sections were permeabilized with 0.3% Triton X-100 and blocked with 1% BSA and 5% normal donkey serum for 1 h at room temperature. The brain sections were incubated with primary antibodies (rabbit polyclonal anti-Iba-1 (1:500 dilution) at 4℃ overnight, followed by an incubation for 1 h at room temperature with secondary antibodies (Cy3-conjugated donkey anti-rabbit IgG; Jackson ImmunoResearch Laboratories). The anti-fade mounting medium containing DAPI (VECTASHIELD, Vector laboratories) was used for mounting and counterstaining. Tiled images of each section were captured with a CCD color video camera (Olympus D70) through a 63x objective lens attached to a microscope (Olympus BX51).
Mouse model of neuroinflammation
LPS was administered intraperitoneally (i.p.) to induce neuroinflammation in mice, as previously described [11]. All experiments were carried out on 9~11-week-old male C57BL/6 mice (25~30 g) supplied by Koatech (Pyongtaec, Korea). To evaluate the expression of PTPs in the brain under an inflammatory condition, LPS was injected i.p. at a dose of 5 mg/kg. The brains were collected 48 h after LPS administration. To assess the effect of PTP inhibitors on neuroinflammation, animals were divided into 8 experimental groups. Group 1 was treated with saline and 0.5% DMSO, group 2 was treated with LPS and 0.5% DMSO, and groups 3~8, were treated with LPS and each inhibitor of PTP1B, TC-PTP, SHP2, MEG2, LYP, and RPTPβ diluted in saline containing 5% propylene glycol. DMSO was included in the vehicle because PTP inhibitors were dissolved in DMSO. LPS (5 mg/kg) was administered i.p. for a single challenge. Inhibitors or vehicle were administered intracerebroventricularly (i.c.v.). For histological analysis, the mice were anesthetized 48 h after the LPS injection, and then transcardially perfused with saline and then with 4% paraformaldehyde. Microglial activation was assessed using Iba-1 staining. At least 3 animals were used for each experimental group. Immunohistological intensity analysis of Iba-1 staining was performed using Image J software (NIH, Bethesda, MD, USA) as previously described [12]. The image was set with a binary threshold of 50% of the background level, and then the particles were converted to a subthreshold image area with a size of 100 to 1000 pixels, which shows the Iba-1-positive activated cells. To count the Iba-1 positive cells, 5 squares (300 × 300 µm) were placed around the injection site in the subthreshold image of 6 independent sections, and the cells in the 5 squares were counted and statistically analyzed. Then, the counted cell number was expressed as cell number per square millimeter (cells/mm2).
Statistical analysis
All data are presented as mean±SE from 3 or more independent experiments, unless stated otherwise. The statistical comparisons between the different treatments were performed using either a Student's t-test or a one-way ANOVA in GraphPad PRISM (Graphpad Software. San Diego, CA, USA) and Excel. To determine the statistical significance of more than 2 groups, the values were compared using a one-way ANOVA followed by a Tukey's multiple comparison test (parametric test) or a one-way ANOVA with a Dunn's test (non-parametric test). For the comparison of 3 groups, the unpaired two-tailed Student's t-test was used, followed by a Mann-Whitney correction for nonparametric data.
RESULTS
Expression of PTPs in the inflamed brain
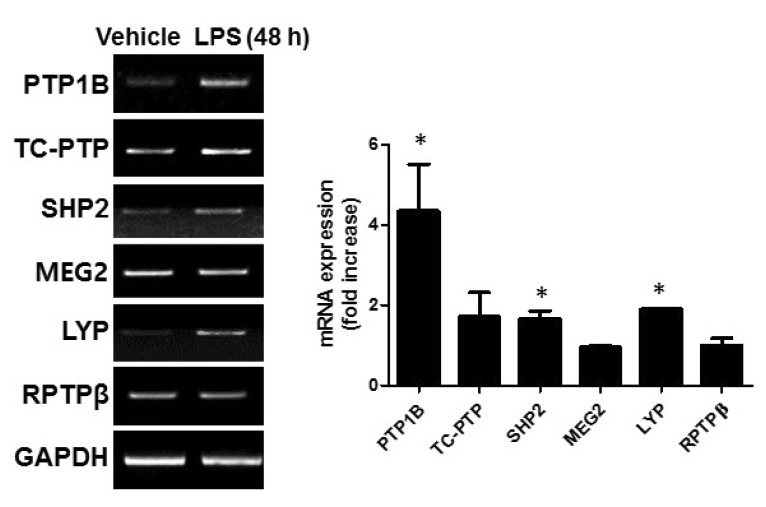
To investigate the role of PTPs in the mouse brain under an inflammatory condition, we first analyzed the mRNA expression of several PTPs. Five non-receptor type of PTPs (subtype NT1-NT4) and 1 receptor type of PTPs were selected in this study (Table 2) because these PTPs were well studied and the specific inhibitors have been developed. For an animal model of neuroinflammation, we used LPS-injected mice. Whole brains were collected 48 h after an i.p. injection of LPS (5 mg/kg). The gene expression levels of PTP1B (also known as PTPN1), TC-PTP (also known as PTPN2, a phosphatase that is highly homologous to PTP1B), SHP2 (also known as PTPN11), MEG2 (also known as PTPN9), LYP (also known as PTPN22) and RPTPβ (also known as RPTPz) were assessed by RT-PCR using gene-specific primers. We found that all PTPs were expressed in the mouse brain. PTP1B expression was increased after 48 h (Fig. 1), which is consistent with our previous report [4]. SHP2 and LYP were slightly increased compared to vehicle control, but LPS stimulation did not alter the expression of the other PTPs (Fig. 1).
