Abstract
Hemorrhagic fever with renal syndrome (HFRS) was considered to be transmitted by Apodemus agrarius and Rattus norvegicus, the principal animal hosts of Hantaan virus and Seoul virus, respectively. The aim of this study is to determine the correlation of HFRS incidence with capture rate and hantavirus infection rate of rodent species in Qingdao City, China. We collected HFRS patients’ information and captured field and residential rodents in Qingdao City, China from 2010 to 2014. The correlations of HFRS incidence to rodent capture rate and hantavirus infection rate of rodents were analyzed statistically. The main findings of this study are that the high HFRS incidence (19.3/100,000) is correlated to the capture rate of field Mus musculus (p = 0.011, r = 0.037); but surprisingly it did not correlated to the capture rate of the principal rodent hosts Apodemus agrarius and Rattus norvegicus and the hantavirus infection rate of these rodent species in the field or residential area. These novel findings suggest that Mus musculus, a nontraditional animal host of hantavirus may play an important role in hantavirus transmission in Qingdao City.
Hantavirus infections occur worldwide, but approximately 90% of hemorrhagic fever with renal syndrome (HFRS) cases has been reported in China1. During 2006–2012, a total of 77,558 HFRS cases have been reported in China with 866 deaths2. Qingdao City located in eastern China is one of multiple high incidence areas of HFRS in China3. Due to its wide geographic distribution, Hantavirus has evolved into different serotypes in different areas of the world4. In China, HFRS is caused by Hantaan virus (HTNV) and Seoul virus (SEOV)4,5,6. The principal rodent host of HTNV is the striped field mouse Apodemus agrarius, and the principal rodent host of SEOV is commensal rodent brown rat Rattus norvegicus7,8. The aim of this study is to determine the correlations of HFRS incidence to rodent species and to hantavirus infection rate in the rodent species in Qingdao City from 2010 to 2014.
Results
HFRS cases in Huangdao District of Qingdao City
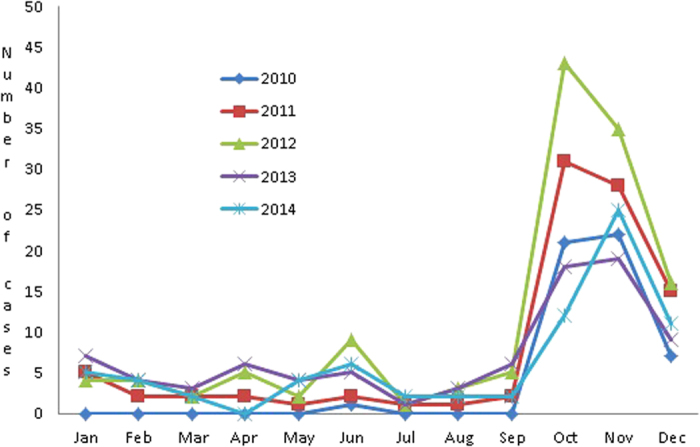
A total of 432 HFRS patients were diagnosed in Huangdao District with 51, 92, 129, 85 and 75 cases, respectively in each year from 2010 to 2014 (Fig. 1). The incidence of HFRS in rural population was 11.4, 20.5, 28.9, 19 and 16.7 every 100,000 population, respectively in each year from 2010 to 2014. The highest prevalence of HFRS occurred in 2012. The profile of HFRS prevalence was similar each year. A low level of HFRS prevalence occurred from January to September each year; a small peak of HFRS prevalence occurred in the spring and summer between April and June in some years; and a high peak of HFRS prevalence occurred each year between October and December (Fig. 2).
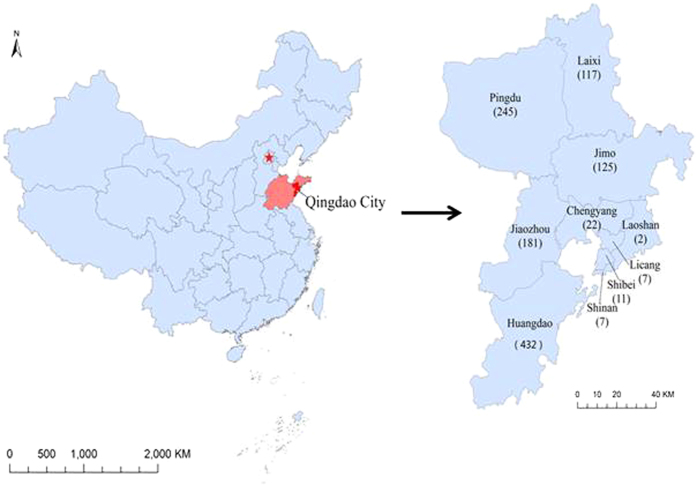
Figure 1. Geographic location of Huangdao District in Qingdao City, China.
