Significance
Immunoglobulin and T-cell receptor genes are assembled in lymphoid cells from gene fragments by the process known as V(D)J recombination, which is initiated by the recombination activating gene (RAG)1/RAG2 recombinase. To ensure that recombination occurs only in the correct cell type and at the right developmental stage, multiple layers of regulation are necessary, including specific modifications of chromatin. We show that nucleosome positioning is another important factor in this regulation. Developmentally regulated changes in nucleosome positioning help to guide RAG1/RAG2 to the correct sites in recombinationally active cells. These changes occur on the scale of hundreds of kilobases, a form of regulation not typically seen in the rest of the mammalian genome.
Keywords: epigenetics, V(D)J recombination, nucleosome positioning, chromatin, lymphocytes
Abstract
We show that the physical distribution of nucleosomes at antigen receptor loci is subject to regulated cell type-specific and lineage-specific positioning and correlates with the accessibility of these gene segments to recombination. At the Ig heavy chain locus (IgH), a nucleosome in pro-B cells is generally positioned over each IgH variable (VH) coding segment, directly adjacent to the recombination signal sequence (RSS), placing the RSS in a position accessible to the recombination activating gene (RAG) recombinase. These changes result in establishment of a specific chromatin organization at the RSS that facilitates accessibility of the genomic DNA for the RAG recombinase. In contrast, in mouse embryonic fibroblasts the coding segment is depleted of nucleosomes, which instead cover the RSS, thereby rendering it inaccessible. Pro-T cells exhibit a pattern intermediate between pro-B cells and mouse embryonic fibroblasts. We also find large-scale variations of nucleosome density over hundreds of kilobases, delineating chromosomal domains within IgH, in a cell type-dependent manner. These findings suggest that developmentally regulated changes in nucleosome location and occupancy, in addition to the known chromatin modifications, play a fundamental role in regulating V(D)J recombination. Nucleosome positioning—which has previously been observed to vary locally at individual enhancers and promoters—may be a more general mechanism by which cells can regulate the accessibility of the genome during development, at scales ranging from several hundred base pairs to many kilobases.
The diverse repertoire of antigen receptors in vertebrates is generated by an ordered series of somatic site-specific DNA rearrangement events, collectively termed V(D)J recombination. Antigen receptor genes are assembled from V, D, and J gene segments organized into seven different loci [T-cell receptor (TCR) α, β, γ, and δ, and Ig H, κ, and λ], each of which undergoes a series of recombination reactions to generate a functional TCR or B-cell receptor (BCR) (1, 2) Rearrangement is lineage-specific, such that BCR genes are fully rearranged only in B cells and TCR genes are assembled only in T cells. Rearrangement also occurs in a preferred temporal order. For example, at the human and murine IgH loci, joining of D to J segments precedes V to DJ joining, and IgH joining precedes joining at Igκ (or Igλ) (3). In addition, recombination at several loci shows “allelic exclusion”: Functional VDJH or VJκ/Jλ joining occurs only on one allele (4).
Recombination is initiated by the lymphocyte-specific recombination activating gene (RAG)1/RAG2 endonuclease. RAG1/2 cleaves at the recombination signal sequences (RSSs) flanking all antigen receptor gene segments. The consensus RSS consists of a heptamer sequence directly adjacent to the coding element and an A/T-rich nonamer separated from the heptamer by a spacer region of conserved length (12 or 23 bp) but relatively nonconserved sequence. The broken ends are then joined with the aid of DNA repair proteins, coding end to coding end and RSS to RSS (5). The observation that the same recombination signal sequence and the same RAG1/2 recombinase are used for all of the antigen receptor assembly events led to the question of how the cell-type specificity and timing of this complex multistep process is regulated.
It has long been understood that one major level of control is the cis-acting accessibility of the various loci (6). Correlations of recombinational accessibility [with sense and antisense transcription, DNase I hypersensitivity, histone modifications, chromatin compaction, chromosome looping, and subnuclear localization of the antigen receptor loci] and the presence or absence of such factors as CTCF/Rad21, Ezh2, YY1, Pax5, and IL7 provide clues as to the changes associated with an accessible state (7). However, the physical nature of the functionally accessible state and the process of establishing it remain poorly understood.
The dynamic packaging of DNA into chromatin provides a mechanism for controlling such diverse processes as transcription, replication, recombination, and DNA repair. Local positioning of nucleosomes can serve to either occlude or reveal a target site. For example, nucleosome depletion is generally observed at the transcriptional start site (TSS) of expressed, compared with silent, genes, and localized nucleosome repositioning is observed at a variety of regulatory loci (8–10). However, large-scale alterations in nucleosome density on the order of 10,000 to 100,000 bp have yet to be observed.
Cis-acting accessibility for V(D)J recombination could in principle be modulated by both large-scale effects and local mechanisms that make an individual RSS accessible to the recombinase. A rosette-like 3D chromatin structure—similar to the multilooped chromosome conformations that have been observed in the beta-globin locus control region, in the Hox cluster, and in olfactory receptor genes (11–13)—has been proposed for the IgH locus, with variable gene segments grouped into distinct chromatin domains (14, 15). Chromatin compaction and looping have also been identified as parameters controlling VDJ recombination (16–19).
V(D)J recombination could also be controlled by locally regulated changes in nucleosome positioning. In vitro studies have confirmed that nucleosome position can influence V(D)J cleavage (20, 21). An RSS assembled into a mononucleosome is refractory to cleavage by the Rag1/2 recombinase, but the precise position of the RSS with respect to the dyad axis governs the extent of inhibition. Histone acetylation and SWI/SNF-dependent nucleosome remodeling can act together to stimulate RSS cleavage within both mononuclesomes (22) and nucleosome arrays (23). In vivo, Brg1, the SWI/SNF ATPase, is found at loci poised to undergo rearrangement (24). Furthermore, the artificial recruitment of Brg1 to a promoter is sufficient to render the nearby locus accessible to the recombinase, consistent with a role for alterations in nucleosome position or occupancy in the regulation of V(D)J recombination (25).
The V(D)J recombinase itself provides a direct mechanistic link between chromatin structure and the ability of a locus to undergo V(D)J recombination. RAG2 contains a PHD finger in its C terminus that recognizes the specific histone H3 tail modification of trimethylation of lysine 4 (H3K4me3) (26, 27). RAG2 binds even more tightly when the nucleosome is symmetrically dimethylated on arginine 2 of histone H3 (H3R2me2s) in addition to bearing the H3K4me3 modification (28). H3K4me3 and H3R2me2s are found at recombinationally accessible antigen receptor loci, such as the IgH D and J segments in pro-B cells, Igκ in pre-B cells, and TCRα in pro-T cells (26, 29–31). Recognition of H3K4me3 by RAG2-PHD is required for efficient V(D)J recombination in vivo (26, 27). In addition, in vitro analysis has revealed that the binding of H3K4me3 by RAG2 activates the recombinase complex by relieving autoinhibition imposed by interaction of the C-terminal domains of RAG1 and RAG2 (32). Because the generation of H3K4me3 is facilitated by RNA pol II and transcription, the requirement for H3K4me3 to activate the recombinase provides an explanation for the critical role transcription through antigen receptor loci plays in V(D)J recombination. Further, it suggests a requirement for an H3K4me3 nucleosome in close proximity to the RSS.
Little is known about local or global chromatin organization of the antigen receptor loci at the level of nucleosome positioning and occupancy. A few studies have addressed this question at individual sites, but no systematic evidence of developmentally regulated nucleosome positioning has emerged. In isolation, the consensus nonamer sequence within the RSS can influence nucleosome position of in vitro assemblies (33), but, when longer flanking DNA, with a variety of other sequences, is included, this nonamer effect is found to be relatively weak and easily overcome (20, 23). When the nucleosome positioning and occupancy of a few, specific TCR gene segments was compared between recombinationally competent and recombinationally incompetent TCRβ and TCRα alleles in primary thymocytes, no consistent positioning of nucleosomes with respect to the RSS was observed (34). However, because the recombinationally incompetent alleles were missing key enhancers and promoters, these results left open the possibility that transcription-dependent depletion or repositioning of nucleosomes might occur and facilitate accessibility of the RSS.
In this study, we compared the chromatin structure of the Ig heavy chain and the T-cell receptor (TCR) alpha loci in three murine cell types rendered recombinationally inactive by loss of a RAG recombinase: (i) RAG2−/− pro–B-cell lines, where the entire IgH locus is poised and available for recombination; (ii) RAG1−/− pro–T-cell lines, lymphoid cells where the TCRα locus is poised and available for recombination and the IgH locus is partly open but the V region does not rearrange; and (iii) recombinationally inactive RAG2−/− mouse embryonic fibroblasts (MEFs), where the entire IgH and TCRα loci are in a closed conformation and unavailable for rearrangement. We demonstrate that nucleosome positioning and occupancy are highly regulated around the RSSs of the V segments of both the IgH and TCRα loci. In addition, we found that nucleosome density varies on the scale of hundreds of kilobases, defining chromosomal domains within the IgH locus in a way that depends on cell type. These findings suggest that developmentally regulated changes in nucleosome location and occupancy play a fundamental role in creating and controlling the accessibility of recombination signal sequences during V(D)J recombination.
