Significance
Tropical African lakes are well-known to house exceptionally biodiverse assemblages of fish and other aquatic fauna, which are thought to be at risk in the future. Although the modern assemblages are well-studied, direct evidence of the origin of this incredible wealth of species and the mechanisms that drive speciation are virtually unknown. We use a long sedimentary record from Lake Malawi to show that over the last 1.2 My both large-scale climatic and tectonic changes resulted in wet–dry transitions that led to extraordinary habitat variability and rapid diversification events. This work allows us to understand the environmental context of aquatic evolution in the most biodiverse tropical lake.
Keywords: tropical climate, cichlid evolution, adaptive radiation, paleoclimate, paleoecology
Abstract
Long paleoecological records are critical for understanding evolutionary responses to environmental forcing and unparalleled tools for elucidating the mechanisms that lead to the development of regions of high biodiversity. We use a 1.2-My record from Lake Malawi, a textbook example of biological diversification, to document how climate and tectonics have driven ecosystem and evolutionary dynamics. Before ∼800 ka, Lake Malawi was much shallower than today, with higher frequency but much lower amplitude water-level and oxygenation changes. Since ∼800 ka, the lake has experienced much larger environmental fluctuations, best explained by a punctuated, tectonically driven rise in its outlet location and level. Following the reorganization of the basin, a change in the pacing of hydroclimate variability associated with the Mid-Pleistocene Transition resulted in hydrologic change dominated by precession rather than the high-latitude teleconnections recorded elsewhere. During this time, extended, deep lake phases have abruptly alternated with times of extreme aridity and ecosystem variability. Repeated crossings of hydroclimatic thresholds within the lake system were critical for establishing the rhythm of diversification, hybridization, and extinction that dominate the modern system. The chronology of these changes closely matches both the timing and pattern of phylogenetic history inferred independently for the lake’s extraordinary array of cichlid fish species, suggesting a direct link between environmental and evolutionary dynamics.
Reconstructing hydroclimate variability in the tropics is important for understanding how ecosystems have evolved and to predict future environmental tipping points that may negatively impact freshwater resources and aquatic ecosystems (1). This understanding is particularly important in Africa, where incredibly biodiverse aquatic ecological assemblages support a food web that is a critical staple for millions of people and access to fresh water is limited (2). However, an enormous data gap exists in African climate and ecosystem history, precluding a fundamental understanding of these systems’ sensitivity to various environmental perturbations. Continuous lacustrine sedimentary records that reveal ancient physical processes and ecosystem dynamics are emerging as a unique tool that can help us understand the implications of hydrologic thresholds and changes in aquatic ecosystem variability. Long and highly resolved paleorecords are also critical for elucidating the dynamics and potential drivers of explosive speciation, which characterizes many of these ancient lakes (3).
Over the Quaternary, the Afrotropics have undergone large-scale gradual and periodic climate changes, including regional wet–dry cycles regulated by the magnitude of insolation forcing related to orbital eccentricity (4, 5). We hypothesize that these events and changes in the variability of lake-level fluctuations influenced the timing of diversification of biodiverse endemic faunas. However, discontinuous and poorly dated records from outcrops provide only intermittent snapshots, and it is difficult to tease apart the influence of climatic versus geomorphic or tectonic processes. Furthermore, to integrate climate history in understanding ecological and evolutionary records, distant sources of climate information, such as marine sediment cores, are of limited value for interpreting in situ processes (6, 7). Thus, long continuous records that preserve ecological, climatic, and sedimentological indicators from within lakes are crucial to disentangling sensitivity thresholds and observing environmental and evolutionary change.
