Abstract
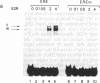
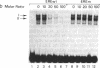
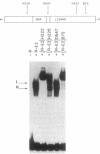
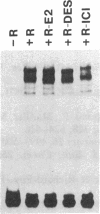
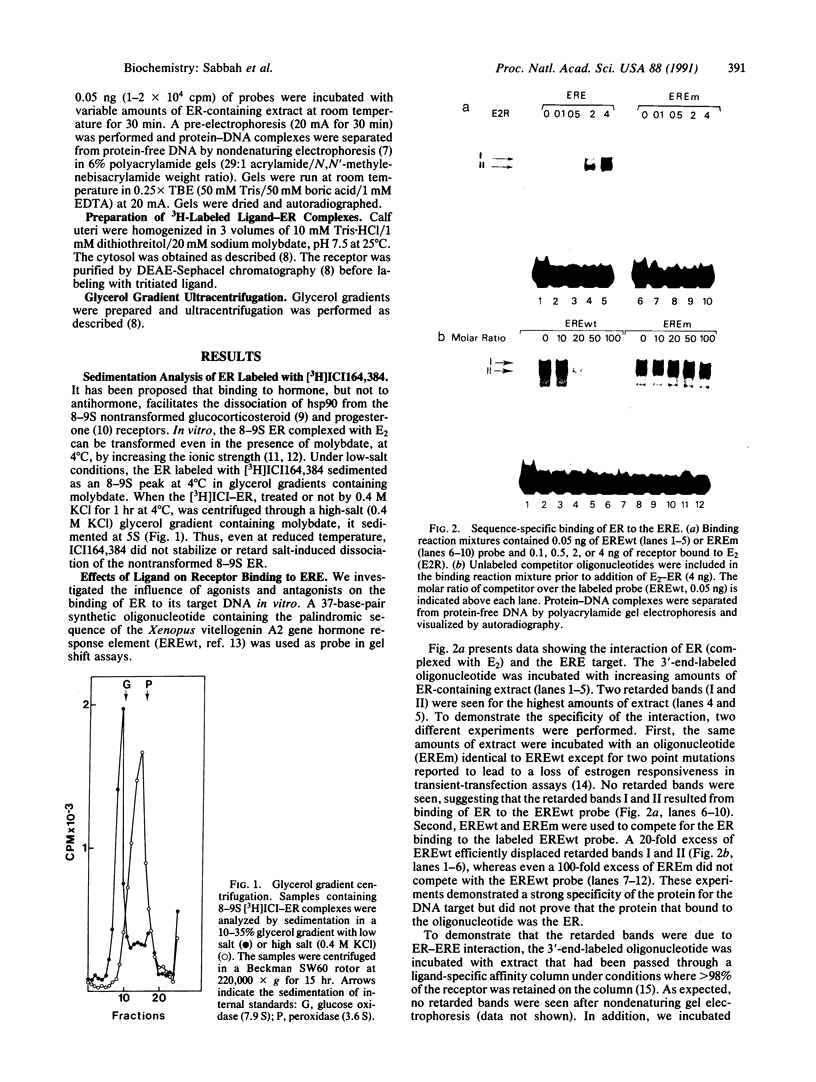
To investigate the molecular mechanism(s) by which the estrogen receptor (ER) modulates transcription, we compared the structures of receptor complexes containing estradiol, the agonist diethylstilbestrol, and the antagonist ICI164,384. The binding of ICI164,384 to nontransformed 8-9S ER does not preclude the salt-induced dissociation of the 90-kDa heat shock protein and releases the transformed homodimeric 5S ER as classically observed in the presence of agonist. We report that calf ER binds to the estrogen response element of the Xenopus vitellogenin A2 gene in either the absence or presence of hormone, agonist, or antagonist. These binding interactions were highly sequence- and receptor-specific. These findings indicate that ligand binding in vitro is not absolutely required for dimerization or specific DNA binding of the ER. As demonstrated by gel retardation assays, the ICI164,384-ER complex bound to the response element displays a slower mobility than complexes formed in the presence of estradiol or agonist. This difference in electrophoretic mobility is suggestive of a conformational change in the complex induced by the ligand. An exchange experiment demonstrated that this alteration of the structure is reversible. We suggest that ICI164,384 induces conformational modifications within the ligand-binding domain of the receptor that do not prevent binding to the response element but could fail to promote subsequent events required for gene transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailly A., Le Page C., Rauch M., Milgrom E. Sequence-specific DNA binding of the progesterone receptor to the uteroglobin gene: effects of hormone, antihormone and receptor phosphorylation. EMBO J. 1986 Dec 1;5(12):3235–3241. doi: 10.1002/j.1460-2075.1986.tb04634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu E. E. Steroid hormone antagonists at the receptor level: a role for the heat-shock protein MW 90,000 (hsp 90). J Cell Biochem. 1987 Oct;35(2):161–174. doi: 10.1002/jcb.240350209. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Brown M., Sharp P. A. Human estrogen receptor forms multiple protein-DNA complexes. J Biol Chem. 1990 Jul 5;265(19):11238–11243. [PubMed] [Google Scholar]
- Chalepakis G., Arnemann J., Slater E., Brüller H. J., Gross B., Beato M. Differential gene activation by glucocorticoids and progestins through the hormone regulatory element of mouse mammary tumor virus. Cell. 1988 May 6;53(3):371–382. doi: 10.1016/0092-8674(88)90157-2. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S. E., White R., Hoare S., Sydenham M., Page M., Parker M. G. Inhibition of estrogen receptor-DNA binding by the "pure" antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6883–6887. doi: 10.1073/pnas.87.17.