Results
We began from an apparent contradiction. RAG2 must be bound to a modified histone tail to be activated for recombination. However, RAG-catalyzed cleavage is greatly inhibited when the RSS resides within a nucleosome. How can these observations be reconciled? This question led us to ask whether nucleosome occupancy around the RSS might be subject to developmental or lineage-specific regulation in such a way as to facilitate V(D)J cleavage in appropriate cell types and suppress V(D)J cleavage in inappropriate cell types.
High-Resolution Nucleosome Mapping of the IgH Locus.
We performed high-resolution nucleosome mapping at the IgH locus (Fig. 1) using custom-designed microarrays (Materials and Methods), which allowed resolution of nucleosome positioning within 5 to 10 bp. To ensure the best opportunity to observe large differences in chromatin structure and nucleosome positioning between two cell types, we chose to compare nucleosome positioning in Abelson-transformed Rag2−/− pro–B-cell lines and in Rag2−/− MEFs, both generated from the same C57BL/6 RAG2−/− inbred mouse strain. The IgH locus in Rag2−/− pro-B cells is poised to undergo recombination, but, in Rag2−/− MEFs, the locus is completely refractory to cleavage even upon overexpression of RAG1/2. Nucleosome positioning was also compared between Rag-deficient pro-B and pro-T cells to determine whether any lineage-specific regulation could be detected.
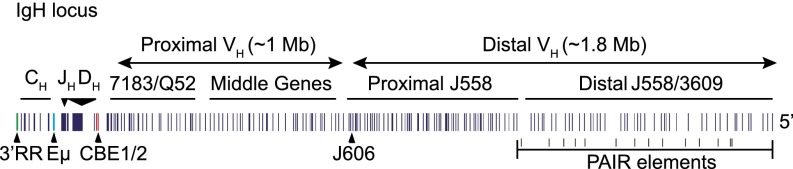
Fig. 1.
Schematic map of the unrearranged murine IgH locus. The relative positions of the CH, JH, DH, proximal VH, middle VH, proximal VH J558, and distal VH J558 gene segments are indicated. The variable gene segments—classified based on sequence, location, and function—are indicated above the locus (50). The IgH 3′ regulatory region (3′RR, green), Eμ enhancer (Eμ, green), CTCF binding elements (CBE1/2, red) (70, 71), and PAX5-dependent regulatory (PAIR) elements (43) are indicated below the locus. The approximate sizes of the proximal and distal VH domains are indicated in the figure.
Isolated chromatin was digested with a range of micrococcal nuclease (MNase) concentrations (Fig. S1), and the DNA derived from the mononucleosome fraction arising from each concentration was pooled, hybridized to the arrays, and analyzed as described (Materials and Methods). Variability in MNase profiles between experiments carried out under different conditions can hinder a direct comparison of absolute levels of nucleosome occupancy between cell types (35). However, the patterns across a locus can be readily compared between cell types, and a comparison of nucleosome occupancy across a locus within an individual cell type can be made (36–39). Thus, our study focused on a comparison of relative values of nucleosome occupancy.
Fig. S1.
Agarose gels illustrating titration of MNase digestion. Chromatin (A) and bare genomic DNA (B) were digested with varying amounts of MNase (Materials and Methods) and resolved on a 2% agarose gel. Then, 500 µg of DNA was loaded onto each lane. Mononucleosomally sized fragments (>100 bp and <200 bp) were isolated from the gel, purified, and used for subsequent microarray analysis.
In Pro-B Cells, V Segment RSSs at IgH Are Accessible and Flanked by a Nucleosome.
To investigate regulation of nucleosome occupancy at RSSs within the IgH locus, we compared the occupancy profiles around the RSS of all IgH V segments in pro-B cells, pro-T cells, and MEFs. Two independent biological samples were evaluated, and the mean occupancy is shown in Fig. 2 (see Fig. S3 for the correlation between these replicates). In RAG2−/− pro-B cells, a strong, narrow peak centered around position −60 bp (relative to the start of the RSS) was observed (Fig. 2A and Fig. S2). The width of this peak was consistent with a single tightly positioned nucleosome (the “−1 nucleosome”) that is directly adjacent to the RSS in pro-B cells poised for V(D)J recombination (Fig. 2B).
Fig. 2.
Lymphoid-specific nucleosome occupancy profiles at murine VH gene segments. (A) The normalized nucleosome occupancy signal around the 195 variable genes from murine IgH variable gene segments was centered on the RSS, averaged per base pair and plotted for the three cell types indicated (see Fig. S2 for comparable analysis of the 110 nonpseudogene VH gene segments). Green, pro-B cells; red, MEFs; blue, pro-T cells. Mean normalized nucleosome occupancy signal from each cell line is scaled (Materials and Methods). Vertical dashed lines represent the start of the RSS (marked as 0). Distances on the x axis are shown in base pairs. Intervals of significance are shown for the −50-bp position, and P values are calculated via Kolmogorov–Smirnov test by comparing the difference between the average nucleosomal profiles of the coding segment (−50 bp) and the intergenic region (+75 bp) flanking the RSS. A schematic representation of the RSS (black triangle) and its flanking VH gene segment (gray rectangle) is shown below the panel. Data represents the mean of two independent biological replicates. (B) Deduced location of positioned nucleosomes (colored ovals) relative to the RSS (black triangles) in the three cell types is represented with the same color scheme as in A.
Fig. S3.
Biological replicates are well-correlated with each other. Scatter plots of MEF, pro-B, and pro-T replicates are given. The local linear polynomial fit of the data is shown in red. The diagonal dashed line (green) indicates a “perfect” correlation between replicates. Pearson (“P”) and Spearman (“S”) correlation coefficients are shown in the top-left corner of each scatter plot.
Fig. S2.
Lymphoid-specific nucleosome occupancy profiles at nonpseudogene, murine VH gene segments. The normalized nucleosome occupancy signal around the 110 nonpseudogene VH segments was centered on the RSS, averaged per base pair, and plotted for the three cell types indicated. Green, pro-B cells; red, MEFs; blue, pro-T cells. Mean normalized nucleosome occupancy signal from each cell line is scaled. Vertical dashed lines represent the start of the RSS (0 to +38). Distances on the x axis are shown in base pairs. Intervals of significance are shown for the −50-bp position, and P values are calculated via Kolmogorov–Smirnov test by comparing the difference between the average nucleosomal profiles of the coding segment (−50 bp) and the intergenic region (+75 bp) flanking the RSS. A schematic representation of the RSS (black triangle) and its flanking VH gene segment (gray rectangle) is shown at the bottom. Data represent the mean of two independent biological replicates.
A nucleosome positioned at −60 bp (±10 bp) would place the heptamer of the RSS on the “shoulder” (entry/exit point) of the nucleosome core particle, with the remainder of the RSS extending into linker DNA. Assuming that adjacent nucleosomes (on the intergenic side of the RSS) are separated by distances similar to those detected for phased nucleosomes at transcription start sites (36, 37), enough room is left for the 39 bp of the RSS (where the RSS is placed at coordinates 0 to +38) to be located off the histone core shoulder. Thus, in pro-B cells, the RSS would be either loosely associated with the nucleosome or in the linker region adjacent to the nucleosome, an ideal location that would allow access to the RAG recombinase (20, 23).
In MEFs, V Segment RSSs at IgH Are Occluded by a Nucleosome.
The pattern of nucleosome occupancy for chromatin derived from MEFs was markedly different from that of Abelson-transformed pro-B cells. In MEFs, the strongly positioned −1 nucleosome observed in pro-B cells is completely absent, and, instead, the coding region adjacent to the RSS is depleted of nucleosomes (Fig. 2 and Fig. S2). The strongest peak in the MEF profile is the “+1” nucleosome. The breadth of the peak is indicative of the presence of a single, but not well-positioned, nucleosome. The MEF +1 nucleosome is located at ∼+75 bp. Therefore, the +1 nucleosome in MEFs overlaps most of the 39 bp of the RSS (Fig. 2B). A series of peaks indicating the presence of additional nucleosomes extends in the direction of the intergenic sequences. Thus, the IgH variable (VH) RSSs seem to be wrapped into localized nucleosomes, flanked by a region within the adjacent coding element where nucleosome occupancy is reduced.
Intermediate Nucleosome Occupancy and Positioning at IgH in Pro-T Cells.