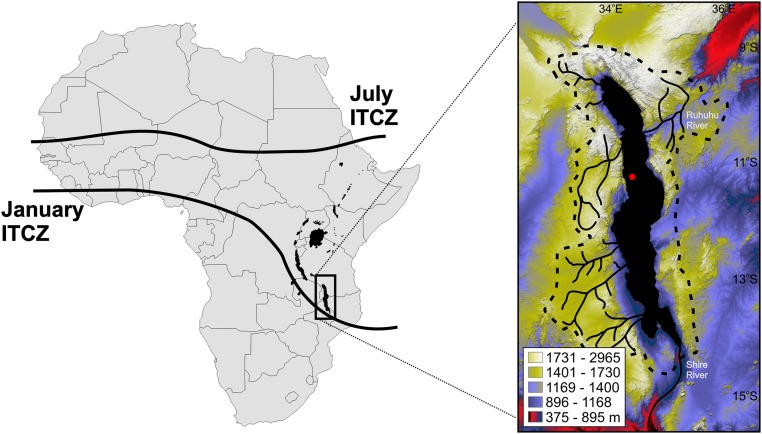
Here we use a suite of paleoecological and mineralogical records from a 380-m drill core (MAL05-1B) from Lake Malawi (Fig. 1; 590-m water depth; 11°18′S, 34°26′E), in southeastern Africa, to reconstruct its ecosystem and limnological history over the last 1.2 My (SI Appendix, Figs. S8–S10 and Table S2) (5). Lake Malawi houses more fish species than any other lake on Earth, including ∼800 cichlid species, and is an iconic “laboratory” for testing questions about rates, modes, and drivers of evolution (8). The lake is deep (706 m), old (>5 My), and permanently stratified with a large watershed (94,000 km2), and preserves an excellent long sedimentological record (9). Furthermore, its location at the southernmost seasonal reach of the Intertropical Convergence Zone (ITCZ) and its effective moisture sensitivity make it a promising archive for reconstructing regional hydroclimate and paleoecology. Previous paleoecological studies have demonstrated that the lake’s biota has undergone dramatic changes in the past 140 ky, which are strongly correlated with water chemistry, availability of clear-water, rocky, and littoral habitats, and water-column stratification changes (4, 10). Here we extend that record for fossil ostracode crustaceans, fish, silicified green algae, lake flies, charred particles, and various mineralogical indicators over the ∼1.2-My core record.
Fig. 1.
Map of Africa with the location of Lake Malawi with respect to July and January ITCZ positions. (Inset) Topographic map of Lake Malawi and its watershed, including the Ruhuhu River. The red dot indicates the core location.
Results and Discussion
Prior studies of the MAL05-1B core from Lake Malawi lacked age control over large portions of the record (5). In this study, we present data with a refined age model that uses paleomagnetic excursion and intensity records, thus allowing us to attribute timing to events and evaluate the frequency of hydroclimate variability (SI Appendix, Figs. S1–S7 and Table S1).
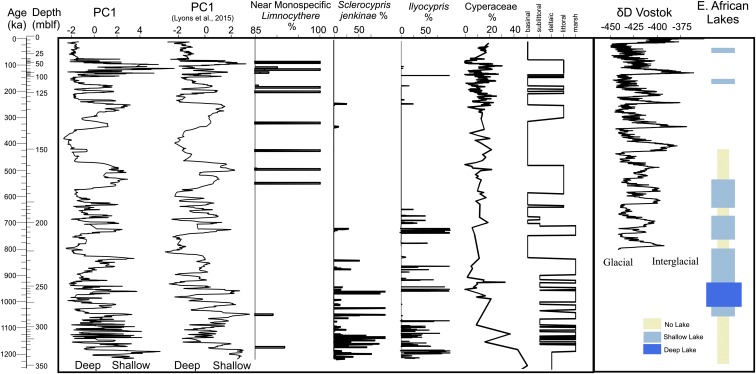
A principal component (PC) analysis of all hydrologically sensitive fossil and mineralogical indicators shows a first-order change in the frequency and amplitude associated with lake state starting about 800 ka (Fig. 2 and SI Appendix, Figs. S8–S10). Before that time, ostracode assemblages dominated by the freshwater Sclerocypris jenkinae and marsh/riverine Ilyocypris (mostly I. alta) are observed. These ostracodes, in combination with abundant freshwater mollusks, sedge pollen, and framboidal pyrite (typical of sediment reduction in areas of high biological oxygen demand), are correlated with negative PC1 values. This assemblage indicates direct river influence with through-flowing, hydrologically open, marsh or shallow lake conditions (11, 12). The intervening wetter/stratified lake phases were relatively brief, with a mean duration of 12.2 ± 3.2 ky for the 10 longest sustained intervals of bottom-water anoxia before the lake state shift at 800 ka.
Fig. 2.
Lake Malawi paleoenvironments with global/regional records for the last 1.2 My. (Left to Right) First principal component of biogenic and mineralogical facies from MAL05-1B from this study, first principal component of physical properties (5) from MAL05-1B (both associated with lake-level variability), percent near monospecific Limnocythere ostracodes, percent S. jenkinae ostracodes, percent Ilyocypris ostracodes, percent Cyperaceae pollen (sedges), and inferred depositional environment from this study. Region/global records are δD from the Vostok ice core (39) and East African Rift Valley lake levels [adapted from Trauth et al. (26)]. mblf, Meters below lake floor.