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisse S., Scheidereit C., Westphal H. M., Hynes N. E., Groner B., Beato M. Glucocorticoid receptors recognize DNA sequences in and around murine mammary tumour virus DNA. EMBO J. 1982;1(12):1613–1619. doi: 10.1002/j.1460-2075.1982.tb01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988 Nov;4(11):309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Sobel N. B., King W. J., Jensen E. V. Immunochemical studies of estrogen receptors. J Steroid Biochem. 1984 Jan;20(1):51–56. doi: 10.1016/0022-4731(84)90188-2. [DOI] [PubMed] [Google Scholar]
- Groyer A., Schweizer-Groyer G., Cadepond F., Mariller M., Baulieu E. E. Antiglucocorticosteroid effects suggest why steroid hormone is required for receptors to bind DNA in vivo but not in vitro. Nature. 1987 Aug 13;328(6131):624–626. doi: 10.1038/328624a0. [DOI] [PubMed] [Google Scholar]
- Guiochon-Mantel A., Loosfelt H., Ragot T., Bailly A., Atger M., Misrahi M., Perricaudet M., Milgrom E. Receptors bound to antiprogestin from abortive complexes with hormone responsive elements. Nature. 1988 Dec 15;336(6200):695–698. doi: 10.1038/336695a0. [DOI] [PubMed] [Google Scholar]
- Horigome T., Golding T. S., Quarmby V. E., Lubahn D. B., McCarty K., Sr, Korach K. S. Purification and characterization of mouse uterine estrogen receptor under conditions of varying hormonal status. Endocrinology. 1987 Dec;121(6):2099–2111. doi: 10.1210/endo-121-6-2099. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Schorpp M., Wagner U., Ryffel G. U. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986 Sep 26;46(7):1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Klock G., Strähle U., Schütz G. Oestrogen and glucocorticoid responsive elements are closely related but distinct. Nature. 1987 Oct 22;329(6141):734–736. doi: 10.1038/329734a0. [DOI] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Lees J. A., Fawell S. E., Parker M. G. Identification of two transactivation domains in the mouse oestrogen receptor. Nucleic Acids Res. 1989 Jul 25;17(14):5477–5488. doi: 10.1093/nar/17.14.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. M., Berthois Y., Jensen E. V. Binding of antiestrogens exposes an occult antigenic determinant in the human estrogen receptor. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2533–2537. doi: 10.1073/pnas.85.8.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudgil V. K., Hurd C. Transformation of calf uterine progesterone receptor: analysis of the process when receptor is bound to progesterone and RU38486. Biochemistry. 1987 Aug 11;26(16):4993–5001. doi: 10.1021/bi00390a017. [DOI] [PubMed] [Google Scholar]
- Murdoch F. E., Meier D. A., Furlow J. D., Grunwald K. A., Gorski J. Estrogen receptor binding to a DNA response element in vitro is not dependent upon estradiol. Biochemistry. 1990 Sep 11;29(36):8377–8385. doi: 10.1021/bi00488a026. [DOI] [PubMed] [Google Scholar]
