Abstract
To analyze the relationship of blood metabolite concentrations and body condition score (BCS) with persistent bacterial uterine infection, specifically that caused by Trueperella pyogenes and anaerobic bacteria, uterine bacteriological swabs (n = 128) were collected from 64 Holstein cows at 5 (W5) and 7 (W7) weeks postpartum, and the percentage of neutrophils in the endometrium was evaluated. Blood glucose, total cholesterol (T-cho), blood urea nitrogen (BUN), non-esterified fatty acid (NEFA), and β-hydroxybutyric acid concentrations were analyzed at 3 weeks (W-3) and 1 week (W-1) prepartum and W3, W5, and W7 postpartum. BCS were evaluated at W-3, W3, and W7. Blood glucose concentrations at W-3 and W-1 in cows with persistent bacterial infection were lower (P = 0.05) than in the rest of the cows. Total BUN concentrations in cows with persistent bacterial infection were lower (P < 0.01) than those in other cows, although the association between the pre or postpartum time and status of infection was not significant. Total NEFA concentrations in cows with persistent bacterial infection were similar to those in uninfected cows and cows positive for infection at W5 but not W7. Total BCS in cows with persistent bacterial infection were lower (P < 0.01) than those in cows positive for infection at both W5 but not W7 and W7 but not W5; however, the association between the pre or postpartum time and status of infection was not significant. Glucose concentrations at W-3 and W-1 negatively correlated with persistent bacterial infection at W5 and W7 (P < 0.01). BUN concentrations at W3 (P < 0.01), W5 (P < 0.05), and W7 (P < 0.05) and BCS at W3 (P < 0.01) negatively correlated with persistent postpartum bacterial infection. Decreased prepartum blood glucose concentrations might be an important risk factor for persistent postpartum bacterial uterine infection in dairy cows.
Keywords: Bacterial infection, Blood metabolites, Cow, Uterus
Most of the infectious and metabolic diseases of dairy cows that affect their well-being and profitability occur during the transition period [1, 2], which spans from 3 weeks before parturition to 3 weeks after [3]. Bacterial contamination of the uterus most commonly occurs during the first week postpartum and persists for 2 weeks in 90% of animals [4, 5]. Concurrent alteration in the uterine defense system might cause uterine diseases. Several studies report that 40% of cows experience metritis within 2 weeks of calving, and 15% experience persistent endometritis 3–6 weeks postpartum [4, 5]. Infections caused by Trueperella pyogenes, Fusobacterium necrophorum, and Proteus spp. have been associated with purulent vaginal discharge, and, when considered independently, T. pyogenes exhibited an increased risk of occurrence of fetid vaginal mucus odor [6].
The problem faced by transitioning dairy cows is that decreased dry matter intake (DMI) during the transition period results in minimal glucose absorption from the digestive tract because of the limited synthesis and availability of propionate in the rumen; consequently, the high glucose demand cannot be met by the feed intake [7]. Propionate concentration is also restricted by the decreased feed intake; therefore, cows rely on the breakdown of skeletal muscle and adipose tissues to supply amino acids and glycerol for gluconeogenesis. Low insulin concentrations enable extensive mobilization of long-chain fatty acids from the adipose tissues, thus triggering an increase in the concentrations of circulating non-esterified fatty acids (NEFAs). Circulating NEFA concentrations also increase in response to the simultaneous increase in energy demand and inadequate feed intake [8]. This negative energy balance is detected clinically through evaluation of alterations in blood metabolite concentrations, such as increased NEFA and β-hydroxybutyric acid (BHBA) concentrations, which are indicative of lipid mobilization and fatty acid oxidation, respectively [9].
Metabolic profile analysis has been used to investigate possible relationships between dietary energy intake and fertility in dairy cows; however, no clear pattern has emerged [10]. Marked loss of body condition between the dry and post-calving periods is associated with unfavorable changes in body condition during early lactation, increased incidence of postpartum metabolic and reproductive diseases, decreased total cholesterol levels during the first month of lactation, and longer intervals to first breeding after calving in Holstein dairy cows [11]. Plasma glucose concentrations can be used to monitor the energy balance in cows, with low glucose concentrations during the postpartum period reported to be associated with a significant decrease in reproductive efficiency [12]. The plasma concentrations of BHBA and NEFA can be used to monitor the degree of body fat mobilization after the onset of lactation. However, plasma BHBA concentration is not correlated with fertility indicators in dairy cows [13]. Plasma NEFA concentrations during the dry and early lactation periods have been reported to be correlated with postpartum ovarian function; however no such trend has been observed in the later postpartum period [14]. Body condition scores (BCS) are used as a means of appraisal of body fat and muscle content in dairy cattle. Variation in BCS is an indirect measure of fat metabolism, and, consequently, it correlates to the other metabolic parameters, particularly NEFA and BHBA [15].