Table 2. The list of PTPs inhibitors used in this study.
| Chemical name of inhibitors | Target PTPs | Subtype (synonyms) | References |
|---|---|---|---|
| Non-receptor PTP | |||
| (S)-4-(((S)-1-(l2-azanyl)-3-(4-(difluoro(phosphono) methyl)phenyl)-1-oxopropan-2-yl)amino)-3-((S)-3-(4-(difluoro(phosphono)methyl)phenyl)-2-pentadecanamidopropanamido)-4-oxobutanoic acid | Protein tyrosine phosphatase type 1B (PTP1B) | NT1 (PTP-1, PTPN1) | [9] |
| ((4-((S)-3-(((S)-1-amino-6-(4-ethylbenzamido)-1-oxohexan-2-yl)amino)-2-((S)-2-(2-(((1R,2R,5S)-2-isopropyl-5-methylcyclohexyl)oxy)acetamido)-3-phenylpropanamido)-3-oxopropyl)phenyl)difluoromethyl)phosphonic acid | T-cell phosphatase (TC-PTP) | NT2 (PTP-2, PTPN2) | [16] |
| ((4-((S)-3-(((S)-1-(((S)-1-amino-3-(2-(4-hydroxy-3-methoxyphenyl)acetamido)-1-oxopropan-2-yl)amino)-5-(3-iodobenzamido)-1-oxopentan-2-yl)amino)-6-hydroxy-3-iodo-1-methyl-2-(3-(2-oxo-2-((4-(thiophen-3-yl)phenyl) amino)acetamido)phenyl)-1H-indole-5-carboxylic acid | Src homology domain2-containing PTP2 (SHP2) | NT3 (SH-PTP2, PTPN11) | [33] |
| ((4-((S)-3-(((S)-1-(((S)-1-amino-3-(2-(4-hydroxy-3-methoxyphenyl)acetamido)-1-oxopropan-2-yl)amino)-5-(3-iodobenzamido)-1-oxopentan-2-yl)amino)-2-(3-bromo-4-methylbenzamido)-3-oxopropyl)phenyl)difluoromethyl) phosphonic acid | Megakaryocyte-PTP2 (MEG2) | NT3 (PTPN9) | [15] |
| 3-((3-Chlorophenyl)ethynyl)-2-(4-(2-(cyclopropylamino)-2-oxoethoxy)phenyl)-6-hydroxybenzofuran-5-carboxylic Acid | Lymphoid specific-tyrosine phosphatase (LYP) | NT4 (LYP) | [14] |
| Receptor PTP | |||
| 2-(3-(2-(3-bromo-5-iodobenzamido)acetamido)phenyl)-6-hydroxy-3-iodo-1-methyl-1H-indole-5-carboxylic acid | Receptor-type tyrosine protein phosphatase beta (RPTPβ) | (PTPRZ1 VE-PTP) | [13] |
Fig. 1. PTP expression in the mouse brain after LPS injection. The mRNA expression of PTPs in the brain 48 h after LPS injection (5 mg/kg) were measured using RT-PCR. The band intensity from 3~4 independent experiments was measured and normalized to GAPDH expression. *p<0.05 vs. vehicle control.
