Abstract
The majority of poultry genetic resources are maintained in situ in living populations. However, in situ conservation of poultry genetic resources always carries the risk of loss owing to pathogen outbreaks, genetic problems, breeding cessation, or natural disasters. Cryobanking of germplasm in birds has been limited to the use of semen, preventing conservation of the W chromosome and mitochondrial DNA. A further challenge is posed by the structure of avian eggs, which restricts the cryopreservation of ova and fertilized embryos, a technique widely used for mammalian species. By using a unique biological property and accessibility of avian primordial germ cells (PGCs), precursor cells for gametes, which temporally circulate in the vasculature during early development, an avian PGC transplantation technique has been established. To date, several techniques for PGC manipulation including purification, cryopreservation, depletion, and long-term culture have been developed in chickens. PGC transplantation combined with recent advanced PGC manipulation techniques have enabled ex situ conservation of poultry genetic resources in their complete form. Here, the updated technologies for avian PGC manipulation are introduced, and then the concept of a poultry PGC-bank is proposed by considering the biological properties of avian PGCs.
Keywords: Cryopreservation, Poultry genetic resources, Primordial germ cells, Transplantation
Cryopreservation of animal germplasm enables sustainable and economical maintenance of genetic resources for the livestock industry and research. In mammals, an ex situ conservation strategy is methodologically possible by integrating key reproductive technologies such as cryopreservation of semen, ova and embryos, artificial insemination, in vitro fertilization, somatic nuclear transfer, and embryo transfer. Indeed, these technologies are used not only for ex situ preservation in domesticated animals, particularly in cattle, and experimental animals, but also for human infertility treatment. In the case of oviparous animals like birds, cryopreservation of intact embryos is the most simple as well as straightforward ex situ conservation strategy. However, in birds, this is impossible at present because of the large yolk-laden structure of their eggs. Although semen of some poultry such as chicken, goose, duck, and turkey can be cryopreserved successfully [1, 2], the post-thaw fertility of poultry semen lags behind other species, varying among breeds, lines, or individuals [3,4,5]. In the case of poultry, particularly the chicken, the current use of ex situ conservation is only limited to industrially as well as commercially valuable breeds or lines through the collection of frozen semen. By contrast, noncommercial breeds including indigenous breeds are exclusively maintained by in situ populations. However, in situ conservation of poultry genetic resources always carries the risk of loss owing to unexpected infectious disease outbreaks such as highly pathogenic avian influenza, and accidents. In addition to these risks, the periodic reproduction of in situ populations makes them costly to feed, and requires special facilities including a poultry house and farm. Moreover, cryobanking of semen is insufficient as an ex situ conservation strategy in birds because genes on the W chromosome and mitochondrial DNA cannot be maintained as the male is the homogametic (ZZ) sex. As an alternative, avian primordial germ cells (PGCs; Fig. 1), the first germ cell population established during early development, can be incorporated into the gonads [6] and differentiated into functional gametes following transplantation to recipient embryos [7, 8]. This technological development of avian PGC transplantation provides insight into ex situ conservation because PGCs enable the capture of the entire genetics of the stock. The practicality of the poultry PGC-bank is affected by the efficiency of each step of PGC manipulation, and the overall success rate in regenerating donor-derived progeny is critical to ensure an adequate effective population for genetic restoration. Because of the recent development of two innovative techniques for long-term culture of chicken PGCs in vitro [9, 10] and constant production of sterile chicken recipient embryos enabling the production of only donor-derived offspring [11], establishment of poultry PGC-bank programs will become more realistic. In this review, I introduce the updated technologies for avian PGC manipulation then propose the concept of poultry PGC-bank by considering the biological properties of avian PGCs.
Fig. 1.
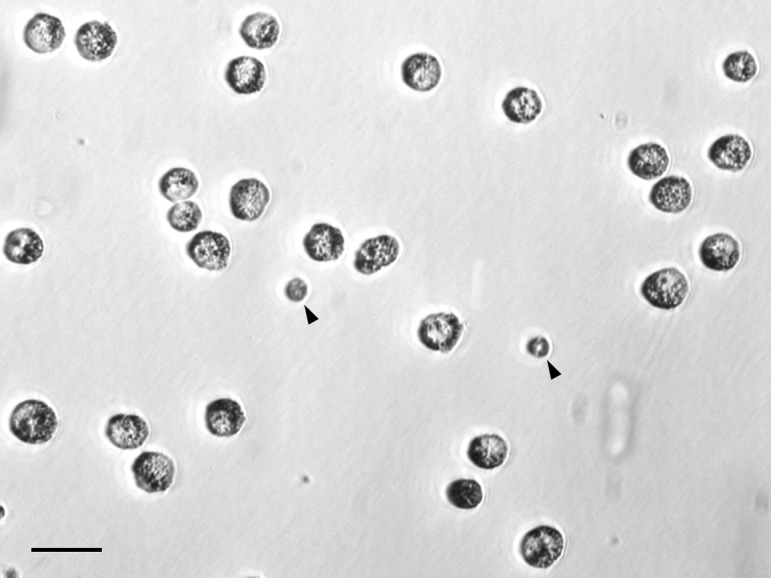
Chicken primordial germ cells (PGCs) isolated from early embryonic blood. Arrow heads indicate erythrocytes. Scale bar, 20 μm.