The hypothesis in the present study is that variations in the pre and postpartum concentrations of certain metabolic parameters could serve as indicators of persistent uterine bacterial infection in postpartum dairy cows. While the severity of uterine infection is dependent, in part, on the bacterial species present, the establishment and persistence of uterine infection is influenced by several risk factors. Infections by T. pyogenes and anaerobic bacteria are correlated with increases in endometrial inflammation and disease severity [6]. Identification of risk factors for postpartum uterine bacterial infection will improve our understanding of the underlying mechanisms of pathogenesis of clinical and subclinical endometritis [16]. Early identification of cows at risk for endometritis could not only allow targeted intervention, but also serve as a basis for change in management practices for the prevention of this disease [16].
Few publications have described the relationships between metabolic disorders, BCS, and chronic postpartum uterine infection under field conditions. The objective of the present study was to investigate the association of pre and postpartum variations in blood metabolite concentrations and BCS with the incidence of persistent bacterial colonization, specifically by T. pyogenes and anaerobic bacteria, in the uteri of Holstein cows.
Materials and Methods
Animals and uterine sampling
The study was conducted in 64 cows at three dairy farms in the Iwate Prefecture, Japan. The mean parity of the cows was 2.6 ± 0.1 (range, 1 to 6). The cows were examined at 3 weeks (W-3) and 1 week (W-1) before calving and at 3 (W3), 5 (W5) and 7 (W7) weeks after calving. The vaginal mucus discharge scores were evaluated using sterile Metricheck devices (Simcro Tech, Hamilton, New Zealand) at W5 and W7. Clear mucus was scored 0; mucus containing flecks of white pus was scored 1; discharge composed of less than 50% white mucopurulent material was scored 2; discharge composed of more than 50% white or yellow pus was scored 3; and discharge composed of more than 50% white or yellow pus with a fetid odor was scored 4 [5]. Uterine swab samples (n = 128) were collected from all of the cows at W5 and W7 using cytobrushes (Puritan Medical Products, Guilford, ME, USA) adapted for use in cattle, as described by Ghanem et al. [17]. The ovarian status was assessed by evaluation of the plasma progesterone levels. Cows with plasma progesterone concentrations ≥ 1.0 ng/ml at W5 and W7 were considered to be in the luteal phase, while those with progesterone concentrations < 1.0 ng/ml at W5 and W7 were considered to either be in the follicular phase or not have resumed postpartum ovarian activity. The cows were fed a total mixed ration comprising corn silage, grass silage, and concentrates. The study protocol was approved by the Institutional Animal Care and Use Committee of the Iwate University.
Bacterial culture at 5 and 7 weeks postpartum
Sterile swabs were rolled against the cytobrushes, placed in transport medium on-site, and transported to the laboratory for analysis. The samples were inoculated on blood, chocolate, and deoxycholate-hydrogen sulfide-lactose (DHL) agar media and incubated at 35.0 ± 1.0°C under aerobic conditions. The samples were also inoculated on BHK agar medium and incubated at 35.0 ± 1.0°C under anaerobic conditions. Bacterial colonies grown under aerobic conditions were harvested at 24 and 48 h and those grown under anaerobic conditions were harvested at 48 h. In cases where no colonies were observed at 48 h under any of these conditions, the samples were inoculated on Gifu Anaerobic Medium (GAM) semisolid for enrichment and incubated at 35.0 ± 1.0°C under aerobic conditions. Bacterial growth was monitored at 24, 48, and 72 h. Semisolid GAM allows bacterial growth under both aerobic (medium surface) and anaerobic (medium interior) conditions. In cases where bacterial growth was observed, one drop of the semisolid GAM was inoculated onto each of the previously mentioned agar media plates and incubated under aerobic or anaerobic conditions. Bacterial species were identified by standard laboratory procedures [18]. Bacterial isolates were classified as either aerobic bacteria requiring oxygen as a terminal electron acceptor and unable to grow in the absence of oxygen or anaerobic bacteria that do not use oxygen for growth and metabolism but, instead, generate energy through fermentation. Facultative anaerobes were defined as isolates able to grow both oxidatively using oxygen and anaerobically by fermentation [19].
Classification of cows according to the presence of bacterial infection
The 64 cows were classified according to the status of bacterial infection, determined by bacterial culture, as follows — negative for bacterial infection at both W5 and W7; positive for bacterial infection at W5 and negative at W7; positive for bacterial infection at W7 and negative at W5; and positive for bacterial infection at both W5 and W7 (persistent uterine bacterial infection).
