Abstract
Normal cows have 2 peaks in endometrial epidermal growth factor (EGF) concentrations on Days 2–4 and 13–14, and the absence of peaks has been linked to reduced fertility in repeat breeder (RB) cows. However, the timing of the peaks (i.e., day of examinations) was estimated for a few cows per cycle day. Therefore, the present study characterized EGF peaks and examined if the absence of peaks in RB cows indicate either peak loss or changed timing. In Study 1, 20 Holstein cows were examined for EGF concentrations between Days 1 and 6 using repeated biopsy of the uterine endometrial tissues. Sixteen cows exhibited increased EGF concentrations for 2–3 days between Days 2 and 5. All 16 cows exhibited increased EGF concentrations on Day 3. In Study 2, 10 cows were examined for EGF concentrations between Days 11 and 16. Increased EGF concentrations for 2–3 days were found in 7 cows between Days 12 and 15. All 7 cows exhibited increased EGF concentrations on Days 13 and 14. In Study 3, 12 RB cows were examined for endometrial EGF concentrations between Days 1 and 6. Four cows exhibited an increase of EGF concentrations on Days 3 and 4, whereas 8 cows (66.7%) exhibited low EGF concentrations throughout the study period. In conclusion, Days 3 and 13–14 are suitable days to examine a cyclic change of endometrial EGF concentrations. Further, low EGF concentrations on Day 3 in RB cows indicated an absence, but not altered timing, of the EGF peak.
Keywords: Biopsy, Endometrial epidermal growth factor (EGF), High yielding cows, Repeat breeding, Uterus
Epidermal growth factor (EGF) concentrations in the uterine endometrium exhibit a cyclic change during the estrous cycle, and the loss of a cyclic EGF profile, in the presence of an apparently normal estrous (ovarian) cycle, may reduce fertility in dairy cows [1]. In normal cows, endometrial EGF concentrations peak twice on Days 2–4 and 13–14 with reduced EGF concentrations around Day 7, whereas the 2 peaks in the endometrial EGF profile are not observed in approximately 70% of repeat breeder (RB) Holstein cows [2, 3]. The relationship between the EGF profile and fertility has also been demonstrated by an embryo transfer experiment. Recipient cows with low EGF concentrations on Day 3 exhibited a lower conception rate than those with EGF concentrations within the normal range (33.3 vs. 76.9%) [4]. This EGF profile pattern persisted between the estrous cycles in both fertile and RB cows [3]. In the fertile controls, the endometrial EGF concentrations exhibited a normal pattern over 3 consecutive estrous cycles. In the RB cows, the alteration in the endometrial EGF concentrations persisted between the estrous cycles when the cows were not treated [3]. The normalization of the EGF profile resulted in the restoration of fertility in RB cows [3, 5]. Thus, these findings support the existence of a potential relationship between the endometrial EGF profile and fertility [1, 3].
However, EGF profiles are currently evaluated based on the information obtained in a preliminary study with a small number of animals [2]. The timing of the 2 peaks during the estrous cycle was estimated using only 31 cows. In this study, the numbers of cows assigned to individual days of the estrous cycle ranged from 0 to 3 as follows: a single cow on Days 3, 6, 16, and 17; 2 cows on Days 2, 4, 5, 7, 8, 10–12, 14, 15, 19, and 20; 3 cows on Day 13; and no cow was assigned to Days 9 or 18. Therefore, an additional study that characterizes EGF concentration profiles with an emphasis on the timing of concentration peaks is necessary before conducting further studies to understand the significance of a normal EGF profile on the fertility of dairy cows.
Similarly, an abnormality in the 2 peaks in RB cows might require characterization. RB cows have been examined for endometrial EGF concentrations on Days 3, 7, and 14, and approximately 70% of RB cows exhibited low EGF concentrations on Days 3 and 14 [2]. This study revealed clearly altered EGF profiles in RB cows. However, the design of the study questioned whether the peaks are lost or have temporally shifted to earlier or later days of the estrous cycle in RB cows, as discussed in our previous report [3].
The present study was designed to characterize the 2 endometrial EGF peaks using an increased number of animals by repeated biopsy of the endometrial tissues. This technique allowed us to reduce the number of animals that are sacrificed to sample the endometrial tissues and enhance the accuracy and reliability of data due to the reduction of individual variation between cows, which can significantly contribute to the data variability. Twenty and 10 cows were subjected to repeated biopsy for 6 consecutive days to cover the potential periods of the first (Study 1) and second (Study 2) EGF peaks, respectively. Next, 12 RB cows underwent repeated biopsy to characterize the alterations in the first peak of the endometrial EGF concentrations (Study 3).