Appropriate Timing of PGC Collection and Transplantation in Chicken
During early development, PGCs originate in a particular region of the embryo that is often located a relatively great distance from where individual germ cells will eventually reside. Unlike other species, in avian and some reptile embryos, PGCs use blood circulation for transport to the gonadal anlage. This unique biological property and accessibility of avian PGCs provides an opportunity to collect and transplant PGCs [12]. It was first reported in 1993 that transfer of chicken PGCs from early embryonic blood to the bloodstream of recipient embryos can result in transmission of the donor genotype to offspring of recipient chickens [7]. Since then, several research groups have attempted to produce germline chimeras using intravascular transplantation of PGCs, particularly in the chicken [8, 9, 11, 13,14,15,16,17,18,19,20,21,22]. Because blood circulation of avian PGCs is transient, appropriate timing of their collection and transplantation remain to be verified. Although several qualitative descriptions of the migration of avian PGCs through blood circulation have been reported [23,24,25], there has been very little quantitative observation. Recent findings of germline-specific molecular markers such as the chicken homologue genes vasa, dead end and dazl enable reliable analysis of chicken PGCs [25,26,27]. Therefore, the distribution and number of immunohistochemically stained chicken PGCs using anti-Vasa antibody were observed to clarify when and where chicken PGCs move from the extraembryonic region to the vasculature, and from the vasculature to the gonadal anlage [28]. The entrance of PGCs from the anterior part of the extra-embryonic region into the vascular network starts at stage 10 (Arabic numerals refer to the staging system of Hamburger and Hamilton, 1951 [29]) and is completed at stage 13. The migration of PGCs to the gonadal anlage begins at stage 15 and is completed at stage 17. However, access to developing vasculature is technically difficult until a chicken embryo reaches stage 13. In addition, the frequency of germline transmission decreases when PGCs are transferred to the chicken embryos at stage 17 or later [21, 30]. Both male and female PGCs collected from 5- to 7-day-old chicken embryos (stages 27–31) also have the ability to differentiate into functional gametes following transplantation [31, 32]. Male germ cells obtained from adult chicken testes, possibly spermatogenic stem cells, retain germline competency after transplantation to recipient embryos [33], but their competency seems lower than PGCs. In contrast to males, the germline competency of female germ cells is readily lost from 15.5 days post-incubation owing to the start of meiosis [34]. Taking these into consideration, the appropriate times for chicken PGC collection and transplantation are stages 13–14 as well as 27–31, and stages 13–16, respectively.
Enrichment of PGCs
Because the PGCs are a very small cell population during early development and comprise less than 0.02% of blood cells and approximately 2% of gonadal cells [35, 36], transfer of intact blood or gonadal cells to recipient embryos results in very low efficiency of producing germline chimeric chickens [37]. Recent investigations of several cell surface antigens of chicken PGCs such as stage-specific embryonic antigen-1 (SSEA-1) and embryonic mouse antigen-1 (EMA-1) [38] enabled enrichment of chicken PGCs by magnetic–activated cell sorting (MACS) or florescence-activated cell sorting (FACS) [36, 39]. The QCR1 antibody [40], which reacts with an epitope in PGCs of Japanese quail (Coturnix japonica) and common pheasant (Phasianus colchicus), but does not react with chicken PGCs, is available for PGC purification in these species [41, 42]. The antigen-antibody reaction-based system allows isolation of PGCs from both blood and gonads. In the future, further investigations of cell surface markers of PGCs in several avian species other than the chicken, Japanese quail, and common pheasant are anticipated. Since avian embryonic blood cells are roughly composed of a small number of PGCs and a huge number of erythrocytes, two approaches can be used to purify PGCs: one is separation of PGCs from erythrocytes by density gradient methods using Ficoll or Nycodenz [6, 35], and the other is erythrocyte lysis using an ammonium chloride-potassium buffer [43]. Among these, the Nycodenz gradient centrifugation method achieves both high recovery and purity rates of PGCs in the chicken and Japanese quail [35]. In addition to these species, availability of this method for duck (Anas platyrhynchos), green pheasant (Phasianus versicolor) and common pheasant has been confirmed (Nakamura et al., unpublished data). A unique PGC enrichment method from gonadal tissues was developed in the chicken using the biological property that PGCs are discharged when embryonic gonads are incubated in Dulbecco’s phosphate-buffered saline without Ca2+ and Mg2+ D-PBS(–) [44]. This is the simplest method currently available to harvest chicken PGCs and is potentially applicable in various avian species. In the future, further improvement of unstable PGC recovery rate is expected.
Long-term Culture of PGCs
Mammalian PGCs can only be propagated as lineage-restricted germ cells for short periods in vitro [45,46,47]. In 2006, Etches and colleagues demonstrated that chicken PGCs can be propagated for the long-term in vitro while maintaining lineage specificity and germline transmission competency [9]. Chicken PGCs isolated from embryonic blood can be expanded in a complex medium containing chicken serum, fetal bovine serum (FBS), fibroblast growth factor 2 (FGF2), and buffalo rat liver (BRL) cell-conditioned medium on a feeder of either BRL cells or Sandoz inbred mouse-derived thioguanine-resistant and ouabain-resistant (STO) fibroblasts [9]. Several research groups revealed that FGF signaling is required for chicken PGC proliferation in vitro [18, 48, 49]. A recent study has suggested that the membrane-bound form of chicken stem cell factor 2 (SCF2), but not the secreted form of chicken SCF1, enhanced the propagation of chicken PGCs in cooperation with FGF2 [50]. However, this ill-defined culture system cannot support efficient propagation of female chicken PGCs. More recently, McGrew and colleagues defined serum-free, feeder-free, and physio-chemically permissive culture conditions for chicken PGCs, ascertaining that FGF2, insulin, and activin are sufficient for the propagation of chicken PGCs [10]. Furthermore, a lower osmolality condition (250 mOsm/kg), one of the characteristics of the defined culture system, also enabled efficient derivation and propagation of both male and female chicken PGCs. Establishment of long-term culture systems of chicken PGCs provides the opportunity to significantly amplify donor PGCs before cryopreservation [51] as well as to manipulate the genome with subsequent cloning [52,53,54] in a manner similar to mouse embryonic stem (ES) cells in the chicken. Further investigations of culture conditions of non-chicken avian PGCs may help to conserve the genetic resources of wild birds ex situ.