Enhancement of PTP1B, TC-PTP, and LYP expression in glial cells following LPS treatment
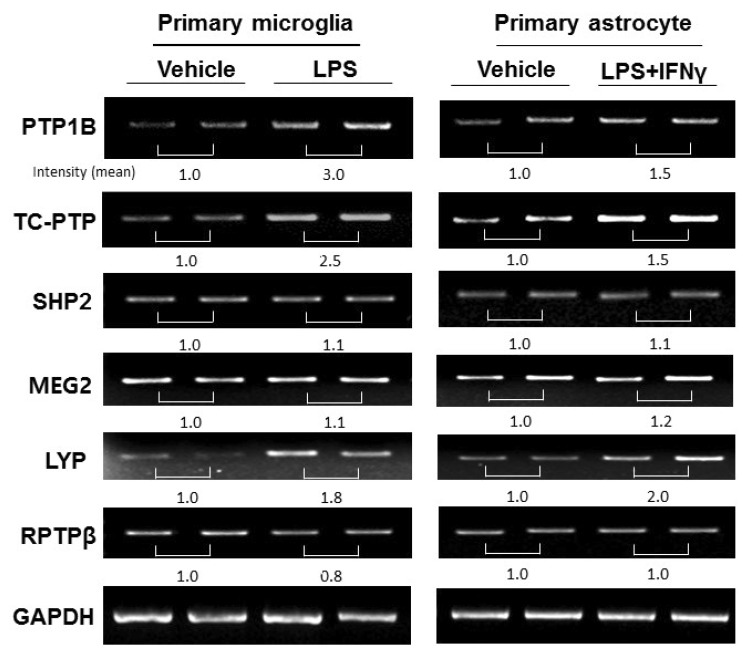
Because microglia are the resident immune cells in the CNS and participate in the initiation and propagation of an inflammatory response, we examined PTPs expression in primary microglia using RT-PCR. PTP1B, TC-PTP, and LYP, but not MEG2, SHP2, or RPTPβ mRNA levels were increased after 24 h of LPS stimulation (100 ng/ml) (Fig. 2). Similarly, PTP1B, TC-PTP, and LYP mRNA were upregulated after LPS and IFNγ stimulation in primary astrocytes (Fig. 2). Taken together, our results indicate that inflammatory stimuli upregulate several PTPs in glia.
Fig. 2. PTP expression in primary microglia and astrocytes after LPS treatment. The mRNA expression of PTPs in primary microglia and astrocytes 24 h after inflammatory stimulation (LPS treatment (100 ng/ml) for microglia; LPS and IFN-γ (10 U/ml) treatment for astrocytes) were measured using RT-PCR. The quantification of each PTP band intensity is normalized by the band intensity of GAPDH.
Inhibition of PTPs suppresses microglial inflammatory activation
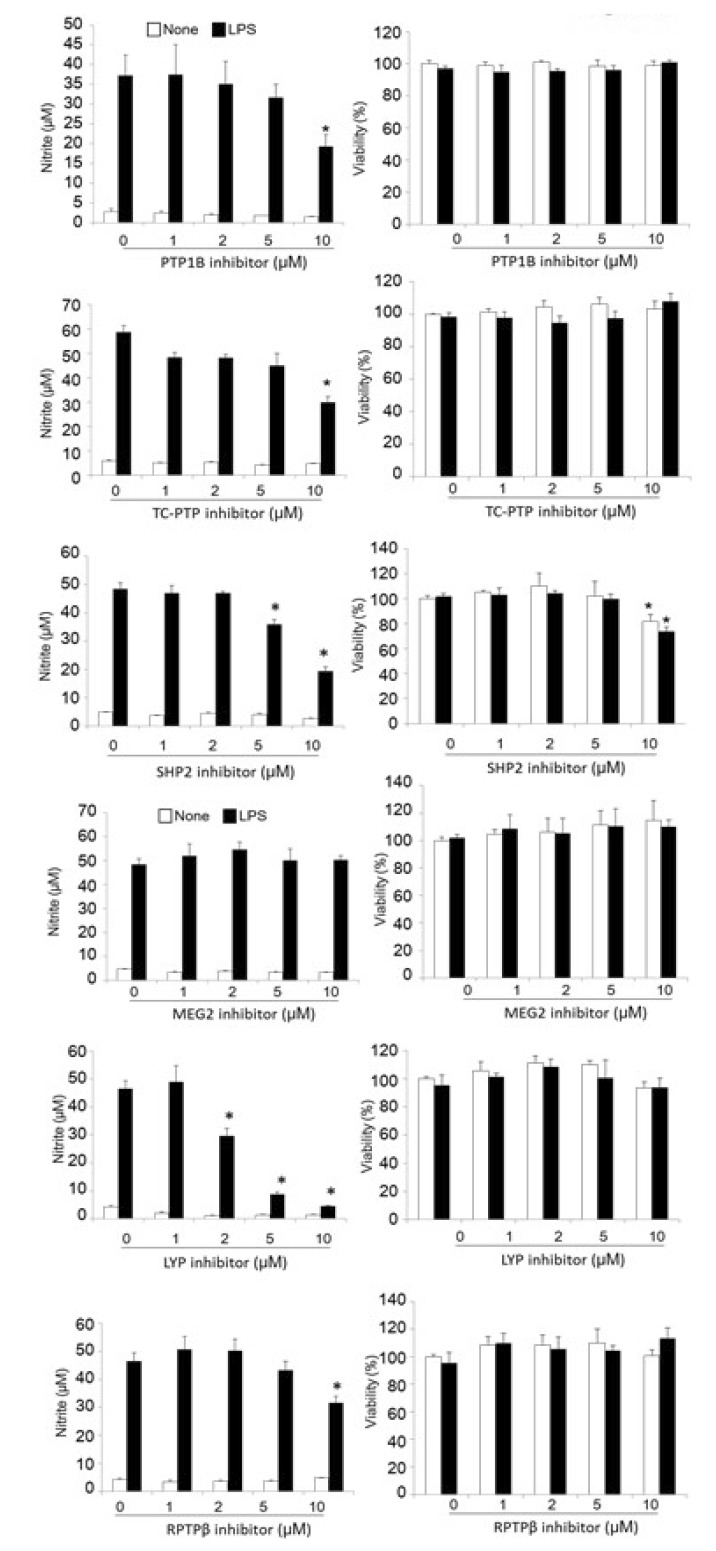
Microglia are the resident immune cells of the brain, and are part of an important defense mechanism. However, uncontrolled inflammatory activation of microglia has been observed in many neurodegenerative diseases [2]. Activated microglia play a central role in neuroinflammation by secreting various neurotoxic factors, such as nitric oxide (NO). Next, we explored the possibility that PTP inhibitors could reduce microglial activation. To test this hypothesis, we used specific inhibitors of PTPs that we previously developed [9,13,14,15,16]. PTPs share a conserved catalytic domain for phosphatase enzyme activity. Nevertheless, specific inhibitors for PTP1B, TC-PTP, SHP2, MEG2, LYP, and