Following a transitional period from 800 to 700 ka, Lake Malawi switched to a very different type of basin, with the disappearance of local riverine influence, freshwater marshes, and indications of open hydrology. In particular, lowstands were characterized by extended periods of saline/alkaline fauna and mineralogical indicators (e.g., monospecific assemblages of Limnocythere ostracodes and calcium carbonate nodules). These lowstands alternated with much more persistent, very deep, stratified lake conditions when deep-water anoxia prevailed over much longer intervals and benthic fauna was absent. These periods had a mean duration of 24.1 ± 16.0 ky for the 10 longest sustained intervals. Stone et al. (10) previously showed that these deep lake phases were stratified, typified by “blue water,” much like highly transparent lake conditions observed in Lake Malawi today. In contrast, the lowstand lakes were well-mixed and highly eutrophic. The three most prominent and longest deep lake phases conspicuously straddle insolation minima on an ∼400-ky beat following the deepening of the basin after 800 ka.
The major patterns of change can be delineated into five paleoenvironmental basin states to better-understand their tempo (Fig. 2). This overall pattern of ecosystem and lake-level change are very closely mirrored by the physical properties and sediment characteristics described by Lyons et al. (5) when cast on the same age model (Fig. 2). However, our data conclusively demonstrate that neither dataset can be simply correlated with lake level nor treated as a stationary record for reconstruction of climate (13) throughout the 1.2-My duration of the record, as the pre–800-ka lake was very different from the modern lake and experienced a much smaller dynamic range of water-level variability.
The change after 800 ka cannot be explained by climate variability alone. Lake conditions before 800 ka suggest a near-continuously open hydrologic system, implying that Lake Malawi had an outlet despite persistent shallower depths, even during lowstands. Following the transition that ended at ∼700 ka, although the lake was much deeper during wet periods, it was frequently a closed basin with water levels that did not reach the modern outlet height, through the Shire River and into the Zambezi River system. The sill elevation of the Shire outlet precludes an open basin at a much lower elevation; similarly, it would be extremely difficult to maintain an open basin with a lake surface elevation hundreds of meters above the current Shire outlet, as is implied in the quantitative paleolake-level reconstruction shown in Lyons et al. (5). It is much more likely that the state change after 800 ka was linked to a secondary overprint of watershed processes of tectonic origin, potentially outside of the current lake margins. A likely candidate is a significant rise in lake surface base level driven by a shift in the outlet position and subsequent increased threshold elevation of the lake. If an outlet were blocked by progressive uplift, this blockage would cause the lake to deepen during wetter climates until it encountered the next-lowest threshold [e.g., the Shire River outlet at ∼477 m asl (meters above sea level)]. The most likely candidate for an earlier outlet is the current influent and rift-antecedent Ruhuhu River (Fig. 1). Uplift at or near the current drainage divide separating the Ruhuhu River from eastward-directed watersheds flowing to the Indian Ocean would explain the incised meanders and eastward-directed tributaries observed within the modern Ruhuhu watershed (SI Appendix, Figs. S12 and S13).
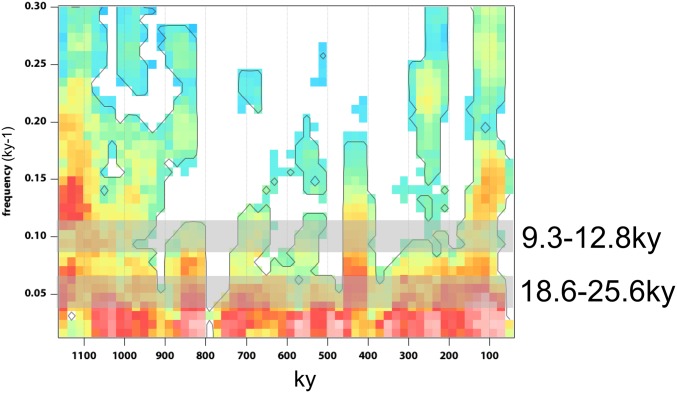
As a result of our improved age model, our record demonstrates a change in hydroclimate variability after 900 ka, suggesting a similar timing to the Mid-Pleistocene Transition (MPT) (14). This frequency change preceded the basin reconfiguration by ∼100 ky. An analysis of evolutive spectrum as well as spectral analysis conducted using the multitaper method on the PC1 hydroclimate reconstruction indicates a change from suborbital hydroclimate variability (∼10 ky) to precessional and suborbital frequencies (∼19–23 ky, 10 ky) across the MPT (Figs. 2 and 3 and SI Appendix, Fig. S11).
Fig. 3.
Evolutive spectrum of PC1 from core MAL05-1B using the multitaper mean method on 80-ky intervals with 20-ky steps. Color background is significant at 50% with 90% significance contour. Gray bars show precessional (18.6 to 25.6 ky) and suborbital frequencies (9.3 to 12.8 ky).
