Significance
Distinct cellular stresses converge on the translation initiation factor, eukaryotic initiation factor 2α (eIF2α) to modulate the rate of protein synthesis. Increased phosphorylation of eIF2α has been described in peripheral neurons from neuropathic and diabetic rats. However, the role of eIF2α phosphorylation in pain has not been reported. Here we show that phosphorylation of eIF2α controls thermal, but not mechanical, sensation via modulation of the activity of a major heat transducer, transient receptor potential vanilloid 1. We also find that chronic inflammation-induced eIF2α phopshorylation contributes to inflammation-induced thermal hypersensitivity. These results demonstrate that eIF2α phosphorylation plays a major role in controlling noxious heat sensitivity.
Keywords: pain, eIF2α, cellular stress response pathway, TRPV1
Abstract
A response to environmental stress is critical to alleviate cellular injury and maintain cellular homeostasis. Eukaryotic initiation factor 2 (eIF2) is a key integrator of cellular stress responses and an important regulator of mRNA translation. Diverse stress signals lead to the phosphorylation of the α subunit of eIF2 (Ser51), resulting in inhibition of global protein synthesis while promoting expression of proteins that mediate cell adaptation to stress. Here we report that eIF2α is instrumental in the control of noxious heat sensation. Mice with decreased eIF2α phosphorylation (eIF2α+/S51A) exhibit reduced responses to noxious heat. Pharmacological attenuation of eIF2α phosphorylation decreases thermal, but not mechanical, pain sensitivity, whereas increasing eIF2α phosphorylation has the opposite effect on thermal nociception. The impact of eIF2α phosphorylation (p-eIF2α) on thermal thresholds is dependent on the transient receptor potential vanilloid 1. Moreover, we show that induction of eIF2α phosphorylation in primary sensory neurons in a chronic inflammation pain model contributes to thermal hypersensitivity. Our results demonstrate that the cellular stress response pathway, mediated via p-eIF2α, represents a mechanism that could be used to alleviate pathological heat sensation.
Response to stress is a major cellular function involved in many physiological and pathological conditions. Cells respond to various forms of stress by activating specific molecular cascades that orchestrate antistress responses or induce apoptosis (1). A key effector of cellular stress responses is the eukaryotic initiation factor 2 (eIF2) (2). Phosphorylation of eIF2 causes a reduction in global translation, allowing cells to conserve energy and modify gene expression to effectively manage stress conditions. Diverse stress signals converge onto eIF2 to integrate stress responses through phosphorylation of the α subunit of eIF2.
eIF2 binds GTP and the initiator methionyl-tRNA (Met-tRNAi) to form the ternary complex (eIF2-GTP-Met-tRNAi). The ternary complex binds the small ribosomal subunit to form the ribosomal preinitiation complex, which scans the 5′UTR of the mRNA for the start codon to initiate mRNA translation (3). On engagement of the initiation codon, GTP is hydrolyzed to GDP (4). The recycling of inactive GDP-bound eIF2 to the active GTP-bound form is catalyzed by the guanine nucleotide exchange factor, eIF2B. Phosphorylation of the α subunit of eIF2 at serine 51 converts eIF2 from a substrate to a competitive inhibitor of eIF2B (4). Because the amount of eIF2B is lower than eIF2, phosphorylation of a small fraction of the eIF2 in the cell is sufficient to strongly inhibit eIF2B activity and translation initiation.
eIF2α is phosphorylated by four eIF2α kinases, each activated in a different stress condition (5–7). PKR (double-stranded RNA-dependent protein kinase) is activated by double-stranded RNA during viral infection; PERK (PKR-like ER kinase) by endoplasmic reticulum stress; GCN2 (general control non–derepressible-2) by nutrient deprivation and UV light; and HRI (heme-regulated inhibitor) by heme deficiency. The eIF2α kinases, except for HRI, are prominently expressed in the mammalian nervous system (8).
Phosphorylation of eIF2α blocks general translation but paradoxically stimulates translation of mRNAs that contain upstream ORFs (uORFs) in their 5′ UTR, such as ATF4 [a cAMP-response element binding protein 2 (CREB-2)] and CHOP (a proapoptotic transcription factor) (9). ATF4 enhances the expression of a related transcription factor, ATF3, which together with ATF4 contribute to stress adaptation by regulating genes involved in metabolism, the cellular redox status, and apoptosis (10, 11). In neurons, an activity-dependent decrease in eIF2α phosphorylation augments long-term potentiation (LTP) and memory via suppression of ATF4 expression (12). Conversely, up-regulation of p-eIF2α is associated with long-term depression (LTD) (13, 14) and several pathophysiological conditions including viral infection, inflammation, and neurodegeneration (15–18). Elevated phosphorylation of eIF2α has been documented in the brain of aged animals (19) and Alzheimer's disease patients and model mice (20, 21). Normalization of p-eIF2α in Alzheimer's disease model mice rescued deficits in protein synthesis, synaptic plasticity, and spatial memory (22). Additionally, phosphorylation of eIF2α is associated with synaptic deficits and neuronal loss in prion-disease model mice (23).
The role of eIF2 in the pain pathway is unknown. An endoplasmic reticulum (ER) stress response is induced in the peripheral nervous system of type I diabetic rats, as phosphorylation of PERK and eIF2α, along with other ER stress markers, is up-regulated (24). Induction of ER stress is accompanied by hypersensitivity, and attenuation of ER stress by an inhibitor of soluble epoxide hydrolase (sEH) down-regulated ER stress markers, p-PERK, p-eIF2α, and reversed mechanical hypersensitivity (24). Despite these intriguing observations, a direct link between eIF2α phosphorylation and nociception has not been established. Using a transgenic mouse model with reduced phosphorylation (by ∼50%) of eIF2α (eIF2α+/S51A), we show that p-eIF2α controls thermal, but not mechanical, sensitivity via the regulation of transient receptor potential vanilloid receptor 1 (TRPV1) activity. Moreover, we find that eIF2α phosphorylation is induced in primary nociceptors in a chronic inflammation model and that it contributes to inflammatory pain hypersensitivity.
Results
p-eIF2α Is Increased in Dorsal Root Ganglia After Chronic Inflammation.