RPTPβ have been shown to be highly specific [8,9]. Table 2 shows the chemical name and the target protein of the inhibitors used in our study. The levels of NO production were determined in the culture media of the LPS-treated microglial cells that were co-treated with inhibitors at different concentrations. LPS-induced NO levels were decreased by inhibitors for PTP1B, TC-PTP, SHP2, LYP, and RPTPβ in a dose-dependent manner. The IC50 value of each PTP inhibitor is listed in Table 3. PTP inhibitors themselves did not alter the basal levels of NO production. No significant cytotoxicity was observed for any of the PTP inhibitors, except that for SHP2 at concentrations of 10 µM, as determined in a MTT assay (Fig. 3 right). It will be interesting to evaluate the effects of these PTP inhibitors on microglial iNOS expression and neuronal loss in LPS-injected mice in the future.
Table 3. Inhibition of LPS-stimulated NO production by PTP inhibitors in microglial cells (IC50, µM).
| Target PTPs | NO reduction IC50 (μM) |
|---|---|
| PTP1B | 10.6 |
| TC-PTP | 17.68 |
| SHP2 | 6.08 |
| MEG2 | 22.92 |
| LYP | 1.87 |
| RPTPβ | 15.1 |
BV-2 microglial cells were treated with LPS (100 ng/ml) in the presence of 1, 2, 5 and 10 µM of PTP inhibitors for 24 h. The nitrite content was measured using the Griess reaction.
Fig. 3. PTP inhibitors suppress LPS-induced NO production in microglial cells. BV-2 microglial cells were treated with LPS (100 ng/ml) in the presence of the indicated concentration of a PTP inhibitors for PTP1B, TC-PTP, SHP2, MEG2, LYP, and RPTPβ for 24 h. The nitrite content was measured using the Griess reaction (left panel), and the cytotoxicity of the PTP inhibitors was assessed in a MTT assay (right panel). The data were expressed as the mean±SEM (n=3). *p<0.05 vs. LPS only; analyzed using one-way ANOVA with Tukey's multiple comparison test.
