Significance
Pseudoboehmite (PB) is a poorly crystallized variant of boehmite, but its crystal structure and morphology have not been clearly understood. In particular, PB gel crystals are formed in an aqueous solution, so the interface between the crystalline surface and water is of crucial importance for understanding its properties and growth mechanism. Here we report the detailed structures of fibrous PB crystals obtained from high-resolution transmission electron microscopy and electron diffraction patterns. Some of the fibers consist of only two layers of an Al–O octahedral double-sheet ribbon structure, which is really a 1D structure. The findings will broaden the understandings in classical but important fields of colloid science and clay mineralogy.
Keywords: 1D material, nanowire, TEM, aluminum oxyhydroxide, pseudoboehmite
Abstract
We report the discovery of a 1D crystalline structure of aluminum oxyhydroxide. It was found in a commercial product of fibrous pseudoboehmite (PB), γ-AlOOH, synthesized easily with low cost. The thinnest fiber found was a ribbon-like structure of only two layers of an Al–O octahedral double sheet having a submicrometer length along its c axis and 0.68-nm thickness along its b axis. This thickness is only slightly larger than half of the lattice parameter of the b-axis unit cell of the boehmite crystal (b/2 = 0.61 nm). Moreover, interlayer splittings having an average width of 1 nm inside the fibrous PB are found. These wider interlayer spaces may have intercalation of water, which is suggested by density functional theory (DFT) calculation. The fibers appear to grow as almost isolated individual filaments in aqueous Al-hydroxide sols and the growth direction of fibrous PB is always along its c axis.
Low-dimensional materials such as quantum dots (1), nanowires (2, 3), and nanosheets (4) have attracted interest in academia and industry. Carbon nanotubes (5), a typical 1D inorganic material, have been investigated widely, but new 1D inorganic materials prepared by a simple and reliable method have rarely been reported.
The aluminum ore bauxite primarily comprises aluminum hydroxide possessing different crystalline forms. The thermal dehydration of aluminum hydroxide produces alumina Al2O3, one of the most important materials in modern industries. γ-Boehmite (γ-AlOOH) (6) is a typical aluminum oxyhydroxide with a crystal structure similar to that of lepidocrocite (γ-FeOOH) (7). These metal oxyhydroxides, represented by γ-MO(OH), where M = Fe, Ni, Mn, Sc, Ti, etc., have attracted interest in fields from energy to medicine. In a nickel–hydrogen battery, the formation of poorly crystalline γ-NiOOH on the cathode has been shown to cause the memory effect of the battery (8). Titanate nanosheets have recently attracted attention in the electronics community because of their semiconducting properties (9). Hydrogen generation by aluminum–water reactions for onboard vehicular hydrogen storage (10) and the radiolytic generation of hydrogen in conjunction with metal corrosion in water in nuclear energy and waste systems (11) both involve aluminum hydroxides as reaction products. Aluminum hydroxide has also been used as a vaccine adjuvant (12) and its biochemical mode of action in a recent medical technology is being investigated (13).
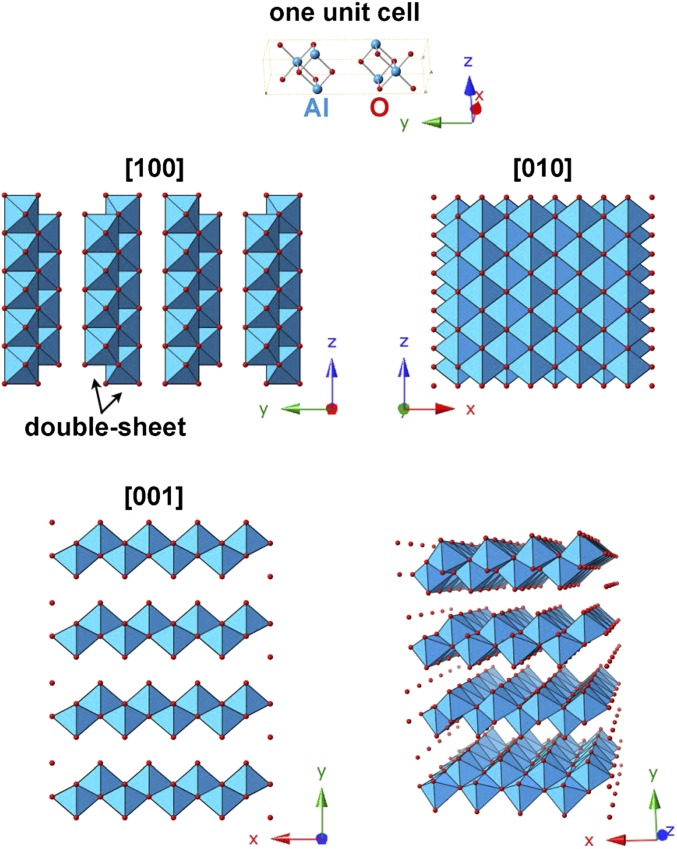
Boehmite is synthesized by aging noncrystalline aluminum hydroxide sol in an aqueous solution at a specific pH, and it has the basic crystal structure of double sheet of a metal–oxygen octahedron layer piled up through hydrogen bonding, as illustrated in Fig. S1. Poorly crystallized boehmite, often called pseudoboehmite (PB), forms in various crystal morphologies, such as thin films, thin platelets, and nanometer-sized rods or fibers. Importantly, the characteristics of alumina Al2O3 for industrial use prepared by thermal dehydration are influenced by the morphology of the starting boehmite crystals.
Fig. S1.
Basic crystal structure of boehmite. The unit cell of boehmite (space group is Amam) and several different view directions are shown. The double sheet of a metal–oxygen octahedron layer can be seen from the [100] direction. Hydrogen atoms are not displayed here.
Fibrous PB has a particularly poor crystalline structure (14, 15), and its crystal structure and morphology have never been clarified even by using the modern crystallography methods such as radial distribution function analyses with synchrotron X-ray diffraction (16, 17) and NMR (18). To solve such a long-standing problem with fibrous PB, we made full use of conventional transmission electron microscopy (TEM) methods, high-resolution TEM (HRTEM) imaging, and electron diffraction (ED), and successfully revealed the detailed morphologies and crystal structures, including determination of the fiber axis, of fibrous PB.
