Significance
Amygdala reactivity and early life stress (ELS) are both strongly implicated in the mechanisms of depression in animal and human models. Despite these mechanistic foundations, amygdala reactivity and ELS have not been investigated as biobehavioral targets for predicting functional remission in depression. We addressed this issue by integrating human imaging and ELS measures within a controlled trial of antidepressant outcomes. We demonstrate that the interaction between ELS and amygdala engagement predicts functional remission on antidepressants with a greater than 80% cross-validated accuracy. In depressed people exposed to high ELS, a greater likelihood of remission was predicted by amygdala hyperreactivity to socially rewarding stimuli, whereas for those with low-ELS exposure, amygdala hyporeactivity to both rewarding and threat-related stimuli predicted remission.
Keywords: amygdala, early life stress, human brain imaging, predictive biomarkers, antidepressant remission
Abstract
Amygdala circuitry and early life stress (ELS) are both strongly and independently implicated in the neurobiology of depression. Importantly, animal models have revealed that the contribution of ELS to the development and maintenance of depression is likely a consequence of structural and physiological changes in amygdala circuitry in response to stress hormones. Despite these mechanistic foundations, amygdala engagement and ELS have not been investigated as biobehavioral targets for predicting functional remission in translational human studies of depression. Addressing this question, we integrated human neuroimaging and measurement of ELS within a controlled trial of antidepressant outcomes. Here we demonstrate that the interaction between amygdala activation engaged by emotional stimuli and ELS predicts functional remission on antidepressants with a greater than 80% cross-validated accuracy. Our model suggests that in depressed people with high ELS, the likelihood of remission is highest with greater amygdala reactivity to socially rewarding stimuli, whereas for those with low-ELS exposure, remission is associated with lower amygdala reactivity to both rewarding and threat-related stimuli. This full model predicted functional remission over and above the contribution of demographics, symptom severity, ELS, and amygdala reactivity alone. These findings identify a human target for elucidating the mechanisms of antidepressant functional remission and offer a target for developing novel therapeutics. The results also offer a proof-of-concept for using neuroimaging as a target for guiding neuroscience-informed intervention decisions at the level of the individual person.
Amygdala reactivity and exposure to early life stress (ELS) are both strongly implicated in depression mechanisms in both animal and human models (1–6). The amygdala plays an important role in emotion processing, including evaluating biologically salient emotional stimuli, generating emotional states and potentiating emotional memories (7). It also plays a central role in the stress response, both promoting downstream hypothalamic–pituitary–adrenal (HPA) axis stimulation and receiving HPA axis feedback (8). Engagement of the stress system can fundamentally change amygdala structure and function, especially as a result of ELS (2, 9–11). Moreover, the amygdala is likely a component of the neural circuit involved in antidepressant action (12, 13), and the antidepressant response is modified by prior stress exposure (10, 14). Despite these mechanistic foundations, amygdala–stress interactions have not been investigated as a prognostic biobehavioral therapeutic target for depression.
Human neuroimaging investigations have demonstrated strong links between depression and amygdala abnormalities in both structure and function (1, 15–21). Structurally, decreased amygdala volumes were found in unmedicated depressed individuals relative to healthy controls, whereas larger amygdala volumes were found in depressed individuals receiving antidepressant treatments (21). Functionally, distinct profiles of amygdala activation, such as hyperengagement of the amygdala to threat and hypoengagement to positive emotion, have been reported in depression (16–19).
Animal models have further highlighted the importance of the amygdala in the pathophysiology of depression and the mechanisms underlying this association. For example, stress paradigms that induce depressive-like behaviors result in hypertrophy of the amygdala, potentially through an increase of dendritic arborizations and spines (1, 9). Parallel physiological changes contribute to a sensitization of amygdala engagement (9). Independently, direct stimulation of the amygdala is sufficient to induce an increase in emotional reactivity and fear-like behaviors (22). These amygdala-mediated behaviors, together with decreased emotional control by medial prefrontal cortex regions, are thought to contribute to the increased negative bias and overly negative evaluation of the self that are fundamental features of human depression (23). Although these rodent models can reproduce depressive-like behaviors, they cannot capture the personal human experience of depression, making it difficult to directly translate findings between animal and human models. Despite this limitation, animal models have significantly aided our understanding of the neural mechanisms by which depression develops. In particular, these models have provided critical insights into a mechanistic pathway through which stress, especially ELS, produces the cascade of neurobiological changes that disrupt emotion regulation and generate depression in adulthood (1, 24, 25).
Similarly, in humans, a history of ELS, particularly abuse or neglect, is a prominent risk factor for developing depression and exacerbating depression severity (3, 4). Mechanisms by which ELS affects adult psychopathology are thought to involve—at least in part—engagement of the HPA axis in the immediate response to the stressor (2, 6, 10, 11, 19, 26). This engagement of the HPA axis and release of stress-related hormones are, in turn, believed to cause structural and functional changes in the neural circuits that underlie emotion processing. The amygdala is one of the most consistently reported regions demonstrating such changes (1, 9, 27). These structural changes outlast the termination of the stressor (9), and may heighten emotional reactivity and fundamentally alter the way these neural circuits respond to subsequent stressors (9).
Not all individuals who experience ELS develop depression. The cumulative risk likely depends on the interaction between the stressor and the degree to which the stressor impacts emotional brain circuits. For example, in rodents, the degree of amygdala hypertrophy resulting from an initial stressor differentiates rodents that go on to develop depressive-like behaviors following a subsequent stressor from those that do not (28). That is, subsequent maladaptive sensitivity to stress depends on the degree to which the early stressor impaired the amygdala. Similarly, in humans, the interaction between the degree of amygdala engagement at baseline and the amount of subsequent stress predicts which people developed an internalizing disorder, including depression, 1–4 years later (29).
Amygdala function and ELS have independently been identified as factors contributing to antidepressant response. Evidence from rodents and humans has implicated the amygdala as a target of antidepressants (12, 13). Congruent with this framework, amygdala engagement in response to both positively and negatively valenced stimuli distinguished antidepressant responders and nonresponders (30). Specifically, preintervention amygdala hypoengagement in response to subliminal fearful and angry faces (a signal of potential threat) and happy faces (a signal of social reward) have separately been associated with better antidepressant response. Moreover, amygdala reactivity in responders to both negatively and positively valenced stimuli increased toward normalization following antidepressant intervention (30). Increasing levels of ELS have also been associated with poorer outcomes to pharmacotherapy, pharmacotherapy combined with psychotherapy, and psychotherapy alone (4, 5, 14).
In summary, these overlapping lines of evidence have demonstrated that: (i) amygdala engagement and ELS independently contribute to antidepressant response, (ii) functional and structural alterations in amygdala circuitry can result as a function of ELS, and (iii) vulnerability to developing depression depends on the interaction between amygdala function and ELS rather than either alone. However, despite these findings, no study has tested whether the interaction of amygdala engagement and ELS could be used as targets for therapeutic response to antidepressants in humans. Addressing this question could provide a new mechanistic understanding for why some individuals respond to antidepressant treatments and others do not, as well as offer new targets for intervention. Given that (i) a large societal cost is associated with untreated depression, (ii) less than a third of individuals typically remit after first-line antidepressants, and (iii) there is currently no test available for predicting remission in practice, these findings have important clinical ramifications.
In the present study, we hypothesized that an individual’s capacity to remit after an antidepressant intervention would depend on ELS history and amygdala reactivity to fearful and happy faces. Defining remission as requiring functional improvement and alleviation of symptoms (31), we tested the following hypotheses:
Hypothesis 1: Combining measures of ELS and preintervention amygdala reactivity to happy faces will predict functional remission better than ELS or amygdala reactivity alone.
Hypothesis 2: Combining measures of ELS and preintervention amygdala reactivity to fearful faces will predict functional remission better than ELS or amygdala reactivity alone.
Hypothesis 3: Combining measures of ELS and amygdala reactivity to both happy and fearful faces will provide the most accurate prediction of functional remission, beyond predictions based on ELS or specific stimulus valence-evoked amygdala reactivity alone.
Results
Participant Characteristics.
Of 102 participants with major depressive disorder (MDD) imaged, 22 dropped out. Eighty were assessed at week 8, of which 10 had no ELS data available. Baseline demographic, clinical breakdown, and dosage information for the three antidepressants are presented in Tables S1 and S2 for the analyzed sample (n = 70) split by ELS status and remission type. Those who achieved “functional remission” (i.e., symptoms returning to healthy range plus functional improvement) and those who did not showed no detected differences in age, education level, duration of MDD episode, social/occupational functioning, depression symptoms, or dose within each treatment arm. This lack of differences remained when further stratified by level of ELS exposure (all P > 0.05).
Table S1.