This is in contrast with high-latitude and North African paleoclimate records that show a transition from 41-ky frequencies associated with obliquity to 100-ky frequencies related to eccentricity across the MPT (Fig. 2) (7, 15). In the high latitudes, changes in obliquity result in large summer insolation changes (3 to 9%) and therefore snow melt and ice-sheet growth. However, the obliquity influence reduces toward the tropics and, at the equator, increased obliquity results in a negligible decrease in local summer insolation (∼−0.4%), likely not sufficient to force hydrological change (16). Furthermore, although there is no indication from the multitaper spectral analysis of a direct 41-ky frequency before the MPT or 100-ky frequency following the MPT as in high-latitude studies, we observe some power associated with combination tones of Milankovitch periods (SI Appendix, Fig. S11). In particular, Quaternary equatorial marine core and deep time records suggest that variability associated with eccentricity or obliquity may be modulated by the strong influence of tropical precession, producing mixed-frequency peaks (17, 18). These frequencies are observed in our record and vary across the MPT, suggesting a modulation by precession of obliquity before the MPT (13.5 ky) and of eccentricity after the MPT (70 ky). This observation implies that teleconnections from high latitudes were overprinted on the strong local insolation control before and after the MPT.
Suborbital frequencies such as those observed at Lake Malawi before the MPT are in theory important climate forcing factors in the tropics (19). Notably, a 40-ky record from Afrotropical Lake Challa illustrates a half-precession frequency, which represents the response of equatorial hydrology to insolation at locations with a double rainy season (20). Although today Lake Malawi at ∼15°S is located outside of the modern equatorial zone and rainfall is unimodal, the observance of half-precession suggests that before the MPT, Malawi received rainfall twice yearly. We suggest that the double rainy season as far south as 15°S before the MPT could result from a wider southern-hemisphere Hadley cell. Observations over the last few decades have recorded such Hadley expansion, suggesting that this phenomenon results from a warmer atmosphere (21, 22). The transition at Malawi following the MPT from suborbital to precession/suborbital variability in hydrologic responses is likely the reverse effect. The MPT in marine records corresponds to a shift in mean δ18O, related to a mean increase in Antarctic ice volume across that boundary (23). Lower Antarctic ice volume before the MPT could result in a more southerly extension of the southern-hemisphere Hadley cell, resulting in a double rainy season in a wider area in the southern tropics relative to today, and thus a more symmetrical Hadley circulation about the equator.
In addition to clarifying the roles of watershed tectonics and pre- versus post-MPT climate variability in the African subtropics, our refined chronology allows the dynamics of the Lake Malawi cichlid fish radiation to be understood in a new context. The strong correspondence between lake-level history, basin configuration, and the rhythm of insolation variability, especially the 400-ky eccentricity modulation of the magnitude of precession cycles, has imposed a first-order structure on Lake Malawi ecosystems over the course of our record. Shallower base level before 800 ka, probably associated with a lower outlet, prevented deep lake formation even at times of high insolation. An easterly directed outlet, perhaps via the Ruhuhu River to either the Rovuma or Rufiji River systems, would explain the strong phylogenetic relationship of the Malawi cichlids with rivers in this region as opposed to the more southerly connections that exist today (24, 25). After 800 ka with the formation of a deeper topographic basin, insolation thresholds became much more important in dictating lake persistence.
We hypothesize that thresholds exist for lake persistence related to insolation magnitude that can also be applied to other East African lake systems based on basin morphometry. Lakes with high surface area-to-volume ratios may be unable to persist when eccentricity is low, and precessional maxima are therefore lower than during high-eccentricity intervals. In other words, there may be a threshold effect imposed by the long-term (400-ky) eccentricity cycles that effectively make lakes insensitive to precessional forcing at the lowest amplitude intervals. Lake basins in this state would remain either low or dry during these periods, with deep lakes only developing during extreme precession maxima, as is the case in the Eastern Rift Valley today (26). However, in steep-sided rifts with low surface area-to-volume such as Lake Malawi, basin geometry requires 70% of the water volume to be evaporated before significant lake-level regression occurs (5). In this case, the lake remains higher unless a much larger change in forcing drives lake-level changes. Thus, the threshold is only crossed during eccentricity maxima when summer insolation reaches sufficiently low values to cause very severe precipitation reductions. Such lakes only dry out during extreme insolation lows, resulting in the Lake Malawi megadroughts of the early Late Pleistocene (27). This would result in an insensitivity to summer insolation variability during eccentricity minima; however, in contrast, here lakes remain deep during these times, as is the case for the past ∼70 ky, and at ∼400-ky intervals before today.