Results and Discussion
Structure of Fibrous PB.
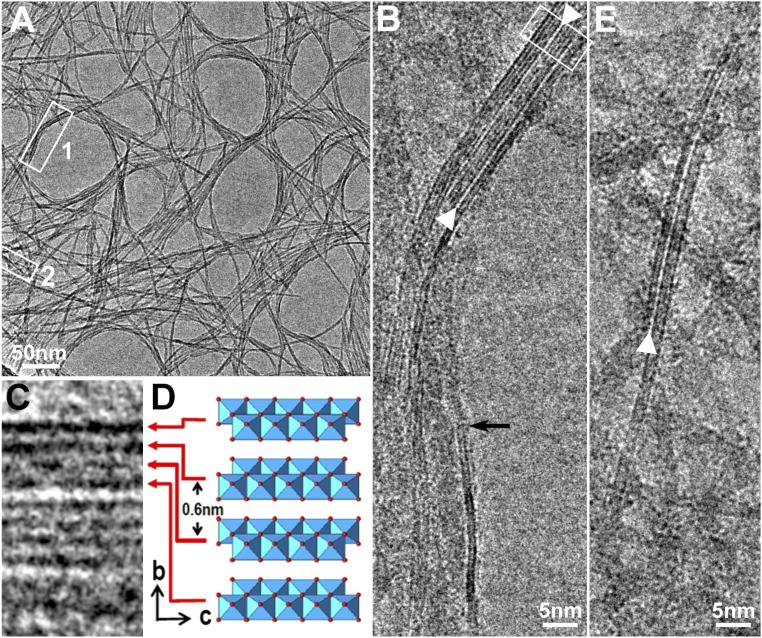
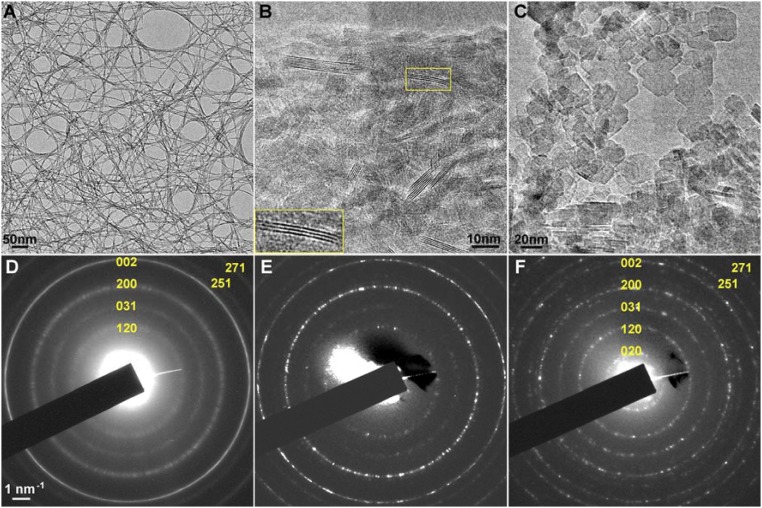
Fig. 1A shows TEM images of fibrous PB forming a lacy network of randomly dispersed nanometer-sized fibers prepared by drying a diluted sol of boehmite “F1000” (KAWAKEN Fine Chemical Co., Ltd.). The Debye–Scherrer ED (DS-ED) ring patterns shown in Fig. S2 A and D demonstrate that the crystal structure of PB is γ-AlOOH, as reported in the literature (17). However, the width of the DS-ED rings is generally broadened with some relatively sharper rings apparent as well, and the intensities of these rings differ slightly from those of the well-crystallized PB sample. The differences can be explained by the crystallite size of the fibrous PB F1000 sample. Such differences in crystal size and morphology from the other two samples, provided by the KAWAKEN Fine Chemical Co., namely nanorod “10A” and platelet “Powder,” were confirmed as shown in Fig. S2 B and C, respectively. DS-ED patterns of the three samples (Fig. S2 D–F) can be easily distinguished from each other in terms of the ring width and intensity.
Fig. 1.
TEM images of fibrous PB. (A) An electron micrograph shows a thin lacy film of fibrous boehmite samples F1000 from KAWAKEN Fine Chemical Co. (B) An enlarged image of a rectangular region 1 in A shows a ribbon-like crystalline structure. The contrast of the two dark lines suddenly disappeared and changed to a gray ribbon-like contrast, as shown by the arrow, indicating the twisting of the fibrous PB nanoribbon. (C) The dark lines in an enlarged image of the upper rectangle part in B are the (020) lattice image. (D) The corresponding Al–O hydroxides double sheet of crystal model of γ-AlOOH. (E) Another ribbon-like image of a rectangular region 2 in A. The wider separation indicated by the arrowheads in B and E suggests some species like H2O are intercalated between the adjacent Al–O octahedral double sheet.
Fig. S2.
Measurements of lattice parameters from DS-ED patterns. Typical DS-ED patterns recorded from three types of PB samples (offered by KAWAKEN Company) and their corresponding TEM images. (A) Quasi-1D fibrous PB crystals with a thickness of 0.7–3 nm form a random network. (B) TEM image of nanorod 10A: PB nanometer-sized rod-like crystallites of 1.2–6-nm thickness and 4–5-nm length. (C) TEM image of platelet Powder PB: platelet crystallites with diameters of 20–30-nm and 5–10-nm thickness. (D) The corresponding DS-ED pattern of a network of PB fibers in A. (E) The corresponding DS-ED pattern of nanorod 10A PB in B. (F) The corresponding DS-ED pattern of platelet Powder PB in C. The positions of the DS-ED rings of the platelet PB crystals accord with those of the fibrous PB but their intensity distributions and the ring widths differ slightly. The intensity of each ED ring is not uniform and also the widths of the rings are broadened, especially in A. Their origins are essential for knowing details of crystallography and crystal morphologies of the fibrous PB.
