Significance
The long-term cooling trend of the Cenozoic is punctuated by shorter-term climatic events, such as the inception of permanent ice sheets on Antarctica at the Eocene−Oligocene Transition (∼33.7 Ma). Taking advantage of the excellent state of preservation of coccolith calcite in equatorial Atlantic deep-sea cores, we unveil progressive tropical warming in the Atlantic Ocean initiated 4 million years prior to Antarctic glaciation. Warming preceding glaciation may appear counterintuitive, but we argue that this long-term climatic precursor to the EOT reinforced cooling of austral high latitudes via the redistribution of heat at the surface of the oceans. We discuss this new prominent paleoceanographic and climatic feature in the context of overarching pCO2 decline and the establishment of an Antarctic circumpolar current.
Keywords: Eocene−Oligocene climate transition, coccolith-based proxies, Atlantic equatorial SSTs, Cenozoic pCO2, meridional temperature gradient
Abstract
Growth of the first permanent Antarctic ice sheets at the Eocene−Oligocene Transition (EOT), ∼33.7 million years ago, indicates a major climate shift within long-term Cenozoic cooling. The driving mechanisms that set the stage for this glaciation event are not well constrained, however, owing to large uncertainties in temperature reconstructions during the Eocene, especially at lower latitudes. To address this deficiency, we used recent developments in coccolith biogeochemistry to reconstruct equatorial Atlantic sea surface temperature (SST) and atmospheric pCO2 values from pelagic sequences preceding and spanning the EOT. We found significantly more variability in equatorial SSTs than previously reported, with pronounced cooling from the Early to Middle Eocene and subsequent warming during the Late Eocene. Thus, we show that the Antarctic glaciation at the Eocene−Oligocene boundary was preceded by a period of heat accumulation in the low latitudes, likely focused in a progressively contracting South Atlantic gyre, which contributed to cooling high-latitude austral regions. This prominent redistribution of heat corresponds to the emplacement of a strong meridional temperature gradient that typifies icehouse climate conditions. Our equatorial coccolith-derived geochemical record thus highlights an important period of global climatic and oceanic upheaval, which began 4 million years before the EOT and, superimposed on a long-term pCO2 decline, drove the Earth system toward a glacial tipping point in the Cenozoic.
The warm, greenhouse Eocene Epoch exhibits climatic patterns that remain difficult to constrain, in part because of unreliable low-latitude sea surface temperature (SST) reconstructions (1). So far, only a handful of sites serve as a reference for climate history at low latitudes during the Eocene and the Eocene−Oligocene Transition (EOT): the nearshore Tanzania in the Indian Ocean [Tanzania Drilling Project (TDP)] (1, 2) and open ocean sites in the equatorial Atlantic [Ocean Drilling Program sites ODP 925 and 929] (3–5). These records exhibit rather conflicting proxy data for the TDP and overall long-term cooling for the Atlantic sites 925 and 929. There is, however, no clear and significant cooling trend from the Early to Middle Eocene that indicates the termination of the Early Eocene Climatic Optimum (Fig. 1A). Furthermore, recent quality-checked SSTs for ODP 925 and 929 sites using the Ring Index indicate relatively similar temperatures between the Late Eocene and the Early Oligocene (5). In contrast, high-latitude sites are consistent in demonstrating appreciable cooling of austral subpolar regions during much of the Eocene (3, 4, 6–8). This apparent lack of substantial temperature change in the intertropical belt during the Eocene and across the EOT remains an enigmatic feature that complicates our understanding of the interplay between seawater temperatures at a global scale, pCO2, and changes in global ocean circulations in the context of new Southern Ocean gateways (9–14). The paucity of low-latitude data, especially in the Pacific Ocean, prevents the reconstruction of the latitudinal temperature gradient, much needed for the successful modeling of processes that drove the greenhouse to icehouse transition (10, 15, 16).
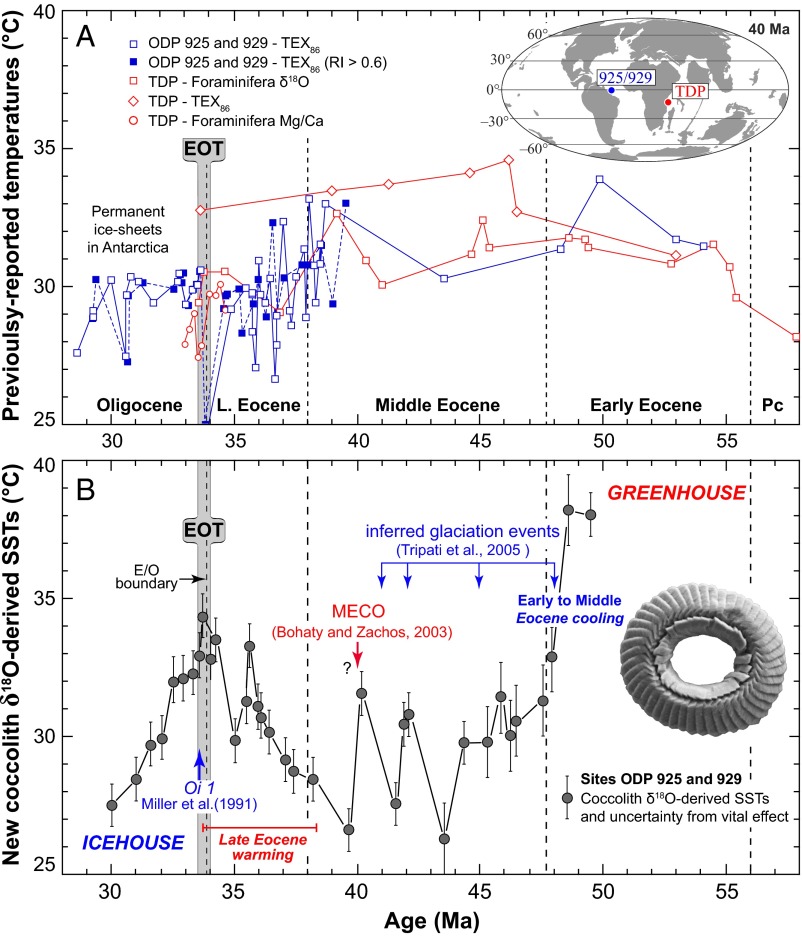
Fig. 1.
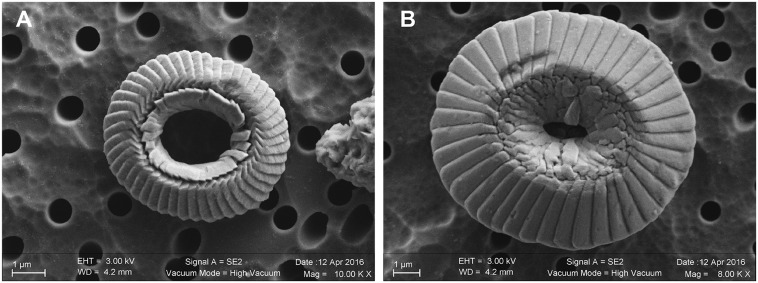
Reconstructed equatorial SSTs during the Early Eocene to the Early Oligocene. (A) Multiproxy temperature estimates from the TDP (in red) indicate a warm and relatively stable Eocene climate (2). The blue curve denotes published TEX86-derived SSTs (3, 4) in the equatorial Atlantic showing relatively similar temperatures in the Eocene and the Early Oligocene. Note that alkenone temperatures (UK’37) from ref. 3 are not presented, as they exceeded the upper limit of the proxy (i.e., >29 °C). For data source ODP 925 and 929, open symbols denote refs. 3 and 4, and filled symbols denote samples with a Ring Index > 0.6 from ref. 5; TDP data are from refs. 2 and 35. (Inset) The location map is modified from ref. 29. (B) Coccolith δ18O-derived temperatures from sites ODP 925 and 929 applying Eq. 1. The raw isotope data are shown in Fig. S4A. A correction of the vital effect of 0.69‰ (SD = 0.11‰) was applied to coccoliths gathered into 3–5-µm fractions (see corresponding biogeochemical discussion in SI Materials and Methods). Disregarding the problem of the vital effect would translate the temperature curve by a constant −3.7 °C offset (see Fig. S4D for SSTs derived from the 5–8-µm fractions). Early to Middle Eocene cooling highlights the termination of the greenhouse period. After climate ups and downs in the Middle Eocene (30–32), including the Middle Eocene Climatic Optimum (MECO), a Late Eocene 6 °C warming predates the pronounced cooling seen coeval with the onset of glaciation during the Early Oligocene. (Inset) The SEM image shows a reticulofenestrid specimen from a sample at 42.1 Ma (see Fig. S5A for an edited version and the scale bar).
