Significance
Oligodendrocytes have been implicated in disease pathology in amyotrophic lateral sclerosis (ALS) using transgenic mouse models. To date there is no human coculture system available to investigate oligodendrocyte involvement in motor neuron (MN) death in ALS. Our data highlight that oligodendrocytes derived from patients with familial and sporadic ALS from induced pluripotent stem cells and induced neural progenitor cells play an active role in MN death. Oligodendrocyte toxicity is mediated through soluble factors and cell-to-cell contact, thus identifying multiple mechanisms of action and therapeutic opportunities. Their pathogenic phenotype can be reversed by achieving superoxide dismutase 1 knockdown in early oligodendrocyte progenitors in both familial and sporadic cases, but not chromosome 9 ORF 72 samples. This study provides important insights for patient subgrouping and timelines for therapeutic approaches.
Keywords: oligodendrocytes, amyotrophic lateral sclerosis, SOD1, C9orf72, lactate
Abstract
Oligodendrocytes have recently been implicated in the pathophysiology of amyotrophic lateral sclerosis (ALS). Here we show that, in vitro, mutant superoxide dismutase 1 (SOD1) mouse oligodendrocytes induce WT motor neuron (MN) hyperexcitability and death. Moreover, we efficiently derived human oligodendrocytes from a large number of controls and patients with sporadic and familial ALS, using two different reprogramming methods. All ALS oligodendrocyte lines induced MN death through conditioned medium (CM) and in coculture. CM-mediated MN death was associated with decreased lactate production and release, whereas toxicity in coculture was lactate-independent, demonstrating that MN survival is mediated not only by soluble factors. Remarkably, human SOD1 shRNA treatment resulted in MN rescue in both mouse and human cultures when knockdown was achieved in progenitor cells, whereas it was ineffective in differentiated oligodendrocytes. In fact, early SOD1 knockdown rescued lactate impairment and cell toxicity in all lines tested, with the exclusion of samples carrying chromosome 9 ORF 72 (C9orf72) repeat expansions. These did not respond to SOD1 knockdown nor did they show lactate release impairment. Our data indicate that SOD1 is directly or indirectly involved in ALS oligodendrocyte pathology and suggest that in this cell type, some damage might be irreversible. In addition, we demonstrate that patients with C9ORF72 represent an independent patient group that might not respond to the same treatment.
Amyotrophic lateral sclerosis (ALS) is the most common adult onset motor neuron (MN) disorder. Patients are initially affected by muscle weakness and fasciculations, rapidly leading to paralysis and eventually death by respiratory failure within 2–5 y from symptom onset. Approximately 10% of patients have a family history of the disease. Mutations in superoxide dismutase 1 (SOD1) (1), TAR DNA-binding protein 43 (TDP43) (2, 3), Fused in sarcoma (FUS) (4, 5), and hexanucleotide repeat expansions in chromosome 9 ORF 72 (C9orf72) (6, 7) are responsible for about 65% of these cases. On the contrary, the etiology of sporadic ALS, affecting about 90% of patients, is still largely unknown. Interestingly, familial and sporadic ALS are clinically indistinguishable, thus leading to the hypothesis that common mechanisms might be involved in disease etiology and progression (8). Nonetheless, the staggering complexity of this disorder and its fast progression have hampered the efforts to find an effective treatment. As a result, riluzole is the only FDA-approved drug for this disease, leading to a modest increase in survival (9).
Although MN degeneration is the most striking event occurring in ALS, in vitro and in vivo murine models of ALS have demonstrated that astrocytes (10, 11) and microglia (12, 13) play a crucial role in MN degeneration during disease progression. Recently, the availability of human samples has confirmed the toxic role of human astrocytes in vitro (14, 15). Elegant studies have shown that oligodendrocytes are also involved in the noncell-autonomous nature of ALS using mouse models of the disease (16–18). In fact, oligodendrocytes are severely affected during disease and their degeneration has been shown to precede MN death in the mutant SOD1 (mSOD1) mouse model (17, 18). Moreover, it has been reported that oligodendrocyte progenitors rapidly proliferate in the spinal cord of mSOD1G93A mice, but fail to replace degenerating oligodendrocytes, thus leaving MN axons demyelinated (17, 18).
Interestingly, removal of mSOD1G37R from only the oligodendrocyte lineage using the Cre-recombinase system under the platelet-derived growth factor alpha receptor (PDGFαR) promoter resulted in a significant delay in disease onset and increase in survival (17). Although the SOD1 mouse models of ALS have greatly helped identify the contribution of individual cell types to disease onset and progression, the complexity of the in vivo system makes it difficult to unravel the role of each cell type leading to MN degeneration. Coculture methods to evaluate oligodendrocytes in vitro may be beneficial to uncover novel therapeutics and may also help determining the timing of disease intervention for maximal therapeutic effect. Because mouse cell models can only be used to reproduce a minority of ALS cases, it remains unknown whether the same observations hold true in a broad spectrum of patients with ALS, including sporadic cases without known genetic cause.
To address these questions, we developed a coculture in vitro model to study both mouse and human ALS oligodendrocytes and their role in MN death. Our data show that oligodendrocytes can be successfully differentiated from mouse neural progenitor cells (NPCs) and human induced pluripotent stem cells (iPSCs), as well as iNPCs (19), from both non-ALS and ALS samples.
We find that oligodendrocytes from ALS samples convey toxicity toward MNs in vitro independent of their origin and that the toxicity can be rescued by reducing SOD1 in the oligodendrocyte precursor cells, but not in differentiated oligodendrocytes. However, the toxicity derived from cells carrying the C9orf72 repeat expansion seems to be SOD1 independent, because no response was seen in those cases.
Our work provides an in vitro coculture model of mouse and human oligodendrocytes for ALS, as well as oligodendrocytes–MN electrophysiology recordings. We demonstrate that oligodendrocytes from ALS samples induce MN death via distinct mechanisms of toxicity mediated by soluble factors and cell-to-cell contact when no sign of oligodendrocyte degeneration can be observed. Finally, this study provides insight into the detrimental role of oligodendrocytes on MNs in ALS and the involvement of SOD1 in different genetic variants of this disease, supporting the finding that C9orf72 mutations define a discrete subgroup of patients with ALS.
Results
Oligodendrocyte Differentiation from Mouse and Human Samples Does Not Differ Between ALS and Controls in Vitro.
Oligodendrocyte degeneration and impaired regeneration have been previously reported as contributors to ALS pathology (17). To study oligodendrocyte differentiation and maturation in ALS samples and their involvement in MN death, we developed a protocol to obtain myelin basic protein (MBP)+ cells from both mouse and human samples in vitro.
Oligodendrocyte progenitors cells (OPCs) were isolated from the cortex of neonate mSOD1G93A mice and WT littermates and cultured in proliferation medium containing PDGF with two alpha chains (PDGFaa). After 48 h, the cultures were stained for the oligodendrocyte progenitor marker NG2, showing 95% NG2+ cells. These cells were then cultured in medium depleted of PDGFaa and supplemented with insulin-like growth factor 1 (IGF-1) for 3 additional days to promote differentiation into MBP+, highly ramified cells (SI Appendix, Fig. S1A). During the differentiation protocol, about 5–10% of the OPCs died. Of the surviving cells, ∼84% were MBP+ and negative for microglia or astrocyte markers (SI Appendix, Figs. S1B and S2 A–D and Table S1). At the end of this 5-d differentiation protocol, cells were harvested and tested for RNA expression of oligodendrocyte, astrocyte, and microglia markers compared with the expression of the same markers in whole spinal cord homogenates as well as microglia and astrocytes isolated from the same preparation (SI Appendix, Fig. S1B). Quantitative PCR (qPCR) data showed that the cell population obtained was highly enriched for cells expressing oligodendrocyte markers and there were no differences between WT and mSOD1 oligodendrocytes in expression levels (SI Appendix, Fig. S3).