Ultra-low Temperature Preservation of PGCs
To data, ultra-low temperature preservation of chicken PGCs has been performed using a slow-freezing method. The most widely used freezing protocol is cryopreservation of chicken PGCs in serum containing media supplemented with 10% dimethylsulfoxide (DMSO) as a cryoprotectant at a cooling rate of −1°C/min until reaching −80°C. This freezing protocol yields a recovery rate of approximately 50%, and over 85% viability of post-thawing chicken PGCs [13, 55, 56]. Several comparative studies have examined the types of cryoprotectant including their concentrations and the cooling rates to investigate a more efficient protocol [22, 55, 57, 58]. To summarize previous studies, chicken PGCs in a medium containing more than 10% serum and either 5–10% DMSO or 10% ethylene glycol as cryoprotectants at a cooling rate of −2°C/min result in higher recovery and viability of post-thawed chicken PGCs than classic freezing protocols. Commercially available serum and DMSO-based cryomedium also results in higher recovery and viability of chicken PGCs after thawing than a common freezing protocol [55]. Due to its easier availability and higher performance, commercial cryomedium particularly CELLBANKER 1 (Nippon Zenyaku Kogyo, Koriyama, Japan) has been used to cryopreserve PGCs from chicken and quail [30, 59,60,61]. In contrast to the slow-freezing method, a vitrification method can theoretically store cells both intracellularly and extracellularly by ice-free solidification. Although a previous study reported lower recovery and viable rates of vitrified PGCs than in frozen-thawed PGCs [56], the vitrification method could improve the recovery and viability of chicken PGCs after exploration of various vitrifying protocols.
Production of High-grade Germline Chimeras Through PGC Transplantation
The ideal host for germ cell transplantation is an infertile recipient. Indeed, several infertile animal models such as germ cell-deficient mutant mice and triploid fishes have been used widely in germ cell transplantation studies to produce only donor-derived sperm or eggs [62, 63]. To date, genetic mutants either lacking germ cells or with deficient gametogenesis have not been reported in birds. ZZZ triploid chickens develop and grow normally but are infertile [64]. ZZZ chicken embryos could potentially be used as recipients for PGC transplantation, but rarely occur. Thus, partial, if not complete, removal of endogenous PGCs prior to transplantation of donor PGCs is an effective approach to increase the efficiency of donor-derived gametes in recipient chickens. Surgical removal of tissues containing PGCs such as blastodermal cells or blood from recipient chicken embryos resulted in increased germline chimerism [65, 66]. Irradiation of chicken embryos with X-rays or gamma-rays resulted in decreased number of endogenous PGCs and thus increased the proportion of donor-derived gametes [67, 68]. However these methods failed to produce stable high-grade germline of chimeric chickens, suggesting recovery of the remaining endogenous PGCs after treatment. Busulfan (1,4-butanediol dimethanesulfonate), a DNA alkylating agent, is the most commonly used drug for removal of endogenous germ cells in mammals. In particular in adult male mice, a single intraperitoneal injection of over 40 mg/kg busulfan enables constant preparation of recipients lacking endogenous germ cells for germ cell transplantation [62, 69, 70]. Although busulfan also has sterilization effects on chicken and Japanese quail germ cells during development, the degree of germ cell depletion is variable owing to less efficient drug delivery [71, 72]. As shown in Fig. 2, Nakamura et al. (2008) developed a unique drug delivery method by utilizing a biological property of the chicken embryo - it lies on the top of the yolk even if the egg is rotated [73]. The principle of this method is as follows: a sustainable emulsion containing busulfan rapidly rises when it is injected into the yolk then contacts with the chicken embryos owing to a lower density than the yolk contents. This drug delivery method enabled a depletion of PGCs at a constant level. A subsequent study revealed that application of busulfan at an early time point could reduce the sterilizing effects of the residual drug on donor PGCs [74]. Finally, an experiment was attempted to transplant donor chicken PGCs to sterilized recipient embryos following administration of busulfan. Of 11 resulting recipients, seven produced only donor-derived gametes (99.5% on average of total recipients) [11]. As described above, the production efficiency of donor-derived gametes was markedly increased using sterile recipients. In the future, production of chicken lines where the germ cells are completely depleted is required for more efficient production of recipient chickens that produce only donor-derived offspring. For example, in combination with transgenesis of cultured PGCs in vitro, generation of Cre/loxP system-mediated germ cell-specific knock-out or ablation avian models via germline chimeras would be the best approach.
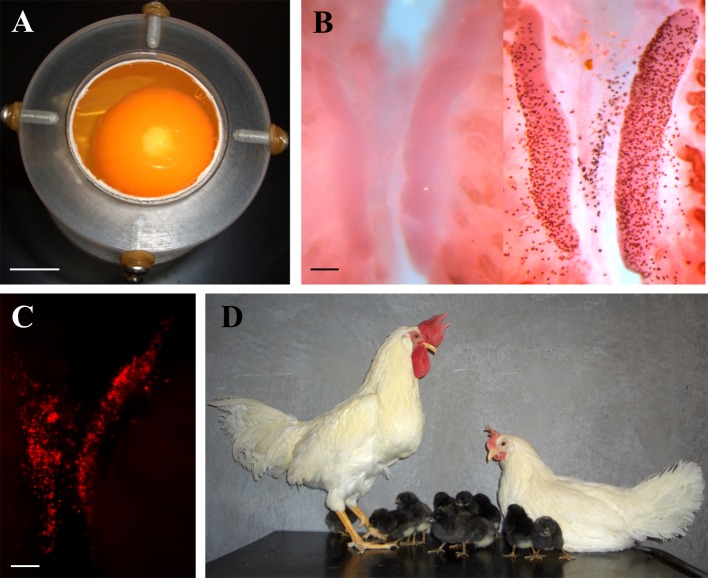
Fig. 2.