The PTP inhibitors limit microglial activation in an in vivo neuroinflammation model
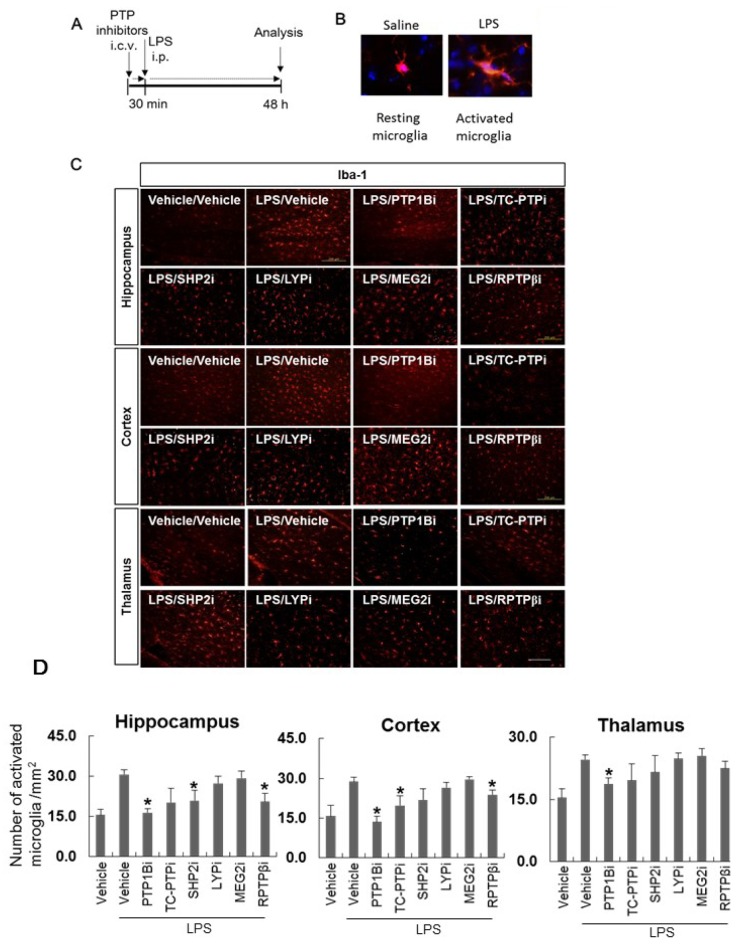
Finally, we examined whether the PTPs inhibitors influence microglial activation in vivo , which is a hallmark of neuroinflammation [17,18,19,20,21]. The brain tissues were collected and stained with anti-Iba-1 antibody, which is a microglia marker, to evaluate the intensity of Iba-1 staining and microglial morphological changes 48 h after i.p. injections of LPS (Fig. 4A). LPS significantly increased the number of Iba-1-positive activated microglia (Fig. 4D). The inhibition of PTP activity via i.c.v. injection of PTP1B, and RPTPβ inhibitors significantly reduced LPS-induced microglial activation in the hippocampus and cortex (Fig. 4C and D). TC-PTP inhibitors reduced LPS-induced microglial activation in the cortex, and SHP2 reduced microglial activation in the hippocampus. As shown in Fig. 4B, microglial morphological change was observed after LPS injection. The number of activated microglia was quantified after delivery of the PTP inhibitors. Taken together, our results show that inhibiting PTP1B, TC-PTP, SHP2, and RPTPβ activity under inflammatory conditions attenuated microglial inflammatory activation in vitro and in vivo.
Fig. 4. PTP inhibitors suppress microglial activation in a mouse neuroinflammation model. (A) C57BL/6 mice were injected i.c.v. with vehicle (saline containing 0.5% DMSO and 5% propylene glycol) or PTP inhibitors (diluted in saline containing 5% propylene glycol). At 30 min after the injection of inhibitors for PTP1B, TC-PTP, SHP2, MEG2, LYP, and RPTPβ, mice were injected i.p. with LPS (5 mg/kg). The mice were anesthetized and transcardially perfused with ice-cold saline 48 h after the LPS injection. (B) Representative pictures of resting microglia and activated microglia stained with anti-Iba-1 antibody (a marker for microglia). (C) The brains were removed and the sections were stained with Iba-1 antibody. Iba-1-positive cells were observed in the mouse hippocampus, cortex, and thalamus. Inhibitors were indicated as PTP1Bi, TC-PTPi, SHP2i, MEG2i, LYPi, and RPTPβi. Scale bar, 200 µm. (D) The graph shows activated microglial cell number per mm2. *p<0.05 vs. LPS and vehicle. More than three brain sections were examined for each experimental group.
DISCUSSION
We demonstrated that all of the PTPs that were examined in this study were expressed in microglia, and the inhibition of the activity of several PTPs decreased LPS-induced microglial activation in an in vitro cellular model. We further showed that i.c.v. administration of small-molecule inhibitors of PTP1B, TC-PTP, SHP2, and RPTPβ attenuated the LPS-induced microglial activation in a mouse neuroinflammatory model.
Neuroinflammation is thought to be a promising therapeutic target for acute brain injury and chronic neurodegenerative diseases such as traumatic brain injury and Alzheimer's disease. The quiescent and resting microglia can be activated by different inflammatory stimuli. Microglial activation is often divided into 2 functional states of polarization, namely, a M1-type (classical/inflammatory activation) and a M2-type (alternative/anti-inflammatory activation) [22]. In the present study, we focused on the classical activation state of microglia, which might be induced by a proinflammatory response such as LPS exposure. Excessive production of NO and proinflammatory cytokines in activated microglia that are stimulated by inflammatory signals have been implicated with the pathogenesis of several neurodegenerative diseases [23]. The activation of microglia also involves changes in their morphology (reviewed in [2]). Inflammatory reactions result in a thicker, less branched, and hypertrophic morphology of microglia. These morphological changes, which occur during the transition from a typical resting state to an activated phenotype, are associated with an increased production of neurotoxic proinflammatory mediators such as NO and TNFα [1,24]. In our neuroinflammatory model, the number of activated microglia was dramatically increased by LPS, and this LPS-induced microglial activation was attenuated by PTP inhibitors in the hippocampus and cortex.