On the left is the map of China, in Which Qingdao City was marked as dark. On the right is the map of Qingdao City with Huangdao district. The maps were constructed using ArcGIS10.1 software (http://resources.arcgis.com/en/home/).
Figure 2. Monthly distribution of HFRS cases in Huangdao District of Qingdao City from 2010 to 2014.
All 432 patients were from rural areas and 86.7% (379/432) of patients were farmers. Of the patients, 97.5% (421/432) had exposure history to rodents within 3 months before onset of illness. The sex ratio of the patients was 2.5 to 1 (308 male to 124 female). The age of the patients ranged from 5 to 85 years old with median age of 50 years old. Majority of the patients (85.4%, 369/432) occurred between 31–70 years old and more than half (56.9%, 246/432) occurred between 41 and 60 years old.
Correlation between HFRS incidence and annual rodent capture rate
The captured animals included rodents and shrews. Only rodents were analyzed for correlation with the incidence of HFRS. The rodent capture rate was 1.6% to 7.1% in field areas and 1.4% to 5.1% in residential areas (Table 1). The captured rodent species in the order of average annual capture rate from high to low were Apodemus agrarius (1.6%), Rattus norvegicus (1.0%), Mus musculus (0.8%), and Rattus rattus (0.3%) in the field area and were Rattus norvegicus (1.4%), Mus musculus (1.4%), and Rattus rattus (0.2%) in the residential area (Table 1). The rodent species and density varied in different years with the peak in 2012 (Table 1) for both field and residential rodents. The capture rate of rodents increased from 2010 to 2012 and declined from 2012 to 2014 annually (Table 1). Mus musculus and Rattus norvegicus were consistently present at a high frequency in both field and residential areas; Rattus rattus had relatively high frequency in both residential and field areas in 2010 and 2011, but disappeared from both areas from 2012 to 2014. Apodemus agrarius was not captured in 2010 and 2011, but started to appear in high numbers in the fall of 2012 and decreased in the summer of 2014 in the field (Table 1). Apodemus agrarius was not captured in residential areas in 2010 and 2011 and very few Apodemus agrarius were captured from 2012 to 2014.
Table 1. Comparison of annual human HFRS incidence and rodent capture rate in Huangdao District, 2010–2014.
| Year | HFRS incidence | Capture rate of field rodents (%) |
Capture rate of residential rodents (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Apodemus agrarius | Rattus norvegicus | Rattus rattus | Mus musculus* | Total* | Apodemus agrarius | Rattus norvegicus | Rattus rattus | Mus musculus | Total | ||
| 2010 | 11.4 | 0 | 0.9 | 0.5 | 0.2 | 1.6 | 0 | 0.7 | 0.2 | 0.5 | 1.4 |
| 2011 | 20.5 | 0 | 2.1 | 1.2 | 0.9 | 4.2 | 0 | 0.9 | 0.65 | 0.4 | 2 |
| 2012 | 28.8 | 3.7 | 1.5 | 0 | 1.9 | 7.1 | 0.04 | 2.3 | 0 | 2.1 | 5.1 |
| 2013 | 19 | 2.5 | 0.2 | 0 | 0.5 | 3.2 | 0.04 | 1.7 | 0 | 2.1 | 4.8 |
| 2014 | 16.7 | 1.7 | 0.3 | 0 | 0.7 | 2.7 | 0.02 | 1.5 | 0 | 2.1 | 4.1 |
| Average | 19.3 | 1.6 | 1 | 0.3 | 0.8 | 3.8 | 0.02 | 1.4 | 0.2 | 1.4 | 3.5 |
Rodent snaps in each year were 5427, 2758, 5228, 11383, 4191 for 2010, 2011, 2012, 2013 and 2014, respectively. The capture rate each year was calculated by the number of rodents captured/snap number × 100.
*Indicated that a significant correlation between HFRS incidence rate and the total capture rate of field rodents (r = 0.938, P = 0.018) and the capture rate of Mus musculus (r = 0.037, p = 0.011).