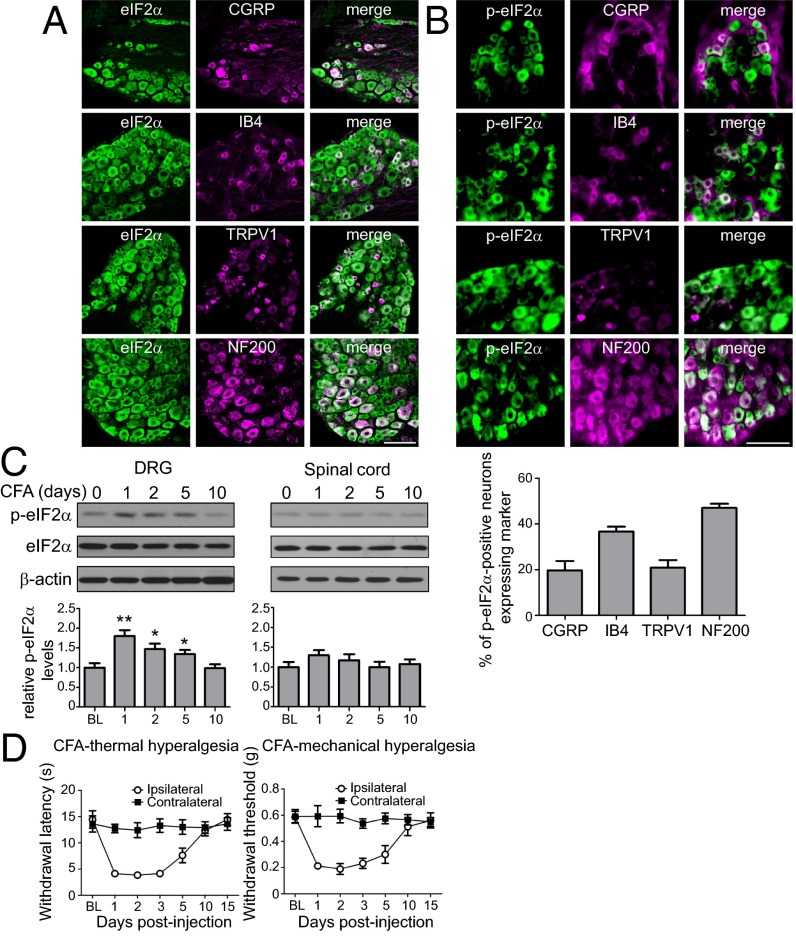
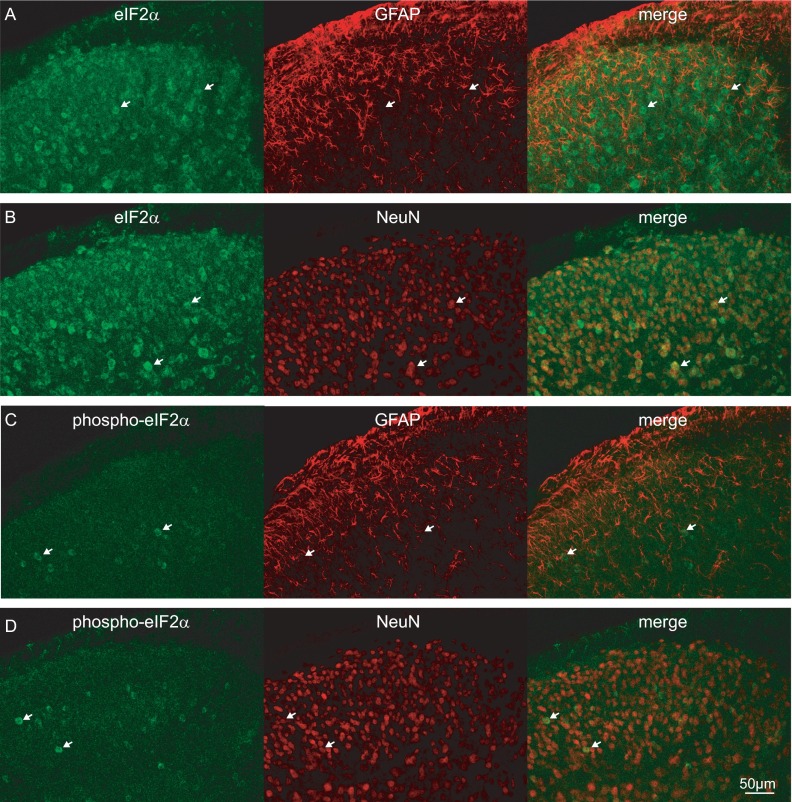
First, we examined the distribution of eIF2α and its phosphorylated form, p-eIF2α, in dorsal root ganglia (DRGs) and spinal cord. Immunostaining revealed neuronal expression of eIF2α and p-eIF2α in peptidergic (CGRP-positive), nonpeptidergic (IB4-positive), TRPV1-positive small diameter, and NF200-positive large diameter neuronal cell bodies in DRGs (Fig. 1 A and B). Colocalization analysis showed that 19.7% of p-eIF2α–positive cells express CGRP, 36.6% express IB4, 20.8% express TRPV1, and 47% express NF200 (Fig. 1B, Bottom). In the dorsal horn of the spinal cord, eIF2α and p-eIF2α were found in neurons, as they colocalized with the neuronal marker NeuN, but not with the astrocyte marker, glial fibrillary acidic protein (GFAP) (Fig. S1). However, in the spinal cord p-eIF2α signal was rather weak and detected only in a small fraction of NeuN-positive neurons (Fig. S1).
Fig. 1.
eIF2α is expressed in DRG neurons and its phosphorylation is increased in an inflammatory pain model. The distribution of total and p-eIF2α in mouse lumbar DRG was examined using immunostaining. Total (A) and p-eIF2α (Β) were costained with CGRP, IB4, TRPV1, and NF200. Percent of p-eIF2α–positive neurons expressing the markers is shown in B (Bottom). (C) Mice were injected (intraplantar) with CFA, and levels of eIF2α phosphorylation were measured in DRGs at different time points after injection using Western blot analysis (n = 4 mice per condition). (D) CFA induces thermal (Left) and mechanical (Right) hypersensitivity as assessed in radiant heat paw withdrawal and von Frey assays, respectively (n = 5 males and 4 females per assay). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01 by Bonferroni post hoc test following one-way ANOVA. (Scale bar, 100 μm.) For distribution of eIF2α and p-eIF2α in the spinal cord, see Fig. S1.
Fig. S1.
eIF2α is expressed in neurons in the spinal cord. Immunofluorescence images of total-eIF2α (A and B) and phospho-eIF2α (C and D) costained with GFAP and a neuronal marker (NeuN) in transverse sections of the mouse lumbar spinal cord (white arrows show the position of total and phospho-eIF2α–positive cells). Total and phospho-eIF2α were not expressed in astrocytes but occurred in neurons. Total-eIF2α was expressed in the gray matter of the spinal cord. The phospho-eIF2α signal was rather low and expressed in fewer cells.
To determine whether phosphorylation of eIF2α is affected by chronic inflammation, we injected complete Freund’s adjuvant (CFA) s.c. into the mouse hind paw (intraplantar injection) and measured the levels of p-eIF2α in lumbar DRGs and dorsal horn of the spinal cord. Levels of p-eIF2α were increased in DRGs, but not in the spinal cord, 1 d after the onset of inflammation, decreased subsequently, and returned to normal after 10 d (Fig. 1C). The alterations in p-eIF2α concurred with the inflammation-induced changes in thermal and mechanical thresholds (Fig. 1D), raising the possibility that the increase in eIF2α phosphorylation mediates the inflammatory hypersensitivity.
P-eIF2α Controls Thermal Sensitivity.
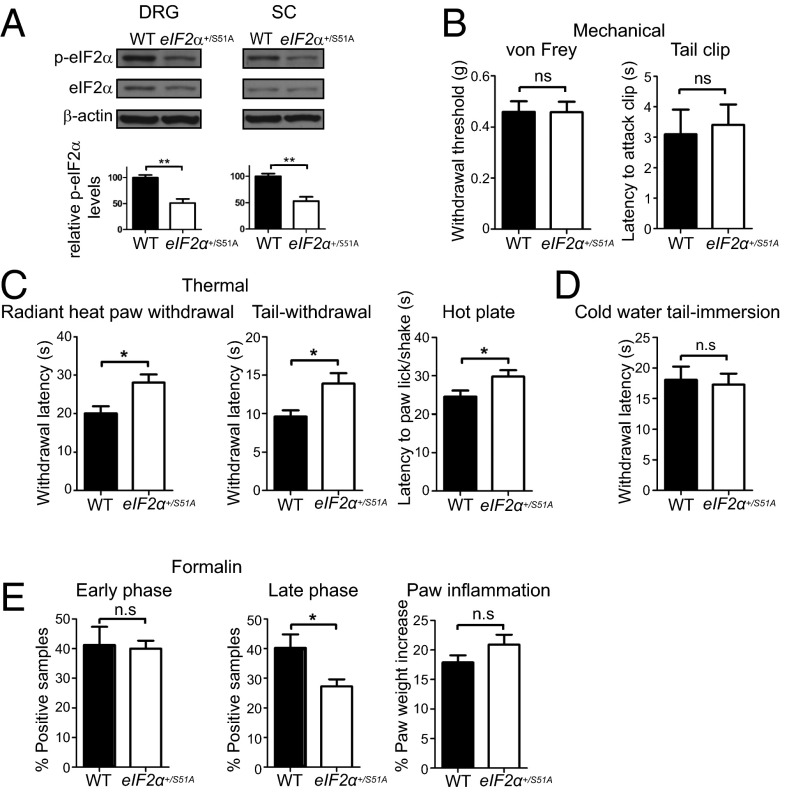
Having established that p-eIF2α is increased in DRGs in response to chronic inflammation, we investigated its role in nociception. To this end, we used a transgenic knock-in (KI) mouse model (25), in which serine-51 is mutated to a nonphosphorylatable alanine residue in one allele (eIF2α+/S51A, homozygous KI mice are not viable), leading to a ∼50% reduction in basal eIF2α phosphorylation (Fig. 2A). Mechanical sensitivity in the von Frey and tail clip tests did not differ between eIF2α+/S51A mice and their WT littermates (Fig. 2B). However, thermal withdrawal and nocifensive behavior latencies were significantly prolonged in eIF2α+/S51A mice compared with WT mice in the radiant heat paw withdrawal, hot water tail withdrawal, and hot-plate tests (40.2 ± 9.7%, 44.0 ± 13.1%, and 21.6 ± 6.6% increase, respectively; Fig. 2C), indicating reduced sensitivity to noxious heat in eIF2α+/S51A mice. No difference in sensitivity to noxious cold was observed between WT and eIF2α+/S51A mice (Fig. 2D). eIF2α+/S51A mice also exhibited reduced inflammatory pain in the formalin test. Nocifensive (licking/shaking) behavior was significantly reduced (by 31.9 ± 6.0%) in eIF2α+/S51A mice during the late/tonic phase (10–60 min after formalin injection), compared with WT mice, whereas no differences were found in the early/acute phase (0–10 min) between these groups (Fig. 2E). The behavioral differences occurred despite equal degrees of paw edema in the two genotypes (Fig. 2E). Taken together, these results demonstrate that p-eIF2α is up-regulated in DRGs in response to chronic inflammation, and mice with reduced eIF2α phosphorylation exhibit decreased heat sensitivity but not mechanical sensitivity.