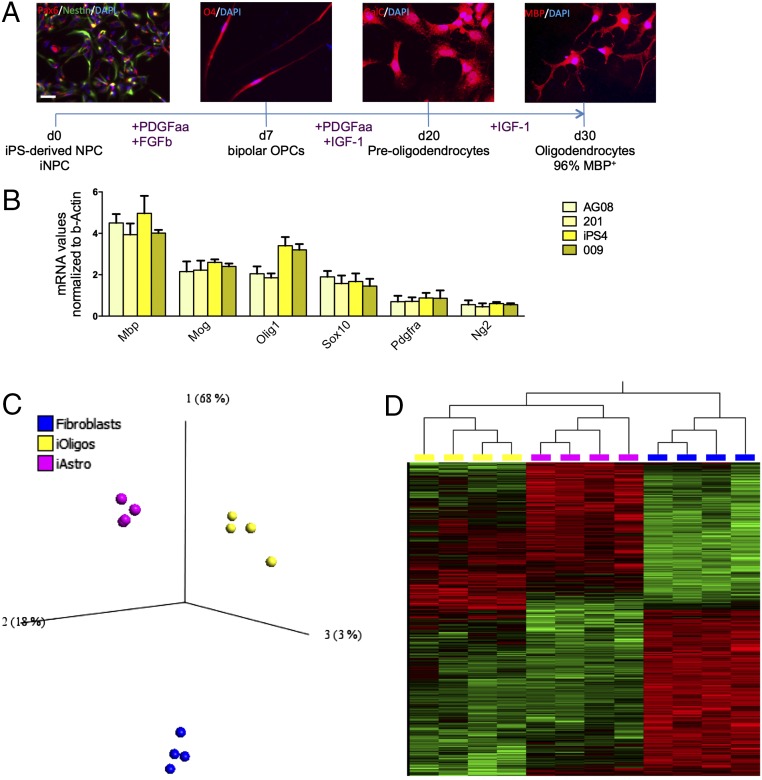
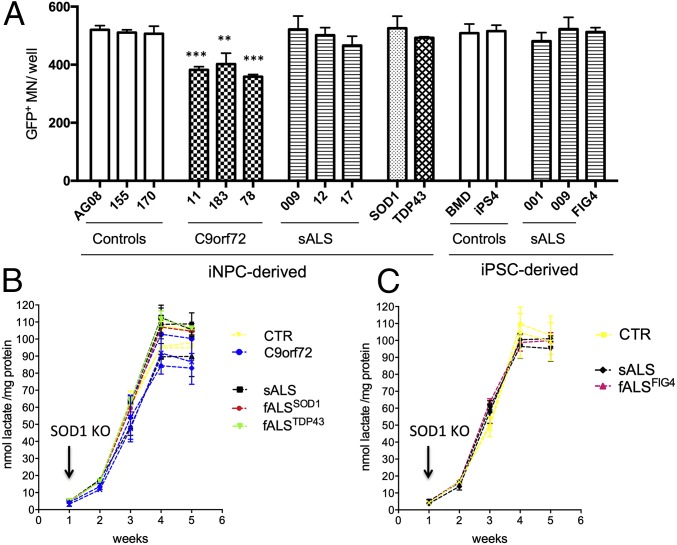
Following successful differentiation of murine cells, an adaptation of the same protocol was tested on human NPCs derived either from iPS colonies (20) or from human skin fibroblasts that were directly converted to iNPCs as previously described (19). NPCs obtained with either method were cultured with low concentrations of fibroblast growth factor 2 (FGF2) and high concentrations of PDGFaa (15 ng/mL) for 7 d. Immunostaining revealed that after only 7 d, the NPCs had considerably changed morphology from triangular to a definite bipolar shape and 82% were already positive for the late OPC marker O4 (Fig. 1A). For the following 13 d, these cells were cultured with reduced amounts of PDGFaa (10 ng/mL) and IGF1 (20 ng/mL). At day 20 of the differentiation protocol, ∼90% of the surviving cells expressed galactosylceramidase (GalC), the enzyme that leads to the production of galactosylceramide, an important component of myelin. For the following 10 d, the cultures were treated with high concentrations of IGF1 (50 ng/mL) without PDGFaa, leading to a definite morphologic change, accompanied by expression of MBP. This differentiation protocol yielded 50–65% survival compared with the initial number of NPCs or iNPCs plated, with 96% of the surviving cell population being positive for MBP (Fig. 1A and SI Appendix, Table S1). MBP+ cells from patients with ALS and controls, derived from iPSC or iNPCs, were analyzed for oligodendrocyte as well as astrocyte and microglia marker expression in comparison with whole spinal cord homogenate (Fig. 1B and SI Appendix, Figs. S2 E–H and S4 A and B). qPCR results showed that the cellular population obtained was highly enriched for cells expressing oligodendrocyte markers, independent of the genotype. Additionally, no difference in marker expression was detected between iPS-derived and iNPC-derived oligodendrocytes, as well as between ALS and control samples in agreement with data recently published (21). Of note, the iPS lines as well as the fibroblasts used for direct conversion were obtained from various sources, i.e., some were purchased from Coriell (https://www.coriell.org/) and some were obtained from national and international collaborators (SI Appendix, Table S2 provides a detailed description of each patient line). Despite the heterogeneous origin of the samples, no significant differences in differentiation patterns, including NPC production, oligodendrocyte yield, or marker expression, were noticed. Of note, the fibroblasts from the two patients with sporadic ALS, 002 and 009, were reprogrammed with both the classical iPS differentiation protocol and direct differentiation to iNPCs, with no differences observed in the ability to generate oligodendrocytes.
Fig. 1.
Efficient differentiation of human neural progenitors into MBP+ oligodendrocytes. Schematic representation of human NPC differentiation into MBP+ oligodendrocytes (A) and expression of oligodendrocyte markers at the end of differentiation determined by qPCR and normalized to β-actin (B). Expression levels are relative to whole spinal cord homogenates. Transcripts were investigated in four lines, two derived from controls (nos. 155 and 170) and two patients (nos. 12 and 17). Error bar = SD, n = 3 per sample. (Scale bar, 30 μm.) PCA reveals that iOligodendrocytes, iAstrocytes, and fibroblasts are three distinct cell populations (C) based on a two-way ANOVA multigroup comparison analysis (P < 0.001). Differentially expressed transcripts were visualized in a heat map, identifying iOligodendrocytes and iAstrocytes as more closely related cell types than they are to fibroblasts, even if significantly different (D).
To further confirm that the cells obtained with this protocol express the gene signature of oligodendrocytes, we performed a small gene expression study limited to four iOligodendrocyte lines from two controls (nos. 155 and 170) and two patients (nos. 12 and 17), four iAstrocyte lines from the same samples, and four fibroblast lines from one of our previously published studies (22). We focused on cell-type–specific gene expression rather than pathology-related transcriptional changes and we found that iOligodendrocytes, iAstrocytes, and human fibroblasts identify three distinct cell populations that significantly differ in gene expression as shown by the principal component analysis (PCA) carried out using the software platform Qlucore (Fig. 1C). Moreover, using a two-way ANOVA multigroup comparison analysis (P < 0.001) 3,361 transcripts were identified as differentially expressed between the three groups. These were visualized in a heat map that demonstrated that the three cell types are clearly distinguishable (Fig. 1D).