Materials and Methods
Animals
Clinically normal lactating Holstein cows (45–60 kg/day/cow) from 4 commercial dairy farms were used. The cows had experienced at least 2 ovulations with a normal length of an inter-estrus interval (18–23 days). The cows were between 2 and 5 in parity and were used for biopsy during the start of the estrous cycle (estrus = Day 0), between 60 and 90 days postpartum.
Clinically normal cows that were housed in the same 4 dairy farms and that had failed to conceive after 3 or more consecutive artificial inseminations (AIs) were recruited for the study as potential RB cows. The cows were then examined for regularity of the estrous cycle, timing of ovulation (< 48 h after the onset of estrus), subclinical inflammation of the uterus by cytology or histology, and oviductal patency. Cows without any abnormality in these examinations were used as RBs. The RB cows were between 2 and 5 in parity and were used for biopsy during the estrous cycle, starting between 130 and 185 days postpartum.
Biopsy of the endometrial tissues
Uterine endometrial tissues were obtained by biopsy using a biopsy instrument (Fujihira, Tokyo, Japan) under caudal epidural anesthesia with 3 ml of 2% lidocaine (2% xylocaine, AstraZeneca, Osaka, Japan) as previously described [2]. At each biopsy, 2 samples of the uterine endometrial tissues of the inter-caruncle region (25–50 mg) were obtained from the middle of 3 sections of the uterine horns, which were equally divided along the longitudinal axis. Tissues were sliced (< 1 mm thick) and examined with a magnifying glass for the presence of a caruncle. If the caruncle was greater than one-third of the tissue, another biopsy was collected. However, if the caruncle was approximately one-third or less of the biopsy, it was dissected out and the remaining tissue was used. The tissues were weighed, frozen in liquid nitrogen, and stored at –80°C within 10 min of collection.
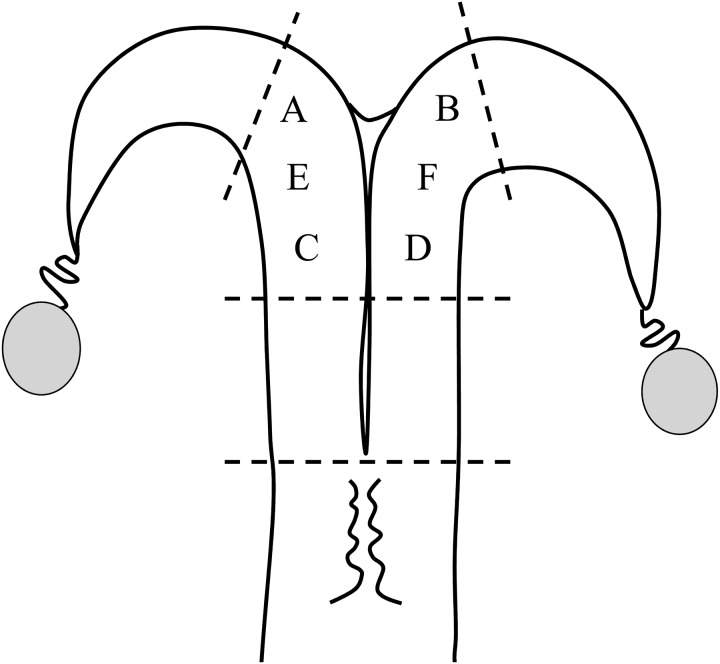
Endometrial tissues were obtained by repeated biopsy for 6 consecutive days, from 6 different positions (A to F) within the middle section of the uterine horns (Fig. 1), regardless of the side of the corpus luteum and dominant follicle. This was to avoid collecting scar tissues and reduce the potential effects of previous biopsies on EGF levels. In half of the animals, repeated biopsy was started at position A and continued in alphabetical order to position F (Fig. 1). In the other half of animals, biopsy was performed from position F to A, in the reverse order.
Fig. 1.
Endometrial biopsy positions. The endometrial tissues were obtained from 6 different positions (A to F) within the middle section of the uterine horns. In half of the animals, repeated biopsy was performed from position A to position F. In the other half of animals, biopsy was performed in the reverse order. Each time, 2 samples of 25–50 mg endometrial tissues were obtained from the inter-caruncle region.