Fig. 2.
Noxious heat sensation is reduced in eIF2α+/S51A mice. (A) eIF2α phosphorylation is reduced in DRGs and spinal cord of eIF2α+/S51A mice. eIF2α+/S51A mice demonstrate no alterations in mechanical sensitivity (B; n = 4 males and 4 females per genotype, P > 0.05), whereas noxious heat sensation is significantly attenuated (C; n = 4 males and 4 females per genotype per assay, P < 0.05). (D) Sensitivity to cold is not changed in eIF2α+/S51A mice (n = 4 males and 6 females per genotype). (E) Nocifensive (licking/shaking) behavior is significantly reduced in formalin test during the late/tonic phase (10–60 min after formalin injection, P < 0.05), whereas no differences are found in the early/acute phase (n = 4 males and 4 females per genotype). Changes in paw weight, indicative of formalin-induced inflammation, are not different in eIF2α+/S51A mice (E, Right; P > 0.05). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01; ns, not significant by Student t test.
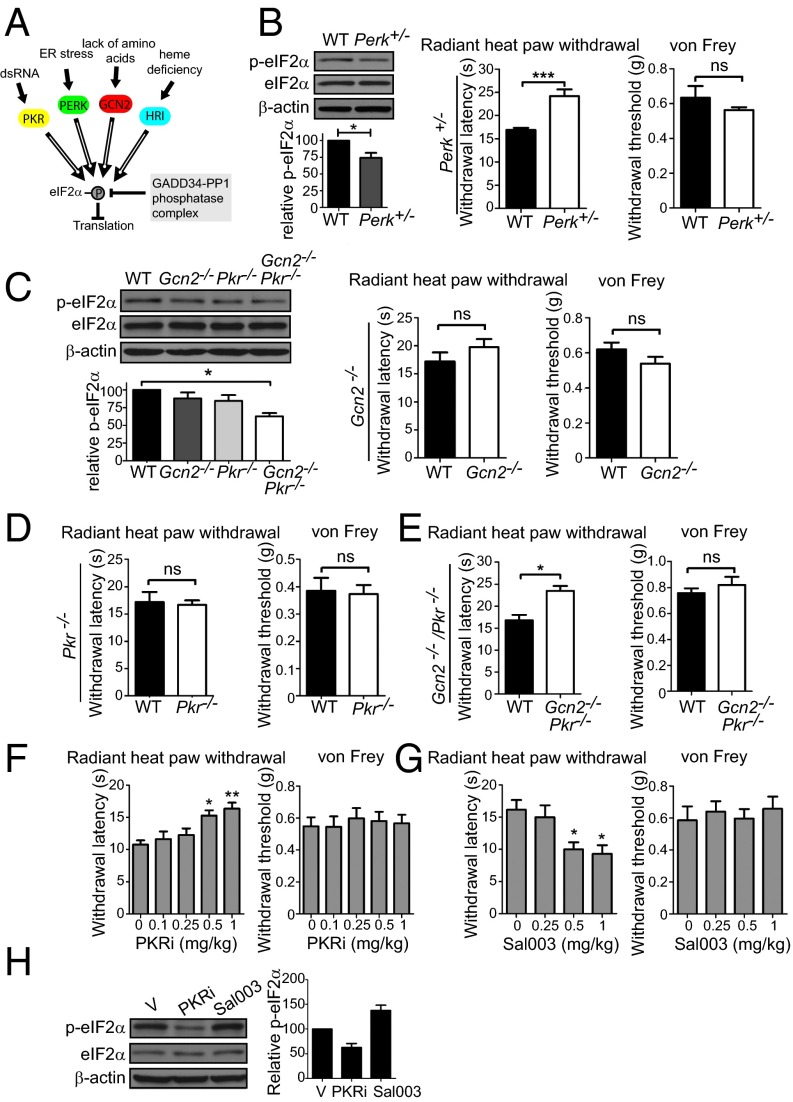
As eIF2α is phosphorylated by four different kinases (Fig. 3A), it was pertinent to study the effect of each kinase on p-eIF2α and thermal threshold. Because HRI expression is very low in the nervous system (12), we examined sensitivity to noxious heat in Perk+/− (Perk−/− exhibits severe postnatal growth retardation) (26), Pkr−/−, and Gcn2−/− mice. Perk heterozygous had reduced p-eIF2α in DRGs and decreased noxious heat sensation (43.0 ±7.7% increase in latency to withdrawal in the radiant heat paw withdrawal test; Fig. 3B). Mechanical thresholds in the von Frey test were not altered in Perk+/− mice, similar to eIF2α+/S51A mice. Gcn2−/− and Pkr−/− mice did not display a significant reduction in p-eIF2α level and sensitivity to noxious heat (Fig. 3 C and D); however, double KO mice for Gcn2 and Pkr (Gcn2/Pkr DKO) exhibited reduced p-eIF2α levels (Fig. 3C) and elevated thermal thresholds (Fig. 3E). This finding suggests a redundant role for these two kinases.
Fig. 3.
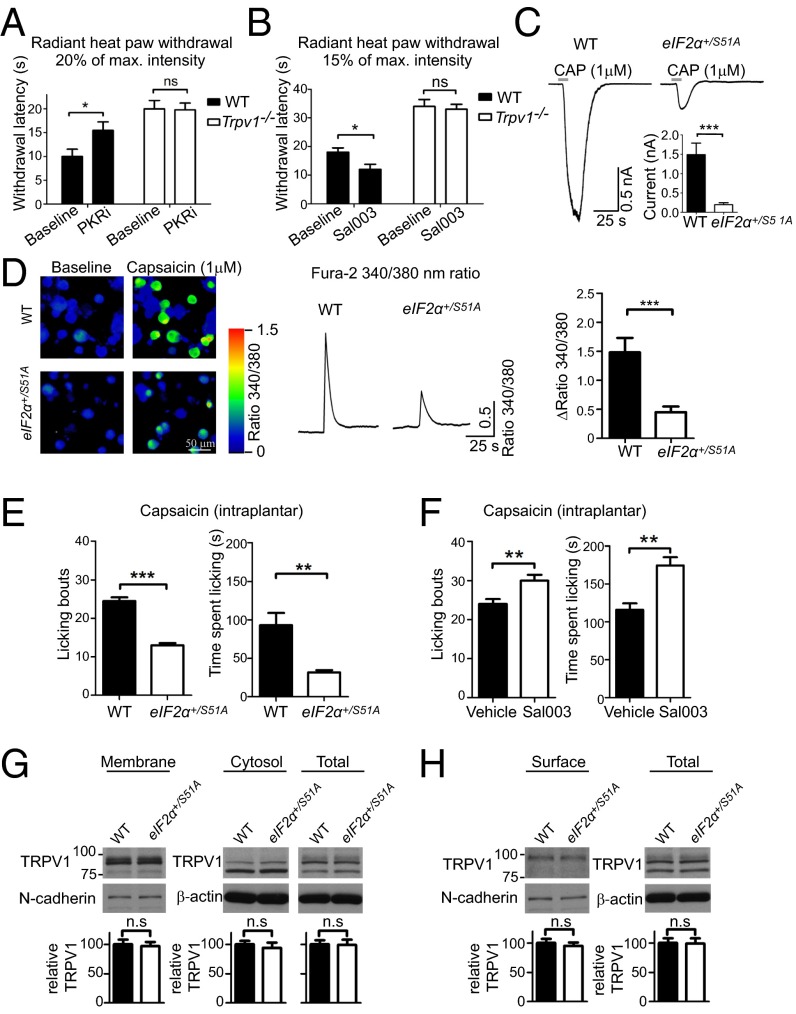
eIF2α kinases control thermal threshold. (A) eIF2α kinases and phosphatase GADD34/PP1 (growth arrest and DNA-damage-inducible 34/protein phosphatase1). (B) Perk+/− mice show a decrease in p-eIF2α (n = 4 mice per genotype, P < 0.05) and in noxious heat sensitivity (n = 4 males and 4 females per genotype, P < 0.001). Gcn2−/− and Pkr−/− mice show no alteration in thermal latencies (C and D, respectively; n = 4 males and 4 females per genotype, P > 0.05). (E) Gcn2−/− Pkr−/− double KO mice show reduced noxious heat sensitivity (n = 3 males and 4 females per genotype, P < 0.05). eIF2α phosphorylation in DRGs of Gcn2−/−, Pkr−/−, and Gcn2−/− Pkr−/− double KO mice is shown in C (n = 3 mice per genotype). PKR inhibitor (PKRi) (F) and Sal003 (G) were injected i.p. for 3 d daily, and thermal and mechanical thresholds were measured 30 min after the last injection (n = 4 males and 4 females per condition). (H) eIF2α phosphorylation in DRGs of mice injected with PKRi (1 mg/kg) and Sal003 (1 mg/kg) is shown (n = 5 mice per drug). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant by Student t test and Bonferroni post hoc test following one-way ANOVA.