As an alternative to the relatively poor preservation of the foraminifera and issues with the calibration of organic-based proxies (1, 5, 17), this study exploits the coccoliths as a source of paleoclimatic information. Coccolithophores, single-celled phytoplankton algae, are a key component of the carbon cycle and the ocean biological pump, and their calcite biominerals, the coccoliths, are a major component of pelagic sediments. Coccoliths are produced in the mixed layer and, as such, are ideally suited to record the chemistry and temperature of ocean surface waters. Their excellent preservation, even in warm and high pCO2 settings (18), may overcome methodological caveats associated with the absence or frequent recrystallization of foraminiferal shells (1, 2, 17). Recent culture and sedimentary studies have produced new coccolith-based proxies that make it possible to exploit this underexplored paleoclimatic archive (SI Materials and Methods) (19–21). In the present study, we used the established temperature dependence of oxygen isotope ratios of coccolith calcite gathered in size-restricted microfractions to calculate SSTs (Eq. 1; see SI Materials and Methods), accounting for local seawater δ18O values (6, 22) (Figs. S1 and S2) and the vital effect (19, 20) (Fig. S3). In sequences postdating the Late Miocene, large isotope vital effects have been reported in coccoliths (21). This was attributed to the consequence of relatively low pCO2 concentrations, as DIC/CO2 aq limitation is thought to be the main cause of the vital effects in these biominerals (20, 21, 23–27). In contrast, studies of laboratory cultures and downcore sediments indicate that the vital effect in coccoliths (regardless of taxonomy) was limited and invariant when pCO2 concentrations in the atmosphere were above ∼500 ppm, as was the case during the time period investigated (28, 29) (see SI Materials and Methods for further explanations on the relation between the environment and the expression of the vital effects in coccolith calcite). Beyond SST reconstruction using the oxygen isotope composition of the coccoliths, the carbon isotope offsets between coccoliths of distinct sizes, concentrated in the 5–8-µm and 3–5-µm microfractions (Δδ13Clarge-small) can be used to derive new pCO2 estimates that may complement existing alkenone-based data (Eq. 2; see Materials and Methods and SI Materials and Methods for details on the biogeochemical calibration for pCO2 reconstruction).
Fig. S1.
Seawater oxygen isotope composition used to calculate temperature estimates in the present study. Reconstructed δ18Osw values are from refs. 2 and 6. EOGM, Eocene−Oligocene Glacial Maximum.
Fig. S2.
A posteriori comparison between the paleotemperature estimates obtained in the present study and δ18Osw values used. (A) SST evolution from reticulofenestrid δ18O, as shown in Fig. 1B. (B) Increments in δ18Osw steps between two consecutive samples converted into temperature equivalents.
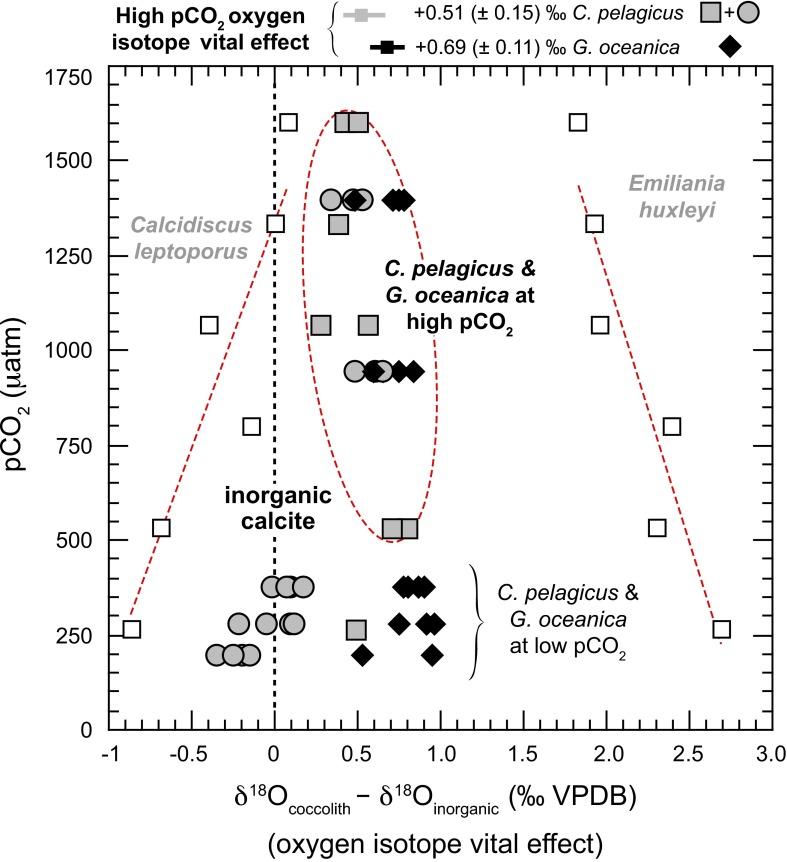
Fig. S3.
Evolution of oxygen isotope vital effect (coccolith δ18O offset from inorganic values) with increasing pCO2 for C. pelagicus (gray symbols) and G. oceanica (black diamonds) in culture. Data source is ref. 19 (black diamonds and gray circles) and ref. 20 (all other datapoints). When grown at high pCO2, the two species of interest precipitate calcite isotopically heavier than the inorganic reference (54) (vertical dashed line). Calcidiscus leptoporus and Emiliania huxleyi isotopic behaviors are indicated, for reference only, with open squares. The datapoints for G. oceanica and C. pelagicus used to calculate the magnitude of the vital effect above a 500-ppm pCO2 threshold are circled in red.
SI Materials and Methods
Constraints on Local Seawater Oxygen Isotope Ratios over the Studied Interval.
Generating SST estimates from the oxygen isotope composition of coccolith calcite requires knowledge of the oxygen isotope ratio of seawater (δ18Osw). The reconstruction of δ18Osw values in deep time is not straightforward and relies on a number of assumptions about the oxygen isotope budget of the global ocean (primarily linked to ice volume) and the effect of the hydrographic regime of the considered area, here the western equatorial Atlantic Ocean. In this study, we use published data (6, 22, 56) to determine, independently of any interpretation relying on our final SST estimates, the absolute values of δ18Osw in the equatorial Atlantic surface waters where the coccoliths have calcified. As it is essential to ensure our reconstructed SST do not reflect the imposed variations in δ18Osw, we will assess the effect of this parameter in the paleotemperature curve over the Early Eocene to Early Oligocene interval.
Over the Meso-Cenozoic, the isotopic ratio of the world ocean (δ18Osw “global”) has primarily evolved as a function of ice volume, and thus has fluctuated between ice-free values of −1.0‰ VSMOW (56) and values closer to zero during periods with more or less developed continental ice sheets, leaving relatively 16O-depleted oceanic waters [0‰ VSMOW in present days (56)]. During the Paleogene, δ18Osw values fluctuated between −1‰ and −0.3‰ (6). Our study interval comprises periods characterized by ice-free oceans in the Early Eocene, by small and/or transient ice caps in the Middle and Late Eocene, and by permanent glaciation during and after the EOT (6, 12, 30) (Fig. S1). Proxies for assessing global ocean δ18Osw include paired Mg/Ca and δ18O measurements from benthic foraminifera from high southern latitude sites (6), which are driven by deep waters formed from the downwelling of southern ocean surface waters (11). Mg/Ca ratios are used to independently assess temperature of calcification of foraminiferal tests and isolate the respective effect of temperature and δ18Osw variations from foraminiferal δ18O composition. Global ocean δ18Osw evolved from ice-free values (−1.0‰ VSMOW) before the Middle Eocene toward near-zero isotope ratios, thus describing an overarching positive trend during the study interval (Fig. S1). In the Middle and Late Eocene, minor and only fleeting excursions of about +0.3‰ to +0.4‰ are recorded, corresponding to ephemeral ice sheets, which are also inferred from sedimentological evidence (refs. 30 and 57 and references therein). The EOT is marked by a sharp ∼1‰ increase (“Oi 1,” ref. 41), followed by a decrease of 0.4‰ during the earliest Oligocene (from 32.9 Ma to 31 Ma), an evolution that matches the established onset of permanent ice cap on Antarctica and its subsequent partial melting in the earliest Oligocene, respectively (12).
In the present study, SSTs are calculated by using local surface water δ18Osw in the equatorial Atlantic. Therefore, the global ocean value of ref. 6 needs to be corrected by the offset of equatorial Atlantic values from global ocean values. This offset, primarily controlled by the regional evaporation to precipitation ratio, has been quantified for the Early Eocene (22). According to this modeling experiment, surface waters at the equatorial Atlantic are enriched in heavy oxygen isotopes (+1‰ ± 0.2‰) due to more evaporated waters compared with the global ocean, as is still the case in present-day settings (58). Therefore, an equatorial Atlantic δ18Osw value of 0‰ (±0.2‰) is suitable for the Early Eocene. For youngest intervals in our sites, we derived local δ18Osw estimates by taking the global ocean δ18Osw curve of ref. 6 offset by the aforementioned coefficient (22) (Fig. S1).
To date, the only studies that have used tropical δ18O values to derive Paleogene SSTs are from the Tanzanian sites (TDP) (1, 2, 59), but they predate the insightful modeling work by ref. 22 that allowed refinement in δ18Osw estimates. The Early Eocene local value of −0.75‰ used by ref. 2 for the TDP corresponds to an ice-free ocean (−1‰, according to ref. 6) and an offset from global ocean of +0.25‰, which agrees with ref. 22. For the Middle and Late Eocene, global ocean δ18Osw values fluctuated between −1‰ and −0.6‰. By applying the same offset for our studied location, local values are thus comprised between −0.75‰ and −0.35‰. It is worth noting that the figure of −0.5‰ used by ref. 2 for the Middle and Late Eocene is consistent with the mean value for this interval. Consequently, the values used in the present study and in ref. 2 are in line with recent evaluations of refs. 6 and 22. The differences between previously reported SSTs inferred from TDP foraminiferal δ18O and the new estimates from coccolith δ18O at sites ODP 925 and 929 cannot therefore arise from distinct treatment or uncertainties in the δ18Osw used to calculate temperature estimates.