Five-year surveillance indicated that the annual incidence of HFRS and annual rodent capture rate had the same profile (Table 1 and Fig. 3). When rodent capture rate was high, HFRS incidence was also high, and vice versa. Both rodent capture rate and HFRS had a peak in 2012. Statistical analysis with Pearson Correlation showed that the incidence of HFRS from 2010 to 2014 was correlated to the annual capture rate of field rodents (p = 0.018, r = 0.938), but not the capture rate of residential rodents. For individual species of the rodents, the HFRS incidence was correlated to the annual capture rate of Mus musculus from the field (p = 0.011, r = 0.037), but was neither correlated to the annual capture rate of other field rodent species nor all residential rodent species from 2010 to 2014.
Figure 3. Comparison of human HFRS incidence and rodent capture rate in Huangdao District, 2010–2014.
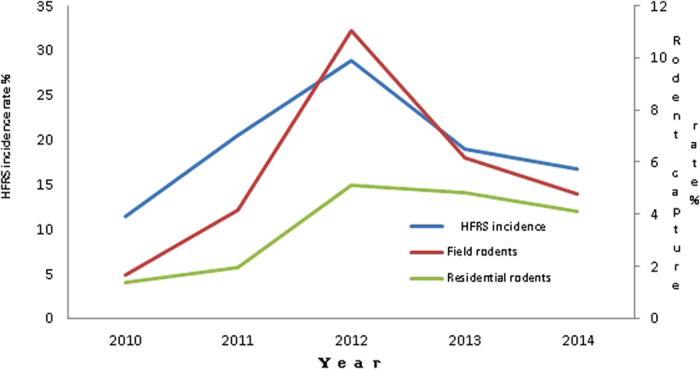
The scale on the left indicated HFRS incidence of 100,000 populations and the scale on the right indicated the annual capture rate of field rodents and residential rodents.
Correlation between HFRS incidence and quarterly rodent capture rate
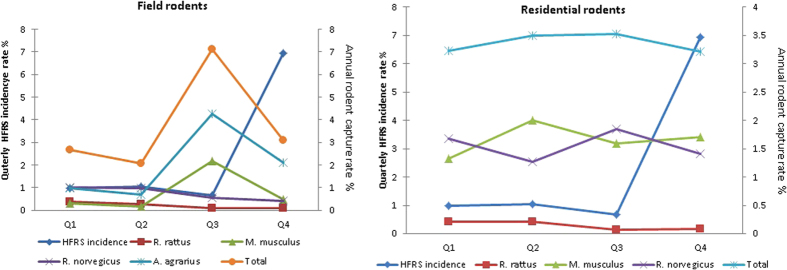
Because rodents were captured quarterly, we further analyzed the correction between the quarterly incidence of HFRS and the quarterly capture rate of rodents. The quarterly capture rate of field rodents varied from 2.7% to 7.1% in different quarters. The peak capture rate occurred in the third quarter and the next highest capture rate was in the fourth quarter. Both Apodemus agrarius and Mus musculus had a peak capture rate in the third quarter (Fig. 4). The quarterly capture rate of residential rodents changed little year-round, and ranged from 3.2% to 3.5% with average capture rate of 3.4% (Fig. 4).
Figure 4. Comparison of quarterly HFRS incidence and quarterly rodent capture rate in Huangdao District, 2010–2014.
The scale on the left indicated quarterly HFRS incidence of 100,000 population and the scale on the right indicated the quarterly capture rate of field rodents and residential rodents.
Statistical analysis indicated that the incidence of HFRS was not correlated to quarterly capture rate of field rodents, residential rodents, or any individual species of rodents from field or residential areas. However, the incidence of HFRS in each quarter was correlated to the capture rate of Apodemus agrarius (p = 0.014, R = 1) and Mus musculus (p = 0.000, r = 1) in the previous quarter.
Hantavirus infection rate in rodents
Detection of hantavirus antigen in the lungs of rodents with hantavirus specific monoclonal antibody by immunofluorescence assay showed that the 5-year infection rate of hantavirus was 1.9% (1/54) to 3.8% (9/240) for residential rodents and 0.9% (1/113) to 6.9% (25/364) for field rodents between 2010 and 2014 (Table 2). The 5-year average infection rate of field rodents (5.7%, 59/1041) was higher than those of the residential rodents (3.4%, 35/1035). Except for one year, the infection rate of field rodents was always higher than residential rodents (Table 2). Statistical analysis showed that the HFRS incidence did not correlate to the annual infection rate of any rodent species.