EGF assay
Uterine endometrial tissue samples were processed using a previously described method [6] with a modification to increase the tissue (g) to extraction solution (ml) ratio from 1:5 to 1:15 [3]. The EGF concentrations in uterine tissue extracts were determined by a double-antibody sandwich EIA using 96-well microtiter plates [2]. An anti-human EGF mouse monoclonal antibody (R & D Systems, Minneapolis, MN, USA) was used for the solid-phase antibody, and an anti-human EGF rabbit antiserum (Biogenesis, Poole, UK) was used for detection with peroxidase-conjugated anti-rabbit IgG goat antibody (Seikagaku, Tokyo, Japan). The sensitivity of the assay was 10 pg/well. The intra- and inter-assay CVs at 50 pg/well were 4.2 and 5.3%, respectively.
Study design
This study was approved by the Institutional Animal Care and Use Committee convened at the Rakuno Gakuen University (approval number: VH21C13).
Study 1
Twenty lactating Holstein cows were used in this study to characterize the first peak of endometrial EGF concentrations during the estrous cycle. The cows were examined for EGF concentrations using endometrial tissues obtained by repeated biopsy for 6 consecutive days between Days 1 and 6. Fifteen cows were then subjected to AI up to 2 times at observed estrus. Pregnancy was diagnosed by transrectal palpation after Day 60. Five cows were not inseminated and were used elsewhere.
Study 2
Ten lactating Holstein cows were used to characterize the second peak of endometrial EGF concentrations. The cows were evaluated for EGF concentrations using endometrial tissues obtained by repeated biopsy for 6 consecutive days between Days 11 and 16. All of the cows were then inseminated and diagnosed for pregnancy as detailed above.
Study 3
Twelve RB cows were examined for EGF concentrations for 6 consecutive days between Days 1 and 6. Next, 6 cows were inseminated and diagnosed for pregnancy as above. The other 6 RB cows were not inseminated and were used elsewhere.
Data analysis
The first (Studies 1 and 3) and second (Study 2) peaks of endometrial EGF concentrations during the estrous cycle were defined as increased EGF concentrations for a single day or for a few consecutive days. Endometrial EGF concentrations > 4.7 and 4.9 ng/g tissue weight (the lower limit of the normal range of EGF concentrations on Days 3 and 14, respectively [3]) were considered as increased levels during the 6-day periods when the first and second peaks, respectively, were expected. The length of the peaks was determined as the number of days on which EGF concentrations increased.
In Studies 1 and 2, the EGF concentrations 1 and 2 days prior to and on the first day of the peak were compared to characterize the transition pattern of EGF concentrations from basal to peak levels. For this analysis, the data derived from 13 and 6 cows in which increased EGF concentrations were first detected on Days 3 and 13, respectively, were used. The EGF concentrations on the last day of the peak and the subsequent two days were also compared to characterize the transition pattern of EGF concentrations from peak to basal levels. This analysis used the data derived from 13 and 4 cows in which the EGF peak terminated on Days 3–4 and 14, respectively.
The proportion of cows with increased endometrial EGF concentrations between Study 1 and 3 was compared by Fisher’s exact test. Endometrial EGF concentrations were compared using a one-way ANOVA with repeated measurements using the Tukey HSD test as post hoc test. All analyses were performed using the computer software SPSS ver. 21 (IBM, Tokyo, Japan).
Results
Study 1
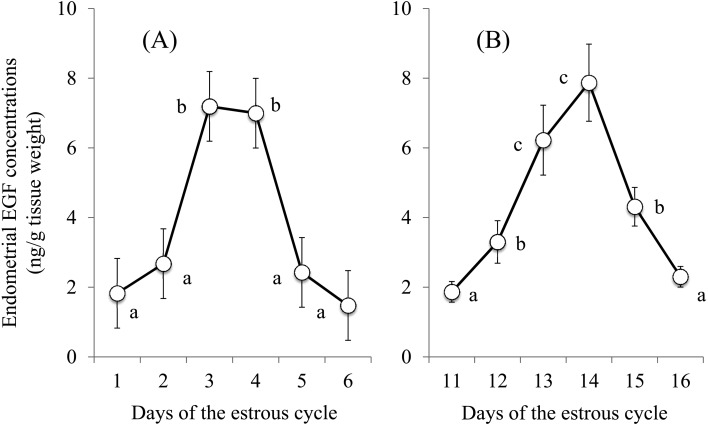
Sixteen cows (80.0%) exhibited increased EGF concentrations (≥ 4.7 ng/g tissue weight) for 2 or 3 consecutive days between Days 2 and 5 (Table 1). Among these 16 cows, 3, 16, 14, and 3 cows exhibited increased EGF levels on Days 2, 3, 4, and 5, respectively. Four cows exhibited no increase (peak) in EGF concentrations (Table 1). The EGF concentrations remained at low levels (≥ 2.5 ng/g tissue weight) from Days 1 to 6, except for a marginal increase on Day 3 in 2 cows (4.4 and 4.5 ng/g tissue weight in Nos. 2 and 11, respectively). As a result, the endometrial EGF concentrations in all 20 cows were increased on Days 3 and 4 compared to that of Days 1, 2, 5, and 6 (P < 0.01) (Fig. 2A).