A highly magnified image of a region enclosed by rectangle 1 in Fig. 1A clarifies the ribbon structure appearing as dark, parallel straight lines that can be seen in the upper portion of the image (Fig. 1B), and a further enlarged image in Fig. 1C (for better visualization, this image has been rotated compared with Fig. 1B). A model of γ-AlOOH projected on (100) (Fig. 1D) shows that the separation between the two layers of the Al–O octahedron double sheet is 0.61 nm. We refer to one Al–O octahedron double sheet as “one layer” in this study for simplicity. By comparing the upper four dark lines in Fig. 1C with those from the model shown in Fig. 1D, it can be seen that these dark lines correspond to the (020) lattice image of the layers that are 0.607 nm apart. Another ribbon was collected in an area surrounded by rectangle 2 located close to rectangle 1, and the enlarged image is reproduced in Fig. 1E. This particular ribbon splits into two pieces with one piece having two layers on the left side and three additional layers on the right side. The contrast of the two dark lines suddenly disappears and changes to a gray ribbon-like contrast, as shown by the arrow in Fig. 1B, indicating the twisting of the fibrous PB ribbon. Such a narrow ribbon is expected to be inherently quite soft and flexible, as will be discussed later.
The anomalous white lines showing the splitting of the ribbons indicated by the arrowheads in Figs. 1B and 1E can be found frequently in the present fibrous PB. The width of the white lines (separation between the two black lines) is roughly 1.0 nm, up to 64% wider than the normal (020) lattice spacing (0.61 nm). The wider layer spacing is likely associated with initiation of cleavage on the (020) planes, suggesting that some species like H2O are intercalated between the adjacent Al–O octahedral double sheet. The water intercalation will be discussed further below.
Atomic Structure of Two-Layered Double Sheet of Fibrous PB.
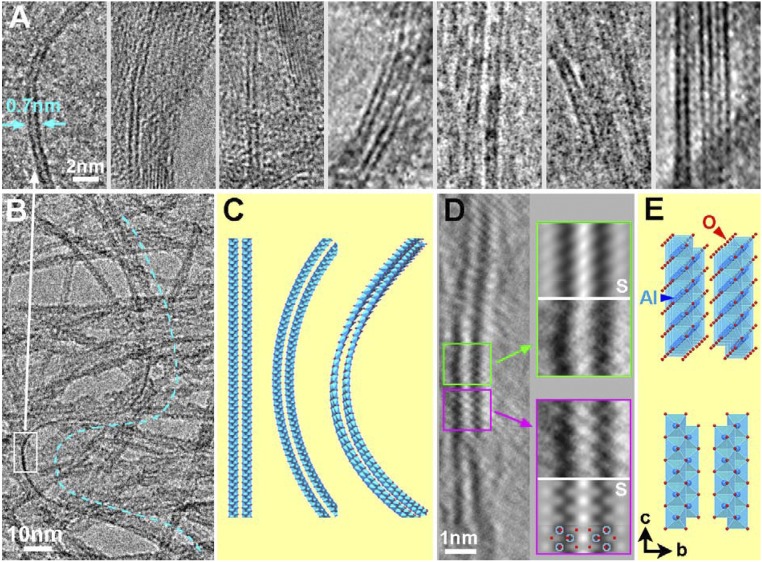
One of the most important results in the present study is the finding of the thinnest fibrous PB ribbon with 1D crystalline structure in a synthetic aluminum oxyhydroxide. The ribbon consists of a pair of Al–O octahedral double-sheet layers (imaged as two dark parallel lines), as shown in Fig. 2A. By measuring the interlayer spacing between two layers of double sheet (i.e., the distances between the two dark parallel lines in Fig. 2A), it is noted that the average distance is about 0.68 nm, as shown in Fig. 2A, which is 12% wider than the normal (020) lattice spacing (b/2 = 0.607 nm) of the boehmite crystal. A similar expansion was observed on double layers of graphene (19). One plausible explanation is the surface effect in nanoscale crystalline systems as demonstrated by density functional theory (DFT) calculation. Another possible explanation is the intercalation of small molecules, such as H2O, between the layers.
Fig. 2.
Two-layered double sheet of fibrous PB. (A) HRTEM images of nanoribbons consisting of two-layered Al–O hydroxide double sheet. The two layers are separated by 0.68 nm. (B) TEM image of a flexible and lengthy double-sheet nanoribbon, highlighted by a dotted line. (C) Schematic models of straight, bent, and twisted double-sheet nanoribbon. (D) HRTEM image with atomic resolution shows local distortion of a two-layered double sheet. The enlarged images of enclosed regions are shown in the right side inserted with the corresponding simulated images (labeled by ”S”) and Al and O atoms overlaid in the lower simulated image. (E) The corresponding models used for simulation in D. The model in the upper part is rotated by 5° along both the b and c axes relative to the model in the lower part.
The long double-sheet nanoribbon is bent and twisted, which is highlighted by a dotted line in Fig. 2B. The winding ribbon proves its softness and flexibility. A rectangular area in Fig. 2B exhibits a clear image of the double-sheet nanoribbon (Fig. 2A). Schematic models of the straight, bent, and twisted double-sheet nanoribbon are illustrated in Fig. 2C. The thinnest fiber is a freestanding ribbon structure consisting of only two layers of Al–O octahedral double sheet terminated with (100) and (010) surfaces, extending in the [001] direction, as shown in Fig. S1. The atomic structure of the fibrous PB nanoribbon can be observed in HRTEM images at atomic resolution (Fig. 2D). It reveals directly the arrangement of Al and O atoms (Al atoms show darker contrast in this image). Differences in the detailed contrast of the two magnified HRTEM images indicated by two rectangles on the right side of Fig. 2D are caused by the bending and twisting along this nanoribbon. The corresponding bending and twisting models are schematically illustrated in Fig. 2E. The model in the upper part is rotated by 5° around both the b and c axes relative to the model in the lower part. This HRTEM image with atomic resolution further reveals the local distortion of such thin PB nanoribbons and proves its flexibility.