Table 2. Annual hantavirus infection rate in rodents in Huangdao District from 2010 to 2014.
| Year | Field rodent infection rate (%) |
Residential rodent infection rate (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A. agrarius | M. musculus | R. norvegicus | R. rattus | Total | A. agrarius | M. musculus | R. norvegicus | R.rattus | Total | |
| 2010 | 0 (0/0) | 0 (0/12) | 4 (2/50) | 11.1 (3/27) | 5.6 (5/89) | 0 (0/0) | 0 (0/26) | 2.9 (1/34) | 3.7 (1 /27) | 2.3 (2/87) |
| 2011 | 0 (0/0) | 0 (0/22) | 0 (0/59) | 3.1 (1/32) | 0.9 (1/113) | 0 (0/0) | 0 (0/11) | 4 (1/25) | 0 (0/18) | 1.9 (1/54) |
| 2012 | 6.8 (13/191) | 7.2 (7/97) | 6.6 (5/76) | 0 (0/0) | 6.9 (25/364) | 0 (0/2) | 2.6 (3/115) | 4.9 (6/123) | 0 (0/0) | 3.8 (9/240) |
| 2013 | 3.4 (10/290) | 7.5 (4/53)) | 35 (7/20) | 0 (0/0) | 5.8 (21/363) | 0 (0/4) | 2.2 (6/271) | 5.4 (12/224) | 0 (0/0) | 3.6 (18/499) |
| 2014 | 5.5 (4/73) | 11.1 (3/27) | 0 (0/12) | 0 (0/0) | 6.3 (7/112) | 0 (0/1) | 1.1 (1/91) | 6.3 (4/63) | 0 (0/0) | 3.2 (5/155) |
| Total | 4.9 (27/554) | 6.6 (14/211) | 6.5 (14/217) | 6.8 (4/59) | 5.7 (59/1041) | 0 (0/7) | 1.9 (10/514) | 5.1 (24/469) | 2.2 (1/45) | 3.4 (35/1035) |
Analysis of the hantavirus infection rate of rodents in each quarter indicated that all rodent species from field and residential areas were infected year-round with the highest infection rate in the first quarter and the second highest infection rate in the fourth quarter for both field and residential rodents (Table 3). Statistical analysis showed that the HFRS incidence rate did not correlated to the quarterly infection rate of any rodent species.
Table 3. Quarterly hantavirus infection rate in rodents in Huangdao District from 2010 to 2014.
| Quarter | Field rodent infection rate (%) |
Residential rodent infection rate % |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A. agrarius | M. musculus | R. norvegicus | R. rattus | Total | A. agrarius | M. musculus | R. norvegicus | R. rattus | Total | |
| Q1 | 7.2 (5/69) | 19.0 (4/21) | 5.6 (4/72) | 15.4 (4/26) | 9.0 (17/188) | 0 (0/0) | 3.1 (3/98) | 8.1 (10/123) | 6.3 (1/16) | 6.3 (14/237) |
| Q2 | 1.9 (1/54) | 7.1 (1/14) | 3.9 (3/76) | 0 (0/20) | 3.0 (5/164) | 0 (0/0) | 0.6 (1/174) | 1.8 (2/111 | 0 (0/19) | 1.0 (3/304) |
| Q3 | 4.4 (12/270) | 4.3 (6/139) | 5.6 (2/36) | 0 (0/6) | 4.4 (20/451) | 0 (0/1) | 1.9 (2/108) | 3.2 (4/125) | 0 (0/3) | 2.5 (6/237) |
| Q4 | 5.6 (9/161) | 8.1 (3/37) | 15.2 (5/33) | 0 (0/7) | 7.1 (17/238) | 0 (0/6) | 3.0 (4/134) | 7.3 (8/110) | 0 (0/7) | 4.7 (12/257 |
| Total | 4.9 (27/554) | 6.6 (14/211) | 6.5 (14/217) | 6.8 (4/59) | 5.7 (59/1041) | 0 (0/7) | 1.9 (10/514) | 5.1 (24/469) | 2.2 (1/45) | 3.4 (35/1035) |
Q1, 2, 3 and 4 indicate Quarter 1, 2, 3 and 4, respectively.