Recently, PTP1B has been reported to be an important proinflammatory molecule in the brain [4]. In the present study, we showed that other PTPs also have important roles in NO production in microglia. In addition, specific inhibitors of PTP1B, TC-PTP, and RPTPβ have an inhibitory effect on microglial activation in a mouse neuroinflammatory model. PTPs represent a super-family of enzymes that play essential roles in normal development and physiology. The reversible phosphorylation of a protein is one of the most powerful ways to orchestrate the function of proteins in a cellular system. PTPs have been suggested as important regulators of inflammatory pathways [25,26]. Imbert et al. reported that pervandate, which is a PTP inhibitor, stimulated the downstream events of the T-cell activation process, including the induction of NF-kB activity [27]. Their study demonstrated a direct connection between PTPs and NF-kB activation through tyrosine phosphorylation of IkB. Among PTPs, PTP1B has emerged as a key player that regulates LPS-induced NF-kB signaling. Our group previously reported that overexpression of PTP1B in cell cultures increases the level of LPS-induced proinflammatory cytokine production, while an siRNA-induced reduction in PTP1B levels inhibits the LPS-induced microglial activation [4] Moreover, a PTP1B-specific inhibitor reduced neuroinflammation in a mouse model. These data implicate PTP1B (and possibly other PTPs) as an attractive target for the treatment of inflammatory brain diseases.
Other PTPs are also associated with inflammation. Levels of TC-PTP peak in response to IFN-γ treatment in THP-1 monocyte cells. The loss of TC-PTP potentiates the IFN-γ-induced phosphorylation of both of the STATs and p38 [28]. The overexpression of SHP2 was observed in rheumatoid arthritis, and knockdown of SHP2 reduced the invasion and adhesion of fibroblast-like synoviocytes. In addition, a signaling pathway that responds to inflammatory cytokines was impaired after SHP2 knockdown, implicating SHP2 as a candidate therapeutic target for inflammatory diseases [29]. A gain-of-function mutation for LYP is related to rheumatoid arthritis, suggesting its function in inflammation, which is consistent with our results [30]. Moreover, clinical use of the newly discovered LYP inhibitor has been proposed for treating a wide range of autoimmune disorders [14].
MEG2 was originally cloned from a megakaryocytic cell line. MEG2 binds to several phosphoinositides and to phosphatidylserine. In addition, MEG2 is implicated in the regulation of homotypic vesicle fusion in hematopoietic cells and events leading to phagocytosis. Based on these known functions of MEG2, we expected it to play an important role in microglia. However, the MEG2 inhibitor showed no protective effects against LPS-induced inflammation in vitro or in vivo.
The role of PTPs in neuronal development and CNS diseases has not been thoroughly studied. Nevertheless, RPTPβ is known to play a negative a role in oligodendrocyte differentiation in demyelinating CNS diseases [31]. Recently, it was shown that inactivation of RPTPβ by an inhibitory ligand promotes remyelination through activation of oligodendrocyte precursor differentiation [32]. Moreover, the RPTPβ inhibitor AKB9785 decreased inflammation in a sepsis model.
There are currently few examples of a direct association of PTPs with neuroinflammatory diseases, and clearly, the credibility of these enzymes as therapeutic targets requires substantial and continued validation. As a prerequisite for the clinical development of therapeutics targeted to PTPs, it is important to precisely determine the cellular and in vivo effects of small-molecule PTPs inhibitors. Unfortunately, as a consequence of the conserved nature of the PTP active sites (i.e., pTyr binding sites), there are currently only a few PTP-specific inhibitors that exhibit the potency and specificity required for biological and pharmacological investigation. On the basis of the observation that PTP substrate recognition requires both pTyr and its adjacent flanking residues [9,14], we have focused on a strategy for developing bidentate PTP inhibitors that bind to both the active site and a unique adjacent peripheral site. Using this approach, we have obtained several small-molecule inhibitors, which were tested using the LPS-induced neuroinflammation model and microglia cell lines in the present study. Our results suggest that small-molecule inhibitors for PTP1B, TC-PTP, SHP2, and RPTPß can serve as anti-inflammatory agents, validating the notion that small-molecule inhibitors can be used as anti-neuroinflammation therapeutics. Since PTP1B and several PTPs are also involved in the regulation of microglial activation pathways, these potent and specific PTPs inhibitors should be useful reagents in helping to define the role of PTPs in the normal physiology of microglia as well as neuroinflammation.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C3331) and RO1 CA69292 from the National Institutes of Health, USA. This work was also supported by the Basic Science Research Program through the National Research Foundation (NRF) funded by the Korean Ministry of Education, Science, and Technology (MEST) (2008-0062282, 2015R1A2A1A10051958, 2013R1A1A2061607, 2016M3C7A1904148).