Demographic and clinical characteristics by group
| Characteristic | Nonremitters | Remitters | All | ||||||
| Mean | SD | n | Mean | SD | n | Mean | SD | n | |
| Age of first visit | |||||||||
| Low ELS | 38.34 | 15.08 | 8 | 28.81 | 7.69 | 9 | 33.29 | 12.37 | 17 |
| Mid ELS | 31.36 | 11.94 | 27 | 27.53 | 6.92 | 7 | 30.57 | 11.12 | 34 |
| High ELS | 35.47 | 15.69 | 16 | 29.99 | 5.48 | 3 | 34.61 | 14.59 | 19 |
| All | 33.75 | 13.69 | 51 | 28.53 | 6.81 | 19 | 32.33 | 12.38 | 70 |
| Years of education | |||||||||
| Low ELS | 13.88 | 2.95 | 8 | 15.00 | 2.69 | 9 | 14.47 | 2.79 | 17 |
| Mid ELS | 14.37 | 2.59 | 27 | 15.14 | 2.54 | 7 | 14.53 | 2.56 | 34 |
| High ELS | 12.69 | 3.14 | 16 | 16.00 | 1.73 | 3 | 13.21 | 3.17 | 19 |
| All | 13.76 | 2.87 | 51 | 15.21 | 2.42 | 19 | 14.16 | 2.81 | 70 |
| Duration of illness | |||||||||
| Low ELS | 10.62 | 7.84 | 8 | 8.67 | 5.89 | 9 | 9.59 | 6.73 | 17 |
| Mid ELS | 10.33 | 8.63 | 27 | 7.29 | 6.26 | 7 | 9.71 | 8.21 | 34 |
| High ELS | 20.00 | 17.75 | 16 | 4.67 | 3.51 | 3 | 17.58 | 17.23 | 19 |
| All | 13.41 | 12.73 | 51 | 7.53 | 5.65 | 19 | 11.81 | 11.52 | 70 |
| Number of early life events | |||||||||
| Low ELS | 0.50 | 0.53 | 8 | 0.67 | 0.50 | 9 | 0.59 | 0.51 | 17 |
| Mid ELS | 3.44 | 1.15 | 27 | 4.00 | 1.00 | 7 | 3.56 | 1.13 | 34 |
| High ELS | 6.50 | 1.03 | 16 | 8.00 | 2.00 | 3 | 6.74 | 1.28 | 19 |
| All | 3.94 | 2.28 | 51 | 3.05 | 2.86 | 19 | 3.70 | 2.46 | 70 |
| HDRS17 total score at baseline | |||||||||
| Low ELS | 21.38 | 3.42 | 8 | 21.11 | 4.81 | 9 | 21.24 | 4.09 | 17 |
| Mid ELS | 21.00 | 4.24 | 27 | 20.57 | 4.24 | 7 | 20.91 | 4.18 | 34 |
| High ELS | 21.38 | 3.52 | 16 | 21.67 | 4.04 | 3 | 21.42 | 3.49 | 19 |
| All | 21.18 | 3.84 | 51 | 21.00 | 4.27 | 19 | 21.13 | 3.93 | 70 |
| QIDS-SR16 total score at baseline | |||||||||
| Low ELS | 12.12 | 2.75 | 8 | 13.11 | 4.54 | 9 | 12.65 | 3.72 | 17 |
| Mid ELS | 14.41 | 2.98 | 27 | 13.71 | 3.59 | 7 | 14.26 | 3.07 | 34 |
| High ELS | 14.00 | 3.90 | 16 | 15.00 | 6.24 | 3 | 14.16 | 4.14 | 19 |
| All | 13.92 | 3.30 | 51 | 13.63 | 4.27 | 19 | 13.84 | 3.56 | 70 |
| SOFAS at baseline | |||||||||
| Low ELS | 58.38 | 9.18 | 8 | 59.56 | 5.88 | 9 | 59.00 | 7.38 | 17 |
| Mid ELS | 63.78 | 10.57 | 27 | 60.43 | 4.72 | 7 | 63.09 | 9.70 | 34 |
| High ELS | 57.56 | 8.09 | 16 | 63.00 | 4.36 | 3 | 58.42 | 7.80 | 19 |
| All | 60.98 | 9.93 | 51 | 60.42 | 5.14 | 19 | 60.83 | 8.85 | 70 |
| Amygdala reactivity to happy faces at baseline | |||||||||
| Low ELS | 0.08 | 0.16 | 8 | −0.08 | 0.13 | 9 | −0.01 | 0.16 | 17 |
| Mid ELS | −0.02 | 0.12 | 27 | 0 | 0.08 | 7 | −0.02 | 0.11 | 34 |
| High ELS | −0.04 | 0.12 | 16 | 0.09 | 0.02 | 3 | −0.02 | 0.12 | 19 |
| All | −0.01 | 0.13 | 51 | −0.03 | 0.11 | 19 | −0.02 | 0.12 | 70 |
| Amygdala reactivity to fearful faces at baseline | |||||||||
| Low ELS | 0.05 | 0.14 | 8 | −0.08 | 0.11 | 9 | −0.02 | 0.14 | 17 |
| Mid ELS | 0.03 | 0.12 | 27 | −0.07 | 0.06 | 7 | 0.01 | 0.11 | 34 |
| High ELS | −0.03 | 0.18 | 16 | 0.05 | 0.17 | 3 | −0.02 | 0.17 | 19 |
| All | 0.01 | 0.14 | 51 | −0.06 | 0.11 | 19 | −0.01 | 0.14 | 70 |
| HDRS17 total score posttreatment | |||||||||
| Low ELS | 9.75 | 4.83 | 8 | 3.89 | 1.83 | 9 | 6.65 | 4.58 | 17 |
| Mid ELS | 9.33 | 3.86 | 27 | 3.86 | 2.54 | 7 | 8.21 | 4.24 | 34 |
| High ELS | 11.88 | 4.60 | 16 | 3.67 | 1.15 | 3 | 10.58 | 5.22 | 19 |
| All | 10.20 | 4.33 | 51 | 3.84 | 1.95 | 19 | 8.47 | 4.76 | 70 |
| QIDS-SR16 total score posttreatment | |||||||||
| Low ELS | 7.00 | 2.62 | 8 | 3.56 | 1.74 | 9 | 5.18 | 2.77 | 17 |
| Mid ELS | 8.37 | 4.29 | 27 | 3.00 | 1.73 | 7 | 7.26 | 4.46 | 34 |
| High ELS | 10.25 | 4.16 | 16 | 2.67 | 1.53 | 3 | 9.05 | 4.77 | 19 |
| All | 8.75 | 4.12 | 51 | 3.21 | 1.65 | 19 | 7.24 | 4.38 | 70 |
| SOFAS posttreatment | |||||||||
| Low ELS | 68.62 | 13.26 | 8 | 80.11 | 7.91 | 9 | 74.71 | 11.96 | 17 |
| Mid ELS | 73.93 | 8.16 | 27 | 82.43 | 5.68 | 7 | 75.68 | 8.40 | 34 |
| High ELS | 68.81 | 9.47 | 16 | 87.00 | 7.21 | 3 | 71.68 | 11.26 | 19 |
| All | 71.49 | 9.64 | 51 | 82.05 | 7.10 | 19 | 74.36 | 10.14 | 70 |
Table S2.
Dosage information
| Characteristic | Nonremitters | Remitters | All | ||||||
| Mean | SD | n | Mean | SD | n | Mean | SD | n | |
| Escitalopram dose | |||||||||
| Low ELS | 10.00 | 0.00 | 3 | 10.00 | 0.00 | 3 | 10.00 | 0.00 | 6 |
| Mid ELS | 11.28 | 3.53 | 8 | 10.00 | 0.00 | 4 | 10.85 | 2.88 | 12 |
| High ELS | 9.00 | 2.24 | 5 | 10.00 | — | 1 | 9.17 | 2.04 | 6 |
| All | 10.33 | 2.87 | 16 | 10.00 | 0.00 | 8 | 10.22 | 2.32 | 24 |
| Sertraline dose | |||||||||
| Low ELS | 100.00 | 70.71 | 2 | 50.00 | — | 1 | 83.33 | 57.74 | 3 |
| Mid ELS | 56.25 | 29.12 | 8 | 50.00 | 0.00 | 3 | 54.55 | 24.54 | 11 |
| High ELS | 58.33 | 20.41 | 6 | 50.00 | — | 1 | 57.14 | 18.90 | 7 |
| All | 62.50 | 32.91 | 16 | 50.00 | 0.00 | 5 | 59.52 | 29.02 | 21 |
| Venlafaxine, XR dose | |||||||||
| Low ELS | 100.00 | 43.30 | 3 | 90.00 | 33.54 | 5 | 93.75 | 34.72 | 8 |
| Mid ELS | 88.64 | 30.34 | 11 | — | — | 0 | 88.64 | 30.34 | 11 |
| High ELS | 90.00 | 33.54 | 5 | 75.00 | — | 1 | 87.50 | 30.62 | 6 |
| All | 90.79 | 31.41 | 19 | 87.50 | 30.62 | 6 | 90.00 | 30.62 | 25 |
| Using dose equivalents* | |||||||||
| Low ELS | 103.12 | 55.80 | 8 | 83.33 | 25.00 | 9 | 92.65 | 42.17 | 17 |
| Mid ELS | 83.33 | 36.53 | 27 | 75.00 | 0.00 | 7 | 81.62 | 32.60 | 34 |
| High ELS | 82.03 | 28.12 | 16 | 75.00 | 0.00 | 3 | 80.92 | 25.81 | 19 |
| All | 86.03 | 37.72 | 51 | 78.95 | 17.21 | 19 | 84.11 | 33.44 | 70 |
XR, extended release.