Table 1. Increased endometrial EGF concentrations between Days 1 and 6 of the estrous cycle in dairy cows a.
| Cow No. | Days after estrus |
Pregnancy |
||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 1st AI | 2nd AI | |
| 1 | – | + | + | – | – | – | + | NA |
| 2 | – | – | – | – | – | – | − | − |
| 3 | – | – | – | – | – | – | − | − |
| 4 | – | – | + | + | + | – | + | NA |
| 5 | – | – | + | + | – | – | + | NA |
| 6 | – | – | + | + | – | – | + | NA |
| 7 | – | – | + | + | – | – | − | + |
| 8 | – | – | + | + | – | – | − | − |
| 9 | – | – | + | + | + | – | − | + |
| 10 | – | – | + | + | – | – | − | + |
| 11 | – | – | – | – | – | – | − | − |
| 12 | – | – | + | + | – | – | + | NA |
| 13 | – | – | + | + | – | – | − | − |
| 14 | – | – | + | + | – | – | − | + |
| 15 | – | – | – | – | – | – | − | − |
| 16 | – | – | + | + | – | – | ND | ND |
| 17 | – | + | + | – | – | – | ND | ND |
| 18 | – | + | + | + | – | – | ND | ND |
| 19 | – | – | + | + | – | – | ND | ND |
| 20 | – | – | + | + | + | – | ND | ND |
a Endometrial EGF concentrations of ≥ 4.7 ng/g tissue weight (+) were considered as increased levels based on a normal range on Day 3 of the estrous cycle determined using data from 99 fertile cows [3]. NA: not applicable. ND: not determined. Cows Nos. 16–20 were not inseminated in this study. Cows with odd and even numbers were subjected to repeated biopsies from position A to F and F to A, respectively (see Fig. 1).
Fig. 2.
Changes in epidermal growth factor (EGF) concentrations in the uterine endometrial tissues between Days 1 and 6 (A) and between Days 11 and 16 (B) of the estrous cycle in 20 and 10 apparently normal dairy cows, respectively. EGF concentrations with different letters (a, b, c) differ significantly (P < 0.01).
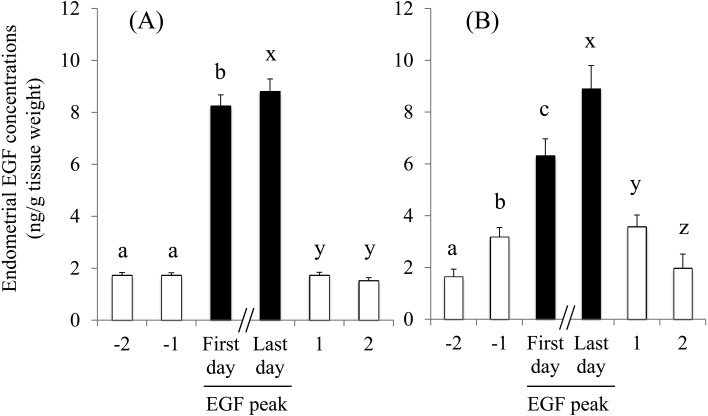
We compared the EGF concentrations 1 and 2 days prior to and on the first day of the first EGF peak in 13 cows (Nos. 4–10, 12–14, 16, 19, and 20) (Fig. 3A). We also compared the EGF concentrations on the last day of the first peak and the following 2 days in 13 cows (Nos. 1, 5–8, 10, 12–14, 16, and 17–19) (Fig. 3A). The EGF concentrations 1 and 2 days prior to the first day of the EGF peak were at equivalent levels but abruptly increased on the following day (the first day of the peak) (P < 0.01). Similarly, the EGF concentrations decreased from peak to basal levels within 1 day and remained at basal levels the following day.
Fig. 3.