We asked whether nucleosome occupancy at IgH in RAG1−/− pro-T cells would be similar to that in MEFs, pro-B cells, or neither. Although V(D)J recombination is cell type-specific, with IgH genes fully rearranging in B cells and not in T cells, some amount of IgH D-to-J rearrangement is observed in developing T cells (3). Moreover, low levels of activating histone modifications are present across the IgH D-J region in pro-T cells (24). Thus, in pro-T cells, at least the D-J region of the IgH locus exists in an intermediate state of accessibility: neither refractory to recombination as it is in MEFs nor fully poised as it is in pro-B cells.
When mononucleosomes prepared from a RAG1−/− pro–T-cell line were analyzed, the nucleosome density averaged over all VH gene segments revealed a pattern intermediate to that in MEFs or pro-B cells (Fig. 2A and Fig. S2). In pro-T cells, as in pro-B cells, the region of nucleosomal depletion in the coding region flanking the RSS was absent, and a single strong peak of nucleosome density was observed (−1 nucleosome). However, this peak was broader than in pro-B cells and was shifted toward the RSS, such that the peak was centered at about −40 bp (Fig. 2A). A series of phased nucleosomes then extended over the intergenic region. The shifted position of the −1 nucleosome in the pro-T cells placed it in an intermediate position relative to the RSS compared with MEFs and pro-B cells. A nucleosome centered at −40 (±10 bp) would fully encompass the RSS, rather than allowing the RSS to be in the more accessible position of the histone core shoulder or “naked” linker DNA (Fig. 2B).
The broader peak observed here in pro-T compared with pro-B cells could be a reflection of a nucleosome that is less positioned. For example, a broader peak is consistent with a nucleosome occupying different fixed positions on the two alleles in a given cell or within the cell population, a nucleosome that can slide within a region, or overlapping rotationally related nucleosome positions. Because the peak represents data averaged from all VH gene segments, the broader peak could also arise from tightly positioned nucleosomes that are present at different distances from the RSS for different gene segments. We therefore proceeded to a detailed analysis of nucleosome profiles corresponding to the individual gene segments.
Comparative Analysis of Individual Gene Segments.
To look in more detail at nucleosomal profiles around the RSS at individual gene segments, we plotted the microarray data as heat maps, where each row represents one of the individual VH gene segments and encompasses 500 bp upstream and downstream of the RSS. The mean signal from these heat maps is shown in Fig. 3 (see Fig. S4 for the heat maps from each biological replicate). The individual gene segments generally followed the same overall pattern of nucleosome occupancy as observed for the average of all VH segments (Fig. 3A). Again, the striking pattern of cell type-specific and lineage-specific nucleosome positioning was readily observed. In general, the RSS resided adjacent to a strongly positioned −1 nucleosome in pro-B cells (Fig. 3A, Middle), whereas it was covered by a nucleosome in MEFs (Fig. 3A, Top). Specifically, many segments in MEFs featured depletion of nucleosomes in the coding region adjacent to the RSS, where the −1 nucleosome resided in pro-B cells. In addition, when comparing nucleosome occupancy across the IgH locus in MEFs to that seen in pro-B cells, a higher level of nucleosome occupancy was observed over the RSS and the intergenic region than was present over the coding DNA. For pro-T cells, the pattern of phased nucleosomes extending into the intergenic region was also observed across the heat map (Fig. 3A, Bottom). Once again, nucleosome density was enriched in the coding region immediately adjacent to the RSS and extending across the RSS in the majority of VH segments.
Fig. 3.
Distinct nucleosome occupancy around the RSS of IgH variable gene segments in cells poised for V(D)J recombination. (A) Heat map representation of nucleosome occupancy distribution from −500 to +500 bp from the start of the RSS in MEFs, pro-B cells, and pro-T cells. Each horizontal line in the heat map represents normalized intensity values from an individual IgH variable gene segment. Gene segments are arranged from bottom to top in the DJ-proximal to DJ-distal direction of the IgH locus. A schematic representation of the RSS (black triangle) and its flanking VH gene segment (gray rectangles) is shown below the panel. The scale for the color gradient of occupancy values is shown on the bottom right, with the presence of a nucleosome represented in red and absence of nucleosome represented in blue. Regions that are absent and give no signal are shown in white. All of the data shown represent the mean of two independent biological replicates (see Fig. S4 for the independent trials). (B) Comparison of nucleosome occupancy at gene segments within distinct V gene segment families. Heat map representation of normalized nucleosome occupancy of IgH variable family genes in MEFs, pro-B cells, and pro-T cells. Gene segments are segregated by gene families. Family names of genes are labeled on the left. Each horizontal line in the heat map represents mean normalized nucleosome occupancy distribution (red, high; blue, low) from −500 to +500 bp relative to the start of the RSS. A schematic representation of the RSS (black triangle) and its flanking VH gene segment (gray rectangles) is shown below the panel. (C) Nucleosome occupancy differences at proximal and distal J558 gene segments in pro-B cells. Heat map representation of nucleosome occupancy around the RSS of proximal J558 and distal J558 genes is shown. (D) Box plots of the heat map data in C. Differences in occupancy in the proximal J558 compared with distal J558 genes over the coding segment (−100 to 0 bp) (Left) and the intergenic region (100 to 200 bp) (Right) flanking the RSS. P values, calculated using the Kolmogorov–Smirnov test (as implemented in the R package “stats”), are indicated. Notches in the box plots indicate the 95% confidence interval of the median.
Fig. S4.
Distinct nucleosome occupancy around the RSS of IgH variable gene segments in cells poised for V(D)J recombination is well-correlated between biological replicates. Heat map representation of nucleosome occupancy distribution from −500 to +500 bp from the start of the RSS (0 to +38) in two independent populations of MEFs (MEF.1 and MEF.2), pro-B cells (pro-B.1 and pro-B.2), and pro-T cells (pro-T.1 and pro-T.2). Gene segments are segregated by gene families. Family names of genes are labeled on the left. Each horizontal line in the heat map represents mean normalized nucleosome occupancy distribution (red, high; blue, low) from −500 to +500 bp relative to the start of the RSS. Distances on the x axis are shown in base pairs. A schematic representation of the RSS (black triangle) and its flanking VH gene segment (gray rectangles) is shown below each panel. The scale for the color gradient of occupancy values is shown on the bottom right with the presence of a nucleosome represented in red and absence of nucleosome represented in blue. Regions that are absent and give no signal are shown in white.
Real-time quantitative PCR (qPCR) of the VH7183.16 RSS with primer pairs designed to amplify subnucleosomal length fragments recapitulated this general trend (Fig. S5). In particular, we found the −1 positioned nucleosome adjacent to the RSS in the coding flank of pro-B cells whereas, in MEFs, the RSS was occluded by a nucleosome. More extensive and detailed tiling qPCR of multiple gene segments was precluded by the extensive sequence similarity among gene segments.
Fig. S5.
Quantitative real-time PCR confirms the nucleosome occupancy profile at the VH7183.16 gene segment. The mapping of nucleosomal positioning is described in Materials and Methods. Data are presented as the mean ± SD of at least three independent biological replicates. Pro-B cells (green line), MEFs (red line), and pro-T cells (blue line) are presented. Diagram of the PCR amplicons is shown below the graph. PCR products are 87 ± 2 bp in size and are spaced 10 ± 9 bp apart. A schematic representation of the RSS (black triangle) and its flanking 7183 #16 gene segment (gray rectangle) is also shown.
Although the individual gene segments generally followed the overall pattern of the averaged segments, some distinctions within gene families are notable. For example, in pro-B cells, the J558 and 7183 families generally showed the strongest occupancy at the −1 nucleosome position (Fig. 3B, Middle). Further inspection of the J558 gene segments showed two distinct patterns of nucleosome occupancy (Fig. 3C) that generally correlated with the division between proximal J558 and distal J558 gene segments as previously defined based on sequence and evolutionary conservation. The distal J558 VH gene segments had not only a robust signal at the −1 position, but also a high nucleosome density spread out over the RSS and 200 bp of the intergenic sequence (Fig. 3C, Top). By contrast, the proximal J558 VH gene segments had a nucleosome tightly restricted to the −1 position and extensive nucleosome depletion over the 200 bp of intergenic sequence (Fig. 3C, Bottom). Differences in histone modification and factor binding have also been reported for these two regions (40, 41). Although the nucleosome occupancy in the −1 position was similar between proximal and distal J558 gene segments (P = 0.141) (Fig. 3D, Left), the nucleosome occupancy in the intergenic region was significantly different between the proximal and distal J558 gene segments (P = 1.063 × 10−6) (Fig. 3D, Right), suggesting that nucleosome occupancy may be one of several factors that affects frequency of recombination and gene segment use. In this regard, the J606 family of gene segments, which are less frequently used than the VH7183 and J558 families (40, 41), had a less tightly positioned nucleosome at position −1 and a generally more fuzzy pattern, and the SM7 family of “middle” genes displayed a distinct pattern from other middle gene segments (Fig. 3B).
V Segment RSSs at the TCRα Locus also Exhibit Regulated Nucleosome Positioning.