Converted to equivalent dosage in venlafaxine (7.5× escitalopram; 1.5× sertraline; 1× venlafaxine).
Predictors of Functional Remission.
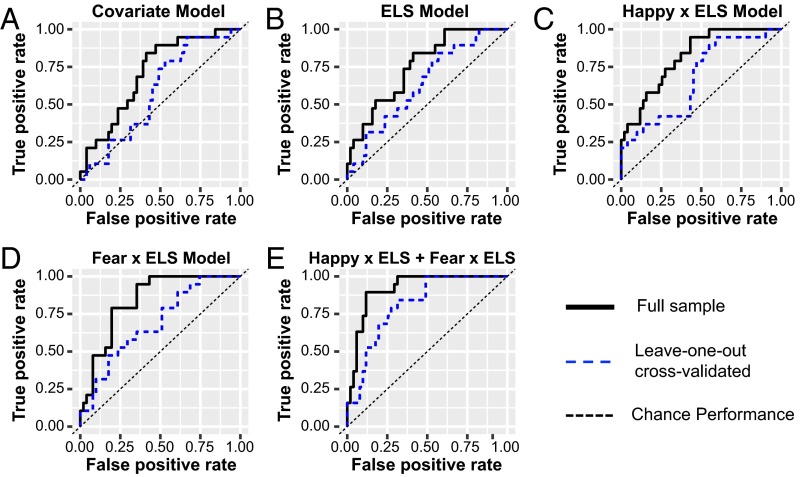
Fig. 1 and Table 1 show the performance of each classification model with and without leave-one-out cross-validation in predicting functional remission.
Fig. 1.
ROC curves showing the relative performance of candidate regression models in predicting functional remission. ROC curves model performance in predicting functional remission for 70 participants using the following predictors: (A) demographic/clinical measures; (B) demographic/covariate measures and ELS; (C) demographic/covariate measures and the interaction between amygdala reactivity to happy faces and ELS; (D) demographic/covariate measures and the interaction between amygdala reactivity to fearful faces and ELS; and (E) demographic/clinical covariates, the interaction between ELS and amygdala reactivity to happy faces, as well as the interaction between ELS and amygdala reactivity to threatening faces.
Table 1.
Model performance summary
| Model | Covariates* | ELS | Happy × ELS | Fear × ELS | Happy × ELS + fear × ELS |
| Full sample | |||||
| AUC | 0.71 | 0.75 | 0.82 | 0.84 | 0.92 |
| Sensitivity (chance rate) | 0.84 (50) | 0.84 (50) | 0.95 (50) | 0.95 (50) | 0.89 (50) |
| Specificity (chance rate) | 0.59 (50) | 0.59 (50) | 0.57 (50) | 0.65 (50) | 0.88 (50) |
| PPV (remission rate) | 0.91 (27) | 0.91 (27) | 0.97 (27) | 0.97 (27) | 0.96 (27) |
| NPV (nonremission rate) | 0.43 (73) | 0.43 (73) | 0.45 (73) | 0.50 (73) | 0.74 (73) |
| Leave-one-out cross-validated | |||||
| AUC | 0.59 | 0.64 | 0.67 | 0.69 | 0.81 |
| Sensitivity (chance rate) | 0.95 (50) | 0.84 (50) | 0.94 (50) | 0.47 (50) | 0.84 (50) |
| Specificity (chance rate) | 0.33 (50) | 0.43 (50) | 0.41 (50) | 0.82 (50) | 0.69 (50) |
| PPV (remission rate) | 0.94 (27) | 0.88 (27) | 0.95 (27) | 0.80 (27) | 0.92 (27) |
| NPV (nonremission rate) | 0.35 (73) | 0.36 (73) | 0.38 (73) | 0.50 (73) | 0.50 (73) |
NPV, negative predictive value; PPV, positive predictive value.
Covariates include age, years of education, baseline depression severity, and MDD episode duration.
Covariate Model.
To serve as a basis of comparison for more complex models, a regression model consisting solely of clinical and demographic variables was used to classify functional remission (Table S3). In the first step, age, years of education, baseline depression severity, and MDD episode duration were included as predictors based on their previous associations with treatment success (30, 32). This model overall showed a trend toward significance (χ2 = 73.07, df = 4, P = 0.067) (Table S3). Receiver operating characteristic (ROC) analyses revealed an area under the curve (AUC) of 0.71 with 84% sensitivity and 59% specificity (Fig. 1A and Table 1).
Table S3.
Hierarchical regression estimating functional remission—Covariate model
| Model predictors | B (95% CI) | SE | Wald | P value | Model fit | Model gain per step | ||
| χ2 (df) | P value | Δχ2 (Δdf) | P value | |||||
| Step 1 | ||||||||
| Intercept | −1.3 (−2.01 to 0.59) | 0.36 | −3.59 | 0.000 | 73.06 (4) | 0.067 | 8.79 (5) | 0.067 |
| Age | −0.02 (−0.09 to 0.04) | 0.03 | −0.69 | 0.487 | ||||
| Years of education | 0.21 (−0.02 to 0.44) | 0.12 | 1.75 | 0.079 | ||||
| MDD duration | −0.07 (−0.17 to 0.03) | 0.05 | −1.41 | 0.158 | ||||
| HDRS17 | −0.05 (−0.20 to 0.10) | 0.08 | −0.65 | 0.517 | ||||
| Step 2 | ||||||||
| ELS | −0.99 (−1.91 to 0.08) | 0.47 | −2.12 | 0.034 | 68.01 (5) | 0.017 | 5.88 (1) | 0.025 |
CI, confidence interval.
Consistent with prior findings (4, 5, 14), adding ELS to the model significantly increased overall performance (Δχ2 = 5.88, Δdf = 1, P = 0.025) (Table S3). Within this model, the likelihood of achieving functional remission was negatively associated with ELS status [unstandardized beta coefficient (B) = −0.99, Wald statistic (W) = −2.12, P = 0.034] when holding covariates at a fixed value. ROC analyses demonstrated that this model performed modestly in predicting functional remission: AUC of 0.75; sensitivity of 84%; and specificity of 59% (Fig. 1B and Table 1). These metrics dropped to 0.64, 95%, and 39%, respectively, using a leave-one-out cross-validation (Table 1).
Happy × ELS Model.
We next tested whether models combining ELS and amygdala reactivity to happy faces would significantly increase model performance (see details in SI Results and Table S4). Briefly, the model that included interactions between ELS and amygdala reactivity to happy faces performed significantly better than the model with ELS and amygdala reactivity without interactions, even at a conservative Bonferroni level (Δχ2 = 8.03, df = 1, P = 0.005) (Table S4). The interaction model classified functional remission with an AUC of 0.82, sensitivity of 95%, and specificity of 57% (Fig. 1C and Table 1). Leave-one-out cross-validation analyses yielded an AUC of 0.69, sensitivity of 89%, and specificity of 45%, suggesting some caution in making inferences about its generalizability (Fig. 1C and Table 1).
Table S4.