Changes in endometrial epidermal growth factor (EGF) concentrations prior to and after the peaks between Days 1 and 6 (A) and Days 11 and 16 (B). Data from 13 and 6 cows in which the increased EGF concentrations were first detected on Days 3 (A) and 13 (B), respectively, were used to compare EGF concentrations 1 and 2 days prior to and on the first day of the EGF peaks. Data from 13 and 4 cows in which EGF concentrations started to decrease prior to or on Days 5 (A) and 15 (B), respectively, were used to compare EGF concentrations on the last day of the peak and the following 2 days. The EGF concentrations with different letters differed significantly (a, b, c, and x, y, z, P < 0.01).
Among 15 cows (No. 1–15) that were inseminated, 9 (60.0%) became pregnant during the study (Table 1). Four of 8 (50.0%) and 5 of 7 (71.4%) cows in which repeated biopsy had been performed from A to F, and F to A (Fig. 1), respectively, became pregnant. None of the 4 cows that exhibited no increase (peak) in EGF concentrations during the 6-day period became pregnant with 2 AIs.
Study 2
Seven cows (70.0%) exhibited an increase in EGF concentrations (≥ 4.9 ng/g tissue weight) for 2 to 3 consecutive days between Days 12 and 15 (Table 2). In these 7 cows, 1, 7, 7, and 3 cows exhibited increased EGF concentrations on Days 12, 13, 14 and 15, respectively. Three cows exhibited no increase in EGF concentrations during the 6-day period. As a result, the EGF concentrations were at the lowest (basal) levels on Day 11 and gradually increased to the highest levels on Days 13 and 14, followed by a gradual decrease to the basal levels on Day 16 (Fig. 2B).
Table 2. Increased endometrial EGF concentrations between Days 11 and 16 of the estrous cycle in dairy cows a.
| Cow No. | Days after estrus |
Pregnancy |
||||||
| 11 | 12 | 13 | 14 | 15 | 16 | 1st AI | 2nd AI | |
| 1 | – | – | + | + | – | – | + | NA |
| 2 | – | – | + | + | + | – | + | NA |
| 3 | – | – | + | + | + | – | − | − |
| 4 | – | + | + | + | – | – | − | + |
| 5 | – | – | + | + | – | – | − | + |
| 6 | – | – | + | + | – | – | + | NA |
| 7 | – | – | – | – | – | – | − | − |
| 8 | – | – | + | + | + | – | − | − |
| 9 | – | – | – | – | – | – | − | − |
| 10 | – | – | – | – | – | – | − | − |
a Endometrial EGF concentrations of ≥ 4.9 ng/g tissue weight (+) were considered as increased levels based on a normal range on Day 14 of the estrous cycle determined using data from 99 fertile cows [3]. NA: not applicable. Cows with odd and even numbers were subjected to repeated biopsies from positions A to F and from positions F to A, respectively (see Fig. 1).
We compared the EGF concentrations 1 and 2 days prior to and following the first day of the EGF peaks in 6 cows (Nos. 1–3, 5, 6, and 8) (Fig. 3B). We also compared the EGF concentrations on the last day of the peak and the following 2 days in 4 cows (Nos. 1 and 4–6) (Fig. 3B). The EGF concentrations 2 days prior to the first day of the peak in 6 cows (Nos. 1–3, 5, 6, and 8) were at the lowest (basal) levels and increased to the highest levels over 2 days, with intermediate EGF levels 1 day prior to the first day of the peak (P < 0.01). Similarly, the EGF concentrations decreased from the highest to the basal levels over 2 days, with intermediate levels of EGF 1 day after the last day of the second peak (P < 0.01).
Five (50.0%) of 10 cows became pregnant during the study period (Table 2). Two of 5 (40.0%) and 3 of 5 (60.0%) cows in which repeated biopsy had been performed from position A to F, and F to A (Fig. 1), respectively, became pregnant during the study. The 3 cows that exhibited no increase (peak) in EGF concentrations during the 6-day period did not become pregnant.
Study 3
In 12 RBs, 4 cows (33.3%) exhibited an increase in EGF concentrations on Days 3 and 4, whereas the remaining 8 cows (66.7%) exhibited no increase (peak) in EGF concentrations between Days 1 and 6 (Table 3). The proportion of RB cows with an increase in EGF concentrations during the 6-day period was smaller than that of apparently normal cows in Study 1 (P < 0.05). The EGF concentrations of 4 cows exhibited an increase in EGF concentrations of 7.1 ± 0.34 and 6.3 ± 0.78 ng/g tissue weight on Days 3 and 4, respectively. Two of 6 cows became pregnant in the study.