Diagnosis of cytological endometritis at 5 and 7 weeks postpartum
Samples for cytological analysis were prepared by rolling the cytobrushes on sterile glass slides and immediately fixing the slides on-site using a cytofixative agent (Cytokeep II, Alfresa Pharma, Osaka, Japan). The slides were then transported to the laboratory within 3 h and stained using the Diff-Quik stain (Sysmex, Kobe, Japan) for 20 sec. The percentage of neutrophils (PMN) was evaluated as described by Ghanem et al. [17]. The threshold values for PMN indicating subclinical endometritis were ≥ 6% at W5 and ≥ 4% at W7 [20].
Blood sampling and plasma metabolite measurements
Blood samples were collected from the coccygeal vein at W-3, W-1, W3, W5, and W7 using vacuum heparinized tubes and transported on ice to the laboratory. Within 3 h of collection, plasma was separated by centrifugation at 2000 × g for 15 min, harvested, and stored at −20°C until further analysis. The blood glucose, total cholesterol (T-cho), blood urea nitrogen (BUN), NEFA, and BHBA concentrations of each of the samples were measured using an operationally-enhanced random access analyzer (Accute, Toshiba Medical Systems, Tokyo, Japan) using kinetic enzymatic assay kits (NEFA-HA Wako test kit, Autokit-3HB Wako test kit for BHBA; Wako Pure Chemical Industries, Osaka, Japan; Glucose HK Riki tech test kit, BUN Riki tech test kit, and T-cho [TCII] Riki tech test kit; Roche Diagnostics, Tokyo, Japan) according to the manufacturers’ instructions. The assay system has previously been verified for use in cattle, and the threshold measurements for the plasma metabolites were as follows: NEFA, ≤ 2.00 μEq/l; BHBA, 1.17–1000 μM; glucose, 2–750 mg/dl; BUN, 0.5–150 mg/dl; and T-cho, 5–800 mg/dl [21]. The intra and inter-assay coefficients of variation were < 5%.
Body condition scores
The BCS of each cow was evaluated on a 5-point scale with increments of 0.25, at W-3, W3, and W7, as described by Ferguson et al. [22].
Statistical analyses
The vaginal mucus scores and PMNs at W5 and W7 were evaluated according to the status of uterine bacterial infection using the Kruskal-Wallis test followed by the Dunn’s test for multiple comparisons. All data were tested for normality by the D’Agostino-Pearson omnibus test. The concentrations of different blood metabolites (glucose, T-cho, BUN, NEFA, and BHBA) at different pre and postpartum time points (W-3, W-1, W3, W5 and W7) were analyzed by two-way analysis of variance (ANOVA) in order to determine any possible associations in cows with different infection statuses — uninfected at W5 and W7, positive for infection at W5 but not W7, positive for infection at W7 but not W5, and persistent bacterial infection. Multiple comparisons and separation of means were performed using the Duncan’s multiple range test (IBM SPSS Statistics version 21, IBM Japan, Tokyo, Japan). In the presence of significant association between the pre or postpartum time and status of infection, the effects of such association were analyzed using the MSTAT-C software (Michigan State University, East Lansing, USA). Correlations between the various blood metabolite concentrations, BCS, and presence of persistent bacterial uterine infection at W5 and W7 were analyzed using the Pearson’s correlation test (GraphPad Prism Version 5.01, GraphPad Software, San Diego, USA). Significance was designated at P < 0.05.
Results
History, PMN, and cervical mucus discharge in cows at W5 and W7
Of the 64 cows included in the present study, 31 exhibited no uterine bacterial infection at either W5 or W7, 11 exhibited bacterial infection at W5 but not W7, 12 exhibited bacterial infection at W7 but not W5, and the remaining 10 exhibited bacterial infection at both W5 and W7 (persistent uterine bacterial infection) (Table 1). Of the 10 cows with persistent uterine bacterial infection, 2 had a history of retained placenta and 1 of dystocia. Among the cows with infection at W5 but not W7 and at W7 but not W5, 1 cow each had a history of dystocia. Of the 31 cows negative for bacterial infection, 1 had a history of stillbirth.
Table 1. Parity, PMN, vaginal mucus score and ovarian status (mean ± SD) in cows with and without persistent bacterial infection at 5 (W5) and 7 (W7) weeks postpartum.