Hierarchical regression estimating functional remission—ELS and amygdala reactivity models
| Model predictors | B (95% CI) | SE | Wald | P value | Model fit | Model gain per step | ||
| χ2 (df) | P value | Δχ2 (Δdf) | P value | |||||
| Reactivity to happy faces and ELS model | ||||||||
| Step 1 | ||||||||
| Intercept | −1.45 (−2.24 to 0.66) | 0.40 | −3.61 | 0.000 | 68.01 (5) | 0.017 | 13.84 (5) | 0.017 |
| Age | −0.03 (−0.09 to 0.03) | 0.03 | −0.92 | 0.358 | ||||
| Years of education | 0.20 (−0.04 to 0.44) | 0.12 | 1.62 | 0.104 | ||||
| MDD duration | −0.07 (−0.18 to 0.04) | 0.06 | −1.28 | 0.201 | ||||
| HDRS17 | −0.05 (−0.21 to 0.10) | 0.08 | −0.69 | 0.492 | ||||
| ELS | −0.99 (−1.91 to 0.08) | 0.47 | −2.12 | 0.034 | ||||
| Step 2 | ||||||||
| Happy reactivity | −0.33 (−0.97 to 0.30) | 0.32 | −1.03 | 0.301 | 66.90 (6) | 0.021 | 1.11 (1) | 0.292 |
| Step 3 | ||||||||
| Happy reactivity × ELS | 1.85 (0.30 to 3.40) | 0.79 | 2.34 | 0.019 | 58.87 (7) | 0.002 | 8.03 (1) | 0.005 |
| Reactivity to fearful faces and ELS model | ||||||||
| Step 1 | ||||||||
| Intercept | −1.45 (−2.24 to 0.66) | 0.40 | −3.61 | 0.000 | 68.01 (5) | 0.017 | 13.84 (5) | 0.017 |
| Age | −0.03 (−0.09 to 0.03) | 0.03 | −0.92 | 0.358 | ||||
| Years of education | 0.20 (−0.04 to 0.44) | 0.12 | 1.62 | 0.104 | ||||
| MDD duration | −0.07 (−0.18 to 0.04) | 0.06 | −1.28 | 0.201 | ||||
| HDRS17 | −0.05 (−0.21 to 0.10) | 0.08 | −0.69 | 0.492 | ||||
| ELS | −0.99 (−1.91 to 0.08) | 0.47 | −2.12 | 0.034 | ||||
| Step 2 | ||||||||
| Fear reactivity | −0.82 (−1.60 to 0.04) | 0.40 | −2.06 | 0.039 | 62.99 (6) | 0.004 | 5.02 (1) | 0.025 |
| Step 3 | ||||||||
| Fear reactivity × ELS | 1.07 (−0.25 to 2.38) | 0.67 | 1.59 | 0.112 | 59.95 (7) | 0.003 | 3.04 (1) | 0.081 |
| Reactivity to happy and fearful faces and ELS model | ||||||||
| Step 1 | ||||||||
| Intercept | −1.45 (−2.24 to 0.66) | 0.40 | −3.61 | 0.000 | 68.01 (5) | 0.017 | 13.84 (5) | 0.017 |
| Age | −0.03 (−0.09 to 0.03) | 0.03 | −0.92 | 0.358 | ||||
| Years of education | 0.20 (−0.04 to 0.44) | 0.12 | 1.62 | 0.104 | ||||
| MDD duration | −0.07 (−0.18 to 0.04) | 0.06 | −1.28 | 0.201 | ||||
| HDRS17 | −0.05 (−0.21 to 0.10) | 0.08 | −0.69 | 0.492 | ||||
| ELS | −0.99 (−1.91 to 0.08) | 0.47 | −2.12 | 0.034 | ||||
| Step 2 | ||||||||
| Fear reactivity | −0.82 (−1.61 to 0.03) | 0.40 | −2.03 | 0.042 | 62.01 (7) | 0.006 | 6.00 (2) | 0.050 |
| Happy reactivity | −0.34 (−1.04 to 0.35) | 0.35 | −0.97 | 0.334 | ||||
| Step 3 | ||||||||
| Fear reactivity × ELS | 1.53 (−0.01 to 3.07) | 0.79 | 1.94 | 0.052 | 47.10 (9) | <0.001 | 14.91 (2) | <0.001 |
| Happy reactivity × ELS | 2.77 (0.76 to 4.77) | 1.02 | 2.71 | 0.007 | ||||
Fear × ELS Model.
We then tested whether models combining ELS and amygdala reactivity to fearful faces would significantly increase model performance beyond each independently (see details in SI Results and Table S4). Briefly, the fear reactivity × ELS interaction model performed only marginally better than a model with fear reactivity and ELS without their interaction (Δχ2 = 3.04, df = 1, P = 0.081) (Table S4). The fear reactivity × ELS model had an AUC of 0.84, sensitivity of 95%, and specificity of 65% (Fig. 1D and Table 1). The leave-one-out cross-validation model was relatively weaker, with an accuracy of 0.72, sensitivity of 47%, and specificity of 90%, suggesting caution in making inferences about its generalizability (Fig. 1D and Table 1).
Happy × ELS + Fear × ELS.
We tested whether combining the additive and interaction effects between ELS, happy reactivity, and fear reactivity would further increase model performance. We focused specifically on the interaction models because both the happy and fear interaction models performed better than the additive models, especially for happy. As predicted, the combined model had additive effects in predicting functional remission (χ2 = 47.10, df = 9, P < 0.001) (Table S4). Adding the fear–ELS and happy–ELS interactions to a model that contained the happy reactivity, fear reactivity, and ELS (as well as covariates) significantly increased the ability to predict functional remission at the Bonferroni-corrected level (Δχ2 = 14.91, df = 2, P < 0.001) (Table S4). The combined model increased the AUC to 0.92, while maintaining high sensitivity (89%) and specificity (88%) (Fig. 1E and Table 1). The leave-one-out cross-validated model similarly maintained high AUC (0.81), sensitivity (84%), and specificity (69%), suggesting good generalizability (Fig. 1E and Table 1). We replicated these results using an alternate analytical approach that uses the total number of ELSs rather than the interval scale measure (SI Results and Table S5).
Table S5.
Hierarchical regression estimating functional remission—Total number of ELS events and amygdala reactivity models
| Model predictors | B (95% CI) | SE | Wald | P value | Model fit | Model gain per step | ||
| χ2 (df) | P value | Δχ2 (Δdf) | P value | |||||
| Reactivity to happy and fearful faces and total number of ELS events model | ||||||||
| Step 1 | ||||||||
| Intercept | −0.82 (−1.89 to 0.25) | 0.55 | −1.5 | 0.134 | 71.67 (5) | 0.072 | 10.09 (5) | 0.072 |
| Age | −0.03 (−0.09 to 0.04) | 0.03 | −0.79 | 0.428 | ||||
| Years of education | 0.2 (−0.03 to 0.44) | 0.12 | 1.7 | 0.089 | ||||
| MDD duration | −0.07 (−0.18 to 0.03) | 0.05 | −1.39 | 0.164 | ||||
| HDRS17 | −0.05 (−0.2 to 0.1) | 0.08 | −0.67 | 0.504 | ||||
| ELS | −0.15 (−0.4 to 0.11) | 0.13 | −1.12 | 0.264 | ||||
| Step 2 | ||||||||
| Fear reactivity | −0.85 (−1.6 to 0.1) | 0.38 | −2.21 | 0.027 | 80.83 (7) | 0.017 | 6.93 (2) | 0.031 |
| Happy reactivity | −0.36 (−1.04 to 0.33) | 0.35 | −1.02 | 0.31 | ||||
| Step 3 | ||||||||
| Fear reactivity × ELS | 0.38 (−0.07 to 0.83) | 0.23 | 1.64 | 0.1 | 49.57 (9) | <0.001 | 15.26 (2) | <0.001 |
| Happy reactivity × ELS | 0.86 (0.18 to 1.53) | 0.34 | 2.49 | 0.013 | ||||
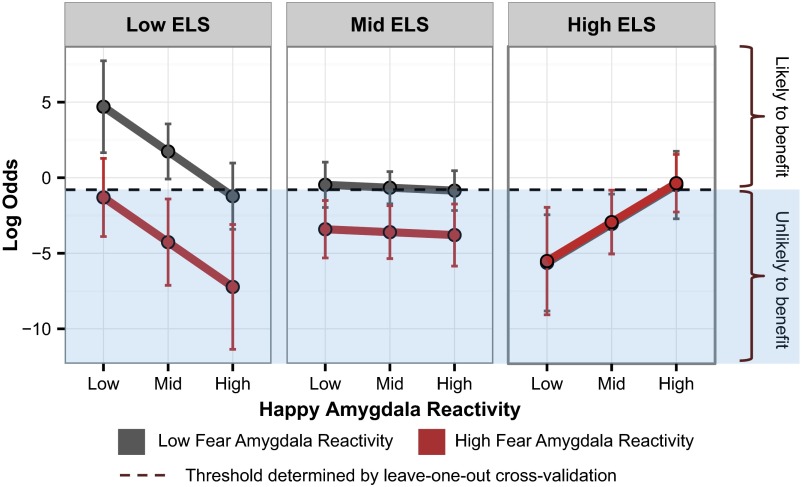
Within this full model, the happy–ELS (B = 2.78, W = 2.70, P = 0.007) and to a lesser extent the fear–ELS (B = 1.53, W = 1.94, P = 0.052) interactions contributed to the prediction. The slope of the relationship between the likelihood of functional remission and amygdala reactivity to both happy and fearful faces became increasingly positive with greater ELS, as computed by our statistical model at mean levels of other predictors. Thus, those with low ELS were predicted to have a greater probability of remitting if they had less baseline amygdala reactivity to both happy (B = −2.96, W = −2.53, P = 0.011) and fearful (B = 3.00, W = −2.53, P = 0.011) faces (Fig. 2), whereas for the mid-ELS group, remission was associated with amygdala reactivity to fearful faces (but not happy faces) such that those with the lowest amygdala reactivity were the most likely to remit (fear: B = −1.47, W = −2.33, P = 0.020; happy: B = −0.19, W = −0.34, P = 0.732). In contrast, the model estimated that those with high ELS were more likely to remit with increasing preintervention amygdala reactivity to happy faces (B = 2.57, W = 2.11, P = 0.027) but showed no association with fear reactivity (B = 0.06, W = 0.08, P = 0.940). The predicted likelihood of remission as a function of amygdala reactivity to both fearful and happy faces across ELS groups is plotted in Fig. 2.