Table 3. Increased endometrial EGF concentrations between Days 1 and 6 of the estrous cycle in repeat breeder dairy cows a.
| Cow No. | Days after estrus |
Pregnancy |
||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 1st AI | 2nd AI | |
| 1 | – | – | + | + | – | – | + | NA |
| 2 | – | – | – | – | – | – | − | − |
| 3 | – | – | – | – | – | – | − | + |
| 4 | – | – | + | + | – | – | − | − |
| 5 | – | – | – | – | – | – | − | − |
| 6 | – | – | – | – | – | – | − | − |
| 7 | – | – | + | + | – | – | ND | ND |
| 8 | – | – | – | – | – | – | ND | ND |
| 9 | – | – | – | – | – | – | ND | ND |
| 10 | – | – | + | + | – | – | ND | ND |
| 11 | – | – | – | – | – | – | ND | ND |
| 12 | – | – | – | – | – | – | ND | ND |
a) Endometrial EGF concentrations of ≥ 4.7 ng/g tissue weight (+) were considered as increased levels based on a normal range on Day 3 that was determined by using data from 99 fertile cows [3]. NA: not applicable. ND: not determined. Cows No. 7–12 were not inseminated in the study. Cows with odd and even numbers were subjected to repeated biopsies from position A to F, and F to A, respectively (see Fig. 1).
Discussion
The present study confirmed the presence and timing of the 2 peaks of endometrial EGF concentrations in dairy cows. Our preliminary study [2] approximated the timing of the first peak between Days 2 and 4 of the estrous cycle. In the present study, increased EGF concentrations were observed between Days 2 and 5 in individual cows, and the mean EGF concentrations exhibited an apparent peak on Days 3 and 4. Minor differences in the timing of the first peak found in the 2 studies might not affect the interpretation of our previous findings on the role of altered EGF profiles on reduced fertility in RB and high-yielding dairy cows [1,2,3, 5, 7]. Rather, these results might improve the reliability of our previous studies since these studies used the endometrial EGF concentrations on Day 3 to evaluate the first peak. All of the 16 cows that exhibited a peak in the present study also exhibited increased EGF concentrations on Day 3 (Study 1), indicating that Day 3 is the optimal time for evaluating the first peak of endometrial EGF concentrations. This timing might be compatible with a close relationship between the endometrial EGF concentrations on Day 3 and the fertility observed in our previous studies [4, 8]. For example, 437 cows were examined for endometrial EGF concentrations on Day 3 and a single embryo was transferred on Day 7 of the same cycle. Recipient cows exhibiting EGF concentrations within a normal range on Day 3 also exhibited a greater conception rate (76.9%) than those with reduced EGF concentrations (33.3%) [4].
The present study confirmed the timing (Days 13 and 14) and duration (2 days) of the second peak of EGF concentrations reported in our previous study [2]. In Study 2, the mean concentrations of endometrial EGF exhibited a peak of 2 days on Days 13 and 14. However, peak levels of EGF concentrations were observed between Days 12 and 15 and lasted for 2–3 days in individual cows.
Interestingly, the transition pattern of EGF concentrations differed between the first and second peaks. In the first peak, a transition of EGF concentrations from basal to peak and peak to basal levels occurred within 1 day. During the second peak, these transitions were slower than those of the first peak and both took 2 days. Although most of our knowledge on the regulation of EGF in the uterus was obtained using mice and rats, estrogen is the primary regulator of EGF production in the endometrium [9,10,11,12]. A significant increase in circulating estrogen concentrations and endometrial estrogen receptor levels during estrus in cows [13] should produce a rapid and synchronized transition of the EGF concentrations of the first peak. This was supported by the effect of exogenous estrogen to increase endometrial EGF concentrations in fertile and RB cows [5]. In fertile cows, 1 mg of estradiol benzoate (EB) produced a significant increase in EGF concentrations. In RB cows with low endometrial EGF concentrations on Days 3 and/or 14 of the estrous cycle, EB increased EGF concentrations dose-dependently between 1 and 5 mg, and 5 mg of EB produced an increase of the endometrial EGF concentrations similar to that found in fertile cows treated with 1 mg of EB.