The microarrays that we used for IgH also included probes to determine nucleosome positioning around the RSSs of all V segments of the murine TCRα locus. The TCRα locus is poised to undergo recombination in RAG-deficient pro-T cells whereas the locus is completely refractory to cleavage in MEFs and pro-B cells. Analyzing data from the same hybridizations and biological samples used for the IgH study, we found that nucleosome occupancy at the Vα locus also revealed lineage-specific positioning that correlates with accessibility and the potential to recombine (Fig. 4A and Figs. S6 and S7). In pro-T cells where the TCRα locus was poised to rearrange, there was a nucleosome positioned at −1 adjacent to the Vα RSSs in most gene segments, a position that would allow the RSS to be on the nucleosome shoulder or in linker DNA, similar to the position seen for the pro-B cells at the IgH locus (compare Fig. 4B with Fig. 2B). In pro-B cells, where we had seen a −1 nucleosome at VH segments, there was not a positioned nucleosome at Vα (Fig. 4B). Instead, there was a relative enrichment of nucleosome density over the RSS and intergenic region compared with the coding segment (Fig. 4A). In MEFs, just as was seen at IgH, there was absence of signal at the −1 position and strong nucleosome density over the RSS (Fig. 4A). These results indicate that regulated nucleosome positioning at the RSS is a more general phenomenon and is not restricted to V gene segments at the IgH locus.
Fig. 4.
Pro-T cell–specific nucleosome occupancy profiles at nonpseudogene, murine TCRα variable gene segments. (A) The normalized nucleosome occupancy signal around the 99 nonpseudogene Vα gene segments was centered on the RSS, averaged per base pair, and plotted for the three cell types indicated (see Fig. S6 for comparable analysis of all 130 Vα gene segments). Green, pro-B cells; red, MEFs; blue, pro-T cells. Mean normalized nucleosome occupancy signal from each cell line is scaled (Materials and Methods). Vertical dashed lines represent the start of the RSS (marked as 0). Distances on the x axis are shown in base pairs. Intervals of significance are shown for the −75-bp position, and P values are calculated via Kolmogorov–Smirnov test by comparing the difference between the average nucleosomal profiles of the coding segment (−75 bp) and the intergenic region (+50 bp) flanking the RSS. A schematic representation of the RSS (black triangle) and its flanking Vα gene segment (gray rectangle) is shown below the panel. Data represent the mean of two independent biological replicates. (B) Deduced location of positioned nucleosomes (colored ovals) relative to the RSS (black triangles) in the three cell types is represented with the same color scheme as in A. The absence of a colored oval indicates the absence of any well-positioned nucleosomes in that particular cell type.
Fig. S6.
Pro-T cell–specific nucleosome occupancy profiles at murine TCRα variable gene segments. The normalized nucleosome occupancy signal around the 130 TCRα variable gene segments was centered on the RSS, averaged per base pair, and plotted for the three cell types indicated. Green, pro-B cells; red, MEFs; blue, pro-T cells. Mean normalized nucleosome occupancy signal from each cell line is scaled (Materials and Methods). Vertical dashed lines represent the start of the RSS (marked as 0). Distances on the x axis are shown in base pairs. Intervals of significance are shown for the −75-bp position, and P values are calculated via Kolmogorov–Smirnov test by comparing the difference between the average nucleosomal profiles of the coding segment (−75 bp) and the intergenic region (+50 bp) flanking the RSS. A schematic representation of the RSS (black triangle) and its flanking Vα gene segment (gray rectangle) is shown at the bottom. Data represent the mean of two independent biological replicates.
Fig. S7.
Distinct nucleosome occupancy around the RSS of TCRα variable gene segments in cells poised for V(D)J recombination. Heat map representation of nucleosome occupancy distribution from −500 to +500 bp from the start of the RSS in MEFs, pro-B cells, and pro-T cells. Each horizontal line in the heat map represents normalized intensity values from an individual Vα gene segment. Gene segments are arranged sequentially from top to bottom according to their position in the locus. Distances on the x axis are shown in base pairs. A schematic representation of the RSS (black triangle) and its flanking Vα gene segment (gray rectangle) is shown at the bottom. The scale for the color gradient of occupancy values is shown on the bottom right, with the presence of a nucleosome represented in red and absence of nucleosome represented in blue. Regions that are absent and give no signal are shown in white. Data represent the mean of two independent biological replicates.
Global Nucleosome Occupancy Correlates with Independently Regulated VH Domains.
Our microarray-based approach allows both for an assessment of local nucleosome occupancy and for a more global appraisal of the nucleosome occupancy across the entire locus IgH locus. The murine VH locus consists of at least two distinctly regulated domains. The DH proximal domain, containing the VH7183 and DQ52 gene families along with a number of smaller middle gene families, spans roughly 900 kb whereas the DH distal domain spans ∼1.4 Mb and contains the J558 and 3609 gene families, among others (Fig. 1). V(D)J recombination in the distal domain is known to be dependent on several transcription factors, including Pax5 (42, 43), Ikaros (44), EZH2 (45), and YY1 (46, 47), as well as the cytokine IL7 (48), but recombination of at least some proximal gene segments occurs in their absence (42, 44, 46, 49). Within the distal domain, a further subdivision has been noted as mentioned above, between the tightly clustered J558 gene segments closer to DH (proximal J558) and those further away (distal J558), with the 3609 gene segments interspersed among the distal segments (40, 50).
When we analyzed global nucleosome occupancy across the entire IgH locus, we observed striking cell type-specific patterns (Fig. 5 and Figs. S8 and S9). In MEFs, where the locus is inaccessible for recombination, nucleosome density across the VH locus was generally uniform (Fig. 5, Top). In contrast, distinct regions of intensity were observed in RAG2−/− pro-B cells (Fig. 5, Middle). The location of these regions (labeled I to IV in Fig. 5) showed a striking correlation with the distinct VH domains inferred from both functional studies and genomic and epigenetic information (15, 40, 51, 52), providing an unexpected visual representation of these regions. The density of nucleosomes across the D, J (region IV), and proximal VH gene segments (region III) was substantially lower than the density over the more distal VH segments (regions I and II). The transition point between high and low density occurred next to the proximal J558 proximal gene segments on the side closer to the DH gene segments (between regions II and III), in keeping with the border inferred from multiple studies (15, 40, 51, 52).
Fig. 5.
Cell type-specific global nucleosome distribution patterns at the IgH locus. Nucleosomal landscape at the murine IgH locus in MEFs (red), pro-B cells (green), and pro-T cells (blue) as visualized on the Integrative Genomics Viewer (IGV) browser. Graphs present density of nucleosomes measured by normalizing enrichments of mononucleosomal DNA compared with MNase-digested genomic DNA. Each graph represents the mean of two independent biological replicates for each cell type presented (see Fig. S8 for data from individual trials). The four distinct domains of the IgH locus as defined by nucleosome density are indicated by gray lines with curved ends and labeled I to IV. The asterisk represents a pro-T cell–specific region of higher nucleosome occupancy within domain III. The relative orientation of the IgH locus is represented schematically at the bottom and is labeled as in Fig. 1.
Fig. S8.
Cell type-specific global nucleosome distribution patterns at the IgH locus are well-correlated between biological replicates. Graphs present density of nucleosomes measured by normalizing enrichments of mononucleosomal DNA with MNase-digested genomic DNA. Each graph represents an individual biological sample. Schematic representation of the IgH locus oriented 3′ to 5′, with 3′ regulatory region on the left and VH region on the right. The four domains of the IgH locus as defined by nucleosome density are indicated by gray lines with curved ends and labeled I to IV. The relative orientation of the IgH locus is represented schematically at the bottom and is labeled as in Fig. 1.
Fig. S9.
Distribution of M-values within various regions of the murine IgH locus: 3′ RR (114,451,123 to 114,497,102); D-J (114,667,034 to 114,720,913); Int, intervening region between VH domain and DH domain (114,720,314 to 114,810,903); Proximal VH (114,813,645 to 115,119,975); Middle VH (115,119,975 to 115,632,833); J558P, proximal J558 VH gene segments (115,632,833 to 116,316,323; J558D, distal J558 VH gene segments (116,300,391 to 117,280,173).
Within the distal VH domain, two distinct regions were notable: (i) The region encompassing the proximal J558 genes (region II) had a nucleosomal density intermediate between the VH proximal domain (region III) and the distal J558 segments (region I), and (ii) the distal J558 region (region I) was marked by its high level of nucleosome occupancy, encompassing all but one of the PAIR elements believed to be involved in the regulation of IgH rearrangement (43, 47). Between these two regions was a short region of transition, with background levels of nucleosome density that were neither enriched nor depleted (Fig. 5 and Fig. S8).
A comparison of the global occupancy patterns at the IgH locus between RAG1−/− pro-T cells (Fig. 5, Bottom) and RAG2−/− pro-B cells (Fig. 5, Middle) served to underscore the distinct domain-like structure of the locus. The overall occupancy pattern in pro-T cells was strikingly similar to that in pro-B cells, with a few notable exceptions (see below). The same distinct domains observed in the pro-B cells were again delineated in the pro-T cells: a low level of density over the middle VH gene segments and an intermediate level over the proximal J558 section (region II), with the highest level over the distal J558 segments (region I).