Production of sterilized recipient embryos by removal of endogenous primordial germ cells (PGCs) using a unique drug delivery method and its application for PGC transplantation. (A) A sustainable emulsion containing busulfan contacts with the chicken embryos rapidly after injection into the yolk. (B) This drug delivery method allowed elimination of PGCs at a constant level. (C) Donor PGCs could repopulate with gonads of sterilized recipient embryos after transplantation. (D) Use of sterile recipients enabled efficient production of chickens that produce only donor-derived offspring. Scale bars, 1 cm (A) and 100 μm (B and C). (Revised: Nakamura et al., 2008; 2010)
Conclusions and Perspectives on a Potential Poultry PGC Bank
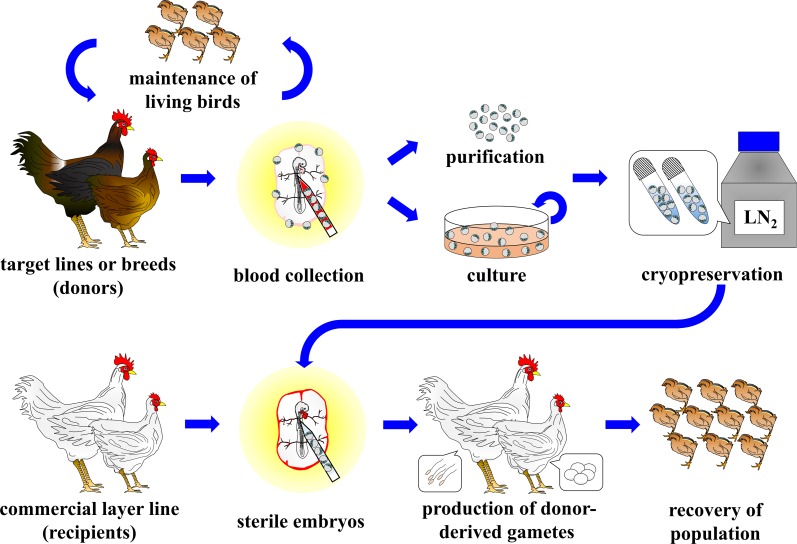
In this review, recent technical advances regarding avian PGC manipulation for use in poultry PGC-bank programs have been introduced. As shown in Fig. 3, the procedures of ex situ conservation of poultry genetic resources consist of five steps; 1) collection of embryonic tissues containing PGCs from target lines or breeds, 2) purification or culture of PGCs, 3) PGC storage in liquid nitrogen, 4) PGC transplantation to sterilized recipient embryos, and 5) recovery of populations by mating of male and female recipients. To date, successful long-term culture of PGCs from various chicken breeds including indigenous breeds has been reported [9, 18, 22, 49]. Therefore, at least in the chicken, the major advantage of PGCs for use as a source of gene banking is that PGCs obtained from a single donor embryo would be amplified significantly in vitro prior to cryopreservation. In addition, transplantation of post-thaw PGCs to sterile recipients of a commercial layer line would be rapidly and significantly multiplied, recovering the population size from only a small flock of recipients.
Fig. 3.
Outline of a poultry PGC-bank program.
The most important aspect of gene banking is the collection of germplasm without losing the present wealth of genetic diversity. To recover a population whilst maintaining sustainable genetic diversity from the germplasm repository, it will be necessary to conserve the germplasm from at least 13 individuals of each sex (ideally 25 individuals of each sex) [75]. Thus, the collection of PGCs from fertilized embryos obtained from various combinations of males and females in each population would be ideal for poultry gene banking. From an industrial point of view, cryopreservation of PGCs could be used as back-up for commercially or industrially important poultry lines or breeds that have been selected for a long time in case of they are lost as a consequence of pathogen outbreaks, genetic problems, breeding cessation, or natural disasters. Additionally, PGC cryobanking enables a dramatic reduction of costs associated with maintenance of live birds.
PGC collection sites should be considered in accordance with the situation. Collection of PGCs from developing gonads has the advantage of recovering a large number of PGCs and ensuring a prolonged period for collection. However all embryos which have the potential to hatch out under ordinary circumstances must be sacrificed for gonadal PGC collection. Therefore, the practical use of gonadal PGCs would be restricted if the availability of fertilized embryos is not limited, such as the case with industrially as well as commercially valuable poultry breeds or lines. However, the availability of fertilized embryos in most noncommercial breeds such as indigenous, rare and/or endangered breeds is restricted by several factors such as small population sizes, low egg production, and seasonal breeding. A unique biological property of avian PGCs that temporally circulate thorough the vascular system would provide the opportunity to combine the reproduction of living birds with collection of PGCs. Indeed, of the 88 fertilized embryos of Gifujidori used for PGC collection, 12 survived to sexual maturity with normal reproductive capacity. Subsequently, six Gifujidori offspring were successfully regenerated (6% of total offspring) by mating male and female recipient chickens that had received frozen-thawed Gifujidori PGCs [59]. In this method, drawing blood from donor embryos involves simple windowing procedures. It was also demonstrated that this procedure is available for combined preservation of living birds with PGCs in Japanese quail, green pheasant, and common pheasant (Nakamura et al., unpublished data).
In fish, male germ cells (type A spermatogonia) and female germ cells (oogonia) that are transplanted to the opposite sex of recipients can differentiate into functional eggs and sperm [63, 76]. Although chicken PGCs can differentiate into functional gametes in the gonads of opposite sex recipients, the efficiency is very low [77]. Histological analysis suggested that female PGCs in male chicken gonads are capable of passing through the first and second meiotic divisions, but rarely complete spermatid elongation [78]. For gene banking purposes, male and female PGCs should be collected separately and cryopreserved, as efficient germline transmission of PGCs requires that the sex of the donor be matched to the sex of the recipient.
If avian PGCs can differentiate into functional gametes in the gonads of xenogeneic recipients, PGC transplantation could be a powerful tool for ex situ conservation of wild birds including endangered species. In avian species, xenogeneic germline transmission between phylogenetically different genera (common pheasant to chicken), family (chicken to guinea fowl (Numida meleagris)) or order (duck to chicken, and houbara bustard (Chlamydotis undulata) to chicken) was reported only in males, but not in females [42, 79,80,81,82]. In future, detailed histological analysis is needed to evaluate the ability of xenogeneic gametogenesis by transfer of donor PGCs carrying a reporter gene to sterilized interspecific recipient embryos.