Next, we examined whether modulation of eIF2α phosphorylation by drugs alters the thermal threshold. eIF2α phosphorylation was decreased by an inhibitor of eIF2α kinase, PKR (PKRi) (27). i.p. administration of PKRi over 3 d reduced noxious heat sensation in a dose-dependent manner, as indicated by the increased withdrawal latency in the radiant heat paw withdrawal test (Fig. 3F), with no effect on mechanical threshold. Conversely, when eIF2α phosphorylation was increased by i.p. administration of Sal003, an inhibitor of the eIF2α phosphatase complex, GADD34/PP1 (growth arrest and DNA-damage-inducible 34/protein phosphatase1) (28) (Fig. 3A), thermal thresholds were decreased (Fig. 3 G and H), whereas mechanical thresholds were not affected. In summary, using genetic and pharmacological approaches, we show that decreasing eIF2α phosphorylation reduces noxious heat sensation, whereas increasing p-eIF2α levels engenders the opposite effect.
TRPV1 Activity Mediates the Effect of Reduced p-eIF2α on Thermal Thresholds.
The strikingly specific impact of eIF2α phosphorylation on noxious heat sensation suggests that mechanisms controlling heat transduction might be selectively controlled. TRPV1 channels transduce noxious heat and are also implicated in inflammation-induced thermal hypersensitivity (29). TRPV1 activity is tightly regulated via gene expression and posttranslational mechanisms (30). We examined the possibility that TRPV1 mediates the effect of eIF2α phosphorylation on heat sensation by studying the impact of PKRi and Sal003 on thermal thresholds in Trpv1−/− mice (29). PKRi increased thermal threshold in WT mice, but not in Trpv1−/− mice (Fig. 4A). Conversely, Sal003 decreased thermal threshold in WT mice, but not in Trpv1−/− mice (Fig. 4B). These data demonstrate that eIF2α phosphorylation controls thermal threshold in a TRPV1-dependent manner. To assess TRPV1 activity, we recorded TRPV1-dependent currents in sensory neurons from eIF2α+/S51A and WT mice. Capsaicin, a specific TRPV1 agonist, elicited significantly smaller currents in dissociated DRG neurons prepared from eIF2α+/S51A compared with WT mice (92% decrease in eIF2α+/S51A neurons; Fig. 4C). For whole-cell recordings, small-diameter (<30 μm) neurons were selected, and only capsaicin-sensitive neurons (∼30% of all tested neurons in WT and eIF2α+/S51A groups) were included in the analysis. Resting membrane potential (Vrest), input resistance (Rin), and membrane capacitance (Cm) were not different between WT and eIF2α+/S51A neurons (WT Vrest −49.74 ± 3.41 mV, eIF2α+/S51A Vrest −46.79 ± 3.99 mV, P = 0.584; WT Rin 686.21 ± 117.41 MΩ, eIF2α+/S51A Rin 517.13 ± 38.41 MΩ, P = 0.193; WT Cm 13.67 ± 2.18 pF, eIF2α+/S51A Cm 14.68 ± 1.95 pF, P = 0.735, n = 8/genotype). Moreover, using calcium imaging, we observed smaller capsaicin-induced calcium transients in cultured eIF2α+/S51A DRG neurons compared with WT neurons (Fig. 4D). The cell body diameter of the responding neurons was not different between the two genotypes (WT 19.18 ± 0.45 μm, n = 53, eIF2α+/S51A 19.32 ± 0.39 μm, n = 88, P = 0.81). Consistent with these results, intraplantar s.c. administration of capsaicin induced significantly less nocifensive behavior in eIF2α+/S51A compared with WT mice (Fig. 4E). Conversely, mice with high p-eIF2α levels, following Sal003 injections, exhibited increased nociceptive responses to capsaicin (Fig. 4F). Despite the reduction in the amplitude of TRPV1-mediated currents in eIF2α+/S51A neurons, Western blot analysis showed that protein levels of TRPV1 in cytosolic and membrane fractions of DRG lysates from eIF2α+/S51A mice were not changed compared with WT mice (Fig. 4G). To examine whether trafficking of TRPV1 to the cell surface is affected by eIF2α phosphorylation, we used a surface biotinylation assay followed by Western blot analysis of TRPV1. We found no differences in TRPV1 amounts on the cell surface (Fig. 4H), indicating that TRPV1 activity, but not protein levels or trafficking to the plasma membrane, is reduced in eIF2α+/S51A neurons. Taken together, these data indicate that TRPV1 is an important mediator of the effect of p-eIF2α on the thermal threshold and suggest that TRPV1 activity is modulated by eIF2α phosphorylation.
Fig. 4.
eIF2α phosphorylation regulates thermal threshold via TRPV1. Modulation of eIF2α phosphorylation in Trpv1−/− mice does not alter heat sensation. PKRi (1 mg/kg for 3 d daily, i.p.) elevates thermal threshold in WT but not in Trpv1−/− mice (A; n = 4 males and 4 females per genotype-drug condition). Sal003 (1 mg/kg for 3 d daily, i.p.) decreases thermal threshold in WT but not in Trpv1−/− mice (B, n = 4 males and 4 females per genotype-drug condition). For the radiant heat paw withdrawal test, the light beam was set to 20% of the maximal intensity in A and to 15% in B. Capsaicin (1 μM) evokes smaller currents (C, n = 12 cells for eIF2α+/S51A and 10 for WT, from three different neuronal cultures per genotype) and smaller calcium transients (D, n = 72 cells for eIF2α +/S51A and n = 46 cells for WT, from four different neuronal cultures per genotype, using Fura-2 340/380-nm ratio) in cultured DRG neurons prepared from eIF2α+/S51A compared with WT mice. Capsaicin (2.5 μg), injected s.c. into the plantar surface of the hindpaw, elicited less nocifensive behaviors in eIF2α+/S51A compared with WT mice (E; n = 4 males and 4 females per genotype or drug), whereas in mice injected with Sal003 (1 mg/kg for 3 d daily, i.p.) capsaicin-induced pain behavior is increased (F). Western blot analysis shows that the TRPV1 protein levels are not altered in membrane and cytosolic fractions, as well as in total lysates of DRGs of eIF2α+/S51A mice (G; n = 4 mice and genotype). TRPV1 surface levels were measured in DRG cultured neurons prepared from eIF2α+/S51A and WT mice using surface biotinylation assay (H; n = 3/group). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 by Student t test and Student t test following two-way (genotype × drug) ANOVA.
PKR-Mediated eIF2α Phosphorylation Contributes to Thermal Hypersensitivity.