In particular, oligodendrocyte marker genes, such as MBP and several enzymes involved in lipoproteins and sphingolipids synthesis, were highly enriched in the iOligodendrocytes and were not found in the corresponding iAstrocytes differentiated from the same iNPCs or fibroblasts (SI Appendix, Table S3).
Gene enrichment analysis carried out using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) (https://david.ncifcrf.gov) also identified membrane and lumen maintenance as well as mitochondrial proteins as the most enriched categories (SI Appendix, Fig. S5), thus identifying iOligodendrocytes as highly metabolic demanding cells with significant membrane remodeling characteristics, as expected from previous studies (23, 24), that were not found in iAstrocytes or fibroblasts.
In conclusion, the method presented here produces high purity MBP+ oligodendrocytes from murine and human samples independent of the method used to establish the NPCs.
Mouse SOD1G93A Oligodendrocytes Induce MN Death in Vitro.
In vivo studies have shown that oligodendrocytes are affected by the pathogenic mechanisms involved in ALS, and removal of mSOD1 from this cell type improves survival in the mSOD1G37R ALS mouse model (17). However, it is still unclear whether oligodendrocytes are actively inducing MN damage or whether their own degeneration is contributing to an inevitable cascade of events leading to MN death. Having established a reliable protocol where ALS and control oligodendrocytes do not seem to differ in differentiation efficiency and survival, we proceeded to test MN viability in this coculture system.
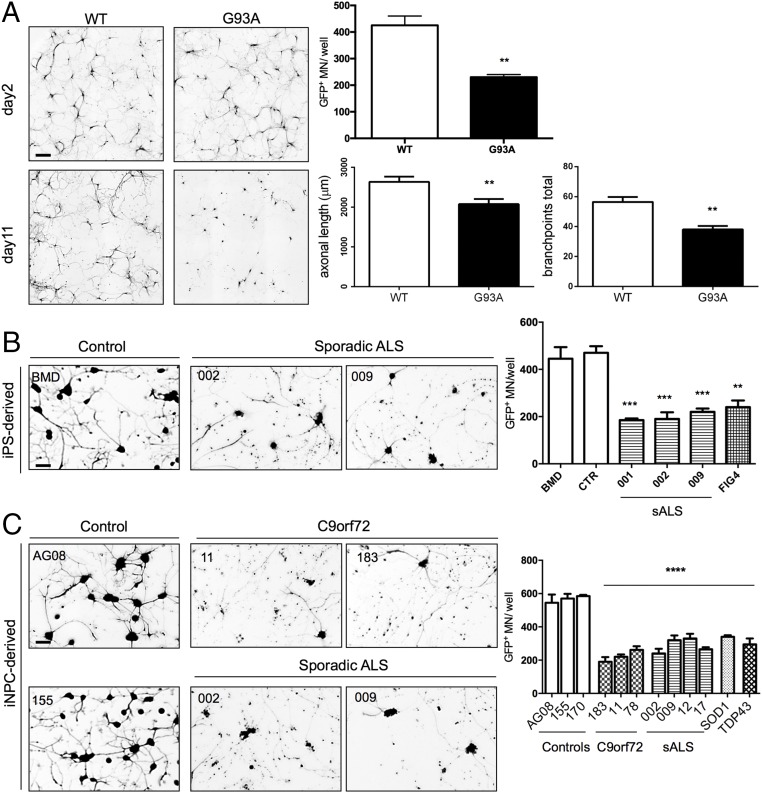
WT Hb9-GFP+ mouse MNs were plated onto 90–95% confluent MBP+ mouse oligodendrocytes. Forty-eight hours postplating, MNs extended long axons, presenting no differences in cell number or branching, regardless of the genotype of the oligodendrocytes (Fig. 2A). However, 11 d postplating, a significant 40% decrease in MN survival, along with decreased axonal length and branching, was detected in the cultures with mSOD1 oligodendrocytes (Fig. 2A).
Fig. 2.
Oligodendrocytes from ALS samples reduce MN survival. Coculture of mouse oligodendrocytes from mSOD1G93A mice and WT Hb9-GFP MNs result in reduced MN survival after 11 d compared with WT oligodendrocyte cocultures. This result is accompanied by reduction in axonal length and branching (A). (Scale bar, 100 μm.) Error bar = SD, n = 6. Coculture of human iPSC- and iNPC-derived oligodendrocytes from sporadic and familial ALS patients results in 50% increased cell death 72 h after plating the MNs (B and C). **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. (Scale bars, 50 μm.) Error bar = SD, n = 3 per line.
Whole cell patch clamp analysis was used to determine whether oligodendrocytes expressing mSOD1 affected the electrophysiological profile of MNs in coculture conditions before cell death was detectable. Electrophysiological recordings were performed at day 7 after MN seeding. This time point was chosen to allow MN maturation and at the same time to determine if MN distress could be detected before cell death. WT MNs in coculture with either WT or mSOD1 oligodendrocytes were excitable and produced action potentials in response to current injection (SI Appendix, Fig. S6A). However, MNs cultured with mSOD1 oligodendrocytes displayed enhanced excitability compared with those cultured with WT oligodendrocytes (SI Appendix, Fig. S6 A and B). To determine if ion channel activity in MNs was also affected by coculture with mSOD1 oligodendrocytes, the cells were voltage clamped in the presence and absence of tetrodotoxin (TTX). Transient TTX-sensitive currents were present but at a lower density in MNs cocultured with mSOD1 oligodendrocytes (SI Appendix, Fig. S6C). However, the sustained, inward TTX-sensitive current was larger in MNs cocultured with mSOD1 oligodendrocytes (SI Appendix, Fig. S6D). This increase in size would result in greater excitability in response to a depolarizing stimulus. The reversal potential was also shifted, suggesting that the identity or ion selectivity of the channels contributing to these sustained currents was altered. The density of TTX-insensitive currents, evoked by voltage-gated potassium channels or through leak channels, was not significantly different between the two coculture conditions (SI Appendix, Fig. S6 E–G). Together, these results indicate that mSOD1 oligodendrocytes can actively induce MN death.
Human ALS Oligodendrocytes Derived from Multiple Genetic and Sporadic Cases Induce MN Death in Vitro.
To determine if human oligodendrocytes from patients with ALS were also able to induce MN death, we performed cocultures of human MBP+ oligodendrocytes and Hb9-GFP+ MNs.
Wild type Hb9-GFP+ MNs were plated onto 60–65% confluent iPS- or iNPC-derived human oligodendrocytes from sporadic and familial ALS cases. After 24 h, the MNs displayed neuritic extensions with no significant differences between ALS and control samples. However, 72 h postplating, a significant difference in survival (50–60% decrease) with a striking axonal beading phenotype was detected in cultures with iPS-derived oligodendrocytes from three patients with sporadic and one with familial ALS carrying a mutation in phosphoinositide phosphatases, also called factor-induced gene 4 (FIG4) (Fig. 2B). Of note, one of the two non-ALS lines was derived from fibroblasts from a patient affected by Becker muscular dystrophy, and no difference in MN survival compared with the control line was detected.