- Piña B., Brüggemeier U., Beato M. Nucleosome positioning modulates accessibility of regulatory proteins to the mouse mammary tumor virus promoter. Cell. 1990 Mar 9;60(5):719–731. doi: 10.1016/0092-8674(90)90087-u. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Redeuilh G., Moncharmont B., Secco C., Baulieu E. E. Subunit composition of the molybdate-stabilized "8-9 S" nontransformed estradiol receptor purified from calf uterus. J Biol Chem. 1987 May 25;262(15):6969–6975. [PubMed] [Google Scholar]
- Redeuilh G., Secco C., Baulieu E. E., Richard-Foy H. Calf uterine estradiol receptor. Effects of molybdate on salt-induced transformation process and characterization of a nontransformed receptor state. J Biol Chem. 1981 Nov 25;256(22):11496–11502. [PubMed] [Google Scholar]
- Redeuilh G., Secco C., Mester J., Baulieu E. E. Transformation of the 8-9 S molybdate-stabilized estrogen receptor from low-affinity to high-affinity state without dissociation into subunits. J Biol Chem. 1987 Apr 25;262(12):5530–5535. [PubMed] [Google Scholar]
- Richard-Foy H., Hager G. L. Sequence-specific positioning of nucleosomes over the steroid-inducible MMTV promoter. EMBO J. 1987 Aug;6(8):2321–2328. doi: 10.1002/j.1460-2075.1987.tb02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah M., Redeuilh G., Baulieu E. E. Subunit composition of the estrogen receptor. Involvement of the hormone-binding domain in the dimeric state. J Biol Chem. 1989 Feb 15;264(5):2397–2400. [PubMed] [Google Scholar]
- Shyamala G., Leonard L. Inhibition of uterine estrogen receptor transformation by sodium molybdate. J Biol Chem. 1980 Jul 10;255(13):6028–6031. [PubMed] [Google Scholar]
- Tora L., Mullick A., Metzger D., Ponglikitmongkol M., Park I., Chambon P. The cloned human oestrogen receptor contains a mutation which alters its hormone binding properties. EMBO J. 1989 Jul;8(7):1981–1986. doi: 10.1002/j.1460-2075.1989.tb03604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling A. E., Bowler J. Novel antioestrogens without partial agonist activity. J Steroid Biochem. 1988 Oct;31(4B):645–653. doi: 10.1016/0022-4731(88)90014-3. [DOI] [PubMed] [Google Scholar]
- Webster N. J., Green S., Jin J. R., Chambon P. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell. 1988 Jul 15;54(2):199–207. doi: 10.1016/0092-8674(88)90552-1. [DOI] [PubMed] [Google Scholar]
- Webster N. J., Green S., Tasset D., Ponglikitmongkol M., Chambon P. The transcriptional activation function located in the hormone-binding domain of the human oestrogen receptor is not encoded in a single exon. EMBO J. 1989 May;8(5):1441–1446. doi: 10.1002/j.1460-2075.1989.tb03526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- el-Ashry D., Oñate S. A., Nordeen S. K., Edwards D. P. Human progesterone receptor complexed with the antagonist RU 486 binds to hormone response elements in a structurally altered form. Mol Endocrinol. 1989 Oct;3(10):1545–1558. doi: 10.1210/mend-3-10-1545. [DOI] [PubMed] [Google Scholar]