Coccolith δ18O compositions used to calculate SSTs are concurrently dictated by the temperature of calcification, the signal sought in our approach, and δ18Osw values of the mineralizing fluid (see Coccolith Oxygen Isotopes and SST Reconstructions for the vital effect problem). To verify that the weight of δ18Osw fluctuations in our temperature curve is only minor, we deconvolved the relative effect of temperature and δ18Osw by using an incremental approach between consecutive samples. Overall, there is no correlation between ΔSST and Δδ18Osw increments (r2 = 0.018 and P value = 0.48). The effect of δ18Osw change on temperature increments can be obtained via the slope of equation tying temperature and oxygen isotope ratios (−0.2‰/°C after ref. 60) and enables us to express δ18Osw variations in a temperature framework (which indicate a conservative temperature effect of 1.4 °C). Therefore, we can confidently assess that there is no influence of the δ18Osw values on our final SST estimates (Fig. S2). More specifically, the magnitude of temperature changes across the Early to Middle Eocene (cooling) and late Middle Eocene to the EOT (warming) cannot be the result of the imposed δ18Osw. For the latter event, which is the key finding of the present study, it further appears that the applied δ18Osw change would, in fact, underevaluate the +6 °C warming.
Background Information on Coccolith Biogeochemistry and the Vital Effects.
Following on from the pioneering work by Dudley et al. (61), 30 years of research on the biogeochemistry of the coccolithophores have led to a better understanding of the mechanisms responsible for the isotopic departure of coccoliths from inorganically precipitated calcite, the so-called “vital effect.” A series of cultures of these phytoplanktonic calcifiers in the laboratory have provided empirical calibrations and a mechanistic understanding of the vital effect that allow circumventing this classical problem in paleoceanography. Furthermore, these studies have progressively highlighted the potential of these biominerals to decipher paleoenvironments in complement to the foraminiferal archive (19–21, 23, 27, 62–66). Examining geologically relevant coccolithophore species exposed to a wide range of ambient DIC concentrations/pCO2, a relaxation of the expression of the vital effects, imprinting both carbon and oxygen isotope systems, has become apparent (19, 20, 23). Diminished vital effects are thought to be the result of alleviated carbon limitation that arguably exists in the aforementioned cultures implemented at present-day and under post-Miocene pCO2 values (20, 21, 23, 25, 27, 65, 67–69). The concept of environmentally driven vital effects (68) is able to reconcile culture and natural environment data and, as such, provides valuable insights in paleoceanography.
Using culture data on extant species, we formalize here a calibration between coccolith calcite δ18O values and temperature, on one hand, and δ13C and DIC/pCO2 levels, on the other hand. Overall, it appears that the coccolith proxies rely on the distinct size of coccolith, and therefore that of the cell, rather than on true interspecies differences. Based on these morphological (size) grounds, the species that serve as analogs of ancient coccoliths encountered in sites ODP 925 and 929 sediments are Gephyrocapsa oceanica, representing the descendant of Reticulofenestra coccoliths within the Noelaerhabdaceae lineage, and C. pelagicus, representing the Coccolithus group. The sizes of these modern forms of coccoliths indeed fall within a 3–5-µm and 5–8-µm range, respectively (14, 70). We must note that G. oceanica has usually slightly smaller cell than Reticulofenestra and, as such, may have a lower carbon demand than its ancestors. This difference in cell volume to surface-area ratio would require size normalization of the vital effect. However, it appears that reticulofenestrid coccoliths retrieved from equatorial sites exhibit very similar sizes to cultured G. oceanica, with only small variations (14); therefore, such a correction was not applied in the present study.
Coccolith Oxygen Isotopes and SST Reconstructions.
Biogeochemical calibration.
Most modern coccolith species precipitate calcite away from equilibrium conditions in the oxygen isotope system, as a result of carbon limitation (54, 71, 72). The range of interspecific coccolith δ18O has been found to be considerable in culture (on the order of 4‰). The Paleogene, however, temporally predates the “Late Miocene threshold” (21) and elevated pCO2 levels induced much more limited oxygen vital effects (Fig. S3). Overall, the culture datasets (19, 20) indicate that a coccolith-integrated coefficient of the vital effect between 0.4‰ and 0.9‰ would need to be applied in Eq. 1 (see Materials and Methods) to transform coccolith δ18O values into SST estimates under a high pCO2 regime. We can calculate a residual oxygen isotope vital effect at pCO2 higher than 500 ppm for each species. For Coccolithus coccoliths, it is 0.51‰ ± 0.15‰, and, for Reticulofenestra coccoliths, it is 0.69‰ ± 0.11‰. These SDs would indicate an uncertainty in coccolith δ18O-derived SST estimates less than 0.8 °C for both species (see below).
Another possible uncertainty in our paleo-vital effect calibration pertains to seawater pH. Indeed, the proposed pH values for the Paleogene ocean are lower than today’s values, as a consequence of higher pCO2 levels (34, 73). The two coccolithophore species serving here as reference have been grown over a wide range of medium pH, and their δ18O analyzed (23). There was no apparent effect on C. pelagicus, which consistently exhibits δ18O values close to inorganic calcite reference, but, for G. oceanica, in contrast, δ18O values decreased by a factor of ∼0.5‰ from pH 8.2 to 7.4. Bearing in mind that these culture data were obtained at the present-day pCO2 level, it can arguably be conceived that decreased oxygen isotope vital effect in the laboratory is the consequence of elevated CO2 aq at low pH (23). Therefore, we did not apply any pH correction on the stable isotope compositions of Paleogene Coccolithus and Reticulofenestra coccoliths.
Sedimentary data also confirm diminished expression of the oxygen isotope vital effect in coccolith calcite compared with present-day (relatively low pCO2) settings, as the range of Δ18Oforaminifera−coccolith offset was only minor during the Paleogene (53, 74, 75). A downcore Pleistocene record (therefore postdating the Late Miocene) provides a paleo-vital effect framework for gephyrocapsid coccoliths and confirms the pCO2 dependence of the oxygen isotope vital effect (25). By extrapolating the oxygen isotope vital effect above a pCO2 value of 500 ppm, it would appear that this isotopic phenomenon is indeed vanished. This field validation of laboratory findings is an important aspect lending support to the robustness of the oxygen isotope paleo-vital effects applied in the present study (19, 20) and, overall, validates the fact that disregarding the vital effect in the numerous attempts to reconstruct SST from bulk or sediment fine fraction δ18O in the Jurassic, Cretaceous, and Paleogene worlds seems to be justified.
Error on SST figures.
Each variable parameter used in Eq. 1 needs to be associated with a specific error, and a cumulative and propagated uncertainty in our SST estimates ought to be calculated (Fig. S4). For coccolith calcite δ18O measurement, the analytical error is 0.1‰ VPDB. For seawater δ18O, it is 0.2‰ VSMOW (see Constraints on Local Seawater Oxygen Isotope Ratios over the Studied Interval). The SD of the oxygen isotope vital effect (18O VE) is 0.11‰ for Reticulofenestra. The error associated with the vital effect free δ18O (i.e., the “δ18Ococcolith – 18O VE” term in Eq. 1) is , hence 0.15. When the extremes of each individual variable are propagated through Eq. 1, it then appears that the conservative uncertainty in SST reconstruction is on the order of 1.6 °C (the error bars reported in Fig. 1B).