An identical phenotype was observed in cultures with iNPC-derived oligodendrocytes, where samples with a wider spectrum of genetic variants were available, i.e., four sporadic ALS samples, three carrying C9orf72 repeat expansion, as well as one carrying SOD1 and one TARDBP mutations (Fig. 2C). MNs showed reduced survival in the presence of these oligodendrocyte lines, with 40–60% of MNs perishing on all lines. Of interest, two sporadic lines, nos. 002 and 009, were reprogrammed with both, the classic iPSC procedure followed by production of NPCs, or the new conversion protocol to make iNPCs directly from fibroblasts as previously described (19). Oligodendrocytes from both iPSCs and iNPCs displayed similar toxicity regardless of the different reprogramming procedure used, thereby further validating the value of direct conversion to generate NPCs.
To determine if oligodendrocyte death was associated with the MN loss observed in vitro, mouse OPCs were transduced with a lentiviral vector carrying the red fluorescent protein (RFP) under the MBP promoter (Lv-MBP-RFP) at the beginning of differentiation, and cell number was determined using the InCell Analyzer 2 h before MN seeding (i.e., 5 d post-RFP infection). Similarly, human oligodendrocytes were transduced 5 d before coculture and RFP+ cell number was determined 2 h prior to MN seeding. RFP+ cell numbers were also determined at the end of coculture and no difference between ALS and control oligodendrocyte numbers was detected in mouse or human cultures (SI Appendix, Fig. S7).
Oligodendrocyte Contribute to MN Death via Soluble Factors and Cell-to-Cell Contact Through Separate Mechanisms.
To test whether MN death required cell-to-cell contact, we tested the effect of mouse and human oligodendrocyte CM onto GFP+ MN monocultures.
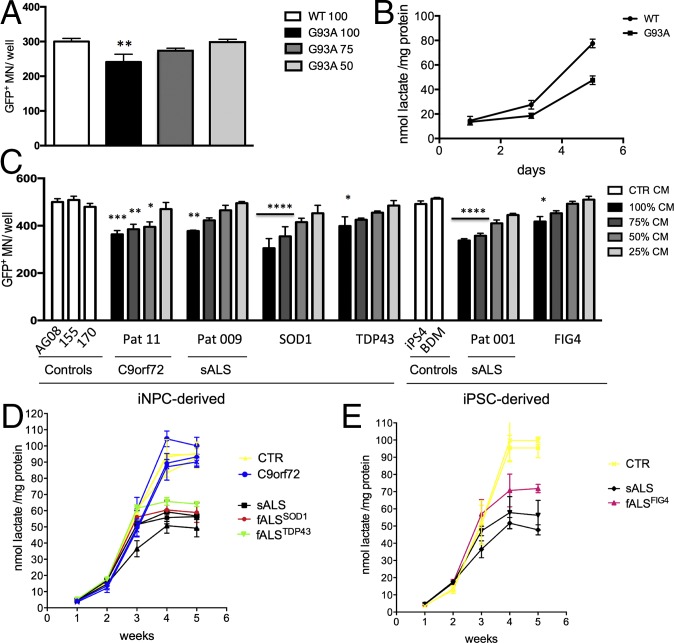
MNs were plated in 96-well plates and conditioned with increasing concentrations of growth medium from mouse WT or mSOD1 oligodendrocytes. After 6 d, we detected a significant (P = 0.013) 20% decrease in cell survival when MNs were treated with 100% CM from oligodendrocytes expressing mSOD1, whereas no significant difference was detected when replacing MN medium with 50% or 75% oligodendrocyte CM (Fig. 3A).
Fig. 3.
Oligodendrocyte CM from ALS samples induces MN death and is associated with decreased lactate levels. Hb9 GFP+ MN treated with increasing percentages of oligodendrocyte CM from the mSOD1G93A mouse model displayed a significant increase in cell death, while increasing percentages of CM from WT oligodendrocytes to MN medium slightly improved, but did not significantly change MN survival (A), thus we are representing the 100% CM condition. Increased mSOD1G93A oligodendrocyte CM-induced MN death was accompanied by significantly lower levels of lactate in the CM (B). As levels of secreted lactate increase in WT cells as they differentiate into oligodendrocytes, mSOD1G93A cells display lower increments, resulting in a significant difference in secreted lactate at the end of the differentiation protocol. Hb9-GFP+ MN treated with CM from human fully differentiated oligodendrocytes from both iNPCs and iPSCs also display increased cell death when treated with increasing percentages of ALS CM (C). Similarly to the mouse data, human ALS cells secrete progressively less lactate than control cells as they differentiate into oligodendrocytes, with exception of samples carrying C9orf72 repeat expansion (D and E). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Error bar = SD, n = 3–4 per line.
Similarly, CM from human ALS oligodendrocytes both familial and sporadic cases, induced a significant increase in MN death of WT GFP+ MNs in monoculture within 4 d (Fig. 3C). The percentage of CM-inducing MN death varied between patients (SI Appendix, Fig. S8), but in general, correlated with the amount of MN death the CM could cause: the higher the MN death, the more the CM had to be diluted to lose toxicity. With complete oligodendrocyte CM replacement, MN death increase ranged between 15% and 40%, with the mildest increase in cell death associated with the CM from the oligodendrocytes carrying a mutation in FIG4, and the highest increase associated with the CM from the oligodendrocytes carrying a SOD1 mutation.
In light of the reported involvement of lactate release impairment in ALS oligodendrocyte pathology (16), we examined the lactate content in the CM of both mouse and human oligodendrocytes throughout differentiation and 1 wk after MBP expression was achieved.
We found that there is no difference in the amount of lactate released by progenitor cells at the beginning of differentiation, regardless of their disease state (Fig. 3 B, D, and E). Lactate secretion increases as progenitor cells differentiate into oligodendrocytes for both mouse and human and reaches a plateau upon expression of MBP.
Interestingly, in ALS samples, the lactate production is reduced starting from day 3 in mouse and week 3 in human samples. These time points correspond to the expression of early oligodendrocyte progenitor markers Ng2 in mouse and GalC in human cells (SI Appendix, Fig. S1A and Fig. 1A). This failure in lactate release developed during differentiation results in significantly lower levels of lactate secreted by mouse and human ALS MBP+ cells compared with controls (Fig. 3 B, D, and E). Intracellular lactate measurements from cell lysates revealed that ALS oligodendrocytes overall produce less lactate (SI Appendix, Fig. S9A) and, in agreement with previous findings, display lower levels of monocarboxylate transporter 1 (MCT1) transcript (SI Appendix, Fig. S9B).
Interestingly, although the CM from C9orf72 mutant oligodendrocytes induced increased MN death, no reduction in lactate intracellular or extracellular content was detected.
To determine whether lower lactate levels in the medium are the only or the main responsibility for MN death in monoculture and coculture, we supplemented mouse and human cultures with 1 or 2 mM lactate to maintain the same range of lactate concentration observed in control oligodendrocyte monocultures (Fig. 3 B, D, and E).
The addition of lactate to the monocultures led to a slight, but significant, increase in MN survival even when MNs were grown in both mouse WT and human control oligodendrocyte CM. The supplementation of 2 mM lactate resulted in complete MN rescue in monocultures treated with SOD1G93A (Fig. 4 A–D) and ALS patient-derived oligodendrocyte CM with exception of C9orf72 samples (Fig. 4 H–L).
Fig. 4.