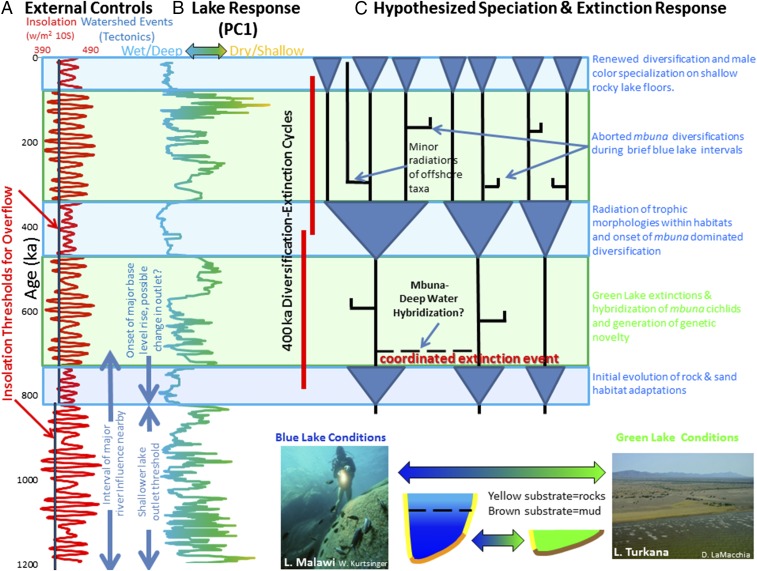
The onset of ∼400-ky cycles at 800 ka generated alternating intervals in Lake Malawi with vastly different lake characteristics (Fig. 4). During times of deep lake persistence, blue lake phases at Malawi were fresh, and strongly stratified with low surface water nutrients and phytoplankton and extensive littoral rocky habitat (4, 10). In contrast, green lake phases were characterized by well-mixed, turbid, saline, shallow lakes, with little submerged rocky substrate (5).
Fig. 4.
Proposed insolation thresholds (vertical bars) for maintaining overflow conditions during pre– and post–800-ka intervals. (A) A higher insolation threshold would have been required to maintain an open lake once the sill outlet elevation rose and the maximum lake depth deepened. (B) The lake responds to insolation variability above threshold values by rising and transforming in state. This is intensified during the post–800-ka period, when deep blue lake conditions prevail most continuously every 400 ky, comparable to modern Lake Malawi. This contrasts with green lakes during insolation lows below the insolation threshold, when the lake was comparable to modern Lake Turkana. (C) Evolutionary responses include major cycles of diversification and extinction every ∼400 ky. Other phylogenetic events that match this chronology discussed in the main text include an initial evolution of rock and sand habitat adaptations ∼800 ka, green lake phase extinctions and hybridization of rock-dwelling (mbuna) littoral cichlids, radiation of trophic morphologies at ∼400 ka, and diversification of modern color specializations during the most recent (current) blue lake phase.
We hypothesize that maintaining blue lake conditions for long periods of time (on the order of >104 y) was critical to allowing rocky habitat-based ecosystems to develop and diversification based on assortative mating to evolve. Molecular genetic data for Lake Malawi cichlids are consistent with this interpretation. We propose that this green–blue lake phasing generated diversification and extinction cycles (28–30) as a first-order control on diversification and extinction within littoral habitats, and is consistent with hypothesized phases of diversification (8) and timing from molecular genetic data (24). An initial burst of diversification around 749 ka (31) corresponds to the earliest evidence of sustained blue lake conditions in Lake Malawi over the last ∼1.2 My, which would have created the rock–sand habitat differentiation thought to have generated initial within-lake adaptations (Fig. 4). Before that, lake-level and other environmental fluctuations appear to have been too frequent to allow diversification, and rocky littoral habitats were uncommon. A subsequent blue lake phase at ∼400 ka may mark the timing of the evolution of distinct trophic morphologies, also consistent with the branch topology of mtDNA trees (24). Finally, most populations of rock-dwelling (mbuna) cichlids expanded during the last 75,000 y, the most recent blue lake phase of the lake (32), consistent with the third phase of male color diversification of Kocher (8). That study also found support for dispersal within regions with genetic diversity accumulating locally following lake-level rise, implying that assortative mating evolved on similar timescales. Conversely, based on comparison with modern lakes, especially those undergoing rapid eutrophication (33, 34), the transition to green lake phases would have generated coordinated episodes of extinction, hybridization, and reduced biodiversity. Haplotype sharing and hybridization were probably the norm during these phases, when assortative mating using visual cues would have been suppressed by more turbid waters (33, 34). Molecular clock data place the onset of mbuna-dominated diversification within the time frame of the penultimate blue lake phase of Malawi (35), consistent with our model outlined in Fig. 4.