Recently, Nakamura et al. (2013) reported that cryopreservation of PGCs and subsequent production of functional gametes following transplantation is also feasible in Japanese quail in which the semen has not been cryopreserved [61]. More recently, a practical trial reported that successful transport of a chicken breed by shipment of cryopreserved PGCs using a dry shipper [30]. In such a situation, it should be possible to run poultry PGC-bank programs that conduct a collection, cryopreservation, depositing, and distribution service. Based on the recent technical advances in poultry PGC manipulation described here, collection, freezing and storage of PGCs from both industrial and indigenous poultry breeds has begun as part of the National Institute of Agrobiological Sciences (NIAS) Genebank projects (NIAS, Tsukuba, Japan). At present, the NIAS Genebank contains 15 chicken breeds, including eight indigenous breeds that are designated as natural treasures of Japan (Gifujiori, Hinaidori, Jitokko, Koeyoshi, Kurokashiwa, Satsumadori, Toumaru and Yakido), and three Japanese quail lines (Nakamura et al., unpublished data). To date, successful transplantation of freeze-thawed immature ovarian tissues to a juvenile recipient has been performed in the Japanese quail [83]. Because of its high production efficiency of donor-derived eggs in recipients, immature poultry ovarian tissues could also be a source for gene banking of female germplasm. Cryopreservation of PGCs together with frozen stocks of semen and immature ovaries will ensure preservation of poultry genetic resources economically and semi-permanently.
Final Notes
My name means “the bright falcon”. The falcon (Falco peregrinus japonensis) is classified as vulnerable (i.e., species facing a high risk of extinction in the wild) by the Japanese Ministry of Environment Red List. PGC culture and xenotransplantation technologies might save falcons from extinction. I do not know whether this idea can be realized, but I intend to establish germ cell manipulation technologies that are universally available across species, to allow conservation of genetic resources of various animal species.
Acknowledgments
I would like to express my thanks to the Society for Reproduction and Development (SRD) for awarding me a 2015 SRD Young Investigator Award. I also would like to give my sincere thanks to Dr Hiroshi Kagami (Faculty of Agriculture, Shinshu University), Dr Keijiro Nirasawa (NARO Institute of Livestock and Grassland Science) and Dr Kazuhiro Rikimaru (Akita Prefectural Livestock Experiment Station) for kind advice concerning my study and especially to Mr Takahiro Tagami (NARO Institute of Livestock and Grassland Science) for his warm-hearted guidance and valuable suggestions for my study. Finally, I would like to express my gratitude to all the members of the Laboratory of Animal Developmental Genetics, Faculty of Agriculture, Shinshu University and Animal Breeding Research Group, Naro Institute of Livestock and Grassland Science. This work was supported by in part by the Grants-in-Aid for JSPS fellows (09J00163) from Japan Society for the Promotion of Science to YN.
References
- 1.Lake PE, Stewart JM. Preservation of fowl semen in liquid nitrogen—an improved method. Br Poult Sci 1978; 19: 187–194. [DOI] [PubMed] [Google Scholar]
- 2.Hammerstedt RH, Graham JK. Cryopreservation of poultry sperm: the enigma of glycerol. Cryobiology 1992; 29: 26–38. [DOI] [PubMed] [Google Scholar]
- 3.Tajima A, Graham EF, Shoffner RN, Otis JS, Hawkins DM. Cryopreservation of semen from unique lines of chicken germ plasm. Poult Sci 1990; 69: 999–1002. [DOI] [PubMed] [Google Scholar]
- 4.Alexander A, Graham J, Hammerstedt RH, Barbato GF. Effects of genotype and cryopreservation of avian semen on fertility and number of perivitelline spermatozoa. Br Poult Sci 1993; 34: 757–764. [DOI] [PubMed] [Google Scholar]
- 5.Chalah T, Seigneurin F, Blesbois E, Brillard JP. In vitro comparison of fowl sperm viability in ejaculates frozen by three different techniques and relationship with subsequent fertility in vivo. Cryobiology 1999; 39: 185–191. [DOI] [PubMed] [Google Scholar]
- 6.Yasuda Y, Tajima A, Fujimoto T, Kuwana T. A method to obtain avian germ-line chimaeras using isolated primordial germ cells. J Reprod Fertil 1992; 96: 521–528. [DOI] [PubMed] [Google Scholar]
- 7.Tajima A, Naito M, Yasuda Y, Kuwana T. Production of germ line chimera by transfer of primordial germ cells in the domestic chicken (Gallus domesticus). Theriogenology 1993; 40: 509–519. [DOI] [PubMed] [Google Scholar]
- 8.Ono T, Matsumoto T, Arisawa Y. Production of donor-derived offspring by transfer of primordial germ cells in Japanese quail. Exp Anim 1998; 47: 215–219. [DOI] [PubMed] [Google Scholar]
- 9.van de Lavoir MC, Diamond JH, Leighton PA, Mather-Love C, Heyer BS, Bradshaw R, Kerchner A, Hooi LT, Gessaro TM, Swanberg SE, Delany ME, Etches RJ. Germline transmission of genetically modified primordial germ cells. Nature 2006; 441: 766–769. [DOI] [PubMed] [Google Scholar]
- 10.Whyte J, Glover JD, Woodcock M, Brzeszczynska J, Taylor L, Sherman A, Kaiser P, McGrew MJ. FGF, Insulin, and SMAD Signaling Cooperate for Avian Primordial Germ Cell Self-Renewal. Stem Cell Rev 2015; 5: 1171–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura Y, Usui F, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Germline replacement by transfer of primordial germ cells into partially sterilized embryos in the chicken. Biol Reprod 2010; 83: 130–137. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura Y, Kagami H, Tagami T. Development, differentiation and manipulation of chicken germ cells. Dev Growth Differ 2013; 55: 20–40. [DOI] [PubMed] [Google Scholar]