There were two notable differences in the global nucleosome density patterns between the pro–B-cell and pro–T-cell lines. The region encompassing the intronic enhancer (Eμ) (located between JH and Cμ) and the 3′ regulatory region (3′RR) (located downstream of IgH constant region genes) contains the two main long-distance cis-regulators of the IgH locus. This region (region IV) had a greatly depressed signal in pro-B cells, but a uniform, neutral density in pro-T cells. Furthermore, in pro-T cells (but not pro-B cells) the region encompassing the 25 proximal members of the VH7183 and VQ52 families (from the first 7183 gene segment 7183-1pg-1 to 7183-14-25) had much higher nucleosome occupancy than the surrounding region, suggesting the existence of a regulatory subdomain (see the region marked by an asterisk in Fig. 5). This higher occupancy (and perhaps a distinct structure it may reflect), when added to the lack of other accessibility factors, might contribute to blocking VH to DJH recombination in pro-T cells, even when the local nucleosome position seems to be in a state analogous to that seen in pro–B-cell lines. We note that a similar global analysis of nucleosome occupancy at TCRα was precluded due to insufficient tiling density across the locus in regions outside of the V segments.
Discussion
Nucleosome Position Is Regulated and Correlates with Accessibility.
The work presented here reveals a previously unidentified level of regulation for the process of V(D)J recombination. We demonstrate that nucleosome positioning around recombination signal sequences of IgH and TCRα V segments is regulated in a cell type-specific and lineage-specific fashion, suggesting that nucleosome positioning modulates accessibility of the RSS to the RAG endonuclease.
That is, at the IgH locus in pro-B cells, the −1 nucleosome is positioned adjacent to the RSS in the coding flank such that the RSS would reside in either the accessible naked DNA or at the relatively accessible entry/exit point of the nucleosome. A similarly positioned −1 nucleosome is observed at the TCRα locus in pro-T cells poised to rearrange this locus, but not in pro-B cells. In MEFs, where no recombination occurs at the endogenous IgH or TCRα loci even when RAG1/2 is expressed and plasmids can undergo recombination, the RSS for all gene families is occluded by a nucleosome, thereby rendering it inaccessible.
A general observation in mammalian genomes has been that active regulatory sites (such as promoters) display specific patterns of nucleosome positioning whereas there is a broad and relatively nonspecific accumulation of nucleosomes across these sites in their inactive state (53). By contrast, at the VH and Vα RSSs, we observed defined, distinct nucleosome positions in different cell types, without a direct correlation with their transcriptional status.
Prior work addressing nucleosome organization at the IgH locus showed increased sensitivity to MNase at the JH locus in CD19+ B-cell precursors, compared with hepatocytes, although the precise positioning and occupancy of nucleosomes relative to the RSS and their accessibility was not described (54). However, before the present work there had been no systematic effort to describe nucleosome positioning across the IgH locus in different cell types. In an alternative model, the RSS could become accessible stochastically, either because of the random distribution of generally immobile nucleosomes in the vicinity of the RSS, thus rendering particular RSSs accessible in individual cells within the population, or because of the presence of a mobile nucleosome in the vicinity of the RSS. In such a model, accessibility would be governed by the many other features of the locus that correlate with recombination, such as expression of lymphoid-specific transcription factors, chromatin modifications, looping, compaction, transcription, etc., that would render the locus “open” or “closed,” but nucleosome positioning itself would play no role. Our data do not support this model. We find that, at least at IgH and TCRα V segments, nucleosome localization is not stochastic but, instead, is tightly correlated with both positive and negative regulation of accessibility.
The correlation of nucleosome position with cell type and recombinational accessibility is consistent with prior biochemical experiments studying V(D)J cleavage of mononucleosomes. We previously showed that an RSS positioned on the nucleosome dyad is not accessible for V(D)J cleavage (20). By contrast, a site closer to the entry/exit points is accessible and can be cleaved at levels approaching that of naked DNA when the nucleosome is acetylated on H3 and nucleosome-remodeling factors such as SWI/SNF are active (22). Thus, in addition to accessibility control by the posttranslational covalent modifications of chromatin (55), based on the current study, it seems highly likely that nucleosome positioning is also a primary mechanism in determining the accessibility of the RSS at the V segments (Fig. 6).
Fig. 6.
Model depicting nucleosomal regulation of V(D)J recombination. In cells that are not poised to undergo V(D)J recombination (top of figure), the RSS of a VH gene segment (red triangle) is occluded by a nucleosome (green sphere), preventing the RAG recombinase (blue) from initiating V(D)J recombination. In cells that are poised to undergo V(D)J recombination (bottom of figure), a nucleosome (green sphere) that is trimethylated on histone H3 at lysine 4 (red lollipop) is positioned adjacent to the RSS (red triangle), facilitating the recruitment and activation of the RAG recombinase (blue).
A Dual Role for Nucleosomes in Regulating Recombination?
We began with an apparent paradox: How can nucleosomes be present at RSSs, so as to allow the PHD finger of Rag2 to bind the K4me3-modified tail of histone H3, without occluding the RSS, and thereby preventing the RAG recombinase from binding and cleaving the RSS? Our data are consistent with a very elegant solution to this paradox: Nucleosome position may be regulated to have the nucleosome sit adjacent to, but not on top of, the RSS. Thus, a properly positioned nucleosome may have a positive effect on V(D)J cleavage, rather than just leaving the RSS in naked DNA. Nucleosomes would therefore play a dual role in regulating V(D)J recombination: (i) governing the access of RAG1/2 to DNA and (ii) activating the endonuclease (Fig. 6). We note the similarity with yeast meiotic recombination where recombination is targeted to the nucleosome-free region at transcriptional start sites adjacent to a positioned nucleosome with the H3K4me3 (and R2me2s) modifications (56).
Lymphoid-Specific Nucleosome Occupancy Pattern?
The similar boundaries between regions of the IgH locus in pro-B and pro-T cells and the similar relative changes in nucleosome occupancy suggest that the microarray method applied here is detecting some important underlying lymphoid-specific physical organization of chromatin. In many regions within the IgH locus, even the more subtle patterns within each domain were remarkably similar between pro-B and pro-T cells. Although these more subtle patterns, if observed within just one cell type, might seem insignificant, their reiteration in two distinct cell types underscores their likely importance to the overall lineage-specific organization of the IgH locus. Of note, although the IgH locus does not undergo complete rearrangement in pro-T cells, they do carry out a low level of IgH D-to-J rearrangement, and changes in chromosomal conformation and contraction of the IgH locus have been observed in pro-T cells (57, 58).
What Governs the Position of the Nucleosome?
The observation that nucleosome positioning is distinct between MEFs and pro-B and pro-T cells raises the question of what is responsible for the position of nucleosomes in a given cell type and for effecting the change in position. The observation of cell type-specific patterns at two different loci underscores that these patterns cannot simply be a reflection of the sequence preferences of the MNase enzyme or histone core. Changes in positioning of the nucleosomes often result from the action of a specific factor, possibly in combination with an underlying sequence that favors or disfavors nucleosome assembly and with changes in histone composition. It remains to be determined which specific factors govern positioning at this locus. Transcription, initiated from the promoter of the locus, is unlikely to play a role for several reasons. (i) Nucleosome localization occurs in MEFs and pro-T cells where the VH locus is not transcribed. (ii) If the positioning at the RSS were the result of positioning at a transcribed V promoter, phased nucleosomes would be seen to extend across the coding region. Instead, phased nucleosomes are seen flanking the positioned nucleosome at the RSS (see Fig. S10 for the nucleosome occupancy profile for the IgH locus ±2 Kb around the RSS). (iii) Even in pro-B cells where there is transcription of IgH, the majority of both sense and antisense transcripts are found in the D, J, and Eμ regions, and not in the V region where the positioned nucleosomes are observed.
Fig. S10.
Cell type-specific nucleosome occupancy profiles around murine VH gene segments. The normalized nucleosome occupancy signal around the 195 variable genes from murine IgH variable gene segments was centered on the RSS, averaged per base pair, and plotted for the three cell types indicated. Green, pro-B cells; red, MEFs; blue, pro-T cells. Mean normalized nucleosome occupancy signal from each cell line is scaled (Materials and Methods). Vertical dashed lines represent the start of the RSS (marked as 0). Distances on the x axis are shown in base pairs and span a range of ±2 kb with respect to the RSS. A schematic representation of the RSS (black triangle) and its flanking VH gene segment (gray rectangle) is shown at the bottom. Data represent the mean of two independent biological replicates.