Lactate is a major component of CM-mediated toxicity, but not in coculture. Monocultures (mc) (A–D) of Hb9 GFP+ MN were treated with 100% WT or SOD1G93A oligodendrocyte CM with addition of 1 or 2 mM lactate, resulting in MN rescue, whereas cocultures (cc) treated with 2 mM lacate (E–G) showed only a minimal increase in MN survival. Similarly, monocultures of Hb9-GFP+ MN treated with 100% CM from human control or ALS oligodendrocytes plus 1 or 2 mM lactate showed increase in MN survival with exception of MNs treated with C9orf72 CM (H–L). Lactate supplementation only marginally, but significantly, rescued MN survival in coculture (M–O). Statistical significance refers to one-way ANOVA with multicomparison test of each treated sample against it own untreated control. (Magnification, 10×.) *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
On the contrary, addition of lactate to cocultures only partially increased MN survival, did not improve the axonal beading phenotype originally observed, and did not affect C9orf72 cultures at all (Fig. 4 E–G and M–O).
These findings indicate that MN survival is likely mediated by both soluble and insoluble factors that require cell-to-cell contact or very close vicinity, and that oligodendrocytes from patients carrying C9orf72 repeat expansions affect MNs via different pathways, compared with other ALS cases.
Mutant SOD1 Irreversibly Causes Oligodendrocyte-Mediated MN Death.
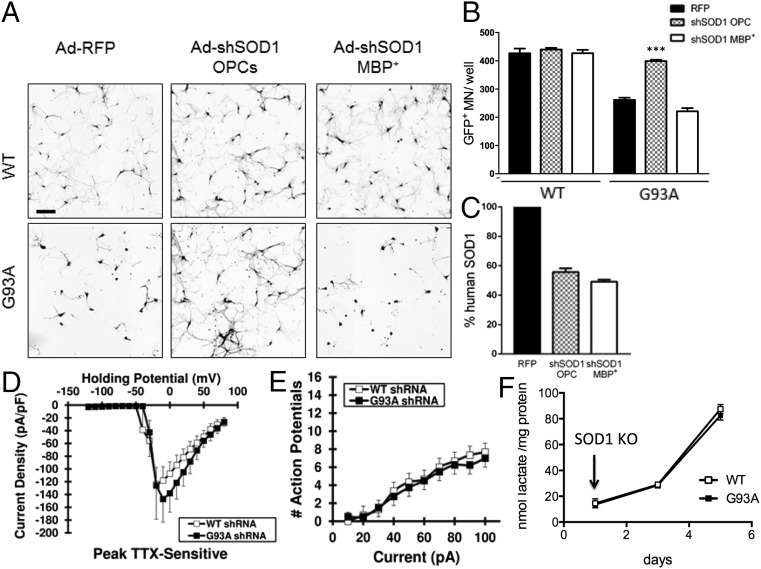
It has been shown that genetic knockdown of mSOD1, specifically in the oligodendrocytic lineage in the mSOD1G37R mouse, resulted in delayed onset and increased survival when the knockdown was driven by PDGFαR, a marker of oligodendrocyte progenitors (17). To test whether mSOD1 knockdown resulted in MN rescue in vitro, as one would expect from the in vivo experiments, and also to determine whether timing is important in this process, mSOD1 knockdown was performed either in primary mouse OPCs or in MBP+ cells (5 d postisolation). Knockdown was achieved by transducing OPCs on the day of isolation or differentiated MBP+ cells 48 h before MN coculture with an adenovirus (Ad) expressing a human SOD1 shRNA. An adenovirus-expressing RFP (Ad-RFP) was used as control. MNs were plated onto fully differentiated oligodendrocytes for all conditions. WT oligodendrocyte cocultures did not affect MN survival, regardless of the treatment (Fig. 5 A and B). Interestingly, mSOD1 knockdown in fully differentiated MBP+ cells did not prevent oligodendrocyte-mediated MN death (Fig. 5 A and B). However, mSOD1 knockdown in OPCs, before differentiation into oligodendrocytes, completely rescued MN survival (Fig. 5 A and B). To confirm that indeed the transgene expression had been decreased in both conditions, human SOD1 levels were measured by ELISA, and the results showed in both cases a 40–50% decrease in mutant protein (Fig. 5C).
Fig. 5.
Knockdown of the human SOD1 transgene in mSOD1G93A oligodendrocyte progenitors, but not finally differentiated oligodendrocytes, results in MN rescue. Knockdown of SOD1 in oligodendrocyte progenitors (before starting differentiation) results in complete rescue in MN survival (A and B) as well as electrophysiological properties (n = 8) (D and E) and secreted lactate levels (F). No difference between mSOD1G93A oligodendroctes infected with Ad-RFP and Ad-shSOD1 at the end of differentiation was detected in coculture with MNs, i.e., no MN rescue is achieved when mSOD1 in knocked down at the end of differentiation before coculture (A and B). SOD1 protein levels were quantified at the end of coculture (C). ***P ≤ 0.001. Error bar = SD, n = 3 per coculture condition. (Scale bar, 100 μm.)
Of interest, the reported increase in MN survival was accompanied by near baseline excitability and transient TTX-sensitive sodium current density, compared with untreated oligodendrocytes (Fig. 5 D and E).
Interestingly, early mSOD1 knockdown also resulted in an increase in lactate content in the oligodendrocyte CM (Fig. 5F), whereas mSOD1 knockdown after full differentiation had no effect (SI Appendix, Fig. S10A).
These results indicate that mSOD1 damage to oligodendrocytes, at least in vitro, is related to their maturation and it is irreversible via transgene knockdown in fully differentiated cells.
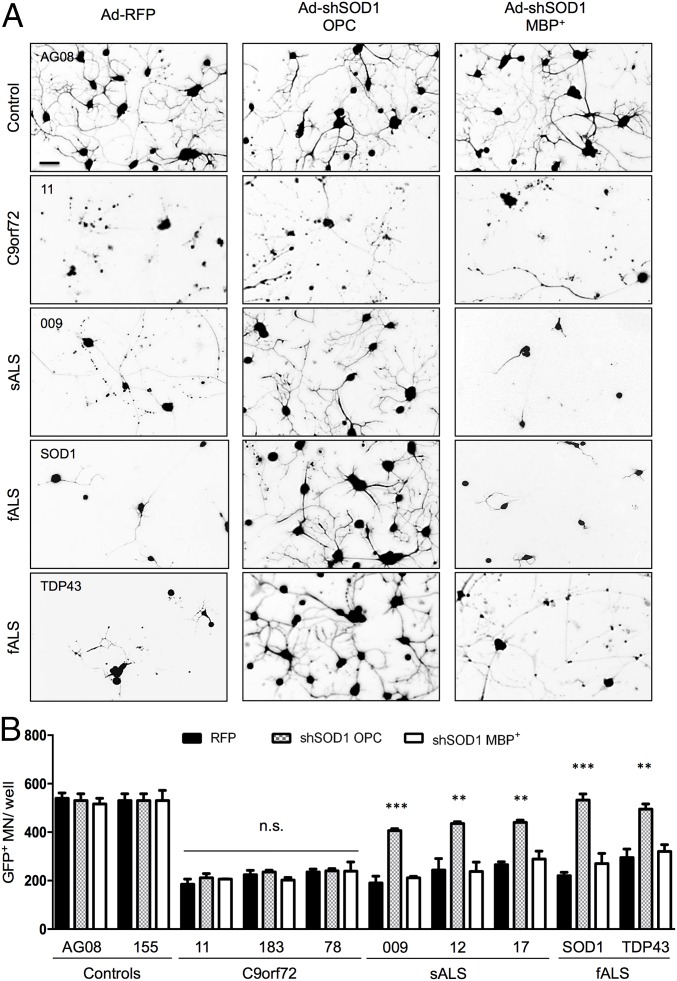
SOD1 Is a Common Target in Oligodendrocytes of Sporadic and Familial ALS Cases with Different Genetic Origins, but Not for C9orf72 Cases.