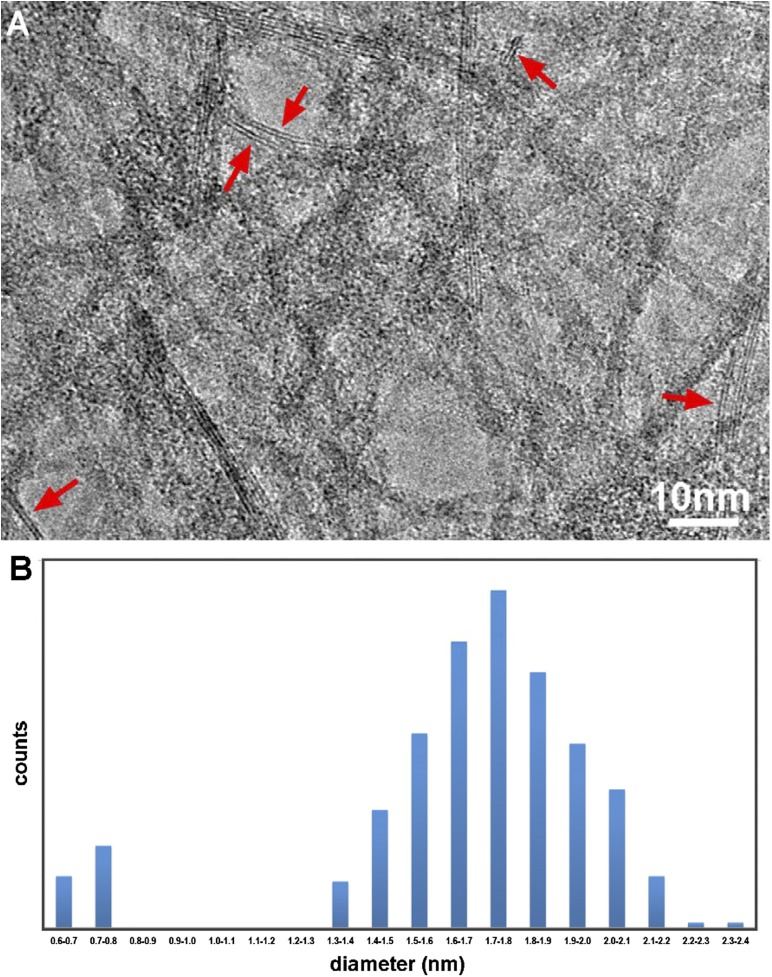
The mean thickness of the PB nanoribbon was measured from large numbers of TEM images. A typical TEM image is shown in Fig. S3A. The arrows indicate occurrence of two-layered double-sheet nanoribbons that happen to be imaged due to their edge on condition. The histogram of fiber thickness shown in Fig. S3B is centered at 1.8 nm, which corresponds to the width of 3–4 layers of the Al–O double sheet. The frequency of the discernible two-layered double-sheet nanoribbons is estimated to be more than 5%. It is noteworthy that, despite frequent appearances of the multilayered double sheets, a single-layered double sheet has never been observed in the present fibrous PB. The lack of a single Al–O octahedral double-sheet nanoribbon structure may be related to the instability or growth of the double sheet itself in aqueous aluminum hydroxides sols, whereas the two-layered nanoribbons are speculated to be nucleated more stably due to the interactions between their two layers. Further investigation is necessary.
Fig. S3.
Mean thickness measurement of PB nanoribbon. (A) A typical TEM image of fibrous PB. Some discernible fibrous PB nanoribbons comprising two layers of Al–O octahedral double sheet are shown by the red arrows. (B) Diameter distributions of fibrous PB measured from large amounts of TEM images. The histogram of diameter distributions reveals a peak centered at 1.7–1.8 nm, which corresponds to the width of 3–4 layers of the Al–O octahedral double sheet.
DFT Calculations.
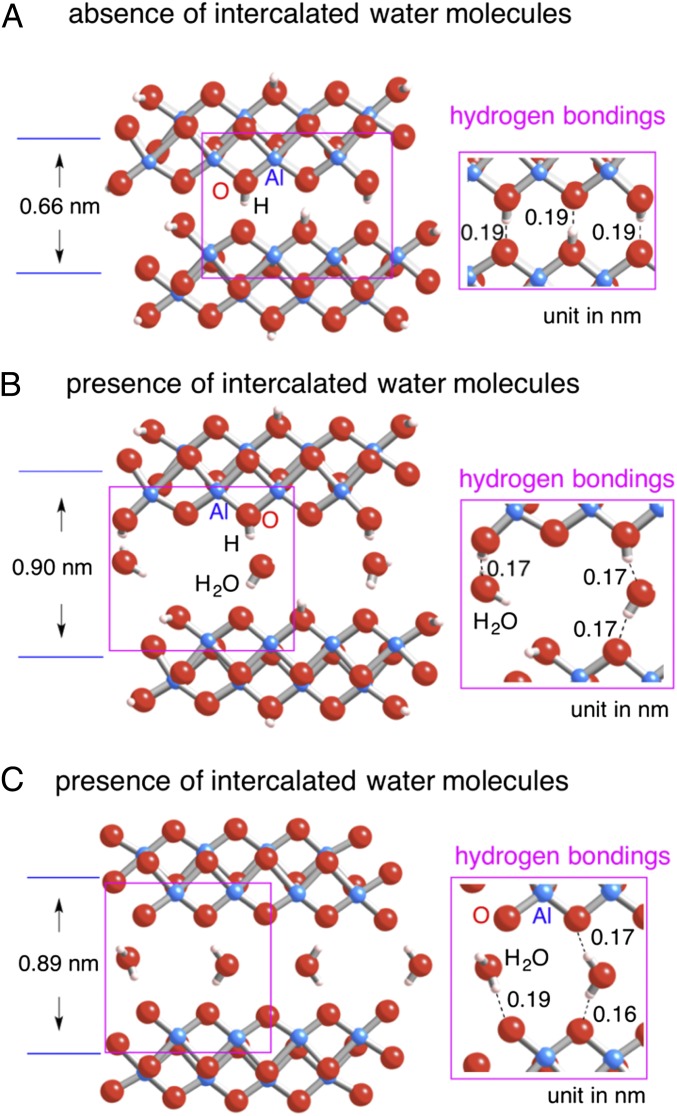
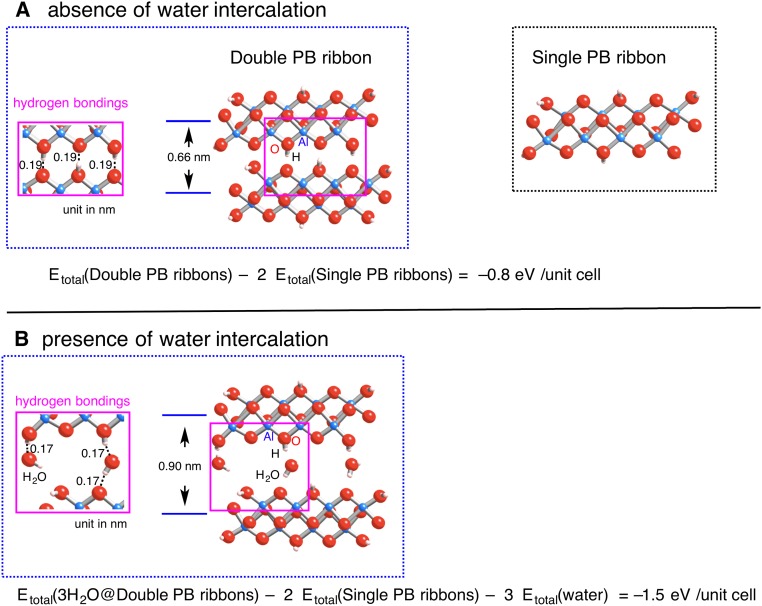
To obtain a better understanding of the structural features of the 1D PB structure observed by HRTEM, spin-polarized DFT calculations were performed under periodic boundary conditions. Two types of bonding patterns of oxygen atom termination were considered: the presence (Fig. 3 A and B) and absence (Fig. 3C) of H-atom saturation. Fig. 3 displays optimized structures for the PB nanoribbons, whose interlayer spacings, corresponding to half the lattice parameter of the b-axis unit cell of the boehmite crystal (blue lines), are given. First, the interlayer spacing of PB nanoribbons without water molecules was calculated to be 0.66 nm (Fig. 3A), which is 8% larger than the normal (020) spacing. Such an expansion fits very well with the TEM observations (0.68 nm for two layers of the Al–O double sheet), demonstrating the interlayer spacing in nanoscale 2D crystalline structure is slightly wider than in the bulk structure. Second, a model including the presence of water in which the adjacent Al–O octahedral double-sheet layers are bound with a monolayer of water molecules was considered. Different interlayer spacings of PB nanoribbons with and without intercalation of a monolayer of water molecules can be found by comparing Fig. 3A and Fig. 3B. The intercalation of only a single layer of water molecules strikingly expands the interlayer spacing of PB nanoribbons from 0.66 to 0.90 nm. In addition, the interlayer spacing is slightly affected by whether H-atom saturation is involved or not: 0.90 nm (Fig. 3B) and 0.89 nm (Fig. 3C) for the presence and absence of H-atom