Recently the impacts of over 18 features were assessed for their ability to predict whether VH segments are active (59). Three distinct chromatin states were identified, two that are associated with active recombination (“A” and “E”) and one with generally inactive gene segments (“Bg”). The A state was characterized by the binding of “architectural proteins” CTCF and RAD21 whereas the E state was characterized by the binding of PAX5, IRF4, and YY1, and the enrichment of “active” chromatin marks H3K4me1, H3K4me2, H3K4me3, and H3K9ac. We found that the single feature of nucleosome position has approximately the same predictive value as the multiple factors contributing to the A/E and Bg classifier. A heat map showing nucleosome occupancy sorted by A/E and Bg state is shown in Fig. S11A. Using the comparison of the difference in nucleosome occupancy between −100 and 0 and +100 and 200 as a measurement of RSS openness, we found a strong correlation with the A, E, and Bg states (Fig. S11B). Further, the quantitation of nucleosome occupancy between −100 and 0 can predict with over 80% accuracy (Materials and Methods) whether a gene segment is in the A or E state. When nucleosome occupancy at −100 to 0 is combined with the recombination information content (RIC) score (a measure of RSS quality) (60, 61), A vs. E states are predicted with over 90% accuracy. As shown in Fig. S11C, nucleosome occupancy between −100 and 0 also correlated well with active vs. inactive gene segments defined by Bolland et al. (59) and, in combination with RIC score, predicted active vs. inactive states with greater than 90% accuracy. Thus, nucleosome position is tightly correlated with these other known factors influencing VDJ recombination and provides a simple, but informative, method for predicting chromatin signatures, as well as active vs. inactive states.
Fig. S11.
Nucleosome positioning is well-correlated with the local chromatin state. (A) Heat map representation of nucleosome occupancy distribution for nonpseudogenes from −500 to +500 bp from the start of the RSS in pro-B cells at the IgH locus was arranged with respect to three states described in Bolland et al. (59): A, E and Background (Bg) states, shown with red, blue and gray colors, respectively, on the left of the heat map. (B) Box plots represent the coding segment (−100 to 0 bp) and intergenic region (100 to 200 bp) flanking the RSS in the heat map for each state. P values calculated via Kolmogorov–Smirnov test are indicated. (C) Box plots represent nucleosome occupancy at the coding segment (−100 to 0 bp) for active and inactive genes (all VH genes), described in Bolland et al. (59). P value calculated via Kolmogorov–Smirnov test are indicated. Accuracy was calculated for A- and E-states as well as for active and inactive state. Accuracy is defined as the ratio of true predictions to the total elements: (TP + TN)/(TP + TN + FP + FN). FN, false negative; FP, false positive; TN, true negative; TP, true positive.
Higher Order Chromatin Structure.
Recent genome-wide studies of nucleosome dynamics and occupancy in differentiating ES cells and in comparing ES cells, iPSCs, NPCs, MEFs, and other somatic cells revealed that differences in occupancy specific to cell type and developmental stage are readily seen locally over small regions of the genome (TSS, specific genes) (62–65). However, large (megabase) domains of differential occupancy between cell types were not observed in mammalian cells although they were observed in yeast. At the IgH locus, by contrast, we observed distinct levels of nucleosome occupancy encompassing broad domains of the IgH variable region. Three distinct levels of occupancy were found in pro-B cells: low (VH proximal and middle genes), medium (proximal J558), and high (distal J558), in addition to a very low occupancy region covering the regulatory domain. A similar, though not identical, pattern was seen in pro-T cells. By contrast, MEFs had a relatively uniform occupancy across the variable domain. Because the patterns were different between MEFs and pro-B cells, sequence alone could not be responsible for these differences. As noted above, transcription was unlikely to play a role in this large-scale control of nucleosome occupancy, given the absence of transcription of the VH locus in pro-T cells. Thus, the IgH locus seems to be subject to a form of regulation not previously seen in the rest of the mammalian genome: large-scale control of nucleosome occupancy.
Long-range nucleosome mapping, then, provides a powerful technique for assessing the chromatin architecture at the antigen receptor loci and an additional tool for understanding the mechanistic impact of the many factors influencing recombination. Moreover, this approach provides a method for locating transitions between these domains, where possible regulatory elements may reside.
The IgH locus, as well as other antigen receptor loci, is subject to developmentally regulated subnuclear relocation, contraction and looping and to changes in histone composition and posttranslational modification. These epigenetic changes and developmentally regulated nuclear and chromatin reorganizations are correlated with recombination. To this list, the work presented here adds two additional layers of regulation. Not only do appropriate regions need to be brought together for V(D)J joining to occur, but our data suggest that finely tuned local nucleosome positioning and appropriate levels of global nucleosome occupancy must be achieved as well.
Materials and Methods
Cell Culture and Nuclei Purification.
Rag2−/− pro-B Abelson-transformed cell lines and Rag2−/− MEFs were generated from Rag2-deficient C57BL/6 mice obtained from The Jackson Laboratory. Rag1−/− p53−/− pro-T cells were as described (60). Cells were cultured as described (24) and grown exponentially until they reached confluence and cross-linked in 1% formaldehyde for 10 min at room temperature. Glycine was then added to a final concentration of 125 mM to quench the formaldehyde. Cells were rinsed twice with cold 1× PBS and resuspended in sucrose buffer (0.3 M sucrose, 2 mM Mg(OAc)2, 3 mM CaCl2, 1% Triton X-100, and 10 mM Hepes, pH 7.8). The homogenate was diluted 1:1 with glycerol buffer (25% glycerol, 5 mM MgOAc2, 0.1 mM EDTA, and 10 mM Hepes, pH 7.8). To isolate the nuclei, the resulting solution was layered on a glycerol-pad buffer and spun at 1,000 × g for 15 min.
MNase Cleavage and Mononucleosomal DNA Purification.
MNase cleavage and purification were performed as described (37, 66). Briefly, chromatin from fixed nuclei was digested in MNase digestion buffer (25 mM KCl, 4 mM MgCl2, 1 mM CaCl2, 12.5% glycerol, and 50 mM Tris⋅HCl, pH 7.4) with titrated amounts of MNase (Worthington) for 5 min at 37 °C (Fig. S1A). Total genomic DNA from the same cells was lightly digested in MNase digestion buffer (25mM KCl, 4 mM MgCl2, 1 mM CaCl2, 12.5% glycerol, and 50 mM Tris⋅HCl, pH 7.4) with titrated amounts of MNase (Worthington) for 5 min at 25 °C (Fig. S1B). The reactions were terminated with 50 mM EDTA/EGTA. MNase-digested samples were treated with 100 µg/mL RNase A (Thermo Fisher Scientific) for 1 h at 37 °C, and the cross-links were then reversed by overnight incubation at 65 °C with 0.2 mg/mL proteinase K (Qiagen) and 1% SDS.
After phenol-chloroform extraction, digested samples from nuclei and total bare genomic DNA were run on a 2% low melting agarose gel (SeaPlaque). Mononucleosomally sized fragments (>100 bp and <200 bp) were isolated from the agarose gel and purified using the QIAquick Gel Extraction Kit (Qiagen).
Microarray Hybridization and Processing.
Tiling genomic DNA microarrays were custom designed (NimbleGen Systems) based on the University of California, Santa Cruz mm9 release of sequence of the IgH locus: murine chr12: 114,341,024 to 117,349,200. The 50-mer probes were selected every 20 bases with no repeat masking, for both forward and reverse. Great care was taken to ensure that the probe design allowed for discrimination between different but similar VH gene segments (and other repetitive elements across the locus). In this manner, we were able to design an array that covers ∼85% of the IgH locus. Probes specific for the murine TCRα locus (chr14: 53,047,642 to 54,843,873) were also included but at lower density. Three replicates for each strand were spotted on the array.
Mononucleosomal DNA and genomic DNA were labeled with Cy3 and Cy5, respectively, and hybridized to the array by the manufacturer or in a micro array facility set up by NimbleGen at Florida State University.
Computational Analysis.
At least two independent biological replicates were assessed for each cell type. The microarray data were normalized using the bioconductor package Ringo developed for two-colored arrays (www.bioconductor.org). The ratios of signal intensities for mononucleosomal and bare genomic DNA were computed for each replicate and log2–transformed to obtain M-values. The profiles were smoothed using local regression [locally weighted scatterplot smoothing (LOWESS)] with 75-bp bandwidth and mean-shifted so that the genome-wide average was equal to zero. Standard R functions were used to compute the correlation between biological replicates and generate the corresponding scatter plots (Fig. S3). The data from individual replicates were combined into replicate sets for each cell type using weighted averaging, which was used for all of the presented analysis, unless noted otherwise. When required, the M-values at the genomic positions not represented on the array were obtained by linear interpolation. The M-values in Fig. 3 were computed directly from probe intensities reported by the array-processing facility. These intensities are provided in the GEO entry corresponding to our study (GSE75018). All analyses including calculating P values, building boxplots, heat maps, and aggregate plots around RSS positions were performed in the R environment (www.r-project.org). P values appearing in Fig. 3D were calculated using the Kolmogorov–Smirnov test (as implemented in the R package “stats”).