One of our previous studies indicated that SOD1 could play an important role in various variants of ALS, not only in cases carrying mutations in SOD1 (14). The availability of several human cell lines gave us the opportunity to test this finding in our coculture model by knocking down human SOD1 in oligodendrocytes from patients affected by sporadic as well as familial ALS. Similar to the murine study, SOD1 was knocked down at the OPC stage (i.e., 7 d into the differentiation protocol) or at the final stage of differentiation (i.e., 30 d post-NPC plating) and MNs were seeded in both cases on fully differentiated MBP+ cells. Oligodendrocytes derived from iNPCs (three non-ALS samples, three sporadic, three samples carrying C9orf72 repeat expansion, one familial SOD1, and one familial TDP43 case) were tested. SOD1 knockdown did not affect survival of MNs on the control lines (Fig. 6). Strikingly, oligodendrocytes from patients with sporadic ALS, as well as the familial cases, were responsive to SOD1 knockdown. MN survival was approximately doubled to 70–80% on sporadic ALS oligodendrocytes, whereas complete rescue was achieved in mutant SOD1 and TDP43 cases (Fig. 6). Again, similar to the mouse oligodendrocytes, this rescue was only observed if the knockdown was performed early during the differentiation of the oligodendrocytes and not after completion of maturation. In contrast, SOD1 knockdown in oligodendrocytes carrying C9orf72 mutations did not ameliorate MN survival in coculture, regardless of the timing of knockdown (Fig. 6). This finding indicates that SOD1 is not involved in the pathway leading to MN death caused by this mutation.
Fig. 6.
Knockdown of human SOD1 in oligodendrocyte progenitors results in MN rescue in patients with sporadic and familial ALS, but not in patients carrying C9orf72 repeat expansions. Knockdown of human WT SOD1 in human oligodendrocyte progenitors obtained from iNPCs results in a significant rescue in MN survival in sporadic and familial ALS cases carrying mutations in SOD1 and TDP43, but not C9orf72 repeat expansion (A and B). SOD1 knockdown was ineffective when performed at the end of differentiation. Error bar = SD, n = 3 per coculture condition per cell line. **P ≤ 0.01, ***P ≤ 0.001. (Scale bar, 50 μm.)
Consistent with these findings, the CM of oligodendrocytes from sporadic as well as familial cases associated with mutations in SOD1, TARDBP, and FIG4 lost its toxicity to MNs when SOD1 knockdown was achieved at the progenitor stage (Fig. 7A). This loss of toxicity/lack of support also correlated with restored levels of lactate in the growth medium (Fig. 7 B and C), which were not achieved when SOD1 shRNA treatment was performed in MBP+ cells (SI Appendix, Fig. S10B).
Fig. 7.
Knockdown of human SOD1 in oligodendrocyte progenitors results in normal levels of lactate in the growth medium throughout their differentiation. Knockdown of human WT SOD1 in human oligodendrocyte progenitors obtained from iNPCs or iPSCs results in rescue of MN monocultures treated with oligodendrocyte CM from sporadic and familial ALS cases carrying mutations in SOD1 and TDP43, but not C9orf72 repeat expansion (A). This result is accompanied by restoration of normal levels of secreted lactate (B and C). n = 3 per culture condition per cell line (A) and lactate measurements at all time points (B and C). **P ≤ 0.01, ***P ≤ 0.001.
Consistent with the lack of MN rescue in coculture after SOD1 knockdown, the CM of oligodendrocytes carrying C9orf72 mutations remained toxic/unsupportive of WT MNs.
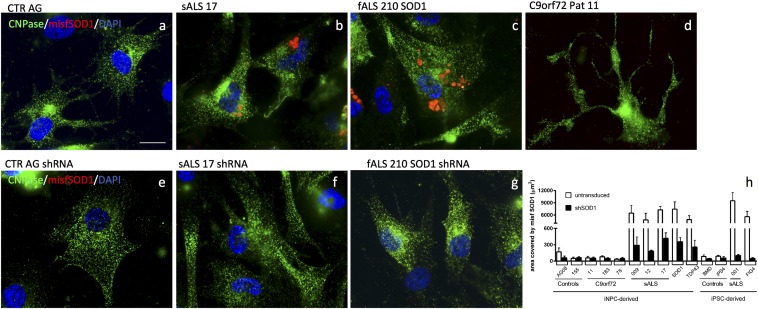
To strengthen the association reported here between sporadic ALS and SOD1, we tested our oligodendrocytes for the presence of misfolded SOD1 with the antibody B8H10 (MediMabs) raised against misfolded SOD1G93A. Several reports have in fact brought to light that spinal cord biopsies from patients with sporadic ALS display considerable levels of WT SOD1 misfolding (25, 26).
Oligodendrocytes derived from patients with familial ALS carrying mutant SOD1, or TDP43, or FIG4, as well as patients with sporadic ALS, displayed B8H10+ signal, indicating presence of misfolded SOD1 (Fig. 8 A–C). Oligodendrocytes carrying C9orf72 repeat expansion did not display misfolded SOD1 (Fig. 8D). Interestingly, SOD1 knockdown at the early stage of differentiation successfully eliminated these aggregates (Fig. 8 E–H) as well as restoring MN survival (Fig. 7A). On the contrary, SOD1 knockdown after differentiation was ineffective in eliminating SOD1 aggregates (SI Appendix, Fig. S10C), thus suggesting that misfolded SOD1 is implicated in the pathogenic mechanism leading to MN death.
Fig. 8.
Oligodendrocytes from patients with sporadic, SOD1, and TDP43-linked ALS display misfolded SOD1. Oligodendrocytes from patients with sporadic, SOD1, and TDP43-linked ALS, but not C9orf72-linked and unaffected individuals, display misfolded SOD1 aggregates (A–D). The pattern is mostly perinuclear (B and C). SOD1 knockdown in progenitor cells successfully eliminates such aggregates as shown via immunocytochemistry (E–G) and ImageJ analysis software (H). (Scale bar, 10 μm.)
Discussion
Oligodendrocytes have been implicated in the pathogenic mechanisms occurring in ALS only recently (16–18). It has been shown that oligodendrocytes are severely affected during disease and their degeneration occurs before MN death. In an attempt to compensate for oligodendrocyte loss, progenitor cells have been reported to be highly proliferative, but also fail to reach maturation. As a result, the motor fibers in both mouse models and in the spinal cords of patients with ALS show signs of evident demyelination (17). Interestingly, neither the ALS mouse model nor patients with ALS show defects in developmental myelination. The data collected in the mSOD1 mouse, in fact, suggest that only the adult-born progenitors are unable to differentiate.
The present study provides an in vitro model of mouse and human oligodendrocyte–MN cocultures to investigate the role of this cell type in ALS. In this study, we analyzed the effect of oligodendrocytes from mSOD1 mice and WT littermates on WT MNs.
Importantly, human oligodendrocytes were differentiated from human fibroblasts reprogrammed using two different methods, i.e., the classical iPSCs reprogramming (27) and the recently published direct conversion from fibroblasts to induced neural progenitor cells (19). This fast reprogramming method enabled us to include a high number of human cell lines. Taking advantage of these two methods, we analyzed oligodendrocytes from non-ALS controls, including a patient with Becker muscular dystrophy, and several patients with ALS, including both sporadic and familial cases, carrying mutations in SOD1, TARDBP, C9orf72, and FIG4.