Fig. 2.
Binary classification of remission. Predicted likelihood of remission (log odds) at varying levels of ELS and amygdala reactivity to emotional faces. The likelihood of remission was generated from the model containing both the interaction between ELS and amygdala reactivity to happy faces and the interaction between ELS and amygdala reactivity to fearful faces, using all 70 participants. The likelihood of remission was calculated at mean levels of covariates at low (mean minus 1 SD), mid (mean), and high (mean plus 1 SD) levels of reactivity to happy faces, as well as low and high levels of reactivity to fearful faces. Individuals with predicted probabilities above the cross-validated threshold for classification (dotted line) would be classified as likely to respond to escitalopram, sertraline, or venlafaxine, whereas those below would not. Error bars show 90% confidence intervals.
Clinical Translational Relevance.
Due to our combined model’s high degree of accuracy, it is relevant to consider its applicability to the clinical setting. Although our results remain speculative, we have demonstrated that in a moderate-sized sample, a single metric may be derived from our combined logistic regression model and used to support decisions about individual patients. In the happy × ELS + fear × ELS model, ROC analyses determined that a log-odds cross-validated threshold of −0.80 (the average threshold across all leave-one-out folds) could be used to distinguish remitters from nonremitters within this sample with a sensitivity of 60% and specificity of 80%. In future applications, a history of ELS coupled with an amygdala scan may be used to identify likely nonremitters (those below the −0.80 threshold; Fig. 2) before beginning a potentially ineffective pharmacotherapy.
SI Results
Happy × ELS Model.
Both the additive model of ELS and preintervention amygdala reactivity to masked happy faces (χ2 = 66.90, df = 6, P = 0.021) (Table S4), as well as the model that included ELS × amygdala reactivity interactions (χ2 = 58.87, df = 7, P = 0.002) (Table S4), significantly predicted postintervention therapeutic outcomes. However, only the interaction model remained significant after Bonferroni correction (P < 0.008). Within the interaction model, the interaction between amygdala reactivity to happy faces significantly contributed to the prediction (B = 1.85, W = 2.34, P = 0.019). Thus, as computed by our statistical model at mean levels of other predictors, the slope of the relationship between the likelihood of remission and amygdala reactivity to happy faces would become increasingly more positive by (1.85 log odds of remission)/(1 SD of amygdala reactivity) with increasing ELS. Thus, although there was a negative relationship between the likelihood of remission and amygdala reactivity to happy faces in patients with lower levels of ELS (B = −1.70, W = −2.06, P = 0.039), there was a positive relationship between the likelihood of remission and amygdala reactivity to happy faces in patients with high ELS (B = 2.00, W = 2.03, P = 0.042). That is, functional remission was associated with amygdala hypoactivation to happy faces in patients with low ELS but hyperactivation to happy faces in patients with high ELS. Happy reactivity was not associated with remission in the mid-ELS group (B = 0.15, W = 0.33, P = 0.74).
Fear × ELS Model.
Both the additive (χ2 = 62.99, df = 6, P = 0.004) (Table S4) and interaction (χ2 = 59.95, df = 7, P = 0.003) (Table S4) models significantly predicted postantidepressant intervention outcomes. Further, both models remained significant after Bonferroni correction (P < 0.008). Moreover, both the additive and interaction models performed marginally better than a model with just covariate predictors and ELS (Δχ2 = 5.02, df = 1, P = 0.025 and Δχ2 = 8.062, df = 2, P = 0.018, respectively) (Table S4). However, neither of the aforementioned tests remained significant post Bonferroni correction. As revealed by the additive model, within the interaction model, greater fear reactivity was marginally associated with worse therapeutic outcome (B = −0.87, W = −1.86, P = 0.063) when controlling for clinical/demographic variables and ELS. Moreover, although the interaction of amygdala reactivity to fearful faces and ELS was a marginal predictor within the model (B = 1.07, W = 1.59, P = 0.111), post hoc analyses revealed that amygdala reactivity to fearful faces was a predictor of remission for those within the low-ELS (B = −1.96, W = −2.11, P = 0.034) group, but this association did not reach significance in the mid- (B = 0.90, W = −1.90, P = 0.057) or high- (B = 0.17, W = 0.25, P = 0.80) ELS groups. Mirroring the main effect, lower fear reactivity in the low-ELS group was associated with greater predicted likelihood of remission.
Happy × ELS + Fear × ELS Using a Continuous Measure of ELS.
In addition to using cross-validation, we also demonstrate the robustness of our primary findings using an additional analytical approach. Specifically, we conducted a secondary analysis using the total number of ELS events (presented in Table S5) rather than using an interval measure of the three degrees of ELS (low, mid, and high). Importantly, these results provide another replication of our findings from the original analysis. First, this model replicates a significant happy × number of ELS events interaction (B = 0.86, W = 2.49, P = 0.013). For each additional ELS event, the slope of the relationship between amygdala reactivity to happy faces and remission status was estimated to increase positively by a log-odds value of 0.82 when keeping all other variables at a constant value. Thus, as was the case with the model using the interval scale measure of ELS, the relationship between amygdala reactivity to happy faces and the likelihood of functional remission was estimated to be negative for individuals with lower levels of ELS (e.g., at 0 ELS events: B = −3.34, W = −2.48, P = 0.013) and increasingly positive for increasing levels of ELS (e.g., at 7 ELS events: B = 2.65, W = 2.05, P = 0.040). Moreover, this alternate model using a continuous variable for ELS events additionally replicated the negative relationship between amygdala reactivity to fearful faces (B = −1.36, W = −2.4, P = 0.015) and functional remission as well as the marginal effect of the fear × ELS interaction (B = 0.38, W = 1.64, P = 0.100).
Discussion
Our study demonstrates that functional remission with antidepressants may be dependent not only on an individual’s ELS history and degree of amygdala engagement during facial emotion viewing tasks independently but on their interaction. Given this information, we could predict with a high degree of accuracy (81%) who would and would not functionally remit following pharmacotherapy with commonly prescribed antidepressants. Given that both amygdala circuitry and ELS are also strongly implicated in the neurobiology of depression, our predictive model provides a potential clinically meaningful target for mechanistic investigations and for novel intervention development.
Previous independent lines of research have shown how both ELS and amygdala reactivity may independently contribute to the pathophysiology of development, maintenance, and functional remission in depression. ELS has been shown to moderate antidepressant outcomes (4, 5, 14, 30). Consistent with these reports, we found that the severity of exposure to ELS in childhood is associated with a greater likelihood of treatment failure across three commonly prescribed antidepressants. Similarly, and consistent with previous evidence for the independent role of amygdala reactivity in predicting antidepressant remission (30), our model suggests that amygdala hyporeactivity to subliminally presented fearful faces may be associated with better functional remission when not controlling for ELS. Our study combines these two lines of investigation to demonstrate that the functional alterations in amygdala circuitry, potentially as a result of stress-induced plasticity, may be moderating the impact of ELS on functional remission.
The present findings advance our current knowledge by demonstrating that information on amygdala function and interactions with exposure to ELS together may be used to form the most accurate predictive model for antidepressant remission, even in a moderate-sized sample. When considering degree of ELS exposure and amygdala functioning together, we identified several preliminary patterns of amygdala reactivity that were associated with likelihood of remission and dependent on ELS status. For example, our results suggest a negative relationship between amygdala reactivity to both fearful and happy faces and the likelihood of achieving functional remission in individuals with low levels of ELS. That is, for individuals free of ELS, our model would predict a greater likelihood of remission if they showed neural insensitivity to salient emotion, reflected in hypoengagement in response to both threatening and socially rewarding stimuli. It is possible that these individuals, in the absence of stress sensitization, can benefit most from the impact of antidepressants on amygdala circuits. This result is consistent with a prior study demonstrating that antidepressants can normalize amygdala function by increasing amygdala reactivity to both happy and fearful faces (30) in those individuals who responded to treatment. Those with low ELS but hypersensitivity to threat might be candidates for psychosocial interventions that focus on improving emotion regulation [e.g., dialectical behavior therapy (33)] or prosocial behaviors (34).