Tiling Real-Time qPCR Analysis.
Mononucleosomal DNA and genomic DNA were collected as described above. At least three independent biological replicates were assessed for each cell type. Real-Time qPCR was performed as described (67) using iQ SYBR Green Supermix (Bio-Rad). A tiling series of primers (Table S1) were used to cover a region of 567 bp, each producing an amplicon around ∼85 bp as outlined in Fig. S5. Nucleosomal DNA enrichment was calculated as the ratio between mononucleosomal DNA and MNase-digested genomic DNA, as described previously (68, 69).
Table S1.
List of primers used for tiling qPCR
| Primer name | Primer sequence |
| Primer 1 forward | GAAGCCTGGACAGTCCCTGA |
| Primer 1 reverse | CGGAGTCTGGTGAACCCAAG |
| Primer 2 forward | GTGGTGTTAGCACCTATTATCCAGACA |
| Primer 2 reverse | TGCAGGTAAAGGGTGTTCTTGG |
| Primer 3 forward | CCGATTCGCCATCTCCAG |
| Primer 3 reverse | AATACAAGGCCGTGTCCTCTGA |
| Primer 4 forward | GAGGACACGGCCTTGTATTACTG |
| Primer 4 reverse | GGTGCCAAGGAGGAGGTTTG |
| Primer 5 forward | TTCAAACCAAAATTGTTCCCTTCT |
| Primer 5 reverse | TAATGGTCACAGGTCAGGCATTT |
| Primer 6 forward | GCCTGACCTGTGACCATTACAA |
| Primer 6 reverse | TGCAAGGACAATATAGCTTCAGTGAT |
Forward and reverse primers used for qPCR analysis of VH7183.16.
Acknowledgments
The authors thank Anne E. Corcoran for insightful discussions and critical reading of the manuscript. This work was supported by National Institutes of Health Grants GM048026 (to M.A.O.), AI083510 (to M.A.O.), GM048405 (to R.E.K.), and DA033773 (to J.H.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE75018).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605543113/-/DCSupplemental.
References
- 1.Little AJ, Matthews AG, Oettinger MA, Roth DB, Schatz DG. The mechanisms of V(D)J recombination. In: Honjo T, Reth M, Radbruch A, Alt FW, editors. Molecular Biology of B Cells. 2nd Ed Academic; London: 2015. [Google Scholar]
- 2.Gellert M. V(D)J recombination: RAG proteins, repair factors, and regulation. Annu Rev Biochem. 2002;71:101–132. doi: 10.1146/annurev.biochem.71.090501.150203. [DOI] [PubMed] [Google Scholar]
- 3.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol. 2006;24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 4.Cedar H, Bergman Y. Choreography of Ig allelic exclusion. Curr Opin Immunol. 2008;20(3):308–317. doi: 10.1016/j.coi.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Schatz DG. V(D)J recombination. Immunol Rev. 2004;200:5–11. doi: 10.1111/j.0105-2896.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- 6.Yancopoulos GD, Alt FW. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- 7.Stubbington MJ, Corcoran AE. Non-coding transcription and large-scale nuclear organisation of immunoglobulin recombination. Curr Opin Genet Dev. 2013;23(2):81–88. doi: 10.1016/j.gde.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Morse RH. Transcription factor access to promoter elements. J Cell Biochem. 2007;102(3):560–570. doi: 10.1002/jcb.21493. [DOI] [PubMed] [Google Scholar]
- 9.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128(4):707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Lorch Y, Maier-Davis B, Kornberg RD. Role of DNA sequence in chromatin remodeling and the formation of nucleosome-free regions. Genes Dev. 2014;28(22):2492–2497. doi: 10.1101/gad.250704.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanzuolo C, Roure V, Dekker J, Bantignies F, Orlando V. Polycomb response elements mediate the formation of chromosome higher-order structures in the bithorax complex. Nat Cell Biol. 2007;9(10):1167–1174. doi: 10.1038/ncb1637. [DOI] [PubMed] [Google Scholar]
- 12.Noordermeer D, et al. The dynamic architecture of Hox gene clusters. Science. 2011;334(6053):222–225. doi: 10.1126/science.1207194. [DOI] [PubMed] [Google Scholar]
- 13.Magklara A, Lomvardas S. Stochastic gene expression in mammals: Lessons from olfaction. Trends Cell Biol. 2013;23(9):449–456. doi: 10.1016/j.tcb.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas JS, Bossen C, Murre C. Transcription and recombination factories: Common features? Curr Opin Cell Biol. 2011;23(3):318–324. doi: 10.1016/j.ceb.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bossen C, Mansson R, Murre C. Chromatin topology and the regulation of antigen receptor assembly. Annu Rev Immunol. 2012;30:337–356. doi: 10.1146/annurev-immunol-020711-075003. [DOI] [PubMed] [Google Scholar]
- 16.Choi NM, Feeney AJ. CTCF and ncRNA regulate the three-dimensional structure of antigen receptor loci to facilitate V(D)J recombination. Front Immunol. 2014;5:49. doi: 10.3389/fimmu.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih HY, Krangel MS. Chromatin architecture, CCCTC-binding factor, and V(D)J recombination: Managing long-distance relationships at antigen receptor loci. J Immunol. 2013;190(10):4915–4921. doi: 10.4049/jimmunol.1300218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fugmann SD. Form follows function: The three-dimensional structure of antigen receptor gene loci. Curr Opin Immunol. 2014;27:33–37. doi: 10.1016/j.coi.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Chaumeil J, Skok JA. A new take on v(d)j recombination: Transcription driven nuclear and chromatin reorganization in rag-mediated cleavage. Front Immunol. 2013;4:423. doi: 10.3389/fimmu.2013.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon J, Imbalzano AN, Matthews A, Oettinger MA. Accessibility of nucleosomal DNA to V(D)J cleavage is modulated by RSS positioning and HMG1. Mol Cell. 1998;2(6):829–839. doi: 10.1016/s1097-2765(00)80297-x. [DOI] [PubMed] [Google Scholar]
- 21.Golding A, Chandler S, Ballestar E, Wolffe AP, Schlissel MS. Nucleosome structure completely inhibits in vitro cleavage by the V(D)J recombinase. EMBO J. 1999;18(13):3712–3723. doi: 10.1093/emboj/18.13.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon J, Morshead KB, Guyon JR, Kingston RE, Oettinger MA. Histone acetylation and hSWI/SNF remodeling act in concert to stimulate V(D)J cleavage of nucleosomal DNA. Mol Cell. 2000;6(5):1037–1048. doi: 10.1016/s1097-2765(00)00102-7. [DOI] [PubMed] [Google Scholar]
- 23.Patenge N, Elkin SK, Oettinger MA. ATP-dependent remodeling by SWI/SNF and ISWI proteins stimulates V(D)J cleavage of 5 S arrays. J Biol Chem. 2004;279(34):35360–35367. doi: 10.1074/jbc.M405790200. [DOI] [PubMed] [Google Scholar]
- 24.Morshead KB, Ciccone DN, Taverna SD, Allis CD, Oettinger MA. Antigen receptor loci poised for V(D)J rearrangement are broadly associated with BRG1 and flanked by peaks of histone H3 dimethylated at lysine 4. Proc Natl Acad Sci USA. 2003;100(20):11577–11582. doi: 10.1073/pnas.1932643100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osipovich O, et al. Essential function for SWI-SNF chromatin-remodeling complexes in the promoter-directed assembly of Tcrb genes. Nat Immunol. 2007;8(8):809–816. doi: 10.1038/ni1481. [DOI] [PubMed] [Google Scholar]
- 26.Matthews AG, et al. RAG2 PHD finger couples histone H3 lysine 4 trimethylation with V(D)J recombination. Nature. 2007;450(7172):1106–1110. doi: 10.1038/nature06431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Subrahmanyam R, Chakraborty T, Sen R, Desiderio S. A plant homeodomain in RAG-2 that binds hypermethylated lysine 4 of histone H3 is necessary for efficient antigen-receptor-gene rearrangement. Immunity. 2007;27(4):561–571. doi: 10.1016/j.immuni.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramón-Maiques S, et al. The plant homeodomain finger of RAG2 recognizes histone H3 methylated at both lysine-4 and arginine-2. Proc Natl Acad Sci USA. 2007;104(48):18993–18998. doi: 10.1073/pnas.0709170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins EJ, Kee BL, Ramsden DA. Histone 3 lysine 4 methylation during the pre-B to immature B-cell transition. Nucleic Acids Res. 2004;32(6):1942–1947. doi: 10.1093/nar/gkh523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan CC, et al. Histone H3R2 symmetric dimethylation and histone H3K4 trimethylation are tightly correlated in eukaryotic genomes. Cell Reports. 2012;1(2):83–90. doi: 10.1016/j.celrep.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abarrategui I, Krangel MS. Noncoding transcription controls downstream promoters to regulate T-cell receptor alpha recombination. EMBO J. 2007;26(20):4380–4390. doi: 10.1038/sj.emboj.7601866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grundy GJ, Yang W, Gellert M. Autoinhibition of DNA cleavage mediated by RAG1 and RAG2 is overcome by an epigenetic signal in V(D)J recombination. Proc Natl Acad Sci USA. 2010;107(52):22487–22492. doi: 10.1073/pnas.1014958107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumann M, Mamais A, McBlane F, Xiao H, Boyes J. Regulation of V(D)J recombination by nucleosome positioning at recombination signal sequences. EMBO J. 2003;22(19):5197–5207. doi: 10.1093/emboj/cdg487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kondilis-Mangum HD, et al. Transcription-dependent mobilization of nucleosomes at accessible TCR gene segments in vivo. J Immunol. 