| Parameters | No. | Parity | PMN, % |
Vaginal mucus score |
Cows in the luteal phase, n (%) |
|||
| W5 | W7 | W5 | W7 | W5 | W7 | |||
| Cows negative for bacterial infection at both W5 and W7 | 31 | 2.3 ± 1.4 | 1.7 ± 3.7 a | 2.9 ± 3.4 a | 0.8 ± 0.7 a | 1.0 ± 0.9 a | 10 (32.3%) | 12 (38.7%) |
| Cows positive for bacterial infection at W5 but not W7 | 11 | 1.8 ± 0.8 | 10.1 ± 12.7 ab | 6.5 ± 12.4 a | 1.5 ± 1.3 ab | 1.3 ± 0.8 ab | 3 (27.3%) | 4 (36.4%) |
| Cows positive for bacterial infection at W7 but not W5 | 12 | 2.7 ± 1.7 | 5.5 ± 9.0 a | 1.9 ± 3.4 a | 1.2 ± 0.8 ab | 1.3 ± 0.8 ab | 7 (58.3%) | 9 (75.0%) |
| Cows positive for bacterial infection at both W5 and W7 | 10 | 3.1 ± 0.8 | 18.8 ± 11.9 b | 21.8 ± 14.6 b | 2.6 ± 1.4 b | 2.6 ± 1.3 b | 6 (60.0%) | 8 (80.0%) |
| P value* | 0.2 | 0.0003 | 0.0001 | 0.001 | 0.008 | |||
PMN: percentage of neutrophils determined by cytological examination. * Significant difference in PMN and vaginal mucus score according to the presence of bacterial infection within each data column (Kruskal-Wallis test). Different superscript letters within the same column indicate significant differences.
The PMN was significantly higher in cows with persistent uterine bacterial infection than that in cows without uterine infection at W5 and W7 (P < 0.001). Cows with persistent uterine bacterial infection exhibited higher vaginal mucus scores than those without uterine infection at W5 and W7 (P < 0.01; Table 1).
Based on the presence of plasma progesterone concentrations ≥ 1.0 ng/ml at W5 and W7, 26 (40.6%) and 33 (51.6%) cows, respectively, were considered to be in the luteal phase (Table 1).
Pre and postpartum blood metabolite concentrations in cows with or without persistent infection at W5 and W7
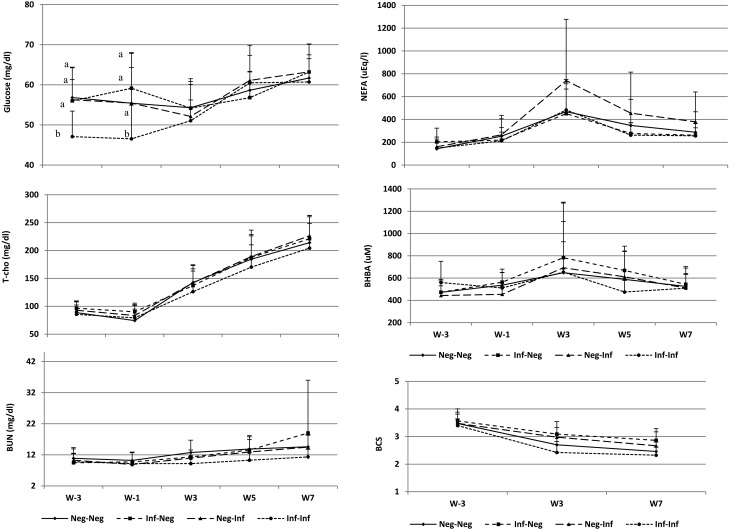
The association between blood glucose concentrations at different times (W-3, W-1, W3, W5 and W7) and the status of uterine bacterial infection (uninfected at W5 and W7, positive for infection at W5 but not W7, positive for infection at W7 but not W5, and persistent bacterial infection) was almost significant (P = 0.05). The blood glucose concentrations at W-3 and W-1 were significantly decreased in cows with persistent bacterial infection compared to those in cows without infection at W5 and W7, positive for infection at W5 but not W7, and positive for infection at W7 but not W5 (P < 0.05). There were no significant differences in the pre and postpartum T-cho concentrations between cows with persistent infection at W5 and W7 and those without persistent infection during the experimental period (from W-3 to W7). The association between the pre or postpartum time and status of infection was also insignificant (P = 0.98), which indicated that the plasma concentration of T-cho had no influence on postpartum uterine bacterial infection. The total BUN concentrations were significantly lower (P < 0.01) in cows with persistent bacterial infection than those in cows without infection at W5 and W7, positive for infection at W5 but not W7, and positive for infection at W7 but not W5; however, the association between the pre or postpartum time and status of infection was not significant (P = 0.32). The total NEFA concentrations in cows with persistent bacterial infection were similar to those in cows without infection as well as cows positive for infection at W5 but not W7; however the association between the pre or postpartum time and status of infection was not significant (P = 0.42). The pre and postpartum BHBA concentrations were not associated (P = 0.88) with persistent postpartum uterine bacterial infection and exhibited no significant differences (P = 0.43) between cows with and without persistent infection during the experimental period (from W-3 to W7) (Fig. 1).
Fig. 1.