- 13.Naito M, Tajima A, Tagami T, Yasuda Y, Kuwana T. Preservation of chick primordial germ cells in liquid nitrogen and subsequent production of viable offspring. J Reprod Fertil 1994; 102: 321–325. [DOI] [PubMed] [Google Scholar]
- 14.Park TS, Jeong DK, Kim JN, Song GH, Hong YH, Lim JM, Han JY. Improved germline transmission in chicken chimeras produced by transplantation of gonadal primordial germ cells into recipient embryos. Biol Reprod 2003; 68: 1657–1662. [DOI] [PubMed] [Google Scholar]
- 15.Tagami T, Matsubara Y, Hanada H, Naito M. Differentiation of female chicken primordial germ cells into spermatozoa in male gonads. Dev Growth Differ 1997; 39: 267–271. [DOI] [PubMed] [Google Scholar]
- 16.Mozdziak PE, Wysocki R, Angerman-Stewart J, Pardue SL, Petitte JN. Production of chick germline chimeras from fluorescence-activated cell-sorted gonocytes. Poult Sci 2006; 85: 1764–1768. [DOI] [PubMed] [Google Scholar]
- 17.Kuwana T, Kawashima T, Naito M, Yamashita H, Matsuzaki M, Takano T. Conservation of a threatened indigenous fowl (Kureko Dori) using the germline chimeras transplanted from primordial germ cells. J Poult Sci 2006; 43: 60–66. [Google Scholar]
- 18.Macdonald J, Glover JD, Taylor L, Sang HM, McGrew MJ. Characterisation and germline transmission of cultured avian primordial germ cells. PLoS ONE 2010; 5: e15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motono M, Yamada Y, Hattori Y, Nakagawa R, Nishijima K, Iijima S. Production of transgenic chickens from purified primordial germ cells infected with a lentiviral vector. J Biosci Bioeng 2010; 109: 315–321. [DOI] [PubMed] [Google Scholar]
- 20.Oishi I. Improvement of transfection efficiency in cultured chicken primordial germ cells by percoll density gradient centrifugation. Biosci Biotechnol Biochem 2010; 74: 2426–2430. [DOI] [PubMed] [Google Scholar]
- 21.Rikimaru K, Ito N, Nakamura Y, Takahashi D, Ono M, Komatsu M, Matsubara K. Identification of germline chimeric chickens produced by transfer of primordial germ cells using a Hinai-dori-specific microsatellite marker. J Poult Sci 2011; 48: 281–291. [Google Scholar]
- 22.Tonus C, Cloquette K, Ectors F, Piret J, Gillet L, Antoine N, Desmecht D, Vanderplasschen A, Waroux O, Grobet L. Long term-cultured and cryopreserved primordial germ cells from various chicken breeds retain high proliferative potential and gonadal colonisation competency. Reprod Fertil Dev 2014; 28: 628–639. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto T, Ukeshima A, Kiyofuji R. The origin, migration and morphology of the primordial germ cells in the chick embryo. Anat Rec 1976; 185: 139–145. [DOI] [PubMed] [Google Scholar]
- 24.Kuwana T, Fujimoto T. Locomotion and scanning electron microscopic observations of primordial germ cells from the embryonic chick blood in vitro. Anat Rec 1984; 209: 337–343. [DOI] [PubMed] [Google Scholar]
- 25.Tsunekawa N, Naito M, Sakai Y, Nishida T, Noce T. Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development 2000; 127: 2741–2750. [DOI] [PubMed] [Google Scholar]
- 26.Aramaki S, Sato F, Kato T, Soh T, Kato Y, Hattori MA. Molecular cloning and expression of dead end homologue in chicken primordial germ cells. Cell Tissue Res 2007; 330: 45–52. [DOI] [PubMed] [Google Scholar]
- 27.Kito G, Aramaki S, Tanaka K, Soh T, Yamauchi N, Hattori MA. Temporal and spatial differential expression of chicken germline-specific proteins cDAZL, CDH and CVH during gametogenesis. J Reprod Dev 2010; 56: 341–346. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura Y, Yamamoto Y, Usui F, Mushika T, Ono T, Setioko AR, Takeda K, Nirasawa K, Kagami H, Tagami T. Migration and proliferation of primordial germ cells in the early chicken embryo. Poult Sci 2007; 86: 2182–2193. [DOI] [PubMed] [Google Scholar]
- 29.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol 1951; 88: 49–92. [PubMed] [Google Scholar]
- 30.Nakamura Y, Rikimaru K, Takahashi D, Komatsu M, Takahashi T, Tagami T. Production of functional gametes following transfer of frozen-thawed primordial germ cells of Hinai-dori fowl after long distance transportation —For diversification of the risk to outbreaks of highly pathogenic avian influenza—. Jpn J Polit Sci 2016; 53: J7–J14. (in Japanese). [Google Scholar]
- 31.Tajima A, Naito M, Yasuda Y, Kuwana T. Production of germ-line chimeras by transfer of cryopreserved gonadal primordial germ cells (gPGCs) in chicken. J Exp Zool 1998; 280: 265–267. [PubMed] [Google Scholar]
- 32.Nakajima Y, Hattori T, Asano A, Ishikawa N, Tajima A. Migration and differentiation of gonadal germ cells under cross-sex germline chimeras condition in domestic chickens. J Reprod Dev 2014; 60: 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung JG, Lee YM, Kim JN, Kim TM, Shin JH, Kim TH, Lim JM, Han JY. The reversible developmental unipotency of germ cells in chicken. Reproduction 2010; 139: 113–119. [DOI] [PubMed] [Google Scholar]
- 34.Smith CA, Roeszler KN, Bowles J, Koopman P, Sinclair AH. Onset of meiosis in the chicken embryo; evidence of a role for retinoic acid. BMC Dev Biol 2008; 8: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao DF, Kuwana T. Purification of avian circulating primordial germ cells by nycodenz density gradient centrifugation. Br Poult Sci 2003; 44: 30–35. [DOI] [PubMed] [Google Scholar]