saturation, respectively. Within the inner space of the PB nanoribbon containing a monolayer of water molecules, the H atoms in water are separated by 0.17 nm from the O atoms in the Al–O octahedral sheet of the PB nanoribbon, and at the same time the O atoms in water are separated by 0.17 nm from the H atoms terminating the O atoms in the Al–O octahedral sheet of the PB nanoribbon. These interatomic separation ranges are suitable for attractive hydrogen-bonding interactions connecting water molecules and the Al–O octahedral sheet in the PB nanoribbon. The water molecules can act as adhesive agents for connecting Al–O octahedral double-sheet layers of the PB nanoribbon as shown in Fig. 1 B and E. Similar adhesion effects of water molecules between a γ-alumina surface and an epoxy resin were also found (20). The DFT results clearly indicate the influence of intercalated water molecules in enhancing interlayer spacings of PB nanoribbons, which provide a hint to understand the splitting observed in the TEM results. The calculated value of the separation, 0.9 nm, seems to support the observation on the large separation of splitting ribbons (∼1.0 nm, indicated by white arrowheads in Fig. 1 B and E) due to water intercalation. Although our calculation model containing three water molecules per unit cell gives rise to an interlayer spacing of 0.9 nm in the b axis, decreasing or increasing the number of water molecules within the Al–O octahedral double-sheet layers would diminish or expand interlayer spacing from 0.9 nm. Actually, the splitting width observed in this study has a distribution from 0.83 to 1.5 nm with an average value of 1.0 nm. However, in this study the occurrence of such kinds of splitting is not so frequent that the intercalated water content does not obviously affect the total amount of absorbed water in the present PB. Moreover, the slight expansion of the interlayer spacing from 0.61 to 0.68 nm could be associated with intercalation of small amount of water. The conclusion presented here suggests that the thicker PB nanoribbons showing the normal interlayer spacing of boehmite (b/2 = 0.61 nm) contain almost no water in their interlayer spaces.
Fig. 3.
Spin-polarized DFT optimized geometries for PB nanoribbons in the absence (A) and presence (B) of a monolayer of water molecules intercalated. Some terminated oxygen atoms are bound with H atoms. Interspace between Al–O octahedral double sheet with intercalated water molecules, surrounded by purple lines, was magnified (B). (C) Terminated oxygen atoms on PB nanoribbons are not bound with H atoms. Interspace between Al–O octahedral double sheet with intercalated water molecules, surrounded by purple lines, was magnified. Note that the separation of a water H atom to an end O atom on a PB nanoribbon (0.19 nm) is slightly longer than those to a normal O atom on a PB nanoribbon (0.16 and 0.17 nm). The unit of interlayer spacing between adjacent Al–O octahedral double sheet, corresponding to the half-lattice parameter of b-axis unit cell of the boehmite crystal (blue lines), and the length of hydrogen bonds (dotted lines in the inserted images), is given in nanometers.
Growth Direction of Fibrous PB.