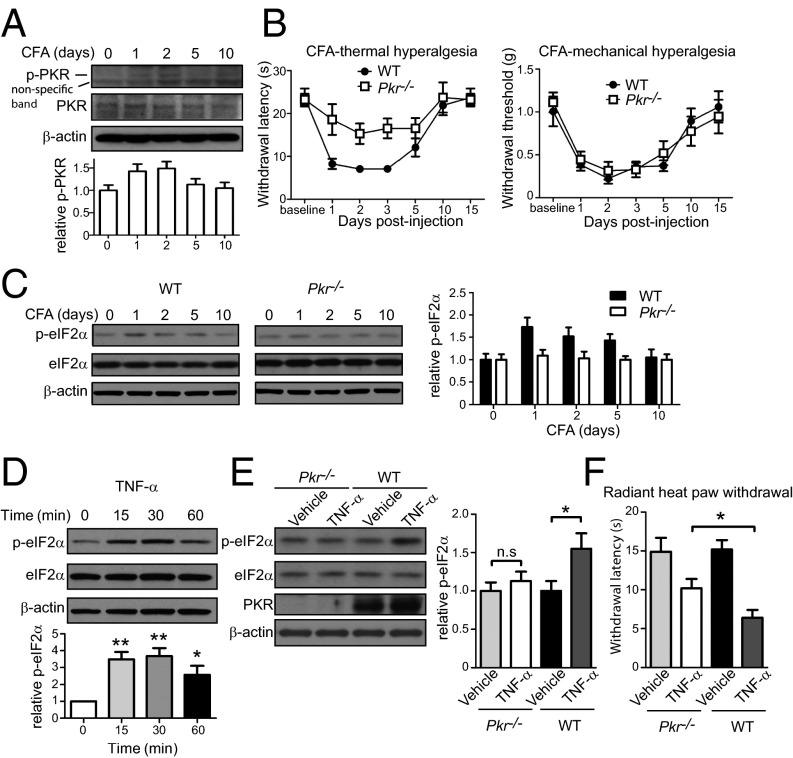
After establishing that eIF2α phosphorylation regulates thermal sensation, we studied whether CFA-induced increase in p-eIF2α contributes to thermal hypersensitivity. eIF2α kinase, PKR, has been implicated in inflammatory responses (31). Thus, we assessed PKR activation in DRGs following CFA injection and found that levels of p-PKR were significantly increased (Fig. 5A), raising the possibility that the increase in p-eIF2α is mediated via PKR activation. Consequently, we assessed thermal and mechanical hypersensitivity of Pkr−/− mice after CFA-induced inflammation. Pkr−/− mice exhibited reduced thermal, but not mechanical, pain hypersensitivity after CFA injection (Fig. 5B). Moreover, p-eIF2α increased after CFA injection in DRGs of WT mice, but not in Pkr−/− mice (Fig. 5C). These data demonstrate that PKR is required for the up-regulation of p-eIF2α following CFA and contributes to thermal hyperalgesia.
Fig. 5.
TNF-α–mediated PKR activation elevates p-eIF2α. (A) Phosphorylation of PKR (Thr451) was measured at different time points after CFA injection (n = 4 mice). (B) CFA-induced thermal hyperalgesia is attenuated in Pkr−/− mice compared with WT mice, whereas mechanical hyperalgesia is not changed (n = 4 males and 4 females per genotype). (C) P-eIF2α in DRGs was measured at different time points after CFA injection in WT and Pkr−/− mice. (D) HEK293 cells were treated with TNF-α (100 ng/mL) for 15, 30, and 60 min, and p-eIF2α was measured (n = 3, P < 0.05). (E) TNF-α (20 ng in 5 μL PBS + 0.5% BSA) was injected i.t. three times every 3 h in WT and Pkr−/− mice, and the p-eIF2α was measured in lumbar DRGs 30 min after the last injection. TNF-α induced an increase in p-eIF2α in WT but not in Pkr−/− mice (n = 5 females per genotype per drug). (F) TNF-α (20 ng, i.t., three injections at 3-h intervals) elicited bigger thermal hyperalgesia in WT mice compared with Pkr−/− mice (n = 4 males and 4 females per genotype per drug, P < 0.05). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 by Bonferroni post hoc test following one-way ANOVA and Student t test following two-way (genotype × drug) ANOVA.
TNF-α induces a robust up-regulation of eIF2α phosphorylation in cultured cells and in the nervous system via activation of PKR (32–36). Because TNF-α is a major proinflammatory cytokine (37–40), which plays a critical role in the pathogenesis of inflammatory pain (41, 42), we examined whether inflammation-induced TNF-α promotes eIF2α phosphorylation. First, we showed that TNF-α elevates p-eIF2α in HEK293 cells, replicating previous studies (Fig. 5D). Importantly, intrathecally delivered TNF-α increased p-eIF2α in DRGs of WT mice, but not Pkr−/− mice (Fig. 5E), supporting the idea that TNF-α stimulates p-eIF2α via PKR. In accordance with previous reports, TNF-α (i.t.) induced heat hyperalgesia in WT mice (37), whereas in Pkr−/− mice this hyperalgesia was significantly attenuated (Fig. 5F). Taken together, these results demonstrate that TNF-α- and PKR-dependent eIF2α phosphorylation contributes to chronic inflammation-induced thermal hypersensitivity.
Discussion
Here we describe a previously unrecognized role for the cellular stress response pathway in nociception. Transgenic mice with decreased eIF2α phosphorylation (eIF2α+/S51A) exhibited reduced responses to noxious heat and attenuated nocifensive behavior in the late phase of formalin test, whereas cold sensitivity and mechanical thresholds were not altered. The noxious heat-specific phenotype was recapitulated in transgenic mice in which the eIF2α kinases PERK and PKR/GCN2 were reduced or knocked out, as well as in response to pharmacological manipulation of eIF2α phosphorylation. Reducing eIF2α phosphorylation with a PKR inhibitor attenuated noxious heat sensitivity, whereas increasing eIF2α phosphorylation with Sal003 had the opposite effect. Our findings indicate that the effect of p-eIF2α on thermal nociception is mediated via modulation of TRPV1 activity. First, we show that pharmacological modulation of eIF2α phosphorylation altered noxious heat sensation in WT mice, but had no effect in mice lacking TRPV1. Second, capsaicin-induced TRPV1-mediated currents and pain behavior were greatly reduced in eIF2α+/S51A mice. Taken together, these data demonstrate that the eIF2α pathway controls noxious heat sensation via TRPV1. Because we found no evidence of alterations in TRPV1 protein levels or trafficking to the membrane, we postulate that the mechanism by which eIF2a phosphorylation affects thermal sensation involves modulation of TRPV1 activity. Under inflammatory conditions, TRPV1 can be sensitized by numerous inflammatory mediators (e.g., bradykinin, nerve growth factor, prostaglandins, serotonin, and histamine), via multiple agonists and modulators [protein kinase A (PKA), protein kinase C (PKC), metabolites of the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome-P450 (CYP)-pathways, phospholipids, protons, and heat, among others], leading to the reduction in the activation threshold and eventually to pain hypersensitivity (43, 44). The modulators, which mediate the sensitization of TRPV1 activity by p-eIF2α, remain to be determined. eIF2α phosphorylation promotes translation of ATF4 mRNA (9, 11). Interestingly, ATF4 is increased in DRGs following facet joint distraction (45). Moreover, ATF4 transcriptionally activates ATF3 expression, which is a well-known cellular marker of nerve injury (46). This evidence suggests that some of the effects of p-eIF2α on thermal thresholds could be mediated by ATF4/ATF3 axis.
We show that TNF-α induces p-eIF2α in WT but not in Pkr−/− mice, indicating that TNF-α up-regulates p-eIF2α via PKR. PKR is activated after CFA injection, suggesting that TNF-α–mediated activation of PKR contributes to p-eIF2α up-regulation and thermal hypersensitivity. Our results do not exclude the involvement of other eIF2α kinases. For example, ER stress induces a robust PERK activation (19), which has a strong effect on eIF2α phosphorylation and thermal sensitivity (Fig. 3B).
A recent study found that hyperglycemia, activation of unfolded protein response (UPR), or dysregulation of calcium homeostasis induce ER stress in primary sensory neurons, as evident by the activation of PERK (and eIF2α), inositol-requiring enzyme 1a (IRE1a), ATF6, MAPK (p38 and JNK), and autophagy (LC3) (24). Whether the ER stress-induced mechanical pain is caused by elevated p-eIF2α or through other mechanisms was not documented. Because elevated p-eIF2α affects thermal, but not mechanical thresholds, it seems unlikely that the effects of ER stress on nociception are mediated via p-eIF2α, but could be attributed to the activation of p38 and JNK or to other ER stress-dependent mechanisms. Increased p-eIF2α was also documented in the sciatic nerve of rats with presumed neuropathic pain (47); however, the impact of this phosphorylation event on nociception has not been investigated.
Recent preclinical studies concluded that modulators of eIF2α phosphorylation might have therapeutic potential in treatment of several cellular stress-related pathologies such as Alzheimer's disease (22, 48), prion diseases (23), diabetes (49), Huntington's disease (50), and amyotrophic lateral sclerosis (51). It will be important to consider the effect of eIF2α phosphorylation on thermal nociception while developing clinically applicable compounds to the latter maladies.
In summary, we uncovered a previously unknown role for the cellular stress response pathway in nociception. This knowledge can be used to develop strategies to treat conditions associated with altered heat sensation, most notably burn pain, and should be considered while introducing eIF2α modulators to clinical practice.
Experimental Procedures
Behavioral Experiments, Electrophysiological Recordings, and Calcium Imaging.