Diversification could have occurred during predominant green phases, but it is probably manifest in Malawian cichlid phylogeny in very different ways. Bursts of diversification almost certainly occurred during brief blue lake interludes in what are otherwise green lake cycles. However, these were probably aborted as the lake quickly reverted to its green lake state. More importantly, introgressive hybridization among lineages in which assortative mating breaks down under turbid green lake conditions may have been an important source of evolutionary novelty. For example, molecular clock data suggest that during an early green lake phase, some deep benthic cichlid clades evolved through mbuna hybridization (36). These taxa today are specialized for low-light feeding, similar to what would have been the shallow water norm during a green lake interval. Other groups that underwent minor radiations during green lake phases are open-water, pelagic predators such as Diplotaxodon, which today show little genetic differentiation around the lake and would not be subject to the most severe effects of green lake habitat loss experienced by the mbuna cichlids (25). More generally, green lake phases may be critical for “priming the pump” of diversification by creating a wide variety of novel morphologic/behavioral phenotypes during hybridization episodes (37) that could be exploited during subsequent blue lake radiations. Our causative model would also help explain why Lake Tanganyika does not appear to show the three-phase evolutionary history proposed by Kocher (8), as that lake, because of its much greater depth, probably persisted as a blue lake with rocky littoral habitats through the 400-ky cycles that so strongly affected Lake Malawi (38).
Methods
Age Model.
Our age model uses all 14C, Ar/Ar, and paleomagnetic reversal and excursion data previously published in Lyons et al. (5). Additionally, a series of new age constraints based on paleomagnetic excursion and intensity data has been added (SI Appendix, Figs. S2–S7 and Table S1). The age model itself was created by fitting the data with a monotonic polynomial spline (SI Appendix, Fig. S1).
Paleoecology.
Sediment samples (n = 1,945) were taken at 16-cm intervals (average intersample resolution ∼570 y). Samples were split into two aliquots; the first was weighed and then disaggregated using deionized water and freeze–thaw treatment (often requiring multiple cycles), and finally washed through 63-μm mesh stainless steel sieves to recover all material sand-sized and greater. The second aliquot was weighed wet, oven-dried at 60 °C for at least 48 h, and reweighed to determine water content. This calculated water content was then used to determine the dry weight of the sieved aliquot.
Wet-sieved residues were counted for the total number of ostracodes per sample (normalized to abundance per g dry weight) and for taphonomic condition (% broken, whole carapace, adult, carbonate-coated, and oxidation/reduction-stained), and each valve was identified to the species level where possible. Additionally, the wet-sieved residues were counted for absolute abundance of other common fossil remains in this same size range [chaoborid fragments, charred particles, fish (including bones, teeth, and scales), algae (Pediastrum and Botryococcus), and Polypodiaceae sori] and the relative abundance of mollusk fragments and both monomineralic and lithic grains in the sand fraction (specifically quartz, mica, pyrite, siderite, vivianite, aragonite needles, and ooids). Because of the variety of data types in the dataset, we used a principal component analysis to reduce dimensionality. Indicators included in this analysis were the relative abundance of the key ostracode taxa Cypridopsines, Limnocythere, Candonopsis, Ilyocypris, and Sclerocypris, their taphonomic condition (broken, whole carapace, adult, and carbonate-coated), the log-transformed concentrations of chaoborid fragments, charred particles, fish (including bones, teeth, and scales), algae (Pediastrum and Botryococcus), and Polypodiaceae sori, and the relative abundance of mollusk fragments and authigenic and terrigenous mineral grains in the sand fraction (quartz, mica, pyrite, siderite, and ooids).
Spectral Analysis and Evolutive Spectrum.
For both the multitaper evolutive spectral analysis conducted on PC1 and presented in the main text of the paper and the spectral analysis in SI Appendix, Fig. S11, multitaper mean spectral analysis with the AR1 (autoregressive) noise spectrum was conducted to look in detail at the relative influence of different frequencies across the Mid-Pleistocene Transition. For this analysis, PC1 was smoothed (five points), divided into pre- and post-MPT time series (0–700 and 800–1,200 ka), and run separately. Data pretreatment to a uniform time step involved interpolating to a 250-y resolution and then resampling to 1,000 y, which is closer to the average resolution before pretreatment.
Supplementary Material
Acknowledgments
We thank all participants involved in the Lake Malawi Drilling Project, in particular Chris Scholz, Tom Johnson, Robert Lyons, and Eric Brown. We also thank Walter Salzburger for valuable discussion of cichlid evolution and Sylvia Dee for spectral analysis code. Initial core processing and sampling were conducted at LacCore at the University of Minnesota. Sample processing was conducted by Devin Gaugler, Chris Johnson, Jeanine Ash, and Claire DeCelles. Funding came from the US National Science Foundation–Earth System History Program (EAR-0602350); International Continental Scientific Drilling Program, American Chemical Society–PRF (54376-DNI8); and Smithsonian Institution. Partial sample preparation and analysis support was received from DOSECC, Chevron and BP summer scholarships, and UA SAGUARO.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.P.S. is a Guest Editor invited by the Editorial Board.