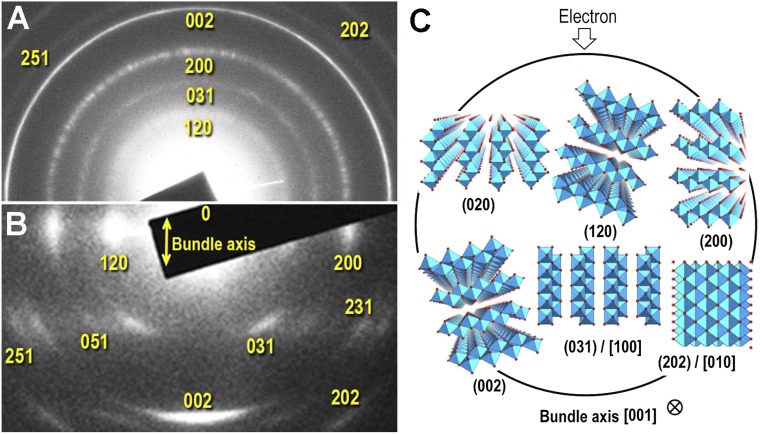
To determine the fiber axial direction of the fibrous PB, we systematically examined the DS-ED patterns of the fibrous PB crystals that tend to agglomerate to different degrees from completely randomly dispersed fibers to well-bundled ones. Two typical DS-ED patterns from these crystals are shown in Fig. 4 A and B. It is noted here that the 020 reflections were not observed in most of the DS-ED patterns. The absence of the 020 reflections was understood as a diffraction phenomenon for an extremely narrow ribbon structure of fibrous PB. Compared with the DS-ED ring pattern from randomly dispersed fibers (Fig. 4A), the pattern for the well-bundled fibers showed characteristic intensity maxima on each DS-ED ring (Fig. 4B). Regardless of which bundled fibers are observed, these maxima always appear at the same positions and can be indexed as labeled in Fig. 4B. The position of the intensity maxima of the 002 reflection always lies on the direction parallel to the bundle axis. This means that the individual fibrous PB crystals grow along the c axis, namely the [001] direction; 200, 002, and 202 reflections appear to belong to h0l reflections from a single crystal of γ-AlOOH, but 120 and 031 reflections do not. In addition, 120 reflections always appear in the same direction as the 200 reflection in the ED pattern, which should not be observed in a single crystal of γ-AlOOH. Such kinds of contradictions can be explained as long as the incident electron beam is perpendicular to the c axis of the fibrous PB crystal. This situation is illustrated in a cross-sectional view of a bundle of fibrous PB in Fig. 4C. This conclusion could explain many other experimental observations regarding the fiber morphology proposed here. An important conclusion is that the appearance of the intensity maxima in the DS-ED pattern (Fig. 4B) is a summation of diffraction intensities from every individual fiber in a bundle.
Fig. 4.
Bundles of fibrous PB. (A) DS-ED pattern recorded from a randomly dispersed thin film of fibrous PB crystals. (B) DS-ED pattern from bundled fibrous PB crystals, where the bundle axis is shown by the double-headed arrow. Both patterns are indexed as those from a bulk crystal of γ-AlOOH. The (002) reflections, however, are relatively strong in both DS-ED patterns in A and B, suggesting the long ribbon-like structure of PB. Broadening of the (200) reflection is caused by an extremely narrow width of the PB ribbon. The intensity maxima, which look like a single crystal, can be explained by a model in which individual fibers form in a bundle, and align parallel to the [001] direction (i.e., c axis) but randomly rotated around this axis. (C) A cross-sectional view of a bundle is illustrated. Some of the fibers aligned in low-index zone axes are indicated by the index numbers shown in C.
Although a bulk boehmite crystal is an electrical insulator, 1D nanoribbon PB crystals could have different physical properties when intercalated with some ion species, as is known for functionalized carbon nanotubes (21, 22) and 2D materials (23). The electronic structure of intercalated 1D PB nanoribbon is of interest in theoretical and experimental investigations, in terms of the nanoribbon size, layer distance between adjacent Al–O octahedral double sheet, and a sol state where the structure is immersed in an aqueous ionic solution.
One of the differences between bulk boehmite and PB is that the latter contains more water. In general, the specific surface area of a crystal is increased with a decrease in crystal size. Therefore, the fibrous PB crystals, which have a larger specific surface area than the bulk boehmite crystals, could adsorb more water on their surfaces (24). The present study could specify the site for the water that goes preferentially into the interlayer spaces of the fibrous PB nanoribbons having splitting or cleavage on the (010) plane. A similar interlayer structure for water intercalation will be taking place in the bundle of the fibrous PB nanoribbons.