See SI Experimental Procedures for details of the experimental procedures. All procedures complied with Canadian Council on Animal Care guidelines and were approved by McGill University's Downtown Animal Care Committee.
Drugs.
PKR inhibitor (PKRi) and Sal003 were purchased from Calbiochem and dissolved in 30% polyethylene glycol in saline. Capsaicin was purchased from Sigma and dissolved in ethanol. TNF-α was purchased from Kamiya Biomedical.
Western Blotting and Immunohistochemistry.
Proteins were resolved on SDS-polyacrylamide gels using standard techniques. See SI Experimental Procedures for details of the experimental procedures and antibodies used.
Statistical Analyses.
All results are expressed as mean ± SEM. All statistical comparisons were made with either the Student t test or a one-way ANOVA followed by between-group comparisons using the Bonferroni post hoc test, unless otherwise indicated, with P < 0.05 as the significance criterion. Power analyses were not possible because we had no a priori expectation of effect size but rather were informed by normative practices in the pain field (52).
SI Experimental Procedures
Mice.
eIF2α+/S51A mice were kindly provided by Randal Kaufman, Sanford-Burnham-Prebys Medical Discovery Institute, La Jolla, CA, and were backcrossed for more than 10 generations to a C57BL/6J background. Gcn2−/− mice, on a 129/SvEv genetic background and Perk+/− mice on a mixed albino Swiss Webster;129/SvEv background were kindly provided by David Ron, Cambridge Institute for Medical Research, Cambridge, UK. Pkr−/− mice (53) were backcrossed for at least eight generations to 129/SvEv mice. Trpv1−/− mice on a C57BL/6J background were obtained from The Jackson Laboratory (B6.129 × 1-Trpv1tm1Jul/J). Pharmacological experiments were performed on naive, adult outbred CD-1 (ICR:Crl) mice. All behavioral experiments were performed on 7- to 12-wk-old mice of both sexes by an experimenter blinded to genotype and drug. Food and water were provided ad libitum, and mice were kept on a 12:12-h light/dark cycle (lights on at 0800 hours). All procedures complied with Canadian Council on Animal Care guidelines and were approved by McGill University's Downtown Animal Care Committee.
CFA.
Following baseline testing for mechanical (von Frey) and thermal sensitivity (radiant heat paw withdrawal), mice were injected s.c. with CFA (50% in 20 µL) into the left hind paw. Mice were tested every second day (days 1, 3, 5, 7, and 9) starting 24 h after injection for mechanical and thermal sensitivity.
Nociceptive Assays.
Nociceptive assays are described in detail in ref. 52. A brief description is as follows:
von Frey test: Mice were placed individually in transparent Plexiglas cubicles (5 × 8.5 × 6 cm) set on a perforated steel floor and habituated for 1 h before testing. Nylon monofilaments (Stoelting #2-#9) were firmly applied to the plantar surface of each hindpaw for 0.5 s. The up-down method of Dixon (54) was used to estimate the 50% withdrawal threshold.
Radiant heat paw withdrawal test: Mice were placed in cubicles (described above) on a glass floor and a focused beam of high-intensity light was aimed at the plantar surface of the hindpaw. The intensity of the light was set to 15% or 20% of maximum (IITC Model 390) with a cutoff value of 40 s. The latency to withdraw the hindpaw was measured to the nearest 0.1 s.
Hot-plate test: Mice were placed into a clear Plexiglas cylinder atop a metal surface (Columbus Instruments) maintained at 50 °C. The latency to lick or shake either hind paw was measured with a stopwatch to the nearest 0.1 s. Only one measurement was made.
Tail clip test: A small alligator clip (force, 700 × g) was applied at 1 cm from the base of the tail. The latency to attack/bite the clip was measured with a stopwatch to the nearest 0.1 s. Upon attack, the clip was removed, and the animals were returned to their cages. Only one measurement was made.
Tail withdrawal test: Mice were lightly restrained inside a cloth/cardboard “pocket” with their tail maintained outside. The distal half of the tail was immersed into 47 °C water and the latency to vigorously withdraw the tail from the water was measured with a stopwatch to the nearest 0.1 s. Two measures were taken at 30-min intervals for a total of eight measures per mouse (four time points).
Cold water tail immersion test: For the cold water tail immersion/withdrawal test, mice were lightly restrained inside a cloth/cardboard “pocket” and the distal half of their tail was immersed in ethanol maintained at −15 °C. The time to vigorous withdrawal of the tail was measured to the nearest 0.1 s with a stopwatch. Each mouse was tested twice at 5-min intervals.
Formalin test: Mice were placed into Plexiglas cylinders on a glass floor and habituated for at least 30 min. Following habituation, all mice were given intraplantar injections of formalin (4%) into the left hind paw and placed back into the cylinders. Cameras recorded the licking behavior over the next 60 min. Video files were sampled at 1-min intervals for the presence or absence of licking behavior in the first 10 s of each interval. Data are expressed as the percent of positive (licking) samples. The early phase was defined as minutes 0–10 and the late phase as minutes 10–60 after injection.
DRG Cultured Neurons.
Electrophysiological recordings and calcium imaging experiments were performed on dissociated DRG neurons. Briefly, DRGs were isolated from 6- to 8-wk-old WT and eIF2α+/S51A littermate mice and incubated in a solution containing dispase (1.37 mg/mL) and collagenase (1.08 mg/mL) for 45 min at 37 °C. After two washes in complete medium [Ham’s F-12 Nutrient Mixture, 10% (vol/vol) FBS, 2 mM l-glutamine, and 1% Pen-Strep], DRGs were mechanically dissociated using three fire-polished Pasteur pipettes with sequentially decreasing diameters. The cells were concentrated by centrifugation (3,000 rpm for 1 min) (Sorvall Legend RT, Heraeus swing bucket rotor; Cat#75006445), resuspended in culture medium, and plated on the glass bottom of the 35-mm dish precoated with a mixture of 100 μg/mL poly-d-lysine and 10 μg/mL laminin in Hanks' balanced salt solution (HBSS). Calcium imaging experiments and patch-clamp recordings of capsaicin-evoked responses were performed 18–48 h after cells plating.
Whole-Cell Recordings from DRG Cultured Neurons.
Cells were patch-clamped in voltage-clamp mode using glass pipettes containing a solution (pH 7.2) comprising (in mM) K-gluconate 120, MgCl2 1, EGTA 10, Hepes 10, and ATP 4, adjusted to 285 mOsmol/kg. Recordings were done in the whole cell mode using an Axopatch-200B amplifier (Axon Instruments). Membrane current (d.c., −2 KHz) was digitized at 10 kHz via a Digidata 1322A interface coupled to a PC running Clampex 8 (Axon Instruments). Whole-cell capacitance and series resistance were compensated electronically, and values of cell input capacitance (Ci) were noted. During recordings, cells were perfused with Hepes solution consisting of 150 mM NaCl, 3 mM KCl, 1 mM MgCl2, 10 mM Hepes, and 10 mM glucose and mannitol to adjust the osmolality of the solution to 312 mosmol/kg. Capsaicin (1 μM final concentration) was applied by rapidly switching the fluid delivery tube facing the cell using a piezoelectric stepper device (SF-77B; Warner Instruments). DRG cultured neurons with a diameter smaller than 30 μm were used for recordings, and about 30% of those showed responses to capsaicin. To measure input resistance (Rin) and membrane capacitance (Cm), a small square current pulse was injected into cells under the current-clamp mode, and Rin and Cm were computed. Only cells that responded to capsaicin were included in the calculations.
Calcium Imaging.
Calcium imaging was performed using dual-wavelength fluorescent calcium indicator FURA-2AM (Invitrogen). Isolated DRG neurons were loaded with 1 μM FURA-2AM mixed with pluronic acid (Sigma) in serum free DMEM for 30 min at room temperature, followed by a 10-min wash. Experiments were conducted at room temperature. Cells were visualized using Imaging workbench software (Photometrics) connected to an Olympus IX71 microscope attached to a Lambda DG-4 fluorescence unit (Sutter), and fluorescence images were captured with a cooled CCD camera (Coolsnap HQ2; Photometrics). Neurons were tested with 1 μM capsaicin. At the end of each experiment, 50 mM KCl solution was applied to depolarize neurons, thereby allowing to distinguish viable neurons from nonneuronal cells or nonfunctioning neurons. No differences in KCl-induced responses were observed between eIF2α+/S51A DRG neurons compared with WT neurons. For analysis, only neurons with a diameter of less than 30 μm that responded to capsaicin were considered.
Western Blotting.