- 36.Mozdziak PE, Angerman-Stewart J, Rushton B, Pardue SL, Petitte JN. Isolation of chicken primordial germ cells using fluorescence-activated cell sorting. Poult Sci 2005; 84: 594–600. [DOI] [PubMed] [Google Scholar]
- 37.Petitte JN, Clark ME, Etches RJ. Assessment of functional gametes in chickens after transfer of primordial germ cells. J Reprod Fertil 1991; 92: 225–229. [DOI] [PubMed] [Google Scholar]
- 38.Karagenç L, Cinnamon Y, Ginsburg M, Petitte JN. Origin of primordial germ cells in the prestreak chick embryo. Dev Genet 1996; 19: 290–301. [DOI] [PubMed] [Google Scholar]
- 39.Kim JN, Kim MA, Park TS, Kim DK, Park HJ, Ono T, Lim JM, Han JY. Enriched gonadal migration of donor-derived gonadal primordial germ cells by immunomagnetic cell sorting in birds. Mol Reprod Dev 2004; 68: 81–87. [DOI] [PubMed] [Google Scholar]
- 40.Aoyama H, Asamoto K, Nojyo Y, Kinutani M. Monoclonal antibodies specific to quail embryo tissues: their epitopes in the developing quail embryo and their application to identification of quail cells in quail-chick chimeras. J Histochem Cytochem 1992; 40: 1769–1777. [DOI] [PubMed] [Google Scholar]
- 41.Ono T, Machida Y. Immunomagnetic purification of viable primordial germ cells of Japanese quail (Coturnix japonica). Comp Biochem Physiol A Mol Integr Physiol 1999; 122: 255–259. [DOI] [PubMed] [Google Scholar]
- 42.Kang SJ, Choi JW, Kim SY, Park KJ, Kim TM, Lee YM, Kim H, Lim JM, Han JY. Reproduction of wild birds via interspecies germ cell transplantation. Biol Reprod 2008; 79: 931–937. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto Y, Usui F, Nakamura Y, Ito Y, Tagami T, Nirasawa K, Matsubara Y, Ono T, Kagami H. A novel method to isolate primordial germ cells and its use for the generation of germline chimeras in chicken. Biol Reprod 2007; 77: 115–119. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima Y, Minematsu T, Naito M, Tajima A. A new method for isolating viable gonadal germ cells from 7-day-old chick embryos. J Poult Sci 2011; 48: 106–111. [Google Scholar]
- 45.Matsui Y, Toksoz D, Nishikawa S, Nishikawa S, Williams D, Zsebo K, Hogan BL. Effect of Steel factor and leukaemia inhibitory factor on murine primordial germ cells in culture. Nature 1991; 353: 750–752. [DOI] [PubMed] [Google Scholar]
- 46.Dolci S, Williams DE, Ernst MK, Resnick JL, Brannan CI, Lock LF, Lyman SD, Boswell HS, Donovan PJ. Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature 1991; 352: 809–811. [DOI] [PubMed] [Google Scholar]
- 47.Durcova-Hills G, Prelle K, Müller S, Stojkovic M, Motlik J, Wolf E, Brem G. Primary culture of porcine PGCs requires LIF and porcine membrane-bound stem cell factor. Zygote 1998; 6: 271–275. [DOI] [PubMed] [Google Scholar]
- 48.Choi JW, Kim S, Kim TM, Kim YM, Seo HW, Park TS, Jeong JW, Song G, Han JY. Basic fibroblast growth factor activates MEK/ERK cell signaling pathway and stimulates the proliferation of chicken primordial germ cells. PLoS ONE 2010; 5: e12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyahara D, Mori T, Makino R, Nakamura Y, Oishi I, Ono T, Nirasawa K, Tagami T, Kagami H. Culture conditions for maintain propagation, long-term survival and germline transmission of chicken primordial germ cell-like cells. J Poult Sci 2014; 51: 87–95. [Google Scholar]
- 50.Miyahara D, Oishi I, Makino R, Kurumisawa N, Nakaya R, Ono T, Kagami H, Tagami T. Chicken stem cell factor enhances primordial germ cell proliferation cooperatively with fibroblast growth factor 2. J Reprod Dev 2016; 62: 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nandi S, Whyte J, Taylor L, Sherman A, Nair V, Kaiser P, McGrew MJ. Cryopreservation of specialized chicken lines using cultured primordial germ cells. Poult Sci 2016(published online). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schusser B, Collarini EJ, Yi H, Izquierdo SM, Fesler J, Pedersen D, Klasing KC, Kaspers B, Harriman WD, van de Lavoir MC, Etches RJ, Leighton PA. Immunoglobulin knockout chickens via efficient homologous recombination in primordial germ cells. Proc Natl Acad Sci USA 2013; 110: 20170–20175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park TS, Lee HJ, Kim KH, Kim JS, Han JY. Targeted gene knockout in chickens mediated by TALENs. Proc Natl Acad Sci USA 2014; 111: 12716–12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oishi I, Yoshii K, Miyahara D, Kagami H, Tagami T. Targeted mutagenesis in chicken using CRISPR/Cas9 system. Sci Rep 2016; 6: 23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Setioko AR, Tagami T, Tase H, Nakamura Y, Takeda K, Tagami T. Cryopreservation of primordial germ cells (PGCs) from White Leghorn embryos using commercial cryoprotectants. J Poult Sci 2007; 44: 73–77. [Google Scholar]
- 56.Kohara Y, Kanai Y, Tajima A. Cryopreservation of gonadal germ cells (GGCs) from the domestic chicken using vitrification. J Poult Sci 2008; 45: 57–61. [Google Scholar]
- 57.Moore DT, Purdy PH, Blackburn HD. A method for cryopreserving chicken primordial germ cells. Poult Sci 2006; 85: 1784–1790. [DOI] [PubMed] [Google Scholar]
- 58.Sawicka D, Chojnacka-Puchta L, Zielinski M, Plucienniczak G, Plucienniczak A, Bednarczyk M. Flow cytometric analysis of apoptosis in cryoconserved chicken primordial germ cells. Cell Mol Biol Lett 2015; 20: 143–159. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura Y, Usui F, Miyahara D, Mori T, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Efficient system for preservation and regeneration of genetic resources in chicken: concurrent storage of primordial germ cells and live animals from early embryos of a rare indigenous fowl (Gifujidori). Reprod Fertil Dev 2010; 22: 1237–1246. [DOI] [PubMed] [Google Scholar]