Discussion
The incidence of HFRS in Huangdao District is very high, ranged from 11.4 /100,000 to 28.9/100,000 from 2010 to 2014. The epidemiology of HFRS from 2010 to 2014 indicated that there are two peaks of HFRS cases in Huangdao District of Qingdao City. A small peak occurred in the spring to summer seasons and a large peak occurred in the fall season between September and December. Previous studies indicated that the spring peek is caused by Seoul virus and the autumn peak is caused by Hantaan virus3.
Human pathogenic hantaviruses are transmitted by rodents8. After infection with hantavirus, rodents carried hantavirus and excreted hantavirus for a long period. In hantavirus infected Apodemus agrarius, the virus has been detected in the blood from 7 to 12 days, in saliva 9–40 days, in urine 9–360 days, in feces 12–40 days, in lung 12–180 days, in kidney 15–18 days, and in liver 12–40 days9,10. Therefore, rodent species distribution, density, and hantavirus infection rate are the main factors affecting the incidence rate of HFRS in an area. HFRS is caused by Hantaan virus and Seoul virus in China and currently these viruses were recognized to be transmitted by Apodemus agrarius and Rattus norvegicus, respectively.
We captured rodents from 2010 to 2014 in Huangdao District, a high HFRS incidence area to determine the correlation between rodent species and HFRS incidence. The five-year surveillance indicated that density of field rodent population has dramatically changed in different seasons with a low density in the first two quarter (January to June), a rapid rise in the third quarter (July to September), and a fall in the fourth quarter (October to December). The seasonal changes of field rodent population density may be caused by the availability of food and weather condition. The density of residential rodents changed little year-round, suggesting that the residential rodents have a stable food source and weather condition at all the time. Apodemus agrarius and Mus musculus are the dominant species of the field rodents. Both of them have a peak population in the third quarter, resulting in a third quarter rise of field rodent population. The major residential rodents are Mus musculus and Rattus norvegicus. Striped field mouse Apodemus agrarius is rarely captured in residential areas, however, commensal rodents Rattus norvegicus, Rattus rattus, and Mus musculus have similar density in field areas and residential areas, suggesting that Apodemus agrarius mainly lives in field areas and Rattus norvegicus, Rattus rattus, and Mus musculus live in both residential areas and field areas.
According to the rodent transmission model of hantavirus, the density and hantavirus infection rate of principal rodent hosts will determine the HFRS incidence in an area with a stable human population. Our study indicated the annual capture rate of field rodents and residential rodents in Huangdao District had the same tendency with the annual incidence rate of HFRS. HFRS annual incidence rate is correlated to the density of field rodents, but not the density of residential rodents, suggesting that the high HFRS incidence is caused by high density of field rodent population. Apodemus agrarius is considered as the principal rodent host of Hantaan virus in China. However, in 2010 and 2011, the HFRS incidence was very higher in the fall season even though Apodemus agrarius was not captured during this time. It suggests that Hantaan was transmitted by other rodent species in 2010 and 2011 when Apodemus agrarius was absent. Statistical analysis indicated that HFRS incidence correlated to the density of Mus musculus in the field area, but not Apodemus agrarius from field. It is surprising that the annual capture rate of Apodemus agrarius, the principal animal host of Hantaan virus in China, is not correlated to the incidence of HFRS. The density of rodent species in the field and residential areas has changed dramatically in different years. Apodemus agrarius was not captured in 2010 and 2011, but had a high density after 2012; in contrast, Rattus rattus had a relatively high density in the 2010 and 2011 and disappeared since 2012. We do not know what cause the fluctuation of these rodent species in the field and residential areas.
Mus musculus was not considered a rodent host of Hantavirus until recently11. Previous studies have demonstrated that Mus musculus carries both Seoul virus and Hantaan virus. We have detected Hantaan virus RNA in Mus musculus captured in field in Huangdao District previously11. Seoul virus RNA was detected in Mus musculus captured on residence area in Shandong, Hunan, Beijing and Inner Mongolia in China12,13,14,15; Mus musculus was antibody positive to hantavirus (9.1%) in South Korea16. Mus musculus might also play an important role in harboring and transmitting PUU-like viruses in Europe17,18. Our study indicated that Mus musculus has a high density in field and residential areas and a high infection rate with hantavirus. We also demonstrated that both the annual and quarterly capture rates of Mus musculus are correlated to the incidence of HFRS. Our study suggests that Mus musculus is important in the transmission of hantavirus to humans in China.