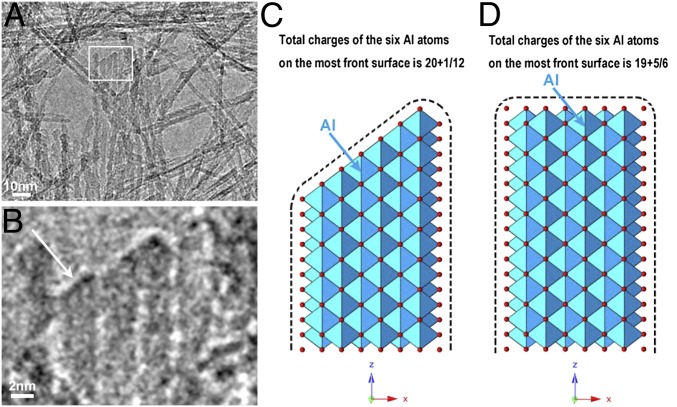
Synthesis of several different types of morphologies of boehmite crystals has been established in the sol–gel reaction of Al-hydroxide solution by controlling the pH, temperature, and reaction time. In contrast with the rod PB and platelet PB, the fibrous PB crystals extend preferentially in the c-axis direction. Such an extremely anisotropic linear growth of the fibrous PB-like carbon nanotubes (5) may be caused by a reactive site on the tip of the nanoribbon, where Al-hydroxide ionic species could be attracted. The amount of surface negative charge was counted to be slightly higher on the (001) surface than on the (100) and (010) surfaces. Therefore, positive Al-hydroxide ion clusters will be continuously attracted more on the (001) plane that accelerates the growth of c-axis direction. The TEM image shown in Fig. 5A and an amplified area (white rectangle area in Fig. 5A) showing one of the terminated fibers (Fig. 5B) will serve to clarify more specifically the growth mechanism of the fibrous PB. The tip shapes of the terminated fibers (indicated by an arrow in Fig. 5B) show a geometrical feature and its possible model is illustrated in Fig. 5C. The knife-edge shape of the tip appears to fit well with the (101) crystal surface and therefore this surface is considered to be a growth front of the fiber. By comparing the (101) surface (Fig. 5C) with the (001) surface (Fig. 5D), it is known that the total amount of charge of the wedge tip ending with the (101) surface is slightly larger than that of the flat (001) surface on the assumption that the aluminum ions are fully saturated with O−2 or OH−1 when counting the amount of the local net charges near the tips. It is reasonable therefore that positively charged Al–O clusters can be attracted and deposited onto the tip surface, and fibrous PB grows along its c axis. The growth should be influenced by the stability of the positively charged multivalent octahedral clusters [Al–O]n, (n = 0 ∼ +3) (25–27). The absence of the monolayered Al–O octahedral double sheet is demonstrated by DFT energy calculation shown in Fig. S4, in which two-layered Al–O octahedral double sheet is 0.8 eV (per unit cell) and 1.5 eV (per unit cell) more energetically favorable than monolayered Al–O octahedral double sheet without and with water intercalation, respectively. This may be a tie into the Al Keggin cluster intermediates and their assembly (25). It could be interesting to explore further in the future.
Fig. 5.
Tip shapes of the fibrous boehmite – F1000. (A) An electron microscope image showing some terminated fibers enclosed by a rectangle; their enlarged image is reproduced in B. (C) A possible structure of the tip of the fibers terminated with the (101) facet of the boehmite crystal. The tip region has a slight excess positive charge comparing to a tip terminated with the (001) facet shown in D. The excess charge would promote an anisotropic crystal growth of the fibrous PB.
Fig. S4.
DFT calculation showing two-layered Al–O octahedral double sheet is 0.8 eV (per unit cell) and 1.5 eV (per unit cell) more energetically favorable than monolayered Al–O octahedral double sheet when water intercalation is absent and present, respectively.
We conclude that a “genuine 1D” nanoribbon of fibrous boehmite has been found in commercial products of PB. Although 1D nanoribbons of two-layered Al–O octahedral double sheet are a minor structure in the present product (more than 5%), it could be synthesized to be a major structure by fully controlling growth processes. It is challenging to form the nanoribbons in other similar metal oxyhydroxides γ-MO(OH). Moreover, the growth mechanism of fibrous PB will broaden deeper understandings in classical but important fields such as nucleation and growth in sol–gel processes, polymorphic transformations, and related aluminum aqueous clusters.
Methods
Three PB samples examined in the present study are F1000: fibers of 0.7–3-nm thickness, 10A: nanometer-sized rod-like crystallites of 1.2–6-nm thickness and 4–5-nm length, and Powder: platelet crystallites with diameters of 20–30-nm and 5–10-nm thickness. All are commercial products (KAWAKEN Fine Chemical Co.) prepared by hydrolysis and peptization of aluminum isopropoxide and their morphologies are controlled by reaction processes. The F1000 is synthesized at the reaction temperature of 150 °C and aged for 6 h with stabilizer of acetic acid (pH 3.4–4.2) (28). The first two samples are aqueous hydroxide sols, and the last one is a dried powder. For the TEM observation, the samples were diluted in water and collected on a TEM grid covered with a holey carbon film. The TEM specimens were examined by a JEM 2010F electron microscope with a Corrected Electron Optical Systems GmbH (CEOS) spherical aberration corrector operated at 120 keV. Many clay minerals that contain hydroxyl groups or water are unstable under electron beam irradiation (29). In this study, the electron beam damage to the specimen was minimized as much as possible [the beam density during the observations ranged from 50 to 150 electrons/(nm2·s)]. A sequence of HRTEM images (up to 20 frames) was recorded with an exposure time of 0.5–1 s for each frame, and after drift compensation, some frames can be superimposed to increase the signal-to-noise ratio for better visualization.
DFT calculations were performed by using the projected augmented wave method, implemented in the Vienna ab initio Simulation Package (VASP 4.6) (30). In the VASP calculations, the Perdew, Burke, and Ernzerhof (PBE) functional was used to describe electronic exchange and correlation (31). Previous studies confirmed that the PBE functional is suitable for describing hydrogen-bond interactions (32–35), together with metal–oxygen bond interactions. All calculations for optimizing PB nanoribbon structures were spin polarized. Kinetic energy cutoff of the plane-wave basis is 400 eV. For the calculations of two Al–O octahedral double-sheet layers with a monolayer of water molecules, we used a square supercell, containing 77 and 68 atoms for the H-atom saturated and unsaturated models, respectively. We allowed full geometry relaxation of PB nanoribbons directed in the c direction, where the lattice parameters in the a- and b directions were fixed at 3 nm to avoid significant interactions of the two Al–O octahedral double-sheet layers with those located on the neighboring unit cells. A Γ-centered 1 × 1 × 2 k-point mesh was used for the geometry optimization. The optimization processes were stopped when the forces on all of the mobile atoms were smaller than 0.01 eV/0.1 nm.
Acknowledgments
We thank Dr. F. Mizukami (National Institute of Advanced Industrial Science and Technology) and Dr. N. Nagai (Kawaken Fine Chemicals Co.) for discussions. Z.L. acknowledges Japan Society for the Promotion of Science KAKENHI Grant JP 25107003.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614059113/-/DCSupplemental.
