Biologists rarely have access to reliable and detailed historical records of environmental conditions, and changes thereof, over extended periods of time. Where such data exist, they can be instrumental for understanding evolutionary processes by providing a framework for interpreting patterns of organismal diversification, supplementing biogeography, molecular evidence, and fossils. In PNAS, Ivory et al. (1) present a long and fully time-calibrated paleoecological record for Lake Malawi in Africa, which is home to the most species-rich extant adaptive radiation on Earth, consisting of ∼800 species of cichlid fishes. The ecological and morphological diversity, as well as the rapidity of species formation, is extraordinary in Malawi cichlids, paralleled only by the cichlid radiations in two other African Great Lakes: Victoria and Tanganyika (2). The reconstruction of the history of Lake Malawi covering the past 1.2 My (1) illuminates the links between external factors shaping the lake’s environment and the evolution of its biota, in particular the cichlids.
Adaptive radiation— the rapid origin of an array of species from a common ancestor as a consequence of adaptation to distinct ecological niches—is thought to be responsible for much of the biological diversity on Earth (3, 4). The most well-known and best-studied—including genomic examinations—examples of adaptive radiation are Darwin’s finches, Anolis lizards, three-spine stickleback fish, Heliconius butterflies, and cichlid fishes in East Africa (5). Such outbursts of life may be triggered by: (i) external, often abiotic, factors creating ecological opportunity (e.g., “empty” or underoccupied ecological niches in newly created environments or “liberated” niches resulting from extinction); and/or (ii) internal or biotic features of the radiating groups (i.e., evolutionary “key-innovations” providing access to previously underused resources) (4).
The Environmental History of Lake Malawi
Lake Malawi initially attained the characteristics of a deep-water lake ∼4.5 Ma but was largely dry between 1.6 Ma and 1 Ma (6), during which much of its original fauna may have gone extinct. Ivory et al. (1) examined fossil crustaceans, fish, algae, lake flies, charred particles, and mineralogical indicators in a 380-m drill core from the lake’s central basin, obtaining a continuous 1.2-My record, a substantial extension of an earlier study covering the most recent 140 ky (7). Combining the new data with geochemical and sedimentological variables (8), Ivory et al. (1) offer impressively detailed and informative insights into the lake’s history.
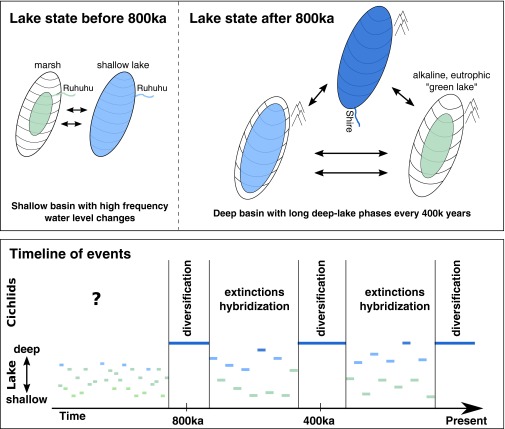
To begin with, Ivory et al. (1) present convincing evidence that between 1.2 Ma and 800 ka Lake Malawi was much shallower than it is today and underwent frequent but low-amplitude water-level changes. During this period, the basin alternated between marshland and a shallow lake, and was under the influence of a through-flowing river, even at lowstands (Fig. 1). The authors further suggest that, by this time, the Ruhuhu River functioned as outlet, through a connection with the Ruvuma or Rufiji river systems that both flow into the Indian Ocean. This finding is consistent with previous molecular data suggesting a close relationship between cichlids from these watersheds and Lake Malawi (9, 10).
Fig. 1.