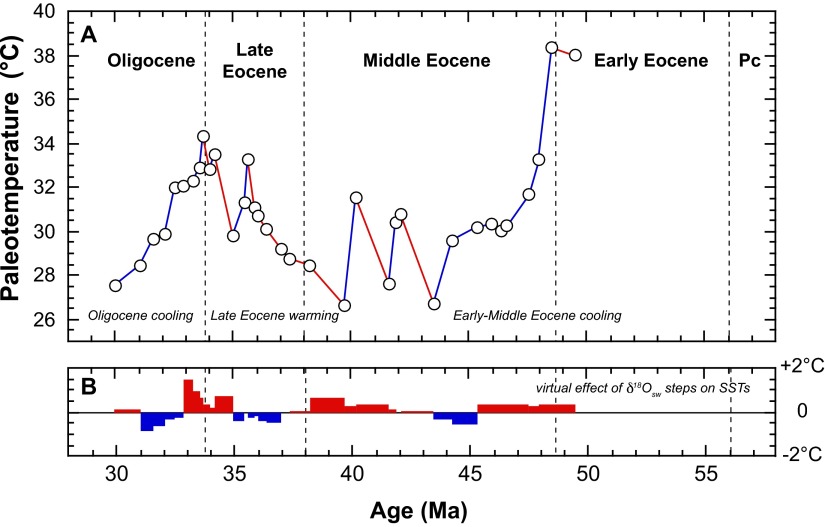
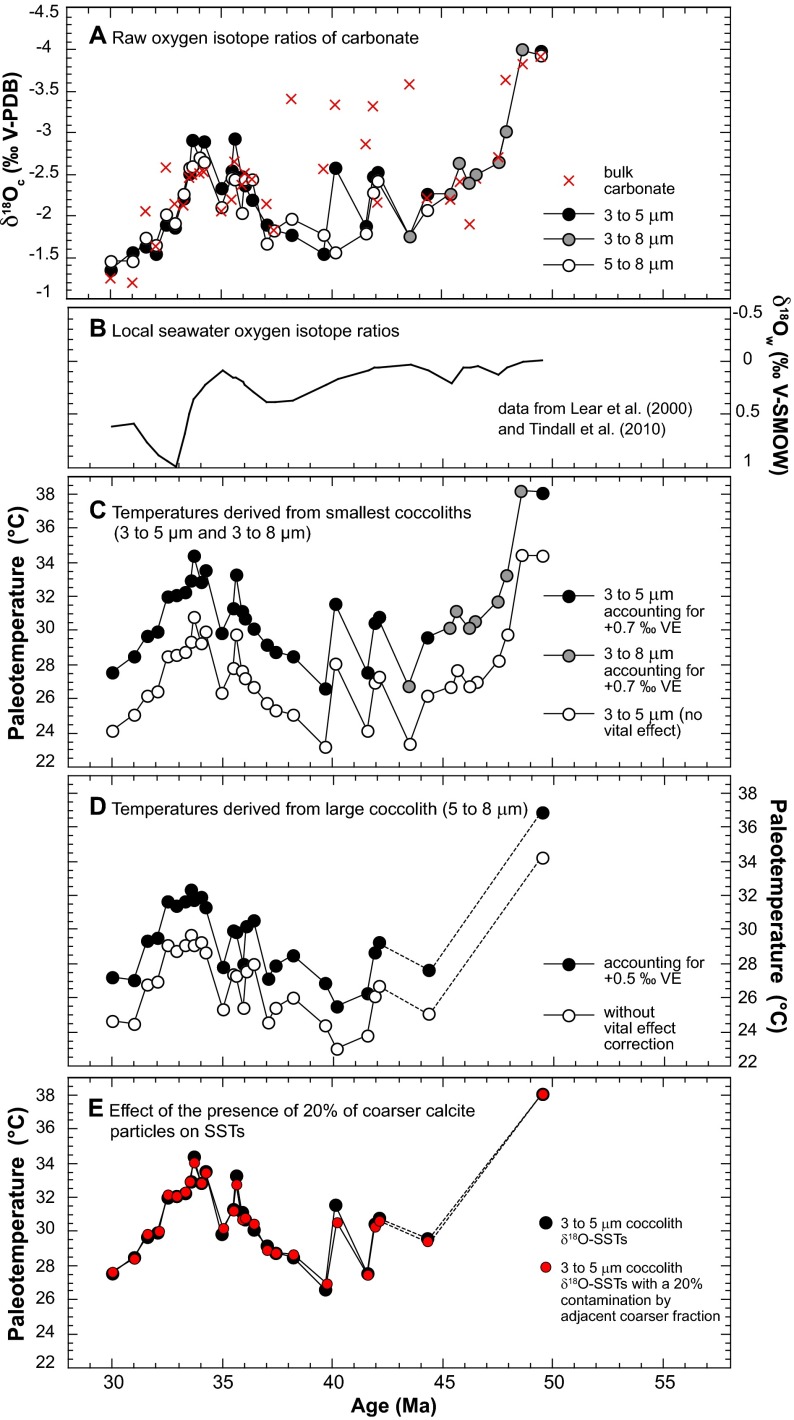
Fig. S4.
(A) Raw bulk and coccolith fraction δ18O values, (B) applied δ18Osw values (6, 22), and (C and D) comparison in the reconstructed SST estimates with correction of the vital effect for (C) small and (D) large coccoliths. (E) The negligible effect of the presence of 20% of coarser calcite particles (from the adjacent 5–8-µm fraction) on our SST reconstruction mainly based on 3–5-µm microfractions (Fig. 1B).
The SST estimates presented in this study derive from well-preserved (reticulofenestrid) coccoliths (Figs. S5−S7), and, especially, they are barren of calcite overgrowth, implying that they convey a pristine δ18O record. Some coarser particles, however, may pervade into the 3–5-µm assemblages due to imperfections in the screen membranes used (see details in ref. 53). In all of the 3–5-µm fractions presented in the present study, the calcite mass of the fraction is composed by, at least, 90 wt% of coccolith calcite (Figs. S6 and S7). Assuming a conservative “contamination” of the 5–8-µm fractions, the δ18O of which is known, we can, by a simple mass balance equation, assess the effect of this purity on our final SSTs. Overall, this effect is only minor, as one may expect on the basis of similar δ18O values observed between the 3–5-µm and 5–8-µm fractions and relative similar vital effects applied to Gephyrocapsa and Coccolithus coccoliths (Fig. S4E).
Fig. S5.
Close-up of coccolith specimens showing the excellent preservation state of the coccoliths: (A) Reticulofenestra sp. sample at 42.1 Ma and (B) C. pelagicus sample at 30.0 Ma. Slight etching of the coccoliths may be the result of the microseparation technique, but the absence of notable calcite overgrowth on the coccoliths is evident. Scale bars are inset.
Fig. S7.
SEM micrograph showing the high abundance of relatively small coccoliths in the 3–5-µm microfractions (example from the Oligocene sample at 30.0 Ma). Scale bar is inset.
Fig. S6.
Cross-polarized micrographs of 3–5-µm microseparated fractions distributed throughout the studied interval and showing a high relative abundance (>80%) of small coccoliths. (A) Oligocene sample (31 Ma). (B) Late Eocene sample (34.2 Ma). (C) Middle Eocene sample (41.9 Ma). Scale bars are inset.
Formalizing a CO2 Proxy Based on Coccolith Carbon Isotopes.
Interspecies Δ13C offset and ambient CO2 (DIC) levels.
The pCO2 proxy presented here (Eq. 2) is based on a compilation of data from two distinct laboratory culture studies performed on different strains of G. oceanica and C. pelagicus (19, 20) (Fig. S8A). These studies have examined the evolution of coccolith δ13C under a wide range of pCO2/DIC levels compatible with Paleogene and Neogene values. Interspecific δ13C composition of coccoliths is primarily the result of differences in the volume to surface-area ratios (themselves scaling cell size) in coccolithophores. These parameters (among others) control carbon limitation and ultimately dictate the magnitude of the vital effect (20, 21). We note that the size of the cultured G. oceanica coccoliths and that of sedimentary specimens gathered into size fractions at site ODP 925 are similar (14); the same observation applies for Coccolithus, thus providing a suitable culture-based calibration for our study case.
Fig. S8.
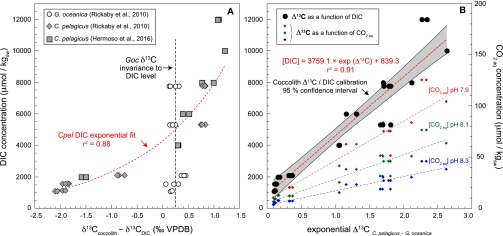
Culture-derived relationship between coccolith carbon isotopes and ambient DIC levels. (A) Offset between the coccolith carbon isotope composition and that of DIC in the culture medium. (Inset) Data are from refs. 19 and 20. (B) Expression of the interspecies (intersize) correlation using an exponential fit between Δ13C (δ13CCpel – δ13CGoc) and DIC concentrations in culture medium (left vertical axis) and, for reference, ambient aqueous CO2 (right vertical axis).
From seawater DIC estimates to atmospheric CO2 concentrations.
In seawater, the concentration of DIC is conservative, meaning that the total amount of inorganic carbon species is not temperature- or pH-dependent, in contrast to aqueous CO2 concentrations (CO2 aq). We then need to account for the following parameters (76) to generate atmospheric pCO2 estimates, noting that points i and iii are common with the alkenone-based proxy: (i) temperature and salinity, as they set the exchange between CO2 g and CO2 aq and between dissolved DIC species; (ii) seawater pH values, as they set the relative proportion of CO2 aq with respect to DIC; and (iii) the air−surface ocean disequilibrium at the considered paleolocation.
Using the CO2CALC software (77), we converted our DIC estimates into atmospheric CO2 concentrations assuming a range of pH between 7.9 and 8.05, as per refs. 34 and 73, using the temperatures from our SSTs derived from coccolith δ18O values (Fig. 1B), and assuming a constant salinity of 35 (Fig. S9 C and D). Last, according to ref. 29, the surface ocean water mass was at near-equilibrium with the atmosphere at sites ODP 925 and 929, so we did not apply any final correction. Compared with the alkenone-based CO2 barometry, we do not make an assumption about the relative contribution of CO2 and HCO3− to photosynthetic carbon fixation; as for calcification, HCO3− (hence DIC) is the ultimate carbon substrate used by the coccolithophores to calcify (78).
Fig. S9.
(A) Raw δ13C values of the 3–5-µm coccolith microfractions, (B) comparison between the estimates of paleo-DIC concentrations from the biogeochemical calibration, and (C and D) pCO2 estimates applying two distinct seawater pH values: (C) 7.9 and (D) 8.05.
Cumulative errors on pCO2 estimates.