References
- 1.Brus LB. Electron–electron and electron‐hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J Chem Phys. 1984;80(9):4403–4409. [Google Scholar]
- 2.Vrbanic D, et al. Air-stable monodispersed Mo6S3I6 nanowires. Nanotechnology. 2004;15(5):635–638. [Google Scholar]
- 3.Zhang Z, et al. Ultrathin inorganic molecular nanowire based on polyoxometalates. Nat Commun. 2015;6:7731. doi: 10.1038/ncomms8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novoselov KS, et al. Electric field effect in atomically thin carbon films. Science. 2004;306(5696):666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 5.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. [Google Scholar]
- 6.Christoph GC, Corbato CE, Hofmann DA, Tettenhorst RT. The crystal structure of boehmite. Clays Clay Miner. 1979;27(2):81–86. [Google Scholar]
- 7.Ewing FJ. The crystal structure of lepidocrocite. J Chem Phys. 1935;3(7):420–424. [Google Scholar]
- 8.Tkalych AJ, Yu K, Carter EA. Structural and electronic features of β-Ni(OH)2 and β-NiOOH from first principles. J Phys Chem C. 2015;119(43):24315–24322. [Google Scholar]
- 9.Sasaki T, Ebina Y, Kitami Y, Watanabe M. Two-dimensional diffraction of molecular nanosheet crystallites of titanium oxide. J Phys Chem B. 2001;105(26):6116–6121. [Google Scholar]
- 10.Petrovic J, Thomas G. Reaction of Aluminum with Water to Produce Hydrogen. US Department of Energy; Washington, DC: 2008. Version 1.0, pp 1–26. [Google Scholar]
- 11.Westbrook M, Sindelar R, Fisher D. Radiolytic hydrogen generation from aluminum oxyhydroxide solids: Theory and experiment. J Radioanal Nucl Chem. 2015;303(1):81–86. [Google Scholar]
- 12.Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82(5):497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 13.Marichal T, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17(8):996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 14.Bugosh J. Colloidal alumina-the chemistry and morphology of colloidal boehmite. J Phys Chem. 1961;65(10):1789–1793. [Google Scholar]
- 15.Sousa Santos H, Sousa Santos P. Pseudomorphic formation of aluminas from fibrillar pseudoboehmite. Mater Lett. 1992;13(4-5):175–179. [Google Scholar]
- 16.Brühne S, Gottlieb S, Assmus W, Alig E, Schmidt MU. Atomic structure analysis of nanocrystalline boehmite AlO(OH) Cryst Growth Des. 2008;8(2):489–493. [Google Scholar]
- 17.Tettenhorst R, Fofmann DA. Crystal chemistry of boehmite. Clays Clay Miner. 1980;28(5):373–380. [Google Scholar]
- 18.Francetič V, Bukovec P. Peptization and Al-Keggin species in alumina sol. Acta Chim Slov. 2008;55(4):904–908. [Google Scholar]
- 19.Iijima S. Coherent imaging of finitely-sized objects and its application to graphitic carbon. Chem Scr. 1978-79;14:117–123. [Google Scholar]
- 20.Semoto T, Tsuji Y, Yoshizawa K. Molecular understanding of the adhesive force between a metal oxide surface and an epoxy resin: Effect of surface water. Bull Chem Soc Jpn. 2012;85(6):672–678. [Google Scholar]
- 21.Zhu WH, et al. Fluorescent chromophore functionalized single-wall carbon nanotubes with minimal alteration to their characteristic one-dimensional electronic states. J Mater Chem. 2003;13(9):2196–2201. [Google Scholar]
- 22.Liu Z, et al. Self-assembled double ladder structure formed inside carbon nanotubes by encapsulation of H8Si8O12. ACS Nano. 2009;3(5):1160–1166. doi: 10.1021/nn9002727. [DOI] [PubMed] [Google Scholar]
- 23.Liu M, et al. An anisotropic hydrogel with electrostatic repulsion between cofacially aligned nanosheets. Nature. 2015;517(7532):68–72. doi: 10.1038/nature14060. [DOI] [PubMed] [Google Scholar]
- 24.Majzlan J, Navrostsky A, Casey WH. Surface enthalpy of boehmite. Clays Clay Miner. 2000;48(6):699–707. [Google Scholar]
- 25.Casey WH. Large aqueous aluminum hydroxide molecules. Chem Rev. 2006;106(1):1–16. doi: 10.1021/cr040095d. [DOI] [PubMed] [Google Scholar]
- 26.Ohlin CA, Villa EM, Rustad JR, Casey WH. Dissolution of insulating oxide materials at the molecular scale. Nat Mater. 2010;9(1):11–19. doi: 10.1038/nmat2585. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong CR, Casey WH, Navrotsky A. Energetics of Al13 Keggin cluster compounds. Proc Natl Acad Sci USA. 2011;108(36):14775–14779. doi: 10.1073/pnas.1111243108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagai N, Mizukami F. Properties of boehmite and Al2O3 thin films prepared from boehmite nanofibers. J Mater Chem. 2011;21(38):14884–14889. [Google Scholar]
- 29.Iijima S, Buseck PR. Experimental study of disordered mica structures by high-resolution electron microscopy. Acta Crystallogr A. 1978;34(5):709–719. [Google Scholar]
- 30.Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B Condens Matter. 1996;54(16):11169–11186. doi: 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- 31.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett. 1996;77(18):3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 32.Yumura T, Yamasaki A. Roles of water molecules in trapping carbon dioxide molecules inside the interlayer space of graphene oxides. Phys Chem Chem Phys. 2014;16(20):9656–9666. doi: 10.1039/c4cp00658e. [DOI] [PubMed] [Google Scholar]
- 33.Ireta J, Neugebauer J, Scheffler M. On the accuracy of DFT for describing hydrogen bonds: Dependence on the bond directionality. J Phys Chem A. 2004;108(26):5692–5698. [Google Scholar]
- 34.Zhao Y, Truhlar DG. Benchmark databases for nonbonded interactions and their use to test density functional theory. J Chem Theory Comput. 2005;1(3):415–432. doi: 10.1021/ct049851d. [DOI] [PubMed] [Google Scholar]
- 35.Rao L, Ke H, Fu G, Xu X, Yan Y. Performance of several density functional theory methods on describing hydrogen-bond interactions. J Chem Theory Comput. 2009;5(1):86–96. doi: 10.1021/ct800237n. [DOI] [PubMed] [Google Scholar]