Our in vitro data indicate that both mouse and human progenitors from non-ALS and ALS samples can efficiently differentiate into oligodendrocytes that express the main typical cellular markers in agreement with a recent report (21).
Although we did not detect any difference in oligodendrocyte survival between ALS and control samples during the time the cells were differentiated and kept in coculture, we did observe that MBP+ cells from mSOD1 mice and patients with ALS, induced MN death. In addition, because the mouse model provided a slower in vitro assay, we were able to perform whole cell patch clamp recordings, which showed that the oligodendrocytes expressing mSOD1 can induce substantial electrophysiological changes in WT MNs before cell death. Similar results have been reported in mSOD1 mice (28), where increased persistent sodium currents were identified as selectively altered and leading to hyperexcitability. The pattern described in this study in vitro, and previously reported in the mouse model (28), is in perfect agreement with the findings that cortical hyperexcitability is one of the first alterations detected in patients with ALS (29), even before disease onset (30). Although the origin of this phenomenon is still unknown, our results suggest that oligodendrocytes are involved in this pathologic mechanism. Moreover, consistent with data from the mouse model (17, 18), both mouse and human ALS oligodendrocytes in vitro display impairment in lactate production and release, along with down-regulation of the lactate transporter MCT1.
Human oligodendrocytes from both sporadic and familial cases carrying different mutations, i.e., SOD1, TDP43, FIG4, and C9orf72, and obtained through different reprogramming protocols, all induced a significant decrease in MN survival within 72 h from MN seeding. Whereas MNs plated on non-ALS oligodendrocytes develop long and highly branched neurites over time, most cells plated onto ALS oligodendrocytes die without extending processes or making neuritic connections. This result is particularly interesting in light of the new finding that oligodendrocytic connexin expression is significantly decreased in the spinal cord of the mSOD1 mouse model, whereas the inhibitory molecule Nogo-A is up-regulated (31).
In contrast to the mechanisms of human astrocyte toxicity against MNs (15, 19), we found that decreased lactate levels account for a large part of the CM-mediated toxicity associated with oligodendrocytes. In fact, not only MN survival improves with ALS oligodendrocyte CM dilution, but addition of lactate to the monocultures completely rescues MN survival in all ALS-derived cases, with exception of samples carrying C9orf72 mutations.
Interestingly, we had previously reported that lactate secretion deficiency is also involved in astrocyte-mediated toxicity in mutant SOD1 astrocytes from the SOD1G93A mouse (32), thus indicating that lactate production and secretion might be an impairment common to multiple cell types. Indeed oligodendrocytes secrete trophic factors and metabolic substrates that promote MN survival. In fact, WT oligodendrocyte CM supports MNs in monoculture as well, if not slightly better than MN medium enriched with several growth factors and supplements.
Similarly to astrocytes (15, 19, 32–34), however, we report that oligodendrocytes affect MNs through two distinct mechanisms of action: soluble factors and cell-to-cell contact, with the latter being more aggressive. Addition of lactate to the cocultures, in fact, does not rescue MNs. Another potential explanation for the data here presented is that, in addition to lactate, the toxic factor(s) secreted by oligodendrocytes are short-range diffusible factor(s) or highly labile soluble factor(s), which need the two cell types to be in a close range. In this case, even if all toxic factors were soluble, they would need the two cell types to be in close vicinity.
Particularly interesting is the finding that SOD1 knockdown in finally differentiated mSOD1 carrying murine oligodendrocytes did not improve MN survival. On the other hand, SOD1 knockdown was effective when performed in the progenitor cells of the very same animals, resulting in restored levels of lactate secretion from oligodendrocytes and rescue of MN survival as well as their electrophysiological properties. Although the aberrant characteristics of mouse ALS oligodendrocytes did not alter classic oligodendrocyte marker expression, the lower levels of lactate in the growth medium revealed that ALS oligodendrocytes are partially dysfunctional. Of note, the difference between secreted lactate levels in control and ALS samples builds up during cell differentiation, indicating that the presence of SOD1 during ALS-derived oligodendrocyte differentiation causes an intrinsic damage interfering with their functionality, thus resulting in a phenotype that is irreversibly deadly to MNs.
Of therapeutic interest is that SOD1 knockdown has the same effect on human oligodendrocytes from various samples from patients with sporadic and familial ALS. Our group previously reported that SOD1 knockdown in human postmortem NPC-derived astrocytes has beneficial effects on MN survival in culture (14). In this current study, a different virus and a different, commercially available, shRNA sequence were used to further strengthen that the effect was specific to SOD1 knockdown rather than potential off-target effects. Again, this study mirrors results that were obtained in the astrocytes differentiated from the postmortem NPCs. The novelty of the data presented here is the finding that in oligodendrocytes, timing for SOD1 suppression is important. Although the mechanisms behind this process are unknown, our study suggests that ALS oligodendrocyte function is affected directly or indirectly by SOD1.
Our microarray data, which is in line with multiple previously published studies, show that even after maturation, oligodendrocytes are highly energy demanding cells, sensitive to endoplasmic reticulum stress (23, 24), both mechanisms that have been implicated in the early phases of ALS (35). Consistent with the hypothesis that metabolic failure might be responsible for the reported dysfunction of ALS oligodendrocytes, we show that these cells are unable to produce and provide metabolic substrates to MNs.
In agreement with observations from postmortem tissues (24), which identified misfolded SOD1 and other protein aggregates predominantly in periaxonal oligodendrocytes in the spinal cord of sporadic ALS cases, our human ALS oligodendrocytes display misfolded SOD1 aggregates, mostly with perinuclear localization.
Importantly, samples carrying C9orf72 repeat expansions did not display misfolded SOD1 aggregates, did not respond to SOD1 knockdown at any time point, and also did not display dysfuction in lactate release. These findings add to the evidence indicating that this mutation defines a specific subgroup of patients with ALS within a neuropathological spectrum (36, 37). Of importance, CM from C9orf72 mutant oligodendrocytes still causes MN death, but likely through different mechanisms compared with sporadic and SOD1-related familial ALS, as they did not react to the same treatment.
The finding that the presence of misfolded SOD1 in sporadic and familial oligodendrocytes, but not C9orf72 samples, correlates with the metabolic ability of oligodendrocytes to produce and secrete lactate suggests that WT SOD1 misfolding might be implicated in sporadic ALS through a dysregulation of metabolic pathways. In support of this hypothesis, using yeast and human cell lines, Reddi and Culotta identified a new role in cellular metabolism for SOD1: to integrate signals from oxygen and glucose to repress respiration within cells (38). The action of SOD1 knockdown, however, is not limited to the restoration of secreted lactate levels, as this approach successfully rescues MNs in cocultures, whereas simple addition of lactate to the medium only marginally improves neuronal survival.
Although more work needs to be done to determine how WT SOD1 is implicated in sporadic ALS, the present results support a potential role of SOD1 in metabolic pathways, which might lead to a cascade of events altering the signaling pathways between oligodendrocytes and MNs.
In conclusion, the present study provides an in vitro model to study the pathogenic features of human ALS oligodendrocytes and their contribution to MN death. The fast direct conversion method proved equally efficient in producing differentiated oligodendrocytes compared with classical reprogramming and allowed for inclusion of multiple ALS samples carrying different mutations.
Indeed, our results indicate that there are not only distinct therapeutic windows to target different cell types involved in ALS pathology, but also different patient populations that might need to be considered separately for future clinical trials.