Blood metabolite concentrations and body condition scores (mean ± SD) in cows of different test groups — negative for infection (Neg-Neg) at 5 (W5) and 7 (W7) weeks after calving, positive for infection at W5 but not W7 (Inf-Neg), positive for infection at W7 but not W5 (Neg-Inf), and persistent uterine bacterial infection at W5 and W7 (Inf-Inf).
Pre and postpartum variations in BCS in cows with and without bacterial infection at W5 and W7
The BCS in cows with persistent bacterial infection were significantly lower (P < 0.01) than those in cows positive for infection at W5 but not W7 and at W7 but not W5; however, the association between the pre or postpartum time and status of infection was not significant (P = 0.25).
Correlation of blood metabolite concentrations and BCS with persistent uterine bacterial infection
The concentrations of blood glucose at W-3 and W-1 were negatively correlated with the incidence of persistent bacterial infection at W5 and W7 (P < 0.01; Table 2). Additionally, the concentrations of BUN at W3 (P < 0.01), W5 (P < 0.05), and W7 (P < 0.05) as well as the BCS at W3 (P < 0.01) were also negatively correlated with the incidence of persistent postpartum bacterial infection. The NEFA and BHBA concentrations were not correlated with persistent postpartum bacterial infection.
Table 2. Pearson correlation coefficients (r) of the relationship between persistence of bacterial infection in the uterus, concentrations of different blood metabolites, and body condition scores.
| W-3 |
W-1 |
W3 |
W5 |
W7 |
||||||
| r | P | r | P | r | P | r | P | r | P | |
| Glucose | –0.44 | 0.0002 | –0.39 | 0.001 | –0.16 | 0.2 | 0.05 | 0.64 | –0.09 | 0.45 |
| T-cho | –0.11 | 0.3 | –0.05 | 0.7 | –0.17 | 0.2 | –0.10 | 0.4 | –0.11 | 0.4 |
| BUN | –0.04 | 0.7 | –0.09 | 0.5 | –0.32 | 0.008 | –0.30 | 0.02 | –0.29 | 0.02 |
| NEFA | –0.15 | 0.2 | –0.13 | 0.3 | 0.03 | 0.8 | –0.13 | 0.3 | –0.01 | 0.9 |
| BHBA | 0.2 | 0.1 | –0.03 | 0.8 | –0.17 | 0.2 | –0.25 | 0.04 | –0.02 | 0.9 |
| BCS | –0.10 | 0.4 | –0.33 | 0.007 | –0.23 | 0.06 | ||||
Values of P < 0.05 indicate significant differences. The blood glucose, total cholesterol (T-cho), blood urea nitrogen (BUN), non-esterified fatty acid (NEFA), and β-hydroxybutyric acid (BHBA) concentrations were analyzed at 3 weeks (W-3) and 1 week (W-1) prepartum and 3, 5, and 7 weeks (W3, W5, and W7, respectively) postpartum. The body condition scores (BCS) were evaluated at W-3, W3, and W7.
Discussion
In the present study, few of the cows without infection exhibited subclinical endometritis at W5 and most cows with bacterial infection exhibited no subclinical endometritis at W7. In a previous study, Ghanem et al. [23] reported that while some cows presented with cytological endometritis without clinical endometritis, some others presented with clinical endometritis without cytological endometritis. The assumption that purulent vaginal discharge is associated with endometrial inflammation has never been validated [20]. Cytological endometritis might reflect the presence of uterine inflammation, leading sometimes, but not always, to the drainage of purulent material into the vagina. It is also unclear whether purulent vaginal discharge can lead to cytological endometritis [20]. In the present study, cows positive for bacterial infection at W5 but not W7 exhibited a PMN of 6.5 ± 12.4 at W7, which indicated that most cows without infection at W7 had subclinical endometritis. This might be due to the ability of the uterus to eliminate infection at W7 regardless of the time taken for the reduction of the PMN in the endometrium after bacterial clearance. This point needs to be further investigated, taking into account the possible role of immune mediators such as interleukins and tumor necrosis factors and their relationship with neutrophils in the uterus.
Several studies have reported contradictory results regarding the relationship between the indicators of energy balance and the reproductive performance of dairy cows [24]. In the present study, the blood glucose concentration at W-3 and W-1 were markedly lower in cows with persistent postpartum bacterial infection than those in the other cows. Interestingly, the prepartum blood glucose concentrations were negatively correlated with the incidence of persistent postpartum bacterial infection, which indicates a negative energy balance in cows exhibiting persistent bacterial infection at W5 and W7. This decrease in glucose concentrations might be due, in part, to inadequate DMI; however, a more important contributor is the increased clearance of glucose from blood as a result of nutrient partitioning, which directs glucose towards the insulin-insensitive tissues, primarily the mammary gland [25]. These findings are supported by those of Nazifi et al. [26], who found that cows with uterine infection suffer disturbances in energy metabolism until 4 weeks postpartum as well as delay in the physiological adaptations required to meet their energy needs. This delay might potentially be confirmed by decreased blood glucose concentrations at 4 and 6 weeks postpartum in cows diagnosed with endometritis at 6 weeks postpartum [21].