Our results suggest that individuals with a high-ELS exposure would have a greater likelihood of functional remission with antidepressants if they had a preexisting hyperengagement in response to socially rewarding stimuli, relatively independent of how they respond to fearful faces. The neurobiological consequences of ELS are believed to arise initially as an adaptive response to the current environmental stressors. For example, in an abusive environment, an increased sensitivity to threat-related emotional signals may enable earlier detection and a potentially increased ability to avoid a negative confrontation with the caregiver (35). However, these short-term benefits are proposed to be associated with long-term functional and structural changes of limbic-related brain regions—perhaps most notably in the amygdala—that manifest as a function of the severity of the ELS exposure (1, 9, 27, 36, 37). Consistent with this framework, in childhood, the severity of ELS exposure has been positively associated with amygdala reactivity to both threatening and happy faces in a dose-dependent manner (26, 38). Similarly, as adults, the severity of prior stress exposure has also been associated with amygdala engagement in response to threatening faces (19). However, for happy faces, there was a marginally significant negative relationship such that greater ELS severity was associated with a lower amygdala response to happy faces (39). Taken together, these findings suggest that whereas initially ELS boosts sensitivity for salient expressions that encompass both positive and negative emotions, later in life this may progress into a bias toward threat-relevant stimuli, potentially with decreased sensitivity to happy faces. It is possible that “high-ELS exposure” individuals who are able to maintain amygdala hyperengagement to socially rewarding stimuli are endowed with additional resilience factors that enable them to mount a more effective response to antidepressants (37).
Although additional replication is required, these findings suggest that targets of antidepressant response may (at least in part) be tied to the neurobiology of depression itself. This study also makes a leap forward in demonstrating the possibility of a single composite brain-stress predictive metric relevant to informing therapeutic decisions that may be derived and used before fully understanding underlying neural mechanisms. Such a metric also offers a viable target for future mechanistic investigations and for studies aimed at developing novel interventions. Moreover, these findings provide additional support for the need to consider a life-course approach to the prevention and treatment of mental disorder (40).
Findings from this study should be appreciated within the context of certain limitations. Although our study sample would be considered large for neuroimaging measures and represents a statistical advance in power from the few previous neuroimaging prediction studies, it is relatively small when stratifying by ELS subgroups. Although we have undertaken steps to demonstrate the generalizability of our findings, including using cross-validation techniques and replicating our findings using an alternate analytical approach (SI Methods), replication and assessment of generalizability in an independent sample would be an important next step. Due to stratifying the sample by ELS, our analyses were not sufficiently powered to test whether the ELS interactions with amygdala reactivity predictions would be further dependent on the specific type of antidepressant therapy. Future investigations are needed to determine whether the interaction between ELS and amygdala reactivity is a differential predictor of antidepressant outcomes. Finally, due to the practical trial design, the study was necessarily limited to antidepressants in common use in each of the participating countries. It would similarly be important to verify the predictive role of ELS and amygdala reactivity with additional antidepressants and second-generation antipsychotics with antidepressant actions that have distinct mechanisms of action, as well as psychotherapy.
Our results advance our understanding of how ELS and amygdala engagement function synergistically to predict subsequent remission from depression with antidepressants. Moreover, we have demonstrated that a single brain-stress metric may be derived from our predictive model and used to support decisions about individual patients. In short, metrics based on combined brain and life experience data hold promise for developing a neuroscience-informed approach to mental disorder and its management.
SI Methods
Image Acquisition.
Magnetic resonance images were acquired using a 3.0-T Signa HDx scanner (GE Healthcare). Acquisition was performed using an eight-channel head coil and an echo-planar imaging (EPI) sequence with the following parameters: repetition time (TR), 2,500 ms; echo time (TE), 27.5 ms; matrix, 64 × 64; field-of-view (FOV), 24 cm; flip angle, 90°. Forty contiguous axial/oblique slices with a slice thickness of 3.5 mm were acquired to cover the whole brain in each volume. For each paradigm, 120 volumes were collected (one for every two face stimuli) with a total scan time of 5 min and 8 s as previously published (53, 32). Three dummy scans were acquired at the start of every acquisition.
Structural MRI 3D T1-weighted images were acquired in the sagittal plane using a 3D spoiled gradient-echo (SPGR) sequence (TR, 8.3 ms; TE, 3.2 ms; flip angle, 11°; TI, 500 ms; number of excitations (NEX), 1; array spatial sensitivity encoding technique (ASSET), 1.5; frequency direction, superior/inferior). T1 is the time for 63% of longitudinal relaxation to occur in magnetic resonance imaging. A total of 180 contiguous 1-mm slices were acquired, covering the whole brain with a 256 × 256 matrix with an in-plane resolution of 1 × 1 mm, resulting in 1-mm3 isotropic voxels. The 3D SPGR sequence was collected for use in normalization of the fMRI data to standard space.
fMRI Emotion Paradigm.
A total of 240 faces were grouped in blocks of 8 faces for the same emotion, with each emotion block repeated five times and presented in a pseudorandom order. Emotion expressions were presented in a backward-masked design to prevent conscious awareness (30). Specifically, each face was presented briefly (16.7 ms), followed immediately by a neutral face perceptual mask for 150 ms and an interstimulus interval of 1233.3 ms. Neutral masked face stimuli were offset slightly by 1° in random directions to control for the possible detection of emotions based on perceptual features as done previously (30). No specific behavioral responses were required during scanning due to the inclusion of subliminal presentations. Following our established procedure (30), we ensured continuous active attention to stimuli by monitoring eye gaze via the goggle system for stimulus presentation.
A hemodynamic response convolved boxcar function was used to model the blood oxygenation level-dependent (BOLD) response for each of the emotion blocks. A general linear model was specified for each participant. Contrasts were created in the first-level fixed-effect analysis for the contrast of each emotion vs. neutral. The left and right amygdala regions of interest (ROIs) were defined by an anatomical mask using the Automated Anatomical Labeling (AAL) atlas (54). The ROIs were therefore the same for all participants. Average parameter estimates were extracted from each ROI volume independently and entered into regression analyses.
Processing of Functional Imaging Data.
The fMRI data were preprocessed and analyzed using SPM8 software (www.fil.ion.ucl.ac.uk/spm). Motion correction was performed by realigning and unwarping the fMRI images to the first image of each task run. Following realignment and unwarping, quality control diagnostics were completed on the time-series data for each run. Data volumes that were associated with extreme (i) movement (framewise displacement from one time point to the next) and (ii) changes in BOLD signal intensity (as indexed by the mean squared difference in signal intensity over the entire volume from one time point to the next divided by the mean signal across the volume averaged across the full time series) were censored (temporally masked) to reduce the influence of motion and related artifacts. Framewise displacement was calculated as the sum of the absolute values of the differentiated realignment estimates as in Power et al. (55). Volumes were censored using established thresholds of framewise displacement greater than or equal to 0.3 mm and scaled signal intensity differences greater than 10 (55–57). Censoring was implemented with the time-series difference analysis (TSDiffAna) utility (imaging.mrc-cbu.cam.ac.uk/imaging/DataDiagnostics) and in-house scripts. A temporal mask was then created for each censored volume (as well as subsequent volume) and used as regressors of no interest in the first-level statistical models (55, 56). For normalization to stereotactic Montreal Neurological Institute space, the T1-weighted data were normalized to standard space using the FMRIB nonlinear registration tool, and the fMRI EPI data were coregistered to the T1 data using the FMRIB linear registration tool (58). Normalization warps from these two steps were stored for use in functional-to-standard space transformations. Global signal was estimated using an eroded mask within the ventricles and white matter, and was removed from the motion-corrected fMRI time series. fMRI data were smoothed using an 8-mm Gaussian kernel and high-pass–filtered using a cutoff period of 128 s.
Statistical Analyses.
To test hypothesis 1 and hypothesis 2, a hierarchical regression analysis examined relative improvements in predictive performance when including additive and interactive effects of early life stress and amygdala reactivity to happy or threat-related faces as follows:
Step 1: Clinical and demographic covariates (age, years of education, depression severity, and depressive episode duration).
Step 2: Main effects of ELS group.
Step 3: Main effects of amygdala reactivity to happy or threat-related faces (additive test).
Step 4: Two-way interaction between ELS and amygdala reactivity to happy or threat-related faces (interactive test).
To test hypothesis 3, an additional hierarchical regression analysis examined the relative improvements in predictive performance when including both amygdala reactivity to happy and threat-related face predictors within the same model as follows:
Step 1: Clinical and demographic covariates (age, years of education, depression severity, and depressive episode duration).
Step 2: Main effects of ELS group.
Step 3: Main effects of amygdala reactivity to happy together with main effects of threat-related faces (additive test).
Step 4: The two two-way interactions between ELS and amygdala reactivity to happy and fearful/threatening faces (interactive test).