Data deposition: Raw ostracod data are included in figure form in SI Appendix, and tabulated data have been archived at the National Center for Climate Data (https://www.ncdc.noaa.gov/data-access/paleoclimatology-data/datasets); all core metadata are available at www.ngdc.noaa.gov/geosamples/showsample.jsp?imlgs=imlgs0195811.
See Commentary on page 11654.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611028113/-/DCSupplemental.
References
- 1.Intergovernmental Panel on Climate Change (IPCC) In: Climate Change 2013: The Physical Science Basis. Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Stocker TF, et al., editors. Cambridge Univ Press; New York: 2013. [Google Scholar]
- 2.Thieme ML, et al. Freshwater Ecoregions of Africa and Madagascar: A Conservation Assessment. Island; Washington, DC: 2005. [Google Scholar]
- 3.Salzburger W, Van Bocxlaer B, Cohen AS. Ecology and evolution of the African Great Lakes and their faunas. Annu Rev Ecol Evol Syst. 2014;45:519–545. [Google Scholar]
- 4.Cohen AS, et al. Ecological consequences of early Late Pleistocene megadroughts in tropical Africa. Proc Natl Acad Sci USA. 2007;104(42):16422–16427. doi: 10.1073/pnas.0703873104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyons RP, et al. Continuous 1.3-million-year record of East African hydroclimate, and implications for patterns of evolution and biodiversity. Proc Natl Acad Sci USA. 2015;112(51):15568–15573. doi: 10.1073/pnas.1512864112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupont LM, Jahns S, Marret F, Ning S. Vegetation change in equatorial West Africa: Time-slices for the last 150 ka. Palaeogeogr Palaeoclimatol Palaeoecol. 2000;155(1–2):95–122. [Google Scholar]
- 7.deMenocal PB, Ruddiman WF, Pokras EM. Influences of high‐ and low‐latitude processes on African terrestrial climate: Pleistocene eolian records from equatorial Atlantic Ocean Drilling Program site 663. Paleoceanography. 1993;8(2):209–242. [Google Scholar]
- 8.Kocher TD. Adaptive evolution and explosive speciation: The cichlid fish model. Nat Rev Genet. 2004;5(4):288–298. doi: 10.1038/nrg1316. [DOI] [PubMed] [Google Scholar]
- 9.Ebinger C. Tectonic development of the western branch of the East African rift system. Geol Soc Am Bull. 1989;101(7):885–903. [Google Scholar]
- 10.Stone JR, Westover KS, Cohen AS. Late Pleistocene paleohydrography and diatom paleoecology of the central basin of Lake Malawi, Africa. Palaeogeogr Palaeoclimatol Palaeoecol. 2011;303(1):51–70. [Google Scholar]
- 11.Holmes JA, Hales PE, Street-Perrott FA. Trace-element chemistry of non-marine ostracods as a means of palaeolimnological reconstruction: An example from the Quaternary of Kashmir, northern India. Chem Geol. 1992;95(1):177–186. [Google Scholar]
- 12.Wilkin R, Barnes H. Formation processes of framboidal pyrite. Geochim Cosmochim Acta. 1997;61(2):323–339. [Google Scholar]
- 13.Johnson TC, et al. A progressively wetter climate in southern East Africa over the past 1.3 million years. Nature. 2016;537(7619):220–224. doi: 10.1038/nature19065. [DOI] [PubMed] [Google Scholar]
- 14.Ferretti P, Crowhurst SJ, Hall MA, Cacho I. North Atlantic millennial-scale climate variability 910 to 790 ka and the role of the equatorial insolation forcing. Earth Planet Sci Lett. 2010;293(1):28–41. [Google Scholar]
- 15.Clark PU, et al. The middle Pleistocene transition: Characteristics, mechanisms, and implications for long-term changes in atmospheric pCO2. Quat Sci Rev. 2006;25(23):3150–3184. [Google Scholar]
- 16.Lee SY, Poulsen CJ. Tropical Pacific climate response to obliquity forcing in the Pleistocene. Paleoceanography. 2005;20(4):PA4010. [Google Scholar]
- 17.Rodríguez-Tovar FJ, Pardo-Igúzquiza E. Strong evidence of high-frequency (sub-Milankovitch) orbital forcing by amplitude modulation of Milankovitch signals. Earth Planet Sci Lett. 2003;210(1):179–189. [Google Scholar]
- 18.Clemens SC, Prell WL, Sun Y. Orbital‐scale timing and mechanisms driving Late Pleistocene Indo‐Asian summer monsoons: Reinterpreting cave speleothem δ18O. Paleoceanography. 2010;25(4):PA4207. [Google Scholar]
- 19.Berger A, Loutre M-F, Mélice J. Equatorial insolation: From precession harmonics to eccentricity frequencies. Clim Past. 2006;2(2):131–136. [Google Scholar]