The error bars reported on the pCO2 estimates (Fig. 2B) denote the cumulative error associated with the biogeochemical calibration (taking the confidence limits of the exponential function), uncertainty in SST estimates (seawater δ18O values are given ±0.2‰ VSMOW after ref. 22), and the analytical error in the δ13C measurements [ = 0.07‰ VPDB]. The uncertainty in our DIC evaluation is thus rather small (<75 ppm) and has little effect on our pCO2 reconstruction. It corresponds, on average, to less than 9% of the reconstructed values. The effect of uncertainty in temperature (±0.8 °C) is negligible (<10 ppm). Consequently, the curves describing paleo-CO2 and paleo-DIC estimates are broadly parallel (Fig. S9 C and D), the latter being uninfluenced by external parameters (SST) of the calibration. This point is of particular importance in our study, considering that we compare our new pCO2 and SSTs, and thus avoid circularity. The temperature effect in our pCO2 estimates is not significant (±30 ppm over the entire studied interval), confirming that both parameters are decoupled. The effect of salinity, for reference, is small. Taking a salinity value of 33 would only affect the CO2 values by a negligible CO2 amount, on the order of 10 ppm, compared with the salinity of 35 taken in our derivations. In contrast, the pH effect is a much greater source of uncertainty in the final pCO2 estimates. Rather than propagating errors bars on a favored pH assumption, we have chosen to show distinct curves in Fig. S9 C and D, either using pH 8.05 or 7.9 (34, 73).
Fig. 2.
Evolution of atmospheric carbon dioxide concentrations during the Middle Eocene to Early Oligocene. (A) Previously reported data indicating a decline in pCO2 throughout the study interval (14, 16, 29, 35). Note that the data from ref. 35 established from boron isotope composition of foraminifera from the TDP (solid diamonds) are those recently reevaluated by ref. 16. (B) Our pCO2 coccolith-derived estimates with the pH assumed to be 8.05 (according to ref. 34) are compatible with previous reports, but they reveal a clear and a more progressive negative trend. The Late Eocene warming (Fig. 1B) does not coincide with pCO2 upheaval compared with a long-term record that would indicate fleeting reestablishment of the greenhouse period. Across the EOT (gray shaded area), these estimates are compatible with the modeled pCO2 threshold at 750 ppm, compatible with glaciation in Antarctica (55).
Results
Equatorial SSTs.
The new coccolith-derived SST curve at equatorial sites ODP 925 and 929 decreases across the Early to Middle Eocene transition from 38 °C to 30 °C (Fig. 1B). Middle Eocene coccoliths record temperature fluctuations of ∼4 °C from 47 Ma to 38 Ma, corroborating previous records that show an interval of climate instability (30–32) (Fig. 1B). During the Late Eocene (38 Ma to 34 Ma), the coccolith SST curve fluctuates less and exhibits a clear 6 °C warming trend. This protracted warming is distinct from the short-lived warmth at 40 Ma, commonly referred to as the Middle Eocene Climatic Optimum (MECO) (32). At the EOT, 33.7 Ma in the record, equatorial ocean temperatures were as high as they were during the late Early Eocene and subsequently underwent pronounced cooling with the development of permanent Antarctic ice sheets throughout the Early Oligocene.
From a more methodological viewpoint, we note that the use of large (5–8 µm) or small (3–5 µm) coccolith δ18O values and associated treatments of the vital effects give relatively similar SST estimates (Fig. S4). Nevertheless, our SST curve is mainly based on assemblages gathered in microfractions comprised between 3 µm and 5 µm, as these fractions are purer (Reticulofenestra-dominated, Figs. S5–S7) and less “contaminated” by foraminiferal fragments and nannoliths such as Discoaster sp. that are prone to diagenesis.
The equatorial Atlantic SST estimates from the oxygen isotopes of diagenetically screened coccoliths (this study) and organic compounds (TEX86) from the same sites exhibit similar ranges, but the trends differ (Fig. 1). Recently published SSTs derived from TEX86 taking into account the Ring Index do not significantly vary before and across the EOT (5). The differences in SSTs in stratigraphically coeval sequences of the TDP in the Indian Ocean also remain difficult to interpret, especially as organic and inorganic-based proxy data conflict (Fig. 1A). Existing TEX86-derived estimates for the equatorial Pacific Ocean indicate relatively colder temperatures across the EOT, but the paucity of data and the lack of record spanning the Early to Late Eocene hamper a meaningful comparison of the long-term SST trends between oceanic basins (3).
Coccolith-Derived Atmospheric CO2 Levels.
The pCO2 estimates obtained from coccolith inorganic δ13C values of distinct sizes show a progressive and long-term decrease of about 500 ppm over the late Middle Eocene to Early Oligocene (Fig. 2). This trend corresponds to a drop of ∼55 ppm of CO2 per million-year period, which matches the long-term Cenozoic pCO2 decline (28, 33). The atmospheric CO2 concentrations presented in this study derive from concentrations of dissolved inorganic carbon (DIC) (Eq. 2; SI Materials and Methods and Figs. S8 and S9). Therefore, assumption of seawater pH (among other parameters; see SI Materials and Methods) is required to generate final atmospheric pCO2 estimates and associated errors. Use of a pH value of 8.05, according to the evaluation of ref. 34 in the tropics, provides pCO2 concentrations that fall within the range of previous estimates (14, 16, 28, 29, 35, 36). For reference, choosing a pH of 7.9 (37) would offset our curve by a factor of approximately +400 ppm without altering the trend (Fig. S9 C and D).
It is well established that the decrease in global temperatures that occurred during the Eocene and more broadly in the Cenozoic arose, in part, from declining pCO2 concentrations (28, 38). However, in terms of rate, our Late Eocene pCO2 record does not depart from this long-term overarching trend (Fig. 2). Although all previous reports agree on this decrease in pCO2 during the Late Eocene, we must mention one exception to this, provided by recent work by ref. 16 showing higher Late Eocene pCO2 values than in the Middle Eocene (Fig. 2).
Discussion and Implications for the Long-Term Trigger of Antarctic Glaciation
Inception of a Meridional Temperature Gradient.
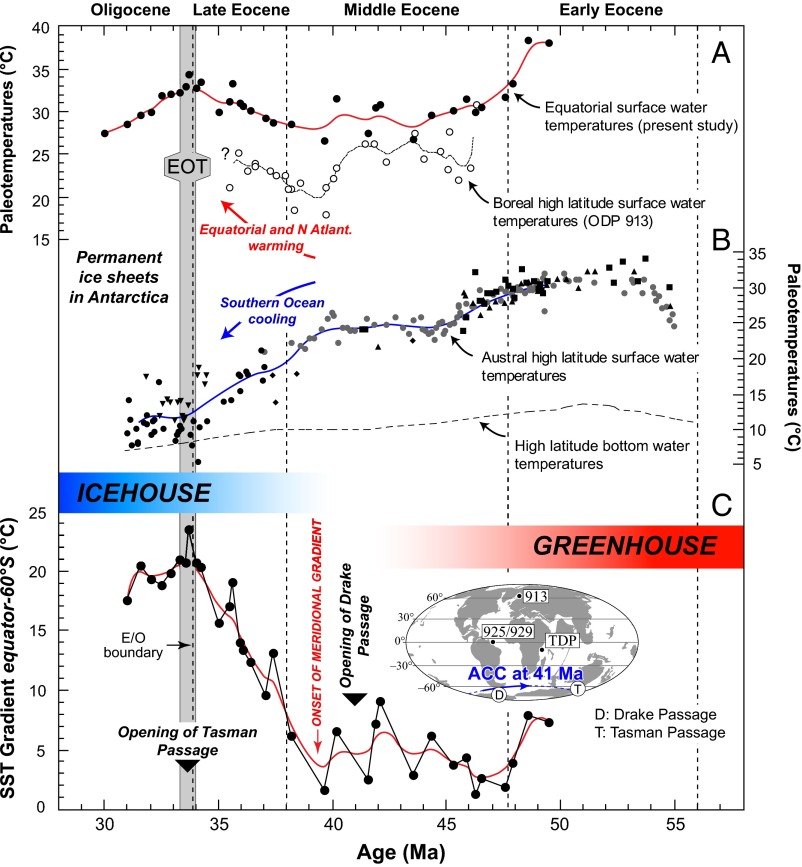
The Early Eocene recorded high temperatures at both low and high latitudes (Fig. 3 A and B), providing evidence for a small meridional temperature gradient between the equator and the Southern Ocean during this interval (ΔSST < 8 °C). Such a small temperature difference between low and high latitudes characterizes the greenhouse climate of the Early and early Middle Eocene (15, 39, 40). A low equator to subpolar temperature gradient persisted through most of the Middle Eocene, as ocean temperatures cooled at similar rates at both high and low latitudes (Fig. 3). Despite this apparently global cooling and continued decline in pCO2, greenhouse conditions persisted during the Middle Eocene. During the late Middle Eocene and the Late Eocene, as the temperature trends diverged with low-latitude warming and accentuation of cooling in the austral subpolar regions (Fig. 3 A and B), this interval thus corresponds to the inception and progressive development of a meridional temperature gradient, at least in the Atlantic Ocean. During the latest Eocene and Early Oligocene interval, warm tropical and distinctly cooler temperatures in the sub-Antarctic regions testify to a strong latitudinal gradient (∼20 °C around the EOT) akin to present-day values. Together with the development of permanent polar ice caps in Antarctica, from an oceanographic perspective, such a strong temperature gradient confirms firmly established icehouse conditions at the Eocene−Oligocene boundary.
Fig. 3.