In contrast, the mechanism triggering the second peak remains poorly understood, although increased estrogen activity due to the secretion of estrogen from developing dominant follicles and/or an increase in the ratio of estrogen/progesterone activity from changes in the levels of estrogen and progesterone receptors might be involved. Changes in estrogen receptor α levels in the bovine endometrium have been studied throughout the estrous cycle [13]. The estrogen receptor α protein levels in the uterine luminal epithelium were highest at estrus and during the mid-luteal phase, which occurs between Days 10 and 14, when the second peak of the endometrial EGF concentration initiates. Although the levels of estrogen receptor α in the deep epithelial gland and stroma did not exhibit a clear cyclic change, the receptor concentrations in these regions were high throughout the estrous cycle. Progesterone receptor mRNA was expressed predominantly in the stroma [13, 14] and superficial gland [13]. In the stroma of the bovine uterus, where the EGF protein localizes [15], the levels of progesterone receptor mRNA were the highest between estrus and Day 8 and decreased between Days 8 and 10, reaching the lowest levels during the mid- and late-luteal phases (Days 12 to 16) [13]. The progesterone receptor protein levels in the stroma exhibited a similar but less clear change. The levels were high from estrus to the early luteal phase and decreased to the lowest levels by the late luteal phase. These changes in the concentrations of estrogen and progesterone receptors could increase the ratio of estrogen/progesterone action and enhance EGF production in the endometrium. However, the estrogenic action could increase more slowly, to a lesser extent, and in a less synchronized manner compared to the time of estrus. This effect could partially explain a slower transition in EGF concentrations during the second peak than that of the first peak.
Except for 4 cows, the endometrial EGF concentrations of RB cows exhibiting a 2-day peak between Days 3 and 4 were low throughout the consecutive days from Day 1 to 6 of the estrous cycle (Study 3). In addition, we examined the endometrial EGF concentrations on Day 7 in more than 500 RB cows in our previous studies [2, 3, 5]; no cows exhibited peak levels of EGF concentrations. Together, EGF concentrations might remain low between Days 1 and 7 in affected RB cows, but could also be low in high-yielding cows. Thus, at least for the first peak, an abnormal EGF profile in subfertile cows can be represented by the absence of the peak, but not the altered timing of an increase in EGF concentrations.
Repeated biopsy of endometrial tissues is a method that has been used to study the physiology of the bovine endometrium that includes changes in EGF [2, 3], the IGF system [16, 17], prostaglandin H-synthase [18], collagens [19], and receptors for estrogen, progesterone [13, 17], and oxytocin [13, 20] during the estrous cycle. The technique elicits no or negligible effects on the cyclicity [18,19,20] and fertility [7, 17] of cows. Repeated biopsy allows us to collect endometrial samples daily or more frequently during the estrous cycle in the same animals, significantly reducing the number of animals to be sacrificed for sampling. Furthermore, an enhanced understanding of the endometrial production of regulatory components for uterine function is provided by repeated measurements during a cycle in the same animal [20]. The present study used repeated biopsy for 6 consecutive days and there was no apparent detrimental effect on estrous cyclicity, fertility, and EGF concentrations in all 3 studies. The conception rate at the first AI and the pregnancy rate after the second AI were 33.3 and 60.0% in Study 1 and 30.0 and 50.0% in Study 2. These values were similar to those of herd mates at comparable days postpartum. We used repeated biopsy in 2 different orders based on a technical concern. Biopsies of uterine endometrial tissues may not be technically demanding but the difficulty of the procedure could differ, to some extent, depending on the side of the uterine horn. However, the order of biopsies may not have a significant effect on fertility since cows became pregnant regardless of the order of biopsies during the study; although the numbers of cows subjected to biopsies should be increased for conclusive evidence.
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research (Nos. 23580446, 20580350, and 15580284) from the Japan Society for the Promotion of Science.
References
- 1.Katagiri S, Moriyoshi M, Takahashi Y. Low incidence of an altered endometrial epidermal growth factor (EGF) profile in repeat breeder Holstein heifers and differential effect of parity on the EGF profile between fertile Holstein (dairy) and Japanese Black (beef) cattle. J Reprod Dev 2013; 59: 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katagiri S, Takahashi Y. Changes in EGF concentrations during estrous cycle in bovine endometrium and their alterations in repeat breeder cows. Theriogenology 2004; 62: 103–112. [DOI] [PubMed] [Google Scholar]
- 3.Katagiri S, Takahashi Y. Potential relationship between normalization of endometrial epidermal growth factor profile and restoration of fertility in repeat breeder cows. Anim Reprod Sci 2006; 95: 54–66. [DOI] [PubMed] [Google Scholar]
- 4.Katagiri S. Relationship between endometrial epidermal growth factor and fertility after embryo transfer. J Reprod Dev 2006; 52(Suppl): 133–137. [Google Scholar]
- 5.Katagiri S, Takahashi Y. A progestin-based treatment with a high dose of estradiol benzoate normalizes cyclic changes in endometrial EGF concentrations and restores fertility in repeat breeder cows. J Reprod Dev 2008; 54: 473–479. [DOI] [PubMed] [Google Scholar]
- 6.Katagiri S, Moon YS, Yuen BH. The role for the uterine insulin-like growth factor I in early embryonic loss after superovulation in the rat. Fertil Steril 1996; 65: 426–436. [DOI] [PubMed] [Google Scholar]
- 7.Katagiri S, Takahashi Y. Relationship between endometrial concentrations of epidermal growth factor (EGF) and preimplantation embryo development in dairy cattle. In: The 37th Annual Meeting of Society for the Study of Reproduction; 2004; Vancouver. 518.