Although this study might be considered a large patient sample for neuroimaging measures and represents a statistical advance in power from the few previous neuroimaging prediction studies on related topics, our sample size is still relatively small when considering ELS groups. It was with this concern in mind that ELS was treated as a linear interval scale variable (low, 0; mid, 1; high, 2) and not as a factor (dummy-coded) variable in all statistical models. By modeling ELS as a linear variable, the beta coefficient is quantified for a single interaction term for ELS by amygdala reactivity to happy/fearful faces. This beta coefficient thus would represent the estimated change in slope (in log odds) of amygdala reactivity predicting the likelihood of remission for a one-unit increase in ELS severity (i.e., from low to mid and from mid to high ELS). This approach is in contrast to the two interaction terms that would be modeled had we used a dummy-coded ELS variable. Therefore, our interpretation of the predicted likelihood of remission as a function of amygdala reactivity is based on a model that incorporates all points together, and not from a model that calculates different slopes between discrete subgroups of ELS exposure. Covariates (age, years of education, depression severity, and depressive episode duration) were entered as mean-centered continuous variables. To increase interpretability of the regression coefficients, amygdala activation for both fearful and happy faces was entered into the models mean-centered and normalized (dividing by the SD).
Effect Sizes.
The log-odds values (beta coefficients) and confidence intervals provide effect sizes for the contribution of individual predictors to all statistical models. These regression coefficients reflect the expected change in log odds of achieving functional remission with a one-unit increase in the predictor variable. The receiver operating characteristic (ROC) metrics [including area under the curve (AUC), sensitivity, and specificity] presented in Table 1 are provided as an effect size for the model as a whole.
Leave-One-Out Cross-Validation Technique.
To increase the generalizability of the model predictions, predictive performance of the ROC analyses was also examined using cross-validation. Specifically, leave-one-out cross-validation was used to estimate the generalizability of our classifier as well as derive an unbiased threshold that could be prospectively used to classify remitters versus nonremitters. In brief, the leave-one-out cross-validation approach was implemented as follows: For each step of a loop from 1 to n observations, a single observation was left out and a model was fit using the n − 1 observations. The model was then used to predict the value of the observation that was left out. The prediction values of each observation across all loop iterations were then used to derive a prevalidated ROC curve. Additionally, an unbiased threshold that maximized the sum of sensitivity and specificity was derived for each model across the n loops. The average of these values was used to create an unbiased threshold for classifying remitters versus nonremitters. Accuracy statistics (AUC, sensitivity, specificity, positive predictive values, and negative predictive values) are reported for the models derived from all available data as well as those that were derived from cross-validation.
Methods
Overview and Study Design.
Functional imaging data were obtained from 102 participants from the International Study to Predict Optimized Treatment in Depression (iSPOT-D). For a complete description of the randomized iSPOT-D practical trial protocol, clinical assessments, inclusion/exclusion criteria, and diagnostic procedures, see Williams et al. (41). In short, the primary diagnosis of nonpsychotic MDD was confirmed using the Mini-International Neuropsychiatric Interview (42), according to Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition (DSM-IV) criteria (43), and inclusion criteria included a score of ≥16 on the 17-item Hamilton Rating Scale for Depression (44) (HRSD17). Sample size and power were determined as part of the protocol development (45).
All participants were either antidepressant medication-naïve or underwent a washout period of at least 1 wk (five half-lives). Participants were randomly assigned using Phase Forward’s validated, web-based interactive response technology to receive escitalopram, sertraline, or extended-release venlafaxine.
This study was conducted according to the principles of the Declaration of Helsinki 2008. After the study procedures were fully explained in accordance with the ethical guidelines of the Western Sydney Area Health Service Human Research Ethics Committee, participants provided written informed consent.
Early Life Stress.
ELS was assessed using the 19-item Early Life Stress Questionnaire, which assesses exposure to abuse, neglect, family conflict, illness/death, and natural disasters before 18 y of age (46). Participants were split into low- (≤1 event), mid- (2–5 events), and high- (≥6 events) ELS groups. These groups did not differ in demographic factors (all P > 0.05; see Table S1 for ELS distribution details).
Criteria for Functional Remission.
Because childhood adversity produces depressive-anxious symptoms as well as stress-related adjustment problems, we defined functional remission by a combined measure of clinician-rated depression symptom severity using the HRSD17 (44), self-reported symptom severity using the 16-item Quick Inventory of Depressive Symptomatology–Self-Rated (QIDS-SR16) (47), and observer-rated functional capacity using the Social and Occupational Functioning Assessment Scale (SOFAS) (48) at week 8. Remitters were defined as being in the normative range of symptoms (≤7 on the HRSD17 and ≤5 on the QIDS-SR16) and with healthy adjustment (≥10-point improvement from baseline to achieve ≥61 on the SOFAS).
Functional MRI Emotion Paradigm.
Amygdala reactivity to happy and fearful faces was assessed relative to neutral comparison faces using an established masking paradigm (30). Stimuli were drawn from a standardized series of facial expressions (49) modified to be centrally positioned at eye level. Details of the MRI acquisition, preprocessing, and stimuli presentation have been published previously (30) and can be found in SI Methods.
Statistical Analyses.
Hierarchical logistic regression models and ROC analyses implemented in R (50) were used to test the predictive performance of models including ELS and amygdala reactivity measures for functional remission. (Here we define hierarchical regression as a series of successive logistic regression models, adding additional predictors with each step. This is not to be confused with hierarchical linear models, which are used in modeling nested data.) The Wald statistic was used to determine the significance of the contribution of each predictor. To account for multiple statistical tests, we report significance for our six tests at a 0.05/6 ≤ 0.008 Bonferroni-corrected level (three additive and three interactive tests).
To increase the generalizability of the model predictions and reduce the bias caused by model fitting (51), leave-one-out cross-validation was used to derive an unbiased threshold that could be prospectively used to classify remitters versus nonremitters as well as assess the generalizability of the ROC performance metrics. The specific steps of each analysis are detailed in SI Methods. ROC curves were drawn using the Epi package of R (52).
Acknowledgments
We thank Jon Kilner for editorial support. Claire Day was the global trial manager for International Study to Predict Optimized Treatment in Depression from 2009 to 2015. iSPOT-D was sponsored by Brain Resource Company Operations Pty Ltd. A.N.G.-P. and L.M.W. are supported by NIMH Grant R01MH101496, A.N.G.-P. is supported by Grant F32MH108299, and T.H. was partially supported by NIH Grant 5R01EB 001988-21. Clinical trial registration: International Study to Predict Optimized Treatment in Depression (iSPOT-D); registry name: ClinicalTrials.gov; www.clinicaltrials.gov/ct2/show/NCT00693849?term=iSPOT-D&rank=1; registration no. NCT00693849.
Footnotes
Conflict of interest statement: L.M.W. received research funding from Brain Resource Pty Ltd. as an investigator at International Study to Predict Optimized Treatment in Depression sites. A.F.S. has speaking fees from Merck, GSK, and Roche; consultant fees from BrainCells, CeNeRx, CNS Response, Eli Lilly, Forest Labs, Genetech, Gilead, GSK, Jazz, Lundbeck, Merck, Neuronetics, Novadel, Novartis, Pathway Diagnostics, Pfizer, PharmaNeuroBoost, Quintiles, Sanofi-Aventis, Sunovion, Synosia, Takeda, Xytis, Wyeth; equity in Amnestix, BrainCells, CeNeRx, Corcept (co-founder), Delpor, Forest, Merck, Neurocrine, Novadel, Pfizer, PharaNeuroBoost, Somaxon, Synosis, and Titan; and patents on glucocorticoid antagonists and antidepressant response. T.S. has received research funding, from 2015 to the present, from the National Institute of Mental Health, Sunovion Pharmaceuticals, Inc., Elan Pharma International Limited, VA Cooperative Studies Program, Pathway Genomics, and Stanley Medical Research Institute. She has received consultant speaker fees from A/S Lundbeck, Sunovion, and Merck & Co; continuing medical education honoraria from Medscape Education, Global Medical Education, and CMEology; and royalties from Jones and Bartlett and UptoDate. C.B.N. has consultant fees from Xhale, Takeda, Mitsubishi Tanabe Pharma Development America, Taisho Pharmaceutical Inc., Lundbeck, Prismic Pharmaceuticals, Bracket (Clintara), Total Pain Solutions (TPS), and Gerson Lehrman Group (GLG) Healthcare & Biomedical Council. He has equity in Xhale, Celgene, Seattle Genetics, Abbvie, Titan Pharmaceuticals, OPKO Health, Inc., and Bracket Intermediate Holding Corp; a Scientific Advisory Board/Board of Directors fee from Skyland Trail, Bracket (Clintara), and RiverMend Health LLC; and patents for Methods and devices for transdermal delivery of lithium (US 6,375,990 BI) and Method to estimate antidepressant drug therapy by ex vivo assay (US 7,148,027B2). L.M.W. has consult fees from Humana and Advisor Board Fees from Psyberguide.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606671113/-/DCSupplemental.