Materials and Methods
All procedures were performed in accordance with the NIH guidelines on the care and use of vertebrate animals and approved by the Institutional Animal Care and Use Committee of the Research Institute at Nationwide Children's Hospital. Primary cultures of cerebral cortical oligodendrocytes were prepared from c57/bl6 SOD1G93A mice and littermate newborn mice (1–3 d old). Pups were screened for human SOD1 transgene at P1 and three brains from mSOD1 or control mice were pooled together.
Mixed cortical cultures were grown to confluence in DMEM containing 10% (vol/vol) FBS in T75 flasks and oligodendrocyte progenitors and microglia were separated from the astrocyte monolayer through shaking (250 rpm, 37 °C overnight). The following morning the supernatant was collected and plated in an untreated Petri dish for 40 min to allow microglia to attach. OPCs were collected in the supernatant, spun at 200 × g for 4 min, counted and plated in 96-well plates for coculture (30,000 cells per well), or on 1-cm2 coverslips for staining and electrophysiology recordings (150,000 cells per well).
OPCs were cultured in DMEM with 10% (vol/vol) FBS for 4 h. Subsequently, the cells were washed twice with PBS to remove traces of serum and the medium was switched to DMEM/F12 supplemented with 2% (vol/vol) B27 and 20 ng/mL PDGFaa for 48 h. The cells were then cultured without PDGFaa and with IGF1 (20 ng/mL) for 72 h.
Detailed descriptions of all methods, reagents, and information about the cell lines, as well as analysis, are provided in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants RC2-NS69476 and R01-NS644912 (to B.K.K.) and funding from the Robert Packard Center for ALS Research (B.K.K.), the Project A.L.S. (B.K.K.), the ALS Association (B.K.K.), and the Helping Link Foundation (B.K.K.). The authors also received research funding from Seventh Framework Programme (EC Seventh Framework Programme Grant 303101) (to L.F.), The Motor Neurone Disease Association (MNDA) (L.F.), The Muscular Dystrophy Association (MDA) Young Investigator Development Award (to K.M.), and Human Health Services/NIH/National Institute of Neurological Disorders and Stroke (NINDS) Training in Neuromuscular Disease (A.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE87385).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607496113/-/DCSupplemental.
References
- 1.Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;364(6435):362. doi: 10.1038/364362c0. [DOI] [PubMed] [Google Scholar]
- 2.Kabashi E, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40(5):572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 3.Sreedharan J, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwiatkowski TJ, Jr, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323(5918):1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 5.Vance C, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323(5918):1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeJesus-Hernandez M, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renton AE, et al. ITALSGEN Consortium A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7(11):616–630. doi: 10.1038/nrneurol.2011.152. [DOI] [PubMed] [Google Scholar]
- 9.Sojka P, Andersen PM, Forsgren L. Effects of riluzole on symptom progression in amyotrophic lateral sclerosis. Lancet. 1997;349(9046):176–177. doi: 10.1016/s0140-6736(05)60977-4. [DOI] [PubMed] [Google Scholar]
- 10.Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3(6):637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Yamanaka K, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11(3):251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boillée S, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312(5778):1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 13.Frakes AE, et al. Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron. 2014;81(5):1009–1023. doi: 10.1016/j.neuron.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haidet-Phillips AM, et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011;29(9):824–828. doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Re DB, et al. Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron. 2014;81(5):1001–1008. doi: 10.1016/j.neuron.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee Y, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487(7408):443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang SH, et al. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci. 2013;16(5):571–579. doi: 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philips T, et al. Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain. 2013;136(Pt 2):471–482. doi: 10.1093/brain/aws339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer K, et al. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc Natl Acad Sci USA. 2014;111(2):829–832. doi: 10.1073/pnas.1314085111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hester ME, et al. Rapid and efficient generation of functional motor neurons from human pluripotent stem cells using gene delivered transcription factor codes. Mol Ther. 2011;19(10):1905–1912. doi: 10.1038/mt.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livesey MR, et al. Maturation and electrophysiological properties of human pluripotent stem cell-derived oligodendrocytes. Stem Cells. 2016;34(4):1040–1053. doi: 10.1002/stem.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raman R, et al. Gene expression signatures in motor neurone disease fibroblasts reveal dysregulation of metabolism, hypoxia-response and RNA processing functions. Neuropathol Appl Neurobiol. 2015;41(2):201–226. doi: 10.1111/nan.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin W, et al. Interferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 2006;129(Pt 5):1306–1318. doi: 10.1093/brain/awl044. [DOI] [PubMed] [Google Scholar]
- 24.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pokrishevsky E, et al. Aberrant localization of FUS and TDP43 is associated with misfolding of SOD1 in amyotrophic lateral sclerosis. PLoS One. 2012;7(4):e35050. doi: 10.1371/journal.pone.0035050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forsberg K, Andersen PM, Marklund SL, Brännström T. Glial nuclear aggregates of superoxide dismutase-1 are regularly present in patients with amyotrophic lateral sclerosis. Acta Neuropathol. 2011;121(5):623–634. doi: 10.1007/s00401-011-0805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Pieri M, Carunchio I, Curcio L, Mercuri NB, Zona C. Increased persistent sodium current determines cortical hyperexcitability in a genetic model of amyotrophic lateral sclerosis. Exp Neurol. 2009;215(2):368–379. doi: 10.1016/j.expneurol.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Vucic S, Cheah BC, Yiannikas C, Kiernan MC. Cortical excitability distinguishes ALS from mimic disorders. Clin Neurophysiol. 2011;122(9):1860–1866. doi: 10.1016/j.clinph.2010.12.062. [DOI] [PubMed] [Google Scholar]
- 30.Vucic S, Nicholson GA, Kiernan MC. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain. 2008;131(Pt 6):1540–1550. doi: 10.1093/brain/awn071. [DOI] [PubMed] [Google Scholar]
- 31.Cui Y, et al. Extensive dysregulations of oligodendrocytic and astrocytic connexins are associated with disease progression in an amyotrophic lateral sclerosis mouse model. J Neuroinflammation. 2014;11:42. doi: 10.1186/1742-2094-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferraiuolo L, et al. Dysregulation of astrocyte-motoneuron cross-talk in mutant superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain. 2011;134(Pt 9):2627–2641. doi: 10.1093/brain/awr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song S, et al. Major histocompatibility complex class I molecules protect motor neurons from astrocyte-induced toxicity in amyotrophic lateral sclerosis. Nat Med. 2016;22(4):397–403. doi: 10.1038/nm.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almad AA, et al. Connexin 43 in astrocytes contributes to motor neuron toxicity in amyotrophic lateral sclerosis. Glia. 2016;64(7):1154–1169. doi: 10.1002/glia.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filézac de L’Etang A, et al. Marinesco-Sjögren syndrome protein SIL1 regulates motor neuron subtype-selective ER stress in ALS. Nat Neurosci. 2015;18(2):227–238. doi: 10.1038/nn.3903. [DOI] [PubMed] [Google Scholar]
- 36.Ash PE, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77(4):639–646. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper-Knock J, Shaw PJ, Kirby J. The widening spectrum of C9ORF72-related disease; genotype/phenotype correlations and potential modifiers of clinical phenotype. Acta Neuropathol. 2014;127(3):333–345. doi: 10.1007/s00401-014-1251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddi AR, Culotta VC. SOD1 integrates signals from oxygen and glucose to repress respiration. Cell. 2013;152(1–2):224–235. doi: 10.1016/j.cell.2012.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.