The environmental history of Lake Malawi over the past 1.2 Ma and its interrelation with its cichlid adaptive radiation. According to Ivory et al. (1), the lake was characterized by frequent oscillations between marsh and shallow-lake conditions before 800 ka and had a constant outflow (the Ruhuhu River). After 800 ka, this outlet closed resulting in a much deeper and often closed basin (with an outflow via the Shire River only during highstands). Consistent with a recent molecular clock calibration (12), the cichlid radiation is likely to have kicked-off during the first phase of deep-lake conditions ∼800 ka. This event might be equivalent to the first stage of adaptive radiation (14–16), the specializations to macrohabitats. Focusing on the rock-dwelling mbuna lineage, Ivory et al. (1) propose that two further deep-lake phases (∼400 ka; since ∼75 ka) might be responsible for trophic specializations (stage II) and diversification with respect to coloration (stage III). A more general hypothesis, which should be evaluated in the future, is that cichlid evolution was shaped by periods characterized by diversification and large population sizes during deep-lake phases, interspersed by periods characterized by unstable conditions, extinctions and interspecific gene-flow during lowstands.
At about 800 ka, the lake became much deeper and changed into a more or less closed system. That is, with the exception of a few remarkable highstands in lake level, the basin lacked an outflow. This transition cannot be explained by climatic oscillations alone. Instead, Ivory et al. (1) suggest that the Ruhuhu–Indian Ocean connection was cut because of a tectonic uplift, shifting the basin threshold to the current southern outlet, the Shire River. The now much higher basin threshold explains why, after 800 ka, the lake possessed an outlet only during highstands, so-called “blue water” phases characterized by stratification and transparent water as observed today. During lowstands, however, the lake became more saline/alkaline and eutrophic comparable to present-day Lake Turkana (a condition termed “green lake” phase).
Importantly, the transition in lake state at 800–700 ka was accompanied by a substantial change in the frequency and amplitude of lake-level oscillations. The three by far longest (>50 ky) and most pronounced deep-lake phases occurred at 400-ky intervals, coinciding with periods of high levels of solar radiation linked to changes in the eccentricity of the Earth’s orbit around the sun. Outside of these periods, lake-level fluctuation followed mainly a 19- to 23-ky beat that can be attributed to periodic changes in the direction of the Earth’s axis, with some influence of other mechanisms acting on shorter timescales.
Environmental Triggers for Cichlid Adaptive Radiation
So, how is this timeline interrelated with the cichlid adaptive radiation? With regards to the onset of the radiation, an earlier study using mitochondrial DNA sequences estimated an age of 2.44 Ma (11), more than twice the age of the deepest parts of the drill cores investigated in Ivory et al. (1). More recently, however, an examination of an array of nuclear genes using methods that account for genetic diversity present before the radiation suggests that the time to the most recent common ancestor of Lake Malawi’s cichlids may be as little as 700–800 ky (12), corresponding to the first prolonged deep-lake phase. That the cichlid radiation kicked-off after the transition toward a deep lake is further substantiated by its phylogenetic structure. Phylogenetic analyses generally associate the initial divergence events in Lake Malawi cichlids with habitat specializations (9, 11–13). These specializations include adaptations to sandy and rocky shores (benthic habitats), the open water column (pelagic zone), and lineages of both benthic and pelagic cichlids that have adapted to deep-water habitats. It is unlikely that these specializations occurred during the period characterized by marshy or shallow lake conditions before 800 ka, as pelagic habitats or ecosystems associated with rocky shores would have been scarce or nonexistent then.
In their interpretation of cichlid evolution, Ivory et al. (1) focus largely on the rocky-habitat specialized “mbuna” group, hypothesizing that the three sustained periods of deep “blue lake” conditions correspond to the three stages observed in the radiation of this group (14, 15; see also refs. 4 and 16): the first period at ∼800 ka would thus coincide with macrohabitat specializations; the second at ∼400 ka with specializations of trophic morphologies to microhabitat structures; and the current period that started ∼75 ka with diversification with respect to male-breeding coloration.