References
- 1.Gomez-Nicola D, Perry VH. Microglial dynamics and role in the healthy and diseased brain: a paradigm of functional plasticity. Neuroscientist. 2015;21:169–184. doi: 10.1177/1073858414530512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreasson KI, Bachstetter AD, Colonna M, Ginhoux F, Holmes C, Lamb B, Landreth G, Lee DC, Low D, Lynch MA, Monsonego A, O'Banion MK, Pekny M, Puschmann T, Russek-Blum N, Sandusky LA, Selenica ML, Takata K, Teeling J, Town T, Van Eldik LJ. Targeting innate immunity for neurodegenerative disorders of the central nervous system. J Neurochem. 2016;138:653–693. doi: 10.1111/jnc.13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10:217–224. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- 4.Song GJ, Jung M, Kim JH, Park H, Rahman MH, Zhang S, Zhang ZY, Park DH, Kook H, Lee IK, Suk K. A novel role for protein tyrosine phosphatase 1B as a positive regulator of neuroinflammation. J Neuroinflammation. 2016;13:86. doi: 10.1186/s12974-016-0545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- 7.Saura J, Tusell JM, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia. 2003;44:183–189. doi: 10.1002/glia.10274. [DOI] [PubMed] [Google Scholar]
- 8.Shen K, Keng YF, Wu L, Guo XL, Lawrence DS, Zhang ZY. Acquisition of a specific and potent PTP1B inhibitor from a novel combinatorial library and screening procedure. J Biol Chem. 2001;276:47311–47319. doi: 10.1074/jbc.M106568200. [DOI] [PubMed] [Google Scholar]
- 9.Xie L, Lee SY, Andersen JN, Waters S, Shen K, Guo XL, Moller NP, Olefsky JM, Lawrence DS, Zhang ZY. Cellular effects of small molecule PTP1B inhibitors on insulin signaling. Biochemistry. 2003;42:12792–12804. doi: 10.1021/bi035238p. [DOI] [PubMed] [Google Scholar]
- 10.Ock J, Lee H, Kim S, Lee WH, Choi DK, Park EJ, Kim SH, Kim IK, Suk K. Induction of microglial apoptosis by corticotropin-releasing hormone. J Neurochem. 2006;98:962–972. doi: 10.1111/j.1471-4159.2006.03933.x. [DOI] [PubMed] [Google Scholar]
- 11.Jang E, Lee S, Kim JH, Kim JH, Seo JW, Lee WH, Mori K, Nakao K, Suk K. Secreted protein lipocalin-2 promotes microglial M1 polarization. FASEB J. 2013;27:1176–1190. doi: 10.1096/fj.12-222257. [DOI] [PubMed] [Google Scholar]
- 12.Jeon H, Kim JH, Kim JH, Lee WH, Lee MS, Suk K. Plasminogen activator inhibitor type 1 regulates microglial motility and phagocytic activity. J Neuroinflammation. 2012;9:149. doi: 10.1186/1742-2094-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng LF, Zhang RY, Bai Y, Wu L, Gunawan AM, Zhang ZY. Hydroxyindole carboxylic acid-based inhibitors for receptor-type protein tyrosine protein phosphatase beta. Antioxid Redox Signal. 2014;20:2130–2140. doi: 10.1089/ars.2013.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Y, Liu S, Menon A, Stanford S, Oppong E, Gunawan AM, Wu L, Wu DJ, Barrios AM, Bottini N, Cato AC, Zhang ZY. A potent and selective small-molecule inhibitor for the lymphoid-specific tyrosine phosphatase (LYP), a target associated with autoimmune diseases. J Med Chem. 2013;56:4990–5008. doi: 10.1021/jm400248c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S, Liu S, Tao R, Wei D, Chen L, Shen W, Yu ZH, Wang L, Jones DR, Dong XC, Zhang ZY. A highly selective and potent PTP-MEG2 inhibitor with therapeutic potential for type 2 diabetes. J Am Chem Soc. 2012;134:18116–18124. doi: 10.1021/ja308212y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Chen L, Luo Y, Gunawan A, Lawrence DS, Zhang ZY. Acquisition of a potent and selective TC-PTP inhibitor via a stepwise fluorophore-tagged combinatorial synthesis and screening strategy. J Am Chem Soc. 2009;131:13072–13079. doi: 10.1021/ja903733z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jha MK, Suk K. Management of glia-mediated neuroinflammation and related patents. Recent Pat Inflamm Allergy Drug Discov. 2014;8:118–124. doi: 10.2174/1872213x08666140619105915. [DOI] [PubMed] [Google Scholar]