- 20.Verschuren D, et al. CHALLACEA project members Half-precessional dynamics of monsoon rainfall near the East African equator. Nature. 2009;462(7273):637–641. doi: 10.1038/nature08520. [DOI] [PubMed] [Google Scholar]
- 21.Lu J, Deser C, Reichler T. Cause of the widening of the tropical belt since 1958. Geophys Res Lett. 2009;36(3):L03803. [Google Scholar]
- 22.Seidel DJ, Fu Q, Randel WJ, Reichler TJ. Widening of the tropical belt in a changing climate. Nat Geosci. 2008;1(1):21–24. [Google Scholar]
- 23.Pollard D, DeConto RM. Modelling West Antarctic ice sheet growth and collapse through the past five million years. Nature. 2009;458(7236):329–332. doi: 10.1038/nature07809. [DOI] [PubMed] [Google Scholar]
- 24.Joyce DA, et al. Repeated colonization and hybridization in Lake Malawi cichlids. Curr Biol. 2011;21(3):R108–R109. doi: 10.1016/j.cub.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 25.Genner MJ, Turner GF. Timing of population expansions within the Lake Malawi haplochromine cichlid fish radiation. Hydrobiologia. 2015;748(1):121–132. [Google Scholar]
- 26.Trauth MH, et al. High- and low-latitude forcing of Plio-Pleistocene East African climate and human evolution. J Hum Evol. 2007;53(5):475–486. doi: 10.1016/j.jhevol.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Scholz CA, et al. East African megadroughts between 135 and 75 thousand years ago and bearing on early-modern human origins. Proc Natl Acad Sci USA. 2007;104(42):16416–16421. doi: 10.1073/pnas.0703874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCune AR, Thomson KS, Olsen PE. Semionotid fishes from the Mesozoic great lakes of North America. In: Echelle AA, Kornfield I, editors. Evolution of Fish Species Flocks. Univ of Maine at Orono Press; Orono, ME: 1984. pp. 27–44. [Google Scholar]
- 29.Schön I, Martens K. Adaptive, pre-adaptive and non-adaptive components of radiations in ancient lakes: A review. Org Divers Evol. 2004;4(3):137–156. [Google Scholar]
- 30.Schultheiss R, Van Bocxlaer B, Wilke T, Albrecht C. Old fossils–young species: Evolutionary history of an endemic gastropod assemblage in Lake Malawi. Proc Biol Sci. 2009;276(1668):2837–2846. doi: 10.1098/rspb.2009.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer BS, Matschiner M, Salzburger W. 2016. Disentangling incomplete lineage sorting and introgression to refine species-tree estimates for Lake Tanganyika cichlid fishes. bioRxiv:039396.
- 32.Genner MJ, Knight ME, Haesler MP, Turner GF. Establishment and expansion of Lake Malawi rock fish populations after a dramatic Late Pleistocene lake level rise. Mol Ecol. 2010;19(1):170–182. doi: 10.1111/j.1365-294X.2009.04434.x. [DOI] [PubMed] [Google Scholar]
- 33.Seehausen O, Van Alphen JJ, Witte F. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science. 1997;277(5333):1808–1811. [Google Scholar]
- 34.Seehausen O. Hybridization and adaptive radiation. Trends Ecol Evol. 2004;19(4):198–207. doi: 10.1016/j.tree.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Genner MJ, et al. Age of cichlids: New dates for ancient lake fish radiations. Mol Biol Evol. 2007;24(5):1269–1282. doi: 10.1093/molbev/msm050. [DOI] [PubMed] [Google Scholar]
- 36.Genner MJ, Turner GF. Ancient hybridization and phenotypic novelty within Lake Malawi’s cichlid fish radiation. Mol Biol Evol. 2012;29(1):195–206. doi: 10.1093/molbev/msr183. [DOI] [PubMed] [Google Scholar]
- 37.Selz OM, Lucek K, Young KA, Seehausen O. Relaxed trait covariance in interspecific cichlid hybrids predicts morphological diversity in adaptive radiations. J Evol Biol. 2014;27(1):11–24. doi: 10.1111/jeb.12283. [DOI] [PubMed] [Google Scholar]
- 38.Muschick M, et al. Testing the stages model in the adaptive radiation of cichlid fishes in East African Lake Tanganyika. Proc Biol Sci. 2014;281(1795):20140605. doi: 10.1098/rspb.2014.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jouzel J, et al. Orbital and millennial Antarctic climate variability over the past 800,000 years. Science. 2007;317(5839):793–796. doi: 10.1126/science.1141038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.