This hypothesis is compelling, and the question of the degree to which morphological evolution and speciation rates correspond to external factors should certainly be studied in further detail. This undertaking would first of all require a well-resolved phylogeny and much more accurate absolute time calibrations, as it remains unknown if the different morphologies indeed occurred at the same time. More fundamentally, although the stages model seems to apply well to the mbuna, it does not seem to hold for other cichlid lineages [nor for the cichlids from nearby Lake Tanganyika (17)]. For example, among Malawi’s sand-dwelling and pelagic cichlids there are very few closely related species that differ only in male coloration.
We suggest that Ivory et al.’s (1) interpretation can perhaps be applied more broadly as a general time-resolved model where periods of large population sizes and diversification in a deep lake are interspersed by periods of small populations, extinctions, and hybridization/gene-flow during lowstands (Fig. 1). Regardless of the particular pattern of diversification, such profound environmental changes must have left traces in the cichlids’ genomes. Were all lineages affected by climate and water level fluctuations to the same degree? Do periods of deep-lake phases always correspond to bursts of diversification? Were only some lineages subject to hybridization? We believe that it is these questions that should be in the main focus of future studies.
Considerable uncertainty remains with regards to the order in which the different habitat specializations appeared. Given that existing phylogenetic studies either focused on a subset of lineages [e.g., rock vs. sand (15, 18); rock vs. pelagic (12)], or lack the power to resolve the main eco-morphological groups as monophyletic (9), we submit that our knowledge of cichlid evolution in Lake Malawi is still incomplete and will require: (i) extensive sampling of taxa within and around the lake; and (ii) the use of a large number of nuclear DNA markers—ideally, whole genomes—to achieve sufficient resolution and to avoid the confounding effects of shared ancestral genetic polymorphisms. In this context, one particular uncertainty concerns the identity of the founding populations that seeded the radiation. The likely existence of an outlet through the Ruhuhu River before 800 ka (1) strengthens the case for revisiting the hypothesis that cichlids in the catchments to the northeast of Lake Malawi (9, 10) may provide a clue.
In any case, the present work by Ivory et al. (1) illustrates the importance of having a detailed understanding of the environmental context for the interpretation of evolutionary phenomena, such as adaptive radiations, calling for further studies along these lines. With Lake Tanganyika, the next—and somewhat ultimate—target for scientific drilling in East Africa has already been identified (19). A well-resolved paleoecological record for Tanganyika, which harbors the eco-morphologically most diverse cichlid assemblages (albeit counting fewer species than the one in Malawi) (2), would allow scrutinization of the relative contribution of environmental factors on organismal diversification. A comparison between the “buddy” lakes Malawi and Tanganyika appears particularly appealing in light of the amount of evolutionary convergence between their cichlid faunas (20). Above all, deep drilling into the lake floor of Tanganyika would provide information critical for understanding what is perhaps the most-important evolutionary event: the origin of our own species. Because of the much greater age of Tanganyika compared with Malawi [9–12 My vs. 4.5 My (2, 6)], a long Tanganyikan sediment core would deliver an unprecedented view on the environmental history in tropical Africa over the late Neogene, covering the entire time span of human evolution starting from the human-chimp split, in the very region where this event has taken place.
Acknowledgments
The authors’ research is supported by European Molecular Biology Organization Grant ALTF 456-2016 (to M.M.); and the European Research Council, the Swiss National Science Foundation, and the University of Basel (W.S.).
Footnotes
The authors declare no conflict of interest.
See companion article on page 11895.
References
- 1.Ivory SJ, et al. Environmental change explains cichlid adaptive radiation at Lake Malawi over the past 1.2 million years. Proc Natl Acad Sci USA. 2016;113:11895–11900. doi: 10.1073/pnas.1611028113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salzburger W, Van Bocxlaer B, Cohen AS. Ecology and evolution of the African Great Lakes and their faunas. Annu Rev Ecol Evol Syst. 2014;45(1):519–545. [Google Scholar]
- 3.Schluter D. The Ecology of Adaptive Radiation. Oxford Univ Press; Oxford: 2000. [Google Scholar]
- 4.Gavrilets S, Losos JB. Adaptive radiation: Contrasting theory with data. Science. 2009;323(5915):732–737. doi: 10.1126/science.1157966. [DOI] [PubMed] [Google Scholar]
- 5.Berner D, Salzburger W. The genomics of organismal diversification illuminated by adaptive radiations. Trends Genet. 2015;31(9):491–499. doi: 10.1016/j.tig.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Delvaux D. 1995. Age of Lake Malawi (Nyasa) and water level fluctuations. Annual Report (Musée Royal de l’Afrique Centrale, Département de Géologie et Minéralogie, Tervuren, Belgium) pp 139–144.