Meridional temperature gradient revealing the shift from greenhouse to icehouse conditions leading to the EOT. (A) Equatorial [ODP 925 and 929 (present study)] and sub-Arctic [ODP 913 (4)] SSTs show similar warming from 38 Ma to 40 Ma. (B) SST estimates from southern high-latitude sites exhibited more pronounced austral subpolar cooling from 40 Ma (data from refs. 4 and 8). High-latitude bottom water temperatures are from ref. 6. (C) Evolution of the meridional (surface) temperature gradient calculated as the SST difference between the equatorial Atlantic and Southern Oceans. The onset of this gradient, linked to the initiation of ACC, highlights the transition from greenhouse to icehouse, leading to permanent ice sheets on Antarctica after the OI 1 event (41).
The extent to which the Late Eocene warming was emplaced in all equatorial regions remains difficult to establish. Indeed, no reliable equatorial SSTs exist for the Pacific Ocean, and the TDP data are too patchy for the Late Eocene to exhibit a possible warming stage of the tropics of the Indian Ocean (Fig. 1A). The Oi 1 isotopic event (41), and more broadly the EOT interval, are often mentioned to pinpoint the major flip from greenhouse to icehouse conditions in the Cenozoic (12, 42). Our work, however, shows that this prominent climate transition was not an abrupt and sudden event but was emplaced progressively consecutive to a 4-My-long period of change in the latitudinal distribution of oceanic heat.
Major Redistribution of Oceanic Heat During the Late Eocene.
The causes of the Late Eocene oceanographic precursor to permanent Antarctic glaciation need to be discussed taking into account the documented environmental changes that occurred during the Middle Eocene–Early Oligocene interval. Two main climatic drivers are currently put forward to explain substantial polar cooling during this critical period of climate change: the decline in pCO2 levels and the opening of new Southern Ocean gateways (9, 13, 14, 43). During the Late Eocene, pCO2 concentrations continued to decline, an overarching phenomenon that may have contributed to reducing mean annual global temperatures (10). As the Late Eocene is not associated with increased atmospheric CO2 concentrations (Fig. 2), reinvigorated greenhouse conditions and enhanced radiative forcing on temperatures cannot explain the equatorial warming. Rather, we suggest that the Late Eocene paleoceanographic and climatic changes are best explained by a major reorganization of ocean circulation. During the Late Eocene, a reduced supply of southward flowing warm waters from the tropical Atlantic toward the austral subpolar regions can account for accentuated cooling of the South Atlantic and the Southern Ocean. From an oceanic viewpoint, this process would correspond to progressive latitudinal contraction of the subtropical gyre. Isotopic tracers provide evidence for major reorganization in the circulation of deep ocean waters in the late Middle Eocene (11, 12). Meanwhile, the Northern Atlantic Ocean did not experience cooling, as coeval warming during the Late Eocene was reported from site ODP 913 at 70°N (4) (Fig. 3A), which suggests thermal decoupling between the Southern Ocean and the Atlantic Ocean during the Late Eocene. It is likely that the thermal difference between the low- and high-latitude austral surface waters of the Atlantic Ocean fueled a meridional overturning circulation before the establishment of a true thermohaline circulation after the EOT (13).
Considering surface and deep ocean circulation upheaval together, the timing of such profound reorganization of oceanic currents, which began between 41 Ma and 38 Ma, is compatible with the opening of the Drake Passage (44–46). Neodymium isotope data further confirm the influx of SW Pacific surface waters through this newly opened seaway from 41 Ma onward (47). The emplacement of an Antarctic circumpolar current (ACC), in turn, led to the progressive thermal isolation of Southern Ocean and Antarctica, a phenomenon that continued as the gateways widened until the Late Oligocene (43, 46). The ACC is an efficient way to block warm surface waters entrained in subtropical gyres and reduce poleward heat transport. The intensity of the thermal isolation of Antarctica was subsequently reinforced at the Eocene−Oligocene boundary, when the deepening and widening of the Tasman ocean gateway led to permanent polar glaciation in Antarctica (13, 43, 48, 49).
Conclusions
By providing reliable coccolith-derived SSTs for the equatorial Atlantic, this study reveals a clear divergence in temperature trends between the Southern Ocean and the equatorial Atlantic Ocean during the Late Eocene. The emplacement and subsequent entrenchment of a strong Atlantic meridional temperature gradient characterize a climate shift from greenhouse and icehouse conditions (15). This prominent change and meridional ocean reorganization predated the Eocene−Oligocene climate transition by ∼4 million years and was the likely consequence of the emplacement of an ACC. The opening of the Drake Passage led to major changes in ocean circulation, not only in the Southern Ocean, as previously established, but also with major consequences for the meridional Atlantic gyral and deep ocean currents. This precursor event heralding the EOT has to be taken into account to constrain the feedback mechanisms ultimately responsible for the permanent glaciation of Antarctica (50).
The transition from greenhouse to icehouse conditions and the emplacement of a meridional temperature gradient are broad and global phenomena that likely influenced the processes of oceanic versus atmospheric heat transport from the equator to the poles (15). The accuracy of climate models in reproducing the latent heat transport is currently limited by large uncertainties in existing temperatures (10, 51, 52). Better constraints on temperature evolution at low latitudes are crucial in this regard, as most latent heat and moisture originate from tropical regions. Therefore, the documentation of a prominent Late Eocene equatorial warming may further our understanding on how the Earth system shifted from a greenhouse to an icehouse world from a modeling perspective.
Materials and Methods
Ocean Study Sites.
We studied sediment samples consisting of calcareous nannofossil oozes with variable amounts of clays recovered from sites ODP 925A and 929E in the western equatorial Atlantic (map in Fig. 1A, Inset). Over the entire study interval (50 Ma to 30 Ma using the age model by ref. 14), average carbonate content was 60 wt% (shipboard data), and the calcareous nannofossils assemblages are diverse and well preserved, whereas the foraminifera tests were systematically found to be recrystallized.
Coccolith Separation and Isotopic Analyses: Diagenetic Screening.
We applied the protocol described in ref. 53, based on a cascade of microfiltering steps, to obtain size-restricted assemblages. The microfractions between 5 µm and 8 µm were dominated by large coccoliths mostly belonging to the Coccolithus pelagicus group, whereas the 3–5-µm microfractions were found to concentrate relatively smaller reticulofenestrid coccoliths (Figs. S6 and S7). After separation, granulometry, and hence quality and purity, of each fraction was controlled under cross-polarized light using a Zeiss Axioscope microscope under 1575× magnification. The state of preservation of the coccoliths (etching and overgrowth) was checked for all of the microfractions under SEM (Zeiss Supra 55VP) (Fig. S5). Noncoccolith particles, such as discoasters (star-shaped or rosette calcareous nannoliths particularly prone to diagenetic alteration) and foraminiferal fragments, were retained in the coarse fractions (8–20 µm) preceding the 5–8-µm and 3–5-µm microfraction steps. The so-called micarbs corresponding to undetermined micrometric crystals (53) were discarded in the finest (<3 µm) fraction.
Isotopic Measurements.
Oxygen and carbon isotope compositions were measured on 80 µg of microseparated sample residue reacted with purified phosphoric acid at 80 °C on a Kiel IV mass spectrometer at Université Pierre et Marie Curie. Stable isotope values were calibrated relative to the Vienna Pee Dee Belemnite (‰ VPDB) via the NBS-19 international standard. The reproducibility of measurements is ±0.1‰ (1σ) for δ18O and ±0.05‰ (1σ) for δ13C.
SST and pCO2 Reconstructions.
To convert the “coccolith δ18O − seawater δ18O” offsets into temperature estimates, we used Eq. 1 derived from ref. 54.
| [1] |
where SST is in degrees Celsius, δ18Ococco and oxygen isotope vital effect are in ‰ VPDB, and δ18Osw is in ‰ Vienna Standard Mean Ocean Water (VSMOW). The variables of the equation are in bold. Please refer to SI Materials and Methods for the treatment of the oxygen isotope vital effect (18O VE) and the oxygen isotope composition of ancient seawater (δ18Osw).
In the present study, we used a paleo-DIC equation derived from recent coccolithophore culture work with a geological perspective (19, 20). This proxy relies on the interspecies δ13C compositions (Δ13Clarge-small) of coccoliths of distinct sizes recently formalized in ref. 21. The range of measured Δ13Clarge-small in the Eocene–Oligocene sediment is within the range of cultured coccolith Δ13CCpel-Goc values, and the sizes of the coccoliths considered here are similar in both the natural and laboratory studies (14). A notable advantage of the proxy is that it does not require knowledge of δ13C of DIC (or CO2 aq), as is the case for alkenone paleo-CO2 estimates. From the data of refs. 19 and 20 collectively shown in Fig. S8B, the equation tying Δ13CCpel-Goc (hence Δ13Clarge-small) to DIC levels is as follows:
| [2] |
where [DIC] is in micromoles of carbon per kilogram of seawater, and Δ13C = δ13Clarge − δ13Csmall is in ‰ VPDB (coccolith assemblages 5–8 µm and 3–5 µm, respectively). The variable of the equation is denoted in bold.
The obtained DIC estimates were subsequently transformed into pCO2 estimates using Henry’s law. Further details on these methods are provided in SI Materials and Methods.