The quarterly HFRS incidence is statistically correlated with the capture rate of Apodemus agrarius and Mus musculus in the previous quarter. This delayed rodent density-dependent incidence of HFRS needs to be further investigated by capturing rodents monthly in a future study.
The highest hantavirus infection rate of rodents was in March, but the highest HFRS incidence was in October. Statistical analysis indicated that HFRS incidence is not correlated to the infection rate of either field rodents or residential rodents. The fluctuation of rodent infection rate of hantavirus may be not sufficient to affect the incidence of HFRS in humans. Our result suggests that the density of rodent populations rather than hantavirus infection rate of rodents in Huangdao District is more important in transmission of hantavirus. With high density of field rodents, people may have increased chance to contact infected rodents, resulting in a higher risk of infection with hantavirus.
In conclusion, our study indicates that Mus musculus play an important role in hantavirus transmission in Qingdao City, China.
Materials and Methods
Ethics statement
The ethical committee of the Qingdao Center for Disease Control and Prevention has approved all human and animal works and the study was carried out according to the medical research regulations of China. The informed consent was obtained from all patients.
Study site
We collected rodents in rural areas of Huangdao District of Qingdao City in Shandong Province of China located at longitude 119°30′–120°30′ and latitude 35°35′–36°08′. The district consists of low-lying hills with forests and farmlands, which is the typical niche for the presence of Hantavirus animal hosts. The rodents were captured in the same villages, but in different locations of each village every year to avoid disturbing the local rodent density, species, and hantavirus infection rate. The population of the Huangdao district was 843,276 with rural population 447,837 in 2012 (http://baike.baidu.com/view/49758.htm?fromtitle=%E8%83%B6%E5%8D%97%E5%8E%BF&fromid=8061645&type=syn).
HFRS patients
The data of HFRS patients in Qingdao City from January, 1 to December 31 from 2010 to 2014 was collected from the national infectious diseases surveillance and reporting system (http://1.202.129.170). Suspected HFRS patients were diagnosed according to the criteria in the < Diagnostic criteria for epidemic hemorrhagic fever WS278-2008> issued by the Ministry of Health of China in 200819.
Hantavirus reservoir animal surveillance
Rodents were trapped once each quarter in March, June, September, and November, respectively with the first quarter starting in January. Rodents were trapped using snap-traps with peanut bait from 2010 to 2014. The traps were set before sunset inside farmhouses or farmyards (residential areas) and in farmlands 500 meters away from farmhouses (field areas). Two or three snap-traps were set in each residential area and the distance between traps was 10 meters in the field. The trapped animals were collected in the morning and their species identified morphologically. Rodent capture rate was determined by dividing the number of trapped rodents by the number of traps used each year.
Detection of Hantavirus antigens
Rodent lung tissues were harvested aseptically and frozen at −70 °C. Viral antigens in the rodent lung tissues were detected by using indirect immunofluorescence assay (IFA) with anti-Hantavirus monoclonal antibody reacting with both HTNV and SEOV antigens as described previously20.
Statistical analysis
Statistical analysis of correlation between HFRS incidence, rodent capture rate and infection rate was performed with Pearson Correlation, SPSS16.0.
Additional Information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
How to cite this article: Jiang, F. et al. Prevalence of hemorrhagic fever with renal syndrome in Qingdao city, China, 2010–2014. Sci. Rep. 6, 36081; doi: 10.1038/srep36081 (2016).
Acknowledgments
This study was supported by the Qingdao City Public Sector Science and Technology Support Program (Grant no. 14-2-3-29); National Natural Science Funds of China (31570167, 81401368, 81102171). Shandong Province Science and Technology Development Program (2014GSF121004), Natural Science Foundation of Shandong Province, China (ZR2014HP025) and Shandong Medical Science and Technology Development Program (no. 2011HZ055).
Footnotes
Author Contributions F.J., Z.Z., L.D., B.H., Z.X., D.M. and H.S., conceived, designed the research, and collected data, H.-l.W., did statistical analysis, F.J., H.Y. and X.-j.Y. analyzed data and wrote manuscript. All authors read and approved the final manuscript.