- 7.Cohen AS, et al. Ecological consequences of early Late Pleistocene megadroughts in tropical Africa. Proc Natl Acad Sci USA. 2007;104(42):16422–16427. doi: 10.1073/pnas.0703873104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyons RP, et al. Continuous 1.3-million-year record of East African hydroclimate, and implications for patterns of evolution and biodiversity. Proc Natl Acad Sci USA. 2015;112(51):15568–15573. doi: 10.1073/pnas.1512864112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyce DA, et al. Repeated colonization and hybridization in Lake Malawi cichlids. Curr Biol. 2011;21(3):R108–R109. doi: 10.1016/j.cub.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 10.Genner MJ, Ngatunga BP, Mzighani S, Smith A, Turner GF. Geographical ancestry of Lake Malawi’s cichlid fish diversity. Biol Lett. 2015;11(6):20150232. doi: 10.1098/rsbl.2015.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genner MJ, et al. Age of cichlids: New dates for ancient lake fish radiations. Mol Biol Evol. 2007;24(5):1269–1282. doi: 10.1093/molbev/msm050. [DOI] [PubMed] [Google Scholar]
- 12.Meyer BS, Matschiner M, Salzburger W. Disentangling incomplete lineage sorting and introgression to refine species-tree estimates for Lake Tanganyika cichlid fishes. Syst Biol. August 18, 2016 doi: 10.1093/sysbio/syw069. [DOI] [PubMed] [Google Scholar]
- 13.Meyer A. Phylogenetic relationships and evolutionary processes in East African cichlid fishes. Trends Ecol Evol. 1993;8(8):279–284. doi: 10.1016/0169-5347(93)90255-N. [DOI] [PubMed] [Google Scholar]
- 14.Danley PD, Kocher TD. Speciation in rapidly diverging systems: Lessons from Lake Malawi. Mol Ecol. 2001;10(5):1075–1086. doi: 10.1046/j.1365-294x.2001.01283.x. [DOI] [PubMed] [Google Scholar]
- 15.Kocher TD. Adaptive evolution and explosive speciation: The cichlid fish model. Nat Rev Genet. 2004;5(4):288–298. doi: 10.1038/nrg1316. [DOI] [PubMed] [Google Scholar]
- 16.Streelman JT, Danley PD. The stages of vertebrate evolutionary radiation. Trends Ecol Evol. 2003;18(3):126–131. [Google Scholar]
- 17.Muschick M, et al. Testing the stage model in the adaptive radiation of cichlid fishes in East African Lake Tanganyika. Proc Biol Sci. 2014;281(1795):20140605. doi: 10.1098/rspb.2014.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albertson RC, Markert JA, Danley PD, Kocher TD. Phylogeny of a rapidly evolving clade: The cichlid fishes of Lake Malawi, East Africa. Proc Natl Acad Sci USA. 1999;96(9):5107–5110. doi: 10.1073/pnas.96.9.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell JM, Cohen AS, Johnson TC, Scholz CA. Scientific drilling in the East African Rift Lakes: A strategic planning workshop. Sci Drill. 2012;14:49–54. [Google Scholar]
- 20.Kocher TD, Conroy JA, McKaye KR, Stauffer JR. Similar morphologies of cichlid fish in Lakes Tanganyika and Malawi are due to convergence. Mol Phylogenet Evol. 1993;2(2):158–165. doi: 10.1006/mpev.1993.1016. [DOI] [PubMed] [Google Scholar]