Acknowledgments
The authors thank Nathalie Labourdette for running the mass spectrometer, Omar Boudouma for help on the SEM, and Moh Belkacemi and Amélie Guittet for assistance with coccolith microseparation. We are grateful to Jeremy Young for assistance in coccolith identification. The authors also thank Delphine Desmares, Katie Egan, Tristan Horner, and Ros Rickaby for useful discussions. We acknowledge the critical input of two anonymous reviewers and constructive remarks on the manuscript. This study used samples provided by the ODP. F.M. acknowledges funding by the Centre National de la Recherche Scientifique (Grant SYSTER 884402), and M.H. acknowledges funding by the Natural Environment Research Council (Grant NE/H015523/1).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608100113/-/DCSupplemental.
References
- 1.Pearson PN, et al. Warm tropical sea surface temperatures in the Late Cretaceous and Eocene epochs. Nature. 2001;413(6855):481–487. doi: 10.1038/35097000. [DOI] [PubMed] [Google Scholar]
- 2.Pearson PN, et al. Stable warm tropical climate through the Eocene Epoch. Geology. 2007;35(3):211–214. [Google Scholar]
- 3.Liu Z, et al. Global cooling during the Eocene-Oligocene climate transition. Science. 2009;323(5918):1187–1190. doi: 10.1126/science.1166368. [DOI] [PubMed] [Google Scholar]
- 4.Inglis GN, et al. Descent toward the Icehouse: Eocene sea surface cooling inferred from GDGT distributions. Paleoceanography. 2015;30(7):1000–1020. [Google Scholar]
- 5.Zhang YG, Pagani M, Wang Z. Ring Index: A new strategy to evaluate the integrity of TEX86 paleothermometry. Paleoceanography. 2016;31(2):220–232. [Google Scholar]
- 6.Lear CH, Elderfield H, Wilson PA. Cenozoic deep-sea temperatures and global ice volumes from Mg/Ca in benthic foraminiferal calcite. Science. 2000;287(5451):269–272. doi: 10.1126/science.287.5451.269. [DOI] [PubMed] [Google Scholar]
- 7.Hollis CJ, et al. Early Paleogene temperature history of the Southwest Pacific Ocean: Reconciling proxies and models. Earth Planet Sci Lett. 2012;349:53–66. [Google Scholar]
- 8.Plancq J, Mattioli E, Pittet B, Simon L, Grossi V. Productivity and sea-surface temperature changes recorded during the late Eocene-early Oligocene at DSDP Site 511 (South Atlantic) Palaeogeogr Palaeoclimatol Palaeoecol. 2014;407:34–44. [Google Scholar]
- 9.DeConto RM, Pollard D. Rapid Cenozoic glaciation of Antarctica induced by declining atmospheric CO2. Nature. 2003;421(6920):245–249. doi: 10.1038/nature01290. [DOI] [PubMed] [Google Scholar]
- 10.Caballero R, Huber M. State-dependent climate sensitivity in past warm climates and its implications for future climate projections. Proc Natl Acad Sci USA. 2013;110(35):14162–14167. doi: 10.1073/pnas.1303365110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borrelli C, Cramer BS, Katz ME. Bipolar Atlantic deepwater circulation in the middle-late Eocene: Effects of Southern Ocean gateway openings. Paleoceanography. 2014;29(4):308–327. [Google Scholar]
- 12.Cramer BS, Toggweiler JR, Wright JD, Katz ME, Miller KG. Ocean overturning since the late Cretaceous: Inferences from a new benthic foraminiferal isotope compilation. Paleoceanography. 2009;24(4):PA4216. [Google Scholar]
- 13.Kennett JP. Cenozoic evolution of Antarctic glaciation, the circum-Antarctic Ocean, and their impact on global paleoceanography. J Geophys Res. 1977;82(27):3843–3860. [Google Scholar]
- 14.Pagani M, et al. The role of carbon dioxide during the onset of Antarctic glaciation. Science. 2011;334(6060):1261–1264. doi: 10.1126/science.1203909. [DOI] [PubMed] [Google Scholar]
- 15.Pagani M, Huber M, Sageman B. Greenhouse climates. In: Holland H, Turekian K, editors. Treatise on Geochemistry. 2nd Ed. Elsevier; New York: 2014. pp. 281–304. [Google Scholar]
- 16.Anagnostou E, et al. Changing atmospheric CO2 concentration was the primary driver of early Cenozoic climate. Nature. 2016;533(7603):380–384. doi: 10.1038/nature17423. [DOI] [PubMed] [Google Scholar]
- 17.Sexton PF, Wilson PA, Pearson PN. Microstructural and geochemical perspectives on planktic foraminiferal preservation: “Glassy” versus “Frosty.”. Geochem Geophys Geosyst. 2006;7(12):Q12P19. [Google Scholar]
- 18.Prentice K, et al. Trace metal (Mg/Ca and Sr/Ca) analyses of single coccoliths by Secondary Ion Mass Spectrometry. Geochim Cosmochim Acta. 2014;146:90–106. [Google Scholar]
- 19.Rickaby REM, Henderiks J, Young JN. Perturbing phytoplankton: response and isotopic fractionation with changing carbonate chemistry in two coccolithophore species. Clim Past. 2010;6(6):771–785. [Google Scholar]
- 20.Hermoso M, Chan IZX, McClelland HLO, Heureux AMC, Rickaby REM. Vanishing coccolith vital effects with alleviated carbon limitation. Biogeosciences. 2016;13(1):301–312. [Google Scholar]
- 21.Bolton CT, Stoll HM. Late Miocene threshold response of marine algae to carbon dioxide limitation. Nature. 2013;500(7464):558–562. doi: 10.1038/nature12448. [DOI] [PubMed] [Google Scholar]
- 22.Tindall J, et al. Modelling the oxygen isotope distribution of ancient seawater using a coupled ocean-atmosphere GCM: Implications for reconstructing early Eocene climate. Earth Planet Sci Lett. 2010;292(3-4):265–273. [Google Scholar]
- 23.Hermoso M. Control of ambient pH on growth and stable isotopes in phytoplanktonic calcifying algae. Paleoceanography. 2015;30(8):PA2844. [Google Scholar]
- 24.Hermoso M, Candelier Y, Browning TJ, Minoletti F. Environmental control of the isotopic composition of subfossil coccolith calcite: Are laboratory culture data transferable to the natural environment? GeoResJ. 2015;7:35–42. [Google Scholar]
- 25.Hermoso M. Isotopic record of Pleistocene glacial/interglacial cycles in pelagic carbonates: Revisiting historical data from the Caribbean Sea. Quat Sci Rev. 2016;137:69–78. [Google Scholar]
- 26.Rickaby REM, et al. Environmental carbonate chemistry selects for phenotype of recently isolated strains of Emiliania huxleyi. Deep Res Part II. 2016;127:28–40. [Google Scholar]
- 27.Hermoso M, et al. An explanation for the 18O excess in Noelaerhabdaceae coccolith calcite. Geochim Cosmochim Acta. 2016;189:132–142. [Google Scholar]
- 28.Pagani M, Zachos JC, Freeman KH, Tipple B, Bohaty S. Marked decline in atmospheric carbon dioxide concentrations during the Paleogene. Science. 2005;309(5734):600–603. doi: 10.1126/science.1110063. [DOI] [PubMed] [Google Scholar]
- 29.Zhang YG, Pagani M, Liu Z, Bohaty SM, Deconto R. A 40-million-year history of atmospheric CO2. Philos Trans A Math Phys Eng Sci. 2013;371(2001):20130096. doi: 10.1098/rsta.2013.0096. [DOI] [PubMed] [Google Scholar]
- 30.Tripati A, Backman J, Elderfield H, Ferretti P. Eocene bipolar glaciation associated with global carbon cycle changes. Nature. 2005;436(7049):341–346. doi: 10.1038/nature03874. [DOI] [PubMed] [Google Scholar]
- 31.Bohaty SM, Zachos JC, Florindo F, Delaney ML. Coupled greenhouse warming and deep-sea acidification in the middle Eocene. Paleoceanography. 2009;24(2):PA2207. [Google Scholar]
- 32.Bohaty S, Zachos J. Significant Southern Ocean warming event in the late middle Eocene. Geology. 2003;31(11):1017–1020. [Google Scholar]
- 33.Hönisch B, et al. The geological record of ocean acidification. Science. 2012;335(6072):1058–1063. doi: 10.1126/science.1208277. [DOI] [PubMed] [Google Scholar]
- 34.Pearson PN, Palmer MR. Middle Eocene seawater pH and atmospheric carbon dioxide concentrations. Science. 1999;284(5421):1824–1826. doi: 10.1126/science.284.5421.1824. [DOI] [PubMed] [Google Scholar]
- 35.Pearson PN, Foster GL, Wade BS. Atmospheric carbon dioxide through the Eocene−Oligocene climate transition. Nature. 2009;461(7267):1110–1113. doi: 10.1038/nature08447. [DOI] [PubMed] [Google Scholar]
- 36.Heureux AMC, Rickaby REM. Refining our estimate of atmospheric CO2 across the Eocene–Oligocene climatic transition. Earth Planet Sci Lett. 2015;409:329–338. [Google Scholar]
- 37.Pearson PN, Palmer MR. Atmospheric carbon dioxide concentrations over the past 60 million years. Nature. 2000;406(6797):695–699. doi: 10.1038/35021000. [DOI] [PubMed] [Google Scholar]