Tissue extracts for Western blotting were prepared in ice-cold homogenization buffer containing (in mM) 20 Tris⋅HCl, pH 7.4; 150 NaCl; 1 EDTA; 1% Triton, 5 NaF; 1.5 Na3VO4; and protease inhibitor mixture (complete, EDTA-free; Roche Applied Science). Following centrifugation at 12,000 × g for 10 min, the supernatant protein concentration was measured, and equal protein quantities were boiled for 5 min in sample buffer and separated by SDS/PAGE. Following electrophoresis, proteins were transferred to 0.2-μm nitrocellulose membranes. Membranes were blocked in 5% (wt/vol) dry milk powder in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) for 1 h before overnight incubation with primary antibody. The membranes were then washed, incubated for 1 h with HRP-conjugated secondary antibody, washed again, treated with enhanced chemiluminesce reagent (Perkin-Elmer), and exposed to autoradiography films (Denville Scientific). All signals were obtained in the linear range for each antibody, quantified using ImageJ (NIH) and in some experiments normalized to β-actin. The Western blot experiments were performed in triplicate. The antibodies and the dilutions for the Western blots used in these studies are as follows: eIF2α total (1:1,000, Cat#9722S; Cell Signaling Technology), eIF2α S51 (1:1,000, Cat#44728; Invitrogen), TRPV1 (1:1,000; Alomone Laboratories), and β-actin (1:5,000, Cat#A5441; Sigma).
To separate the membrane and cytosolic fractions, DRGs were homogenized in ice-cold homogenization buffer containing (in mM) 30 Tris⋅HCl, pH 7.4; 1 EGTA; 0.1 Na3VO4; 10 NaF; and protease inhibitor mixture (complete, EDTA-free; Roche Applied Science), as previously described (55). Following centrifugation at 23,000 × g for 10 min, the supernatant, containing the cytosolic fraction, was collected. Pellets were resuspended in the homogenization buffer containing 1% Triton. Following sonication (20 pulses of 5 s) and centrifugation at 23,000 × g for 10 min at 4 °C, the supernatant, containing the membrane fraction, was collected. Proteins in the membrane fraction were normalized to N-cadherin (1:1,000, Cat#610920; BD Biosciences).
Cell Surface Biotinylation Assay.
DRG neurons, cultured for 24 h, were subjected to cell surface biotinylation using Cell Surface Protein Isolation Kit (Pierce; Cat#89881), according to the manufacturer’s instructions. Briefly, cells were washed with PBS and biotinylated with Sulfo-NHS-SS-Biotin [sulfosuccinimidyl-2-(biotinamido)ethyl-1,3-dithiopropionate] in PBS for 30 min at 4 °C. After quenching, cells were lysed, and labeled proteins were then isolated by incubating the lysate with NeutrAvidin Agarose beads for 60 min at room temperature. After washing, proteins were eluted by heating the beads for 5 min at 95 °C in SDS/PAGE sample buffer containing 50 mM DTT. The amount of the TRPV1 was analyzed by Western blot with an anti-TRPV1 antibody (Alomone; Cat #ACC-030).
Immunohistochemistry.
Mice were deeply anesthetized with Equithesin (6.5 mg chloral hydrate and 3 mg sodium pentobarbital in a volume of 0.3 mL, i.p., per 100 g body weight) and transcardially perfused with 50 mL vascular rinse [0.1% (wt/vol) sodium nitrite in 0.01M perfusion buffer; for composition, see ref. 56] and 200 mL fixative solution (4% paraformaldehyde, 15% saturated picric acid, in 0.1 M PB, pH 7.4) at room temperature for 30 min. The entire vertebral column was dissected out and postfixed overnight at 4 °C in the same fixative solution. Spinal cords were then extracted trough laminectomy, and L3, L4, and L5 DRG were collected. Specimens were than cryoprotected in 30% sucrose in PB overnight at 4 °C. Lumbar levels (L3-L5) of spinal cords were sectioned on a freezing sledge microtome (Leica) and collected in 0.01M PBS (50-µm-thick free floating transverse sections). DRGs were embedded in an optimum cutting temperature medium compound (OCT; TissueTek). Sixteen-micrometer-thick transverse sections were cut at −20 °C on a cryostat (CM3050 S; Leica) and collected directly on superfrost slides (Fisher Scientific).
Sections or slides were washed in PBS containing 0.2% Triton X-100 (PBS-T; Sigma Aldrich) for 30 min at room temperature, incubated in 50% ethanol, and washed again for 30 min in PBS. To block unspecific staining, sections were treated with PBS-T containing 10% (vol/vol) normal goat serum (NGS) for 1 h at room temperature. Sections were then incubated with primary antibodies diluted in PBS-T containing 5% (vol/vol) NGS, overnight at 4 °C. For isolectin B4 (IB4) staining, sections were incubated with IB4 conjugated to AlexaFluor 568, 1:200 (Molecular Probes), which was added to the secondary antibody solution. Sections were washed in PBS-T for 30 min and incubated with secondary antibodies diluted in PBS-T, at room temperature for 2 h. All steps were carried out under constant shaking and protected from light as required. After 30 min of final washes in PBS-T and 10 min in PBS, sections were mounted on gelatin-coated slides, air-dried, and coverslipped with Aqua Polymount (Polysciences). The slides were stored protected from light at 4 °C until examined by use of the confocal microscope.
Antibodies.
Antibodies used in this study were as follows: eIF2α (1:2,000 for spinal cord, 1:1,600 for DRGs, eIF2α D7D3 XP Rabbit mAb; Cell Signaling; #5324P), Phospho-eIF2α [1:100 for spinal cord, 1:200 for DRGs, Phospho-eIF2α (Ser51) D9G8 XP Rabbit mAb; Cell Signaling; #3398P], GFAP [1:1,000, GFAP (GA5) Mouse mAb; Cell Signaling; #3670], Neuronal Nuclei (NeuN, 1:5,000, Mouse Anti-NeuN Antibody, clone A60, MAB377), calcitonin gene-related peptide (guinea-pig anti-CGRP, 1:800 for DRGs; Sigma-Aldrich; C-8198, lot# 070M4835; or mouse anti-CGRP, 1:1,000; Sigma-Aldrich; C-7113, lot# 032M4862), mouse NF200 (1:1,000; Sigma-Aldrich; N0142, lot# 053M4756), and transient receptor potential cation channel subfamily V member 1 [guinea pig VR1 C terminus (capsaicin receptor), 1:1,600; Neuromics; GP14100, lot# 1401615]. For detection, species-specific secondary antibodies were used: goat anti-rabbit Alexa-Fluor 488 IgG (H+L) (1:400; Invitrogen Molecular Probes A11034), goat anti–guinea pig Alexa-Fluor 568 IgG (H+L) (1:800, Invitrogen Molecular Probes A11075), and goat anti-mouse Alexa-Fluor 568 IgG (H+L) (1:800, Invitrogen Molecular Probes A11031).
Colocalization analysis.
For each group, four L4 DRGs were used, originating from four mice. For each DRG, five sections were counted. For each section, the number of neurons labeled for both p-eiF2α and another marker (IB4, CGRP, NF200, or TRPV1) were counted, as well as the number of single-labeled cells. The mean values per DRG were then calculated. Counts were performed on images from the DRGs obtained with a Zeiss Axioimager M2 microscope. Contrast and brightness were adjusted for optimal detection and neurons were counted using the cell counter plugin of ImageJ (NIH). Only neurons displaying an identifiable nucleus were considered in the study to avoid duplicate counts. Data are expressed as the mean percentage (±SEM) of DRG neurons labeled for either eiF2α or p-eiF2α expressing the other labeling under study.
Acknowledgments
We thank A. Sylvestre, S. Perreault, C. Lister, and I. Harvey for technical assistance and I. Daou for technical advice. This work was supported by Canadian Institutes of Health Research (CIHR) Operating Grant MOP-114994 (to N.S.), an unrestricted gift from the Louise and Alan Edwards Foundation (to J.S.M.), CIHR Grant FDN-143337 (to C.W.B.), and the James McGill Chair Program (C.W.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614047113/-/DCSupplemental.