2010;184(12):6970–6977. doi: 10.4049/jimmunol.0903923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West JA, et al. Nucleosomal occupancy changes locally over key regulatory regions during cell differentiation and reprogramming. Nat Commun. 2014;5:4719. doi: 10.1038/ncomms5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mieczkowski J, et al. MNase titration reveals differences between nucleosome occupancy and chromatin accessibility. Nat Commun. 2016;7:11485. doi: 10.1038/ncomms11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sexton BS, et al. Hierarchical regulation of the genome: Global changes in nucleosome organization potentiate genome response. Oncotarget. 2016;7(6):6460–6475. doi: 10.18632/oncotarget.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sexton BS, et al. The spring-loaded genome: Nucleosome redistributions are widespread, transient, and DNA-directed. Genome Res. 2014;24(2):251–259. doi: 10.1101/gr.160150.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiechens N, et al. The chromatin remodelling enzymes SNF2H and SNF2L position nucleosomes adjacent to CTCF and other transcription factors. PLoS Genet. 2016;12(3):e1005940. doi: 10.1371/journal.pgen.1005940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi NM, et al. Deep sequencing of the murine IgH repertoire reveals complex regulation of nonrandom V gene rearrangement frequencies. J Immunol. 2013;191(5):2393–2402. doi: 10.4049/jimmunol.1301279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feeney AJ. Genetic and epigenetic control of V gene rearrangement frequency. Adv Exp Med Biol. 2009;650:73–81. doi: 10.1007/978-1-4419-0296-2_6. [DOI] [PubMed] [Google Scholar]
- 42.Fuxa M, et al. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 2004;18(4):411–422. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebert A, et al. The distal V(H) gene cluster of the Igh locus contains distinct regulatory elements with Pax5 transcription factor-dependent activity in pro-B cells. Immunity. 2011;34(2):175–187. doi: 10.1016/j.immuni.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Reynaud D, et al. Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nat Immunol. 2008;9(8):927–936. doi: 10.1038/ni.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su IH, et al. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat Immunol. 2003;4(2):124–131. doi: 10.1038/ni876. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, et al. Yin Yang 1 is a critical regulator of B-cell development. Genes Dev. 2007;21(10):1179–1189. doi: 10.1101/gad.1529307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verma-Gaur J, et al. Noncoding transcription within the Igh distal V(H) region at PAIR elements affects the 3D structure of the Igh locus in pro-B cells. Proc Natl Acad Sci USA. 2012;109(42):17004–17009. doi: 10.1073/pnas.1208398109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corcoran AE, Riddell A, Krooshoop D, Venkitaraman AR. Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. Nature. 1998;391(6670):904–907. doi: 10.1038/36122. [DOI] [PubMed] [Google Scholar]
- 49.Degner SC, et al. CCCTC-binding factor (CTCF) and cohesin influence the genomic architecture of the Igh locus and antisense transcription in pro-B cells. Proc Natl Acad Sci USA. 2011;108(23):9566–9571. doi: 10.1073/pnas.1019391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnston CM, Wood AL, Bolland DJ, Corcoran AE. Complete sequence assembly and characterization of the C57BL/6 mouse Ig heavy chain V region. J Immunol. 2006;176(7):4221–4234. doi: 10.4049/jimmunol.176.7.4221. [DOI] [PubMed] [Google Scholar]
- 51.Jhunjhunwala S, et al. The 3D structure of the immunoglobulin heavy-chain locus: Implications for long-range genomic interactions. Cell. 2008;133(2):265–279. doi: 10.1016/j.cell.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebert A, Medvedovic J, Tagoh H, Schwickert TA, Busslinger M. Control of antigen receptor diversity through spatial regulation of V(D)J recombination. Cold Spring Harb Symp Quant Biol. 2013;78:11–21. doi: 10.1101/sqb.2013.78.019943. [DOI] [PubMed] [Google Scholar]
- 53.Schones DE, et al. Dynamic regulation of nucleosome positioning in the human genome. Cell. 2008;132(5):887–898. doi: 10.1016/j.cell.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maës J, et al. Chromatin remodeling at the Ig loci prior to V(D)J recombination. J Immunol. 2001;167(2):866–874. doi: 10.4049/jimmunol.167.2.866. [DOI] [PubMed] [Google Scholar]
- 55.Bergman Y, Cedar H. Epigenetic control of recombination in the immune system. Semin Immunol. 2010;22(6):323–329. doi: 10.1016/j.smim.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brachet E, Sommermeyer V, Borde V. Interplay between modifications of chromatin and meiotic recombination hotspots. Biol Cell. 2012;104(2):51–69. doi: 10.1111/boc.201100113. [DOI] [PubMed] [Google Scholar]
- 57.Hewitt SL, et al. Association between the Igk and Igh immunoglobulin loci mediated by the 3′ Igk enhancer induces ‘decontraction’ of the Igh locus in pre-B cells. Nat Immunol. 2008;9(4):396–404. doi: 10.1038/ni1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sayegh CE, Jhunjhunwala S, Riblet R, Murre C. Visualization of looping involving the immunoglobulin heavy-chain locus in developing B cells. Genes Dev. 2005;19(3):322–327; erratum in Genes Dev (2008) 22(12):1717. doi: 10.1101/gad.1254305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bolland DJ, et al. Two mutually exclusive local chromatin states drive efficient V(D)J recombination. Cell Reports. 2016;15(11):2475–2487. doi: 10.1016/j.celrep.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cowell LG, Davila M, Kepler TB, Kelsoe G. Identification and utilization of arbitrary correlations in models of recombination signal sequences. Genome Biol. 2002;3(12):research0072.1–research0072.20. doi: 10.1186/gb-2002-3-12-research0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee AI, et al. A functional analysis of the spacer of V(D)J recombination signal sequences. PLoS Biol. 2003;1(1):E1. doi: 10.1371/journal.pbio.0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Z, et al. Foxa2 and H2A.Z mediate nucleosome depletion during embryonic stem cell differentiation. Cell. 2012;151(7):1608–1616. doi: 10.1016/j.cell.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teif VB, et al. Genome-wide nucleosome positioning during embryonic stem cell development. Nat Struct Mol Biol. 2012;19(11):1185–1192. doi: 10.1038/nsmb.2419. [DOI] [PubMed] [Google Scholar]
- 64.Hu G, et al. Regulation of nucleosome landscape and transcription factor targeting at tissue-specific enhancers by BRG1. Genome Res. 2011;21(10):1650–1658. doi: 10.1101/gr.121145.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He HH, et al. Nucleosome dynamics define transcriptional enhancers. Nat Genet. 2010;42(4):343–347. doi: 10.1038/ng.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Druliner BR, et al. Chromatin patterns associated with lung adenocarcinoma progression. Cell Cycle. 2013;12(10):1536–1543. doi: 10.4161/cc.24664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lion M, et al. Interaction between p53 and estradiol pathways in transcriptional responses to chemotherapeutics. Cell Cycle. 2013;12(8):1211–1224. doi: 10.4161/cc.24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sekinger EA, Moqtaderi Z, Struhl K. Intrinsic histone-DNA interactions and low nucleosome density are important for preferential accessibility of promoter regions in yeast. Mol Cell. 2005;18(6):735–748. doi: 10.1016/j.molcel.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Petesch SJ, Lis JT. Rapid, transcription-independent loss of nucleosomes over a large chromatin domain at Hsp70 loci. Cell. 2008;134(1):74–84. doi: 10.1016/j.cell.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin SG, Guo C, Su A, Zhang Y, Alt FW. CTCF-binding elements 1 and 2 in the Igh intergenic control region cooperatively regulate V(D)J recombination. Proc Natl Acad Sci USA. 2015;112(6):1815–1820. doi: 10.1073/pnas.1424936112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Featherstone K, Wood AL, Bowen AJ, Corcoran AE. The mouse immunoglobulin heavy chain V-D intergenic sequence contains insulators that may regulate ordered V(D)J recombination. J Biol Chem. 2010;285(13):9327–9338. doi: 10.1074/jbc.M109.098251. [DOI] [PMC free article] [PubMed] [Google Scholar]