In the present study, there were no significant differences in the T-cho concentrations among cows with or without uterine bacterial infection. However, Ruegg et al. [27] reported that the concentration of cholesterol during the early lactation period was inversely related to the loss of body condition. These variations in cholesterol concentrations may also be partly explained by the low DMI during transition, which compromises exogenous cholesterol supply [28].
The concentrations of BUN at W3, W5, and W7 were negatively correlated with persistent postpartum bacterial infection. Low concentrations of BUN (< 9 mg/dl) at 2 and 4 weeks postpartum were reported as being risk factors for the development of endometritis by 5 and 6 weeks postpartum, respectively [21]; another study reported that low BUN concentrations indicated increased potential for deficient protein feeding and decreased production in dairy cows [29]. Huzzey et al. [30] reported that cows with uterine diseases at 3 weeks postpartum consumed less dry matter during the transition period than healthy cows. Although no data regarding DMI were available, it is possible that the DMI in the animals included in our study might have been reduced in the early postpartum period. Since no information was available regarding the chemical composition of the feed in the present study, future research focusing on the effects of animal feed constituents such as rumen-degradable proteins and non-fiber carbohydrates on BUN concentration and uterine infection is warranted.
The results of analysis of our data, generated under field conditions, revealed no associations between the concentrations of NEFA and BHBA at different pre and postpartum time points and the status of uterine infection. In general, the NEFA and BHBA concentrations increase immediately after calving, following which, these values tend to decrease with time. The non-correlation of these factors with persistent uterine infection might be because of the sampling time points evaluated in this study. In contrast to our findings, other studies have reported decreased fertility in cows because of elevated NEFA [31] and BHBA [32, 33] concentrations. Hammon et al. [34] reported significantly higher prepartum concentrations of NEFA and BHBA in cows that developed endometritis postpartum than those in cows that remained healthy postpartum. Elevated prepartum plasma concentrations of NEFA and postpartum plasma concentrations of BHBA were associated with the incidence of uterine disorders later during lactation [16]. Moreover, elevated serum BHBA concentrations during the first 2 weeks postpartum indicated an increased risk of metritis [35], which is a common precursor to clinical and subclinical endometritis [33]. The present study was conducted using similar protocols as some of the recent studies [24, 36]. However, in one of these prior studies, the plasma concentrations of NEFA and BHBA during early lactation showed no association with metritis [24], and Burke et al. [36] reported no difference in NEFA concentrations between cows with subclinical endometritis and those without endometritis. Differences in the experimental design, methodology, and disease definition might have contributed to these discrepancies.
In the present study, the BCS in cows with persistent bacterial infection were significantly lower (P < 0.01) than those in cows positive for infection at W5 but not W7 and at W7 but not W5; however, the association between the pre or postpartum BCS and status of infection was not significant. Moreover, the BCS at W3 were negatively correlated with the persistence of postpartum bacterial infection. Hoedemaker et al. [37] reported that cows with low BCS at parturition were at a higher risk of developing endometritis than cows with BCS > 3.0. In addition, cows with low BCS at calving and during early lactation were reported as being at a higher risk of suffering lameness, non-cycling at W3 or W5, and being culled than cows in better body condition. Notably, Ruegg and Milton [38] reported no association between decreased BCS during lactation and increased incidence of metabolic diseases. Taken together, the results of the present study confirm that variations in the concentrations of select metabolites, specifically blood glucose, during the transition period (W-3 to W-1) might be related to the postpartum persistence of uterine bacterial infection, diagnosed by endometrial cytological examination. These findings highlight the benefits of identifying cows at risk of developing uterine infection around the calving period in terms of early implementation of preventive strategies.
In conclusion, prepartum decrease in blood glucose concentrations might be an important risk factor for postpartum persistent uterine infection diagnosed by cytological examination at W5 and W7. Veterinary practitioners should monitor for pre and postpartum variations in blood metabolite concentrations and BCS in cows since such variations might render cows more susceptible to uterine infection.
Acknowledgments
We gratefully acknowledge IDEXX Laboratories for performing the bacterial examination of the test samples.