References
- 1.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol. 2005;6(4):318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 2.Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110(10):1383–1388. doi: 10.1172/JCI16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinnebusch AG, Ivanov IP, Sonenberg N. Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science. 2016;352(6292):1413–1416. doi: 10.1126/science.aad9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell. 2009;136(4):731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnelly N, Gorman AM, Gupta S, Samali A. The eIF2alpha kinases: Their structures and functions. Cell Mol Life Sci. 2013;70(19):3493–3511. doi: 10.1007/s00018-012-1252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raven JF, Koromilas AE. PERK and PKR: Old kinases learn new tricks. Cell Cycle. 2008;7(9):1146–1150. doi: 10.4161/cc.7.9.5811. [DOI] [PubMed] [Google Scholar]
- 7.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34(Pt 1):7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 8.Trinh MA, Klann E. Translational control by eIF2α kinases in long-lasting synaptic plasticity and long-term memory. Neurobiol Learn Mem. 2013;105:93–99. doi: 10.1016/j.nlm.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harding HP, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 10.Han J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15(5):481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutkowski DT, Kaufman RJ. All roads lead to ATF4. Dev Cell. 2003;4(4):442–444. doi: 10.1016/s1534-5807(03)00100-x. [DOI] [PubMed] [Google Scholar]
- 12.Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61(1):10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trinh MA, et al. The eIF2α kinase PERK limits the expression of hippocampal metabotropic glutamate receptor-dependent long-term depression. Learn Mem. 2014;21(5):298–304. doi: 10.1101/lm.032219.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Prisco GV, et al. Translational control of mGluR-dependent long-term depression and object-place learning by eIF2α. Nat Neurosci. 2014;17(8):1073–1082. doi: 10.1038/nn.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hetz C, Mollereau B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci. 2014;15(4):233–249. doi: 10.1038/nrn3689. [DOI] [PubMed] [Google Scholar]
- 16.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14(9):581–597. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 18.Koromilas AE. Roles of the translation initiation factor eIF2α serine 51 phosphorylation in cancer formation and treatment. Biochim Biophys Acta. 2015;1849(7):871–880. doi: 10.1016/j.bbagrm.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Segev Y, Michaelson DM, Rosenblum K. ApoE ε4 is associated with eIF2α phosphorylation and impaired learning in young mice. Neurobiol Aging. 2013;34(3):863–872. doi: 10.1016/j.neurobiolaging.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Chang RC, Wong AK, Ng HK, Hugon J. Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer’s disease. Neuroreport. 2002;13(18):2429–2432. doi: 10.1097/00001756-200212200-00011. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor T, et al. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60(6):988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma T, et al. Suppression of eIF2α kinases alleviates Alzheimer’s disease-related plasticity and memory deficits. Nat Neurosci. 2013;16(9):1299–1305. doi: 10.1038/nn.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno JA, et al. Sustained translational repression by eIF2α-P mediates prion neurodegeneration. Nature. 2012;485(7399):507–511. doi: 10.1038/nature11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inceoglu B, et al. Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proc Natl Acad Sci USA. 2015;112(29):9082–9087. doi: 10.1073/pnas.1510137112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheuner D, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 26.Zhang P, et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22(11):3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jammi NV, Whitby LR, Beal PA. Small molecule inhibitors of the RNA-dependent protein kinase. Biochem Biophys Res Commun. 2003;308(1):50–57. doi: 10.1016/s0006-291x(03)01318-4. [DOI] [PubMed] [Google Scholar]
- 28.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153(5):1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 30.Planells-Cases R, Valente P, Ferrer-Montiel A, Qin F, Szallasi A. Complex regulation of TRPV1 and related thermo-TRPs: Implications for therapeutic intervention. Adv Exp Med Biol. 2011;704:491–515. doi: 10.1007/978-94-007-0265-3_27. [DOI] [PubMed] [Google Scholar]
- 31.Kang R, Tang D. PKR-dependent inflammatory signals. Sci Signal. 2012;5(247):pe47. doi: 10.1126/scisignal.2003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantuano E, et al. The unfolded protein response is a major mechanism by which LRP1 regulates Schwann cell survival after injury. J Neurosci. 2011;31(38):13376–13385. doi: 10.1523/JNEUROSCI.2850-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma B, Altman JK, Goussetis DJ, Verma AK, Platanias LC. Protein kinase R as mediator of the effects of interferon (IFN) gamma and tumor necrosis factor (TNF) alpha on normal and dysplastic hematopoiesis. J Biol Chem. 2011;286(31):27506–27514. doi: 10.1074/jbc.M111.238501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu S, et al. Translational inhibition of colonic epithelial heat shock proteins by IFN-gamma and TNF-alpha in intestinal inflammation. Gastroenterology. 2007;133(6):1893–1904. doi: 10.1053/j.gastro.2007.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert SJ, Duance VC, Mason DJ. Does protein kinase R mediate TNF-alpha- and ceramide-induced increases in expression and activation of matrix metalloproteinases in articular cartilage by a novel mechanism? Arthritis Res Ther. 2004;6(1):R46–R55. doi: 10.1186/ar1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lourenco MV, et al. TNF-α mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer’s β-amyloid oligomers in mice and monkeys. Cell Metab. 2013;18(6):831–843. doi: 10.1016/j.cmet.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, et al. TNF-α contributes to spinal cord synaptic plasticity and inflammatory pain: Distinct role of TNF receptor subtypes 1 and 2. Pain. 2011;152(2):419–427. doi: 10.1016/j.pain.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohtori S, Takahashi K, Moriya H, Myers RR. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: Studies in murine DRG and spinal cord. Spine. 2004;29(10):1082–1088. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- 39.Schäfers M, Geis C, Svensson CI, Luo ZD, Sommer C. Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur J Neurosci. 2003;17(4):791–804. doi: 10.1046/j.1460-9568.2003.02504.x. [DOI] [PubMed] [Google Scholar]
- 40.Uçeyler N, Tscharke A, Sommer C. Early cytokine gene expression in mouse CNS after peripheral nerve lesion. Neurosci Lett. 2008;436(2):259–264. doi: 10.1016/j.neulet.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 41.Xu ZZ, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16(5):592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107(3):660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morales-Lázaro SL, Simon SA, Rosenbaum T. The role of endogenous molecules in modulating pain through transient receptor potential vanilloid 1 (TRPV1) J Physiol. 2013;591(13):3109–3121. doi: 10.1113/jphysiol.2013.251751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sisignano M, Bennett DL, Geisslinger G, Scholich K. TRP-channels as key integrators of lipid pathways in nociceptive neurons. Prog Lipid Res. 2014;53:93–107. doi: 10.1016/j.plipres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Dong L, Guarino BB, Jordan-Sciutto KL, Winkelstein BA. Activating transcription factor 4, a mediator of the integrated stress response, is increased in the dorsal root ganglia following painful facet joint distraction. Neuroscience. 2011;193:377–386. doi: 10.1016/j.neuroscience.2011.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bráz JM, Basbaum AI. Differential ATF3 expression in dorsal root ganglion neurons reveals the profile of primary afferents engaged by diverse noxious chemical stimuli. Pain. 2010;150(2):290–301. doi: 10.1016/j.pain.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melemedjian OK, et al. Targeting adenosine monophosphate-activated protein kinase (AMPK) in preclinical models reveals a potential mechanism for the treatment of neuropathic pain. Mol Pain. 2011;7:70. doi: 10.1186/1744-8069-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fullwood MJ, Zhou W, Shenolikar S. Targeting phosphorylation of eukaryotic initiation factor-2α to treat human disease. Prog Mol Biol Transl Sci. 2012;106:75–106. doi: 10.1016/B978-0-12-396456-4.00005-5. [DOI] [PubMed] [Google Scholar]
- 49.Back SH, et al. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10(1):13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reijonen S, Putkonen N, Nørremølle A, Lindholm D, Korhonen L. Inhibition of endoplasmic reticulum stress counteracts neuronal cell death and protein aggregation caused by N-terminal mutant huntingtin proteins. Exp Cell Res. 2008;314(5):950–960. doi: 10.1016/j.yexcr.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 51.Saxena S, Cabuy E, Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci. 2009;12(5):627–636. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- 52.Mogil JS, et al. Screening for pain phenotypes: Analysis of three congenic mouse strains on a battery of nine nociceptive assays. Pain. 2006;126(1-3):24–34. doi: 10.1016/j.pain.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Abraham N, et al. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem. 1999;274(9):5953–5962. doi: 10.1074/jbc.274.9.5953. [DOI] [PubMed] [Google Scholar]
- 54.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 55.Gong K, Kung LH, Magni G, Bhargava A, Jasmin L. Increased response to glutamate in small diameter dorsal root ganglion neurons after sciatic nerve injury. PLoS One. 2014;9(4):e95491. doi: 10.1371/journal.pone.0095491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Côté S, Ribeiro-da-Silva A, Cuello AC. Current protocols for light microscopy immunocytochemistry. In: Cuello AC, editor. Immunohistochemistry II. Wiley; Chichester, UK: 1993. pp. 147–168. [Google Scholar]