- 60.Nakamura Y, Usui F, Miyahara D, Mori T, Watanabe H, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Viability and functionality of primordial germ cells after freeze-thaw in chickens. J Poult Sci 2011; 48: 57–63. [Google Scholar]
- 61.Nakamura Y, Tasai M, Takeda K, Nirasawa K, Tagami T. Production of functional gametes from cryopreserved primordial germ cells of the Japanese quail. J Reprod Dev 2013; 59: 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA 1994; 91: 11298–11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okutsu T, Shikina S, Kanno M, Takeuchi Y, Yoshizaki G. Production of trout offspring from triploid salmon parents. Science 2007; 317: 1517. [DOI] [PubMed] [Google Scholar]
- 64.Lin M, Thorne MH, Martin IC, Sheldon BL, Jones RC. Development of the gonads in the triploid (ZZW and ZZZ) fowl, Gallus domesticus, and comparison with normal diploid males (ZZ) and females (ZW). Reprod Fertil Dev 1995; 7: 1185–1197. [DOI] [PubMed] [Google Scholar]
- 65.Naito M, Tajima A, Yasuda Y, Kuwana T. Production of germline chimeric chickens, with high transmission rate of donor-derived gametes, produced by transfer of primordial germ cells. Mol Reprod Dev 1994; 39: 153–161. [DOI] [PubMed] [Google Scholar]
- 66.Kagami H, Tagami T, Matsubara Y, Harumi T, Hanada H, Maruyama K, Sakurai M, Kuwana T, Naito M. The developmental origin of primordial germ cells and the transmission of the donor-derived gametes in mixed-sex germline chimeras to the offspring in the chicken. Mol Reprod Dev 1997; 48: 501–510. [DOI] [PubMed] [Google Scholar]
- 67.Carsience RS, Clark ME, Verrinder Gibbins AM, Etches RJ. Germline chimeric chickens from dispersed donor blastodermal cells and compromised recipient embryos. Development 1993; 117: 669–675. [DOI] [PubMed] [Google Scholar]
- 68.Nakamura Y, Usui F, Miyahara D, Mori T, Ono T, Kagami H, Takeda K, Nirasawa K, Tagami T. X-irradiation removes endogenous primordial germ cells (PGCs) and increases germline transmission of donor PGCs in chimeric chickens. J Reprod Dev 2012; 58: 432–437. [DOI] [PubMed] [Google Scholar]
- 69.Nagano M, Avarbock MR, Brinster RL. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol Reprod 1999; 60: 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell 2007; 12: 195–206. [DOI] [PubMed] [Google Scholar]
- 71.Reynaud G. Transfert de cellules germinales primordiales de Dindon à l’embryon de Poulet par injection intravasculaire. J Embryol Exp Morphol 1969; 21: 485–507. [PubMed] [Google Scholar]
- 72.Aige-Gil V, Simkiss K. Sterilisation of avian embryos with busulphan. Res Vet Sci 1991; 50: 139–144. [DOI] [PubMed] [Google Scholar]
- 73.Nakamura Y, Yamamoto Y, Usui F, Atsumi Y, Ito Y, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Increased proportion of donor primordial germ cells in chimeric gonads by sterilisation of recipient embryos using busulfan sustained-release emulsion in chickens. Reprod Fertil Dev 2008; 20: 900–907. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura Y, Usui F, Atsumi Y, Otomo A, Teshima A, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Effects of busulfan sustained-release emulsion on depletion and repopulation of primordial germ cells in early chicken embryos. J Poult Sci 2009; 46: 127–135. [Google Scholar]
- 75.FAO. Secondary guidelines for development of national farm animal genetic resources management plans: Management of small populations at risk. 1998; pp. 1–210.
- 76.Yoshizaki G, Ichikawa M, Hayashi M, Iwasaki Y, Miwa M, Shikina S, Okutsu T. Sexual plasticity of ovarian germ cells in rainbow trout. Development 2010; 137: 1227–1230. [DOI] [PubMed] [Google Scholar]
- 77.Naito M, Matsubara Y, Harumi T, Tagami T, Kagami H, Sakurai M, Kuwana T. Differentiation of donor primordial germ cells into functional gametes in the gonads of mixed-sex germline chimaeric chickens produced by transfer of primordial germ cells isolated from embryonic blood. J Reprod Fertil 1999; 117: 291–298. [DOI] [PubMed] [Google Scholar]
- 78.Tagami T, Kagami H, Matsubara Y, Harumi T, Naito M, Takeda K, Hanada H, Nirasawa K. Differentiation of female primordial germ cells in the male testes of chicken (Gallus gallus domesticus). Mol Reprod Dev 2007; 74: 68–75. [DOI] [PubMed] [Google Scholar]
- 79.van de Lavoir MC, Collarini EJ, Leighton PA, Fesler J, Lu DR, Harriman WD, Thiyagasundaram TS, Etches RJ. Interspecific germline transmission of cultured primordial germ cells. PLoS ONE 2012; 7: e35664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li ZD, Deng H, Liu CH, Song YH, Sha J, Wang N, Wei H. Production of duck-chicken chimeras by transferring early blastodermal cells. Poult Sci 2002; 81: 1360–1364. [DOI] [PubMed] [Google Scholar]
- 81.Liu C, Khazanehdari KA, Baskar V, Saleem S, Kinne J, Wernery U, Chang IK. Production of chicken progeny (Gallus gallus domesticus) from interspecies germline chimeric duck (Anas domesticus) by primordial germ cell transfer. Biol Reprod 2012; 86: 101. [DOI] [PubMed] [Google Scholar]
- 82.Wernery U, Liu C, Baskar V, Guerineche Z, Khazanehdari KA, Saleem S, Kinne J, Wernery R, Griffin DK, Chang IK. Primordial germ cell-mediated chimera technology produces viable pure-line Houbara bustard offspring: potential for repopulating an endangered species. PLoS ONE 2010; 5: e15824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu J, Song Y, Cheng KM, Silversides FG. Production of donor-derived offspring from cryopreserved ovarian tissue in Japanese quail (Coturnix japonica). Biol Reprod 2010; 83: 15–19. [DOI] [PubMed] [Google Scholar]