References
- 1.Drackley JK. ADSA Foundation Scholar Award. Biology of dairy cows during the transition period: the final frontier? J Dairy Sci 1999; 82: 2259–2273. [DOI] [PubMed] [Google Scholar]
- 2.Goff JP, Horst RL. Physiological changes at parturition and their relationship to metabolic disorders. J Dairy Sci 1997; 80: 1260–1268. [DOI] [PubMed] [Google Scholar]
- 3.Grummer RR. Impact of changes in organic nutrient metabolism on feeding the transition dairy cow. J Anim Sci 1995; 73: 2820–2833. [DOI] [PubMed] [Google Scholar]
- 4.Földi J, Kulcsár M, Pécsi A, Huyghe B, de Sa C, Lohuis JA, Cox P, Huszenicza G. Bacterial complications of postpartum uterine involution in cattle. Anim Reprod Sci 2006; 96: 265–281. [DOI] [PubMed] [Google Scholar]
- 5.Sheldon IM, Lewis GS, LeBlanc S, Gilbert RO. Defining postpartum uterine disease in cattle. Theriogenology 2006; 65: 1516–1530. [DOI] [PubMed] [Google Scholar]
- 6.Williams EJ, Fischer DP, Pfeiffer DU, England GCW, Noakes DE, Dobson H, Sheldon IM. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 2005; 63: 102–117. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds CK, Aikman PC, Lupoli B, Humphries DJ, Beever DE. Splanchnic metabolism of dairy cows during the transition from late gestation through early lactation. J Dairy Sci 2003; 86: 1201–1217. [DOI] [PubMed] [Google Scholar]
- 8.Overton TR, Waldron MR. Nutritional management of transition dairy cows: strategies to optimize metabolic health. J Dairy Sci 2004; 87(E. Supple): E105–E119. [Google Scholar]
- 9.Wathes DC, Cheng Z, Bourne N, Taylor VJ, Coffey MP, Brotherstone S. Differences between primiparous and multiparous dairy cows in the inter-relationships between metabolic traits, milk yield and body condition score in the periparturient period. Domest Anim Endocrinol 2007; 33: 203–225. [DOI] [PubMed] [Google Scholar]
- 10.Wathes DC, Fenwick M, Cheng Z, Bourne N, Llewellyn S, Morris DG, Kenny D, Murphy J, Fitzpatrick R. Influence of negative energy balance on cyclicity and fertility in the high producing dairy cow. Theriogenology 2007; 68(Suppl 1): S232–S241. [DOI] [PubMed] [Google Scholar]
- 11.Kim IH, Suh GH. Effect of the amount of body condition loss from the dry to near calving periods on the subsequent body condition change, occurrence of postpartum diseases, metabolic parameters and reproductive performance in Holstein dairy cows. Theriogenology 2003; 60: 1445–1456. [DOI] [PubMed] [Google Scholar]
- 12.Harrison RO, Ford SP, Young JW, Conley AJ, Freeman AE. Increased milk production versus reproductive and energy status of high producing dairy cows. J Dairy Sci 1990; 73: 2749–2758. [DOI] [PubMed] [Google Scholar]
- 13.Kelly JM, Whitaker DA. Nutritional effects on resumption of ovarian cyclicity and conception rate in postpartum dairy cows. In: Diskin MG (ed.), Fertility in the High-Producing Dairy Cow, Occasional Publication no. 26, British Society of Animal Science; 1999: 209–221.
- 14.Huszenicza G, Haraszti J, Molnár L, Solti L, Fekete S, Ekés K, Yaro AC. Some metabolic characteristics of dairy cows with different post partum ovarian function. Zentralbl Veterinarmed A 1988; 35: 506–515. [PubMed] [Google Scholar]
- 15.Roche JR, Friggens NC, Kay JK, Fisher MW, Stafford KJ, Berry DP. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. J Dairy Sci 2009; 92: 5769–5801. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann TB, Drillich M, Tenhagen BA, Heuwieser W. Correlations between periparturient serum concentrations of non-esterified fatty acids, beta-hydroxybutyric acid, bilirubin, and urea and the occurrence of clinical and subclinical postpartum bovine endometritis. BMC Vet Res 2010; 6: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghanem ME, Higuchi H, Tezuka E, Ito H, Devkota B, Izaike Y, Osawa T. Mycoplasma infection in the uterus of early postpartum dairy cows and its relation to dystocia and endometritis. Theriogenology 2013; 79: 180–185. [DOI] [PubMed] [Google Scholar]
- 18.Oguri T, Nishiyama H, Nagasawa Z. Identification of bacteria [in Japanese]. In: Oguri T (ed.), Clinical microbiology handbook, 4th edition. Tokyo: Miwa Syoten; 2011: 97–136.
- 19.Brooks GF, Carroll KC. Infection caused by anaerobic bacteria. In: Brooks GF, Carroll KC, Butel JS, Morse SA (eds.), Jawetz, Melnick and Adelberg's Medical Miicrobiology. New York: Mc Grow Hill; 2007: 306.