References
- McCaughey C. & Hart C. A. Hantaviruses. Journal of medical microbiology 49, 587–599, doi: 10.1099/0022-1317-49-7-587 (2000). [DOI] [PubMed] [Google Scholar]
- Zhang S. et al. Epidemic characteristics of hemorrhagic fever with renal syndrome in China, 2006-2012. BMC infectious diseases 14, 384, doi: 10.1186/1471-2334-14-384 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu B. & Su H. Qingdao Municipal Center for Disease Control and Prevention, Qingdao 266033, China; Dynamic Analysis on Epidemic of Hemorrhagic Fever with Renal Syndrome from 1979 to 2004 in Qingdao [J]. Disease Surveillance 12 (2005). [Google Scholar]
- Song G. et al. Antigenic difference between viral strains causing classical and mild types of epidemic hemorrhagic fever with renal syndrome in China. Journal of infectious diseases 150, 889–894 (1984). [DOI] [PubMed] [Google Scholar]
- Chen H., Qiu F., Zhao X., Luo C. & Li X. Characteristics of the distribution of epidemic season of hemorrhagic fever with renal syndrome in different regions and different years in China. Chin J Clin Virol 8, 197–203 (1994). [Google Scholar]
- Chen H. & Qiu F. Epidemiologic surveillance on the hemorrhagic fever with renal syndrome in China. Chinese medical journal 106, 857–863 (1993). [PubMed] [Google Scholar]
- Kim K. et al. Cytokeratin 17 Expression is Associated With Poor Prognosis in Gallbladder Adenocarcinoma. Applied immunohistochemistry & molecular morphology: AIMM/official publication of the Society for Applied Immunohistochemistry, doi: 10.1097/PAI.0000000000000307 (2016). [DOI] [PubMed] [Google Scholar]
- Schmaljohn C. & Hjelle B. Hantaviruses: a global disease problem. Emerging infectious diseases 3, 95–104, doi: 10.3201/eid0302.970202 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- CaiMing Q. et al. Etiology and molecular biology analysis of rats infected with HFRS in Liaoning Province. Chinese Journal of Zoonoses 26, 528–531 (2010). [Google Scholar]
- Bai X. & Xu Z. Hemorrhagic fever with renal syndrome. Beijing: People’s Medical Publishing House (2013). [Google Scholar]
- Fang L. Z. et al. Reservoir host expansion of hantavirus, China. Emerging infectious diseases 21, 170–171, doi: 10.3201/eid2101.140960 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L. et al. Spatial structure of rodent populations and infection patterns of hantavirus in seven villages of Shandong Province from February 2006 to January 2007. Chinese medical journal 124, 1639–1646 (2011). [PubMed] [Google Scholar]
- Dai D. F. et al. [Virological surveillance on hemorrhagic fever with renal syndrome in Hunan province in 2006]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 28, 1194–1197 (2007). [PubMed] [Google Scholar]
- Zuo S. Q. et al. [Study on the molecular epidemiology of hantaviruse carried by hosts in northern suburb of Beijing]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 25, 421–424 (2004). [PubMed] [Google Scholar]
- Zhang F. X. et al. [Study on the epidemiological characteristics of hemorrhagic fever with renal syndrome in Inner Mongolia]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 28, 1101–1104 (2007).18396665 [Google Scholar]
- Klein T. A. et al. Hantaan virus surveillance targeting small mammals at Dagmar North Training Area, Gyeonggi Province, Republic of Korea, 2001–2005. Journal of vector ecology: journal of the Society for Vector Ecology 36, 373–381, doi: 10.1111/j.1948-7134.2011.00178.x (2011). [DOI] [PubMed] [Google Scholar]
- Hukic M. et al. [Puumala and Dobrava viruses in the northeastern and central regions of Bosnia]. Acta medica Croatica: casopis Hravatske akademije medicinskih znanosti 57, 373–380 (2003). [PubMed] [Google Scholar]
- Diglisic G. et al. Isolation of a Puumala-like virus from Mus musculus captured in Yugoslavia and its association with severe hemorrhagic fever with renal syndrome. The Journal of infectious diseases 169, 204–207 (1994). [DOI] [PubMed] [Google Scholar]
- Park H. S. et al. PPARgamma neddylation essential for adipogenesis is a potential target for treating obesity. Cell death and differentiation 23, 1296–1311, doi: 10.1038/cdd.2016.6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G. & Chen H. X. Prevention and control of epidemic hemorrhagic fever. (People’s health publishing house, 1998). [Google Scholar]