- 18.Jha MK, Seo M, Kim JH, Kim BG, Cho JY, Suk K. The secretome signature of reactive glial cells and its pathological implications. Biochim Biophys Acta. 2013;1834:2418–2428. doi: 10.1016/j.bbapap.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Jha MK, Jeon S, Suk K. Glia as a link between neuroinflammation and neuropathic pain. Immune Netw. 2012;12:41–47. doi: 10.4110/in.2012.12.2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suk K, Ock J. Chemical genetics of neuroinflammation: natural and synthetic compounds as microglial inhibitors. Inflammopharmacology. 2012;20:151–158. doi: 10.1007/s10787-011-0108-2. [DOI] [PubMed] [Google Scholar]
- 21.Choi DK, Koppula S, Suk K. Inhibitors of microglial neurotoxicity: focus on natural products. Molecules. 2011;16:1021–1043. doi: 10.3390/molecules16021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jha MK, Lee WH, Suk K. Functional polarization of neuroglia: Implications in neuroinflammation and neurological disorders. Biochem Pharmacol. 2016;103:1–16. doi: 10.1016/j.bcp.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Yuste JE, Tarragon E, Campuzano CM, Ros-Bernal F. Implications of glial nitric oxide in neurodegenerative diseases. Front Cell Neurosci. 2015;9:322. doi: 10.3389/fncel.2015.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61:71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- 25.Spalinger MR, McCole DF, Rogler G, Scharl M. Role of protein tyrosine phosphatases in regulating the immune system: implications for chronic intestinal inflammation. Inflamm Bowel Dis. 2015;21:645–655. doi: 10.1097/MIB.0000000000000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bottini N, Peterson EJ. Tyrosine phosphatase PTPN22: multifunctional regulator of immune signaling, development, and disease. Annu Rev Immunol. 2014;32:83–119. doi: 10.1146/annurev-immunol-032713-120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imbert V, Peyron JF, Farahi Far D, Mari B, Auberger P, Rossi B. Induction of tyrosine phosphorylation and T-cell activation by vanadate peroxide, an inhibitor of protein tyrosine phosphatases. Biochem J. 1994;297:163–173. doi: 10.1042/bj2970163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharl M, Hruz P, McCole DF. Protein tyrosine phosphatase non-receptor Type 2 regulates IFN-gamma-induced cytokine signaling in THP-1 monocytes. Inflamm Bowel Dis. 2010;16:2055–2064. doi: 10.1002/ibd.21325. [DOI] [PubMed] [Google Scholar]
- 29.Stanford SM, Maestre MF, Campbell AM, Bartok B, Kiosses WB, Boyle DL, Arnett HA, Mustelin T, Firestein GS, Bottini N. Protein tyrosine phosphatase expression profile of rheumatoid arthritis fibroblast-like synoviocytes: a novel role of SH2 domain-containing phosphatase 2 as a modulator of invasion and survival. Arthritis Rheum. 2013;65:1171–1180. doi: 10.1002/art.37872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman WG, Zhang Q, Liu X, Walker E, Ternan H, Owen J, Johnson B, Greer W, Mosher DP, Maksymowych WP, Bykerk VP, Keystone EC, Amos CI, Siminovitch KA. Rheumatoid arthritis association with the FCRL3 -169C polymorphism is restricted to PTPN22 1858T-homozygous individuals in a Canadian population. Arthritis Rheum. 2006;54:3820–3827. doi: 10.1002/art.22270. [DOI] [PubMed] [Google Scholar]
- 31.Kuboyama K, Fujikawa A, Masumura M, Suzuki R, Matsumoto M, Noda M. Protein tyrosine phosphatase receptor type z negatively regulates oligodendrocyte differentiation and myelination. PLoS One. 2012;7:e48797. doi: 10.1371/journal.pone.0048797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuboyama K, Fujikawa A, Suzuki R, Noda M. Inactivation of protein tyrosine phosphatase receptor type Z by pleiotrophin promotes remyelination through activation of differentiation of oligodendrocyte precursor cells. J Neurosci. 2015;35:12162–12171. doi: 10.1523/JNEUROSCI.2127-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng LF, Zhang RY, Yu ZH, Li S, Wu L, Gunawan AM, Lane BS, Mali RS, Li X, Chan RJ, Kapur R, Wells CD, Zhang ZY. Therapeutic potential of targeting the oncogenic SHP2 phosphatase. J Med Chem. 2014;57:6594–6609. doi: 10.1021/jm5006176. [DOI] [PMC free article] [PubMed] [Google Scholar]