- 20.Dubuc J, Duffield TF, Leslie KE, Walton JS, LeBlanc SJ. Definitions and diagnosis of postpartum endometritis in dairy cows. J Dairy Sci 2010; 93: 5225–5233. [DOI] [PubMed] [Google Scholar]
- 21.Senosy WS, Izaike Y, Osawa T. Influences of metabolic traits on subclinical endometritis at different intervals postpartum in high milking cows. Reprod Domest Anim 2012; 47: 666–674. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson JD, Galligan DT, Thomsen N. Principal descriptors of body condition score in Holstein cows. J Dairy Sci 1994; 77: 2695–2703. [DOI] [PubMed] [Google Scholar]
- 23.Ghanem ME, Tezuka E, Devkota B, Izaike Y, Osawa T. Persistence of uterine bacterial infection, and its associations with endometritis and ovarian function in postpartum dairy cows. J Reprod Dev 2015; 61: 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valergakis GE, Oikonomou G, Arsenos G, Banos G. Phenotypic association between energy balance indicators and reproductive performance in primiparous Holstein cows. Vet Rec 2011; 168: 189. [DOI] [PubMed] [Google Scholar]
- 25.Bell AW, Bauman DE. Adaptations of glucose metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia 1997; 2: 265–278. [DOI] [PubMed] [Google Scholar]
- 26.Nazifi S, Saeb M, Rowghani E, Kaveh K. The influences of thermal stress on serum biochemical parameters of Iranian fat-tailed sheep and their correlation with tri-iodothryonine (T3), thyroxine (T4) and cortisol concentrations. Comp Clin Pathol 2003; 12: 135–139. [Google Scholar]
- 27.Ruegg PL, Goodger WJ, Holmberg CA, Weaver LD, Huffman EM. Relation among body condition score, milk production, and serum urea nitrogen and cholesterol concentrations in high-producing Holstein dairy cows in early lactation. Am J Vet Res 1992; 53: 5–9. [PubMed] [Google Scholar]
- 28.Guretzky NA, Carlson DB, Garrett JE, Drackley JK. Lipid metabolite profiles and milk production for Holstein and Jersey cows fed rumen-protected choline during the periparturient period. J Dairy Sci 2006; 89: 188–200. [DOI] [PubMed] [Google Scholar]
- 29.Rajala-Schultz PJ, Frazer GS, Wittum TE, Saville WJA. Association between milk urea nitrogen and fertility in Ohio dairy cows. J Dairy Sci 2001; 84: 482–489. [DOI] [PubMed] [Google Scholar]
- 30.Huzzey JM, Veira DM, Weary DM, von Keyserlingk MA. Prepartum behavior and dry matter intake identify dairy cows at risk for metritis. J Dairy Sci 2007; 90: 3220–3233. [DOI] [PubMed] [Google Scholar]
- 31.Westwood CT, Lean IJ, Garvin JK. Factors influencing fertility of Holstein dairy cows: a multivariate description. J Dairy Sci 2002; 85: 3225–3237. [DOI] [PubMed] [Google Scholar]
- 32.Taylor VJ, Beever DE, Bryant MJ, Wathes DC. Metabolic profiles and progesterone cycles in first lactation dairy cows. Theriogenology 2003; 59: 1661–1677. [DOI] [PubMed] [Google Scholar]
- 33.Walsh RB, Walton JS, Kelton DF, LeBlanc SJ, Leslie KE, Duffield TF. The effect of subclinical ketosis in early lactation on reproductive performance of postpartum dairy cows. J Dairy Sci 2007; 90: 2788–2796. [DOI] [PubMed] [Google Scholar]
- 34.Hammon DS, Evjen IM, Dhiman TR, Goff JP, Walters JL. Neutrophil function and energy status in Holstein cows with uterine health disorders. Vet Immunol Immunopathol 2006; 113: 21–29. [DOI] [PubMed] [Google Scholar]
- 35.Duffield TF, Lissemore KD, McBride BW, Leslie KE. Impact of hyperketonemia in early lactation dairy cows on health and production. J Dairy Sci 2009; 92: 571–580. [DOI] [PubMed] [Google Scholar]
- 36.Burke CR, Meier S, McDougall S, Compton C, Mitchell M, Roche JR. Relationships between endometritis and metabolic state during the transition period in pasture-grazed dairy cows. J Dairy Sci 2010; 93: 5363–5373. [DOI] [PubMed] [Google Scholar]
- 37.Hoedemaker M, Prange D, Gundelach Y. Body condition change ante- and postpartum, health and reproductive performance in German Holstein cows. Reprod Domest Anim 2009; 44: 167–173. [DOI] [PubMed] [Google Scholar]
- 38.Ruegg PL, Milton RL. Body condition scores of Holstein cows on Prince Edward Island, Canada: relationships with yield, reproductive performance, and disease. J Dairy Sci 1995; 78: 552–564. [DOI] [PubMed] [Google Scholar]