- 38.Kent DV, Muttoni G. Equatorial convergence of India and early Cenozoic climate trends. Proc Natl Acad Sci USA. 2008;105(42):16065–16070. doi: 10.1073/pnas.0805382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huber M, Caballero R. The early Eocene equable climate problem revisited. Clim Past. 2011;7(2):603–633. [Google Scholar]
- 40.Greenwood DR, Wing SL. Eocene continental climates and latitudinal temperature gradients. Geology. 1995;23(11):1044–1048. [Google Scholar]
- 41.Miller KG, Wright JD, Fairbanks RG. Unlocking the Ice House: Oligocene-Miocene oxygen isotopes, eustasy, and margin erosion. J Geophys Res. 1991;96(B4):6829–6848. [Google Scholar]
- 42.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292(5517):686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 43.Kennett JP, Exon NF. Paleoceanographic evolution of the Tasmanian seaway and its climatic implications. In: Exon N, Kennett JP, Malone MJ, editors. The Cenozoic Southern Ocean: Tectonics, Sedimentation, and Climate Change Between Australia and Antarctica. Am Geophys Union; Washington, DC: 2004. pp. 345–367. [Google Scholar]
- 44.Sijp WP, England MH. Effect of the Drake Passage throughflow on global climate. J Phys Oceanogr. 2004;34(5):1254–1266. [Google Scholar]
- 45.Livermore R, Nankivell A, Eagles G, Morris P. Paleogene opening of Drake Passage. Earth Planet Sci Lett. 2005;236(1-2):459–470. [Google Scholar]
- 46.Lagabrielle Y, Goddéris Y, Donnadieu Y, Malavieille J, Suarez M. The tectonic history of Drake Passage and its possible impacts on global climate. Earth Planet Sci Lett. 2009;279(3-4):197–211. [Google Scholar]
- 47.Scher HD, Martin EE. Timing and climatic consequences of the opening of Drake Passage. Science. 2006;312(5772):428–430. doi: 10.1126/science.1120044. [DOI] [PubMed] [Google Scholar]
- 48.Egan KE, Rickaby REM, Hendry KR, Halliday AN. Opening the gateways for diatoms primes Earth for Antarctic glaciation. Earth Planet Sci Lett. 2013;375:34–43. [Google Scholar]
- 49.Bijl PK, et al. Expedition 318 Scientists Eocene cooling linked to early flow across the Tasmanian Gateway. Proc Natl Acad Sci USA. 2013;110(24):9645–9650. doi: 10.1073/pnas.1220872110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coxall HK, Wilson PA, Pälike H, Lear CH, Backman J. Rapid stepwise onset of Antarctic glaciation and deeper calcite compensation in the Pacific Ocean. Nature. 2005;433(7021):53–57. doi: 10.1038/nature03135. [DOI] [PubMed] [Google Scholar]
- 51.Lunt DJ, et al. A model–data comparison for a multi-model ensemble of early Eocene atmosphere–ocean simulations: EoMIP. Clim Past. 2012;8(5):1717–1736. [Google Scholar]
- 52.Ladant J-B, Donnadieu Y, Dumas C. Links between CO2, glaciation and water flow: Reconciling the Cenozoic history of the Antarctic Circumpolar Current. Clim Past. 2014;10(6):1957–1966. [Google Scholar]
- 53.Minoletti F, Hermoso M, Gressier V. Separation of sedimentary micron-sized particles for palaeoceanography and calcareous nannoplankton biogeochemistry. Nat Protoc. 2009;4(1):14–24. doi: 10.1038/nprot.2008.200. [DOI] [PubMed] [Google Scholar]
- 54.Kim S-T, O’Neil JR. Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates. Geochim Cosmochim Acta. 1997;61(16):3461–3475. [Google Scholar]
- 55.Deconto RM, et al. Thresholds for Cenozoic bipolar glaciation. Nature. 2008;455(7213):652–656. doi: 10.1038/nature07337. [DOI] [PubMed] [Google Scholar]
- 56.Shackleton NJ, et al. Paleotemperature history of the Cenozoic and the initiation of Antarctic glaciation: Oxygen and carbon isotope analyses in DSDP Sites 277, 279, and 281. Initial Rep Deep Sea Drill Proj. 1975;29:743–755. [Google Scholar]
- 57.Ehrmann WU, Mackensen A. Sedimentological evidence for the formation of an East Antarctic ice sheet in Eocene/Oligocene time. Palaeogeogr Palaeoclimatol Palaeoecol. 1992;93(1-2):85–112. [Google Scholar]
- 58.LeGrande AN, Schmidt GA. Global gridded data set of the oxygen isotopic composition in seawater. Geophys Res Lett. 2006;33(12):L12604. [Google Scholar]
- 59.Dunkley Jones T, et al. Major shifts in calcareous phytoplankton assemblages through the Eocene-Oligocene transition of Tanzania and their implications for low-latitude primary production. Paleoceanography. 2008;23(4):PA4204. [Google Scholar]
- 60.LeGrande AN, Schmidt GA. Water isotopologues as a quantitative paleosalinity proxy. Paleoceanography. 2011;26(3):PA3225. [Google Scholar]
- 61.Dudley W, Blackwelder P, Brand L, Duplessy J-C. Stable isotopic composition of coccoliths. Mar Micropaleontol. 1986;10(1-3):1–8. [Google Scholar]
- 62.Ziveri P, et al. Stable isotope “vital effects” in coccolith calcite. Earth Planet Sci Lett. 2003;210(1-2):137–149. [Google Scholar]
- 63.Ziveri P, Thoms S, Probert I, Geisen M, Langer G. A universal carbonate ion effect on stable oxygen isotope ratios in unicellular planktonic calcifying organisms. Biogeosciences. 2012;9(3):1025–1032. [Google Scholar]
- 64.Candelier Y, Minoletti F, Probert I, Hermoso M. Temperature dependence of oxygen isotope fractionation in coccolith calcite: A culture and core top calibration of the genus Calcidiscus. Geochim Cosmochim Acta. 2013;100:264–281. [Google Scholar]
- 65.Hermoso M, Horner TJ, Minoletti F, Rickaby REM. Constraints on the vital effect in coccolithophore and dinoflagellate calcite by oxygen isotopic modification of seawater. Geochim Cosmochim Acta. 2014;44:612–627. [Google Scholar]
- 66.Stevenson EI, et al. Controls on stable strontium isotope fractionation in coccolithophores with implications for the marine Sr cycle. Geochim Cosmochim Acta. 2014;128:225–235. [Google Scholar]
- 67.Ennyu A, Arthur MA, Pagani M. Fine-fraction carbonate stable isotopes as indicators of seasonal shallow mixed-layer paleohydrography. Mar Micropaleontol. 2002;46(3-4):317–342. [Google Scholar]
- 68.Hermoso M. Coccolith-derived isotopic proxies in palaeoceanography: where geologists need biologists. Cryptogam Algol. 2014;35(4):323–351. [Google Scholar]
- 69.Rousselle G, Beltran C, Sicre M-A, Raffi I, De Rafélis M. Changes in sea-surface conditions in the Equatorial Pacific during the middle Miocene–Pliocene as inferred from coccolith geochemistry. Earth Planet Sci Lett. 2013;361:412–421. [Google Scholar]
- 70.Henderiks J, Pagani M. Coccolithophore cell size and the Paleogene decline in atmospheric CO2. Earth Planet Sci Lett. 2008;269(3-4):576–584. [Google Scholar]
- 71.Watkins JM, Nielsen LC, Ryerson FJ, DePaolo DJ. The influence of kinetics on the oxygen isotope composition of calcium carbonate. Earth Planet Sci Lett. 2013;375:349–360. [Google Scholar]
- 72.Watkins JM, Hunt JD, Ryerson FJ, DePaolo DJ. The influence of temperature, pH, and growth rate on the δ18O composition of inorganically precipitated calcite. Earth Planet Sci Lett. 2014;404:332–343. [Google Scholar]
- 73.Ridgwell A. A Mid Mesozoic Revolution in the regulation of ocean chemistry. Mar Geol. 2005;217(3-4):339–357. [Google Scholar]
- 74.Stoll HM. Limited range of interspecific vital effects in coccolith stable isotopic records during the Paleocene-Eocene thermal maximum. Paleoceanography. 2005;20(1):PA1007. [Google Scholar]
- 75.Bolton CT, Stoll HM, Mendez-Vicente A. Vital effects in coccolith calcite: Cenozoic climate-pCO2 drove the diversity of carbon acquisition strategies in coccolithophores? Paleoceanography. 2012;27(4):PA4204. [Google Scholar]
- 76.Zeebe RE, Wolf-Gladrow D. CO2 in Seawater: Equilibrium, Kinetics, Isotopes. Elsevier; New York: 2001. [Google Scholar]
- 77.Robbins LL, Hansen ME, Kleypas JA, Meylan SC. CO2calc—A user-friendly seawater carbon calculator for Windows, Max OS X, and iOS (iPhone) US Geol Surv Open File Rep. 2010;2010-1280:1–18. [Google Scholar]
- 78.Holtz L-M, Wolf-Gladrow D, Thoms S. Numerical cell model investigating cellular carbon fluxes in Emiliania huxleyi. J Theor Biol. 2015;364:305–315. doi: 10.1016/j.jtbi.2014.08.040. [DOI] [PubMed] [Google Scholar]