- 8.Katagiri S, Moriyoshi M. Alteration of the endometrial EGF profile as a potential mechanism connecting the alterations in the ovarian steroid hormone profile to embryonic loss in repeat breeders and high-producing cows. J Reprod Dev 2013; 59: 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paria BC, Song H, Dey SK. Implantation: molecular basis of embryo-uterine dialogue. Int J Dev Biol 2001; 45: 597–605. [PubMed] [Google Scholar]
- 10.DiAugustine RP, Petrusz P, Bell GI, Brown CF, Korach KS, McLachlan JA, Teng CT. Influence of estrogens on mouse uterine epidermal growth factor precursor protein and messenger ribonucleic acid. Endocrinology 1988; 122: 2355–2363. [DOI] [PubMed] [Google Scholar]
- 11.Huet-Hudson YM, Chakraborty C, De SK, Suzuki Y, Andrews GK, Dey SK. Estrogen regulates the synthesis of epidermal growth factor in mouse uterine epithelial cells. Mol Endocrinol 1990; 4: 510–523. [DOI] [PubMed] [Google Scholar]
- 12.Falck L, Forsberg JG. Immunohistochemical studies on the expression and estrogen dependency of EGF and its receptor and C-fos proto-oncogene in the uterus and vagina of normal and neonatally estrogen-treated mice. Anat Rec 1996; 245: 459–471. [DOI] [PubMed] [Google Scholar]
- 13.Robinson RS, Mann GE, Lamming GE, Wathes DC. Expression of oxytocin, oestrogen and progesterone receptors in uterine biopsy samples throughout the oestrous cycle and early pregnancy in cows. Reproduction 2001; 122: 965–979. [PubMed] [Google Scholar]
- 14.Xiao CW, Goff AK. Hormonal regulation of oestrogen and progesterone receptors in cultured bovine endometrial cells. J Reprod Fertil 1999; 115: 101–109. [DOI] [PubMed] [Google Scholar]
- 15.Ohtani S, Okuda K, Ohtani M, Yamada J. Immunohistochemically-determined changes in the distribution of insulin-like growth factor-I (IGF-I) and epidermal growth factor (EGF) in the bovine endometrium during the estrous cycle. J Vet Med Sci 1996; 58: 1211–1217. [DOI] [PubMed] [Google Scholar]
- 16.Robinson RS, Mann GE, Gadd TS, Lamming GE, Wathes DC. The expression of the IGF system in the bovine uterus throughout the oestrous cycle and early pregnancy. J Endocrinol 2000; 165: 231–243. [DOI] [PubMed] [Google Scholar]
- 17.Meikle A, Sahlin L, Ferraris A, Masironi B, Blanc JE, Rodríguez-Irazoqui M, Rodríguez-Piñón M, Kindahl H, Forsberg M. Endometrial mRNA expression of oestrogen receptor α, progesterone receptor and insulin-like growth factor-I (IGF-I) throughout the bovine oestrous cycle. Anim Reprod Sci 2001; 68: 45–56. [DOI] [PubMed] [Google Scholar]
- 18.Boos A. Immunohistochemical assessment of prostaglandin H-synthase in bovine endometrial biopsy samples collected throughout the oestrous cycle. Anim Reprod Sci 1998; 51: 261–273. [DOI] [PubMed] [Google Scholar]
- 19.Boos A. Immunohistochemical assessment of collagen types I, III, IV and VI in biopsy samples of the bovine uterine wall collected during the oestrous cycle. Cells Tissues Organs 2000; 167: 225–238. [DOI] [PubMed] [Google Scholar]
- 20.Mann GE, Lamming GE. Use of repeated biopsies to monitor endometrial oxytocin receptors in the cow. Vet Rec 1994; 135: 403–405. [DOI] [PubMed] [Google Scholar]