References
- 1.Heller AS. Cortical-subcortical interactions in depression: From animal models to human psychopathology. Front Syst Neurosci. 2016;10:20. doi: 10.3389/fnsys.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol Psychiatry. 1999;46(11):1509–1522. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- 3.Hammen C, Henry R, Daley SE. Depression and sensitization to stressors among young women as a function of childhood adversity. J Consult Clin Psychol. 2000;68(5):782–787. [PubMed] [Google Scholar]
- 4.Westfall NC, Nemeroff CB. The preeminence of early life trauma as a risk factor for worsened long-term health outcomes in women. Curr Psychiatry Rep. 2015;17(11):90. doi: 10.1007/s11920-015-0625-6. [DOI] [PubMed] [Google Scholar]
- 5.Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am J Psychiatry. 2012;169(2):141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- 6.Penza KM, Heim C, Nemeroff CB. Neurobiological effects of childhood abuse: Implications for the pathophysiology of depression and anxiety. Arch Women Ment Health. 2003;6(1):15–22. doi: 10.1007/s00737-002-0159-x. [DOI] [PubMed] [Google Scholar]
- 7.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Chattarji S, Tomar A, Suvrathan A, Ghosh S, Rahman MM. Neighborhood matters: Divergent patterns of stress-induced plasticity across the brain. Nat Neurosci. 2015;18(10):1364–1375. doi: 10.1038/nn.4115. [DOI] [PubMed] [Google Scholar]
- 10.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52(7):671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 11.Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety—Insights from human genetic studies. Mol Psychiatry. 2010;15(6):574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castro JE, et al. Role of the amygdala in antidepressant effects on hippocampal cell proliferation and survival and on depression-like behavior in the rat. PLoS One. 2010;5(1):e8618. doi: 10.1371/journal.pone.0008618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y. Neuropsychological mechanism underlying antidepressant effect: A systematic meta-analysis. Mol Psychiatry. 2015;20(3):311–319. doi: 10.1038/mp.2014.24. [DOI] [PubMed] [Google Scholar]
- 14.Williams LM, DeBattista C, Duchemin A-M, Schatzberg AF, Nemeroff CB. Childhood trauma predicts antidepressant response in adults with major depression: Data from the randomized international study to predict optimized treatment for depression. Transl Psychiatry. 2016;6:e799. doi: 10.1038/tp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattson WI, Hyde LW, Shaw DS, Forbes EE, Monk CS. Clinical neuroprediction: Amygdala reactivity predicts depressive symptoms 2 years later. Soc Cogn Affect Neurosci. 2016;11(6):892–898. doi: 10.1093/scan/nsw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peluso MA, et al. Amygdala hyperactivation in untreated depressed individuals. Psychiatry Res. 2009;173(2):158–161. doi: 10.1016/j.pscychresns.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang TT, et al. Adolescents with major depression demonstrate increased amygdala activation. J Am Acad Child Adolesc Psychiatry. 2010;49(1):42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheline YI, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 19.Dannlowski U, et al. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Suslow T, et al. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry. 2010;67(2):155–160. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Mol Psychiatry. 2008;13(11):993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tye KM, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471(7338):358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belzung C, Willner P, Philippot P. Depression: From psychopathology to pathophysiology. Curr Opin Neurobiol. 2015;30:24–30. doi: 10.1016/j.conb.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Czéh B, Fuchs E, Wiborg O, Simon M. Animal models of major depression and their clinical implications. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:293–310. doi: 10.1016/j.pnpbp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt MV, Wang XD, Meijer OC. Early life stress paradigms in rodents: Potential animal models of depression? Psychopharmacology (Berl) 2011;214(1):131–140. doi: 10.1007/s00213-010-2096-0. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki H, et al. Early life stress and trauma and enhanced limbic activation to emotionally valenced faces in depressed and healthy children. J Am Acad Child Adolesc Psychiatry. 2014;53(7):800–813.e10. doi: 10.1016/j.jaac.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lupien SJ, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci USA. 2011;108(34):14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blugeot A, et al. Vulnerability to depression: From brain neuroplasticity to identification of biomarkers. J Neurosci. 2011;31(36):12889–12899. doi: 10.1523/JNEUROSCI.1309-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swartz JR, Knodt AR, Radtke SR, Hariri AR. A neural biomarker of psychological vulnerability to future life stress. Neuron. 2015;85(3):505–511. doi: 10.1016/j.neuron.2014.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams LM, et al. Amygdala reactivity to emotional faces in the prediction of general and medication-specific responses to antidepressant treatment in the randomized iSPOT-D trial. Neuropsychopharmacology. 2015;40(10):2398–2408. doi: 10.1038/npp.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shelton RC. Steps following attainment of remission: Discontinuation of antidepressant therapy. Prim Care Companion J Clin Psychiatry. 2001;3(4):168–174. doi: 10.4088/pcc.v03n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saveanu R, et al. The international Study to Predict Optimized Treatment in Depression (iSPOT-D): Outcomes from the acute phase of antidepressant treatment. J Psychiatr Res. 2015;61:1–12. doi: 10.1016/j.jpsychires.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 33.Feldman G, Harley R, Kerrigan M, Jacobo M, Fava M. Change in emotional processing during a dialectical behavior therapy-based skills group for major depressive disorder. Behav Res Ther. 2009;47(4):316–321. doi: 10.1016/j.brat.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Davidson RJ, McEwen BS. Social influences on neuroplasticity: Stress and interventions to promote well-being. Nat Neurosci. 2012;15(5):689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibb BE, Schofield CA, Coles ME. Reported history of childhood abuse and young adults’ information-processing biases for facial displays of emotion. Child Maltreat. 2009;14(2):148–156. doi: 10.1177/1077559508326358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCrory EJ, Viding E. The theory of latent vulnerability: Reconceptualizing the link between childhood maltreatment and psychiatric disorder. Dev Psychopathol. 2015;27(2):493–505. doi: 10.1017/S0954579415000115. [DOI] [PubMed] [Google Scholar]
- 37.McEwen BS, et al. Mechanisms of stress in the brain. Nat Neurosci. 2015;18(10):1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCrory EJ, et al. Amygdala activation in maltreated children during pre-attentive emotional processing. Br J Psychiatry. 2013;202(4):269–276. doi: 10.1192/bjp.bp.112.116624. [DOI] [PubMed] [Google Scholar]
- 39.Dannlowski U, et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum Brain Mapp. 2013;34(11):2899–2909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halfon N, Larson K, Lu M, Tullis E, Russ S. Lifecourse health development: Past, present and future. Matern Child Health J. 2014;18(2):344–365. doi: 10.1007/s10995-013-1346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams LM, et al. International Study to Predict Optimized Treatment for Depression (iSPOT-D), a randomized clinical trial: Rationale and protocol. Trials. 2011;12:4. doi: 10.1186/1745-6215-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 43. American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association Publishing, Arlington, VA), 4th Ed.
- 44.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grieve SM, et al. Brain imaging predictors and the international study to predict optimized treatment for depression: Study protocol for a randomized controlled trial. Trials. 2013;14:224. doi: 10.1186/1745-6215-14-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McFarlane A, et al. The impact of early life stress on psychophysiological, personality and behavioral measures in 740 non-clinical subjects. J Integr Neurosci. 2005;4(1):27–40. doi: 10.1142/s0219635205000689. [DOI] [PubMed] [Google Scholar]
- 47.Rush AJ, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 48.Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: A review of measures of social functioning. Am J Psychiatry. 1992;149(9):1148–1156. doi: 10.1176/ajp.149.9.1148. [DOI] [PubMed] [Google Scholar]
- 49.Gur RC, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115(2):137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 50. R Development Core Team (2008) R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna)
- 51.Tibshirani RJ, Efron B. Pre-validation and inference in microarrays. Stat Appl Genet Mol Biol. 2002;1(1):Article 1. doi: 10.2202/1544-6115.1000. [DOI] [PubMed] [Google Scholar]
- 52.Carstensen B, Plummer M, Laara E, Hills M. 2015. Epi: A Package for Statistical Analysis in Epidemiology. R package version 1.1.71. Available at https://cran.r-project.org/web/packages/Epi/index.html. Accessed September 14, 2015.
- 53.Korgaonkar MS, Grieve SM, Etkin A, Koslow SH, Williams LM. Using standardized fMRI protocols to identify patterns of prefrontal circuit dysregulation that are common and specific to cognitive and emotional tasks in major depressive disorder: First wave results from the iSPOT-D study. Neuropsychopharmacology. 2013;38(5):863–871. doi: 10.1038/npp.2012.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 55.Power JD, et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siegel JS, et al. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum Brain Mapp. 2014;35(5):1981–1996. doi: 10.1002/hbm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andersson JLR, Jenkinson M, Smith S. 2007. Non-Linear Optimisation (FMRIB Centre, Oxford), Tech Rep TR07JA1.