Abstract
Rat oocytes can be produced artificially by superovulation. Because some strains show low sensitivity to superovulation treatment, in vitro maturation is an alternative method to produce numerous matured oocytes. Furthermore, establishment of an in vitro maturation system with simple culture conditions is cost effective and leads to easy handling of oocytes. This study examined developmental ability of rat germinal vesicle (GV) oocytes maturing in vitro under simple culture conditions. Significantly different numbers of ovulated oocytes reached the second metaphase of meiosis (MII) among Jcl:Wistar (17.0), F344/Stm (31.0), and BN/SsNSlc (2.2) rats in whom superovulation was induced by pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin. However, similar numbers of GV oocytes were obtained from ovaries of PMSG-injected Wistar (27.7), F344 (34.7), and BN (24.7) rats. These GV oocytes were cultured in vitro in HTF, αMEM, and a 1:1 HTF + αMEM or TYH + αMEM mixture. High proportions of Wistar and F344 oocytes that matured to MII in αMEM were parthenogenetically activated by strontium chloride treatment (78% and 74%, respectively). Additionally, 10% of matured oocytes of both strains developed into offspring after intracytoplasmic sperm injection and embryo transfer to foster mothers. Although BN oocytes cultured in αMEM could be parthenogenetically activated and developed into offspring, the success rate was lower than that for Wistar and F344 oocytes. This study demonstrated that numerous GV oocytes were produced in rat ovaries by PMSG injection. This simple in vitro maturation system of immature oocytes could be further developed to maintain valuable rat strains experiencing reproductive difficulties.
Keywords: Germinal vesicle oocytes, Intracytoplasmic sperm injection, Second metaphase of meiosis
Embryos are generally produced using oocytes collected from the oviducts of superovulation-induced female laboratory animals. In rats, intraperitoneal injection of pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG) has been used to induce superovulation [1]. It was also reported that the effect of superovulation by PMSG and hCG injection was not dependent on the estrous cycle and age of rats [2, 3]. However, although it is possible to induce superovulation in most rat strains using this method, some strains show negative responses [4].
Numerous variously sized follicles, including oocytes, are contained in the mammalian ovary [5, 6]. Some of these oocytes mature and are then ovulated. In the strains that show negative responses to superovulation, it is important to optimize conditions for the injection of PMSG and hCG to achieve efficient superovulation. The in vitro maturation (IVM) technique [7] can be used as alternative method for producing embryos from such strains. Using this technique, a large number of immature oocytes in antral follicles can mature and be used to produce embryos and offspring. Schroeder and Eppig [8] reported the first mouse offspring obtained from oocytes cultured in vitro. In rat, several IVM studies have been also reported [9,10,11]. It is thought that the addition of growth factors and hormones to the culture medium is required for maturation of mammalian oocytes. The establishment of an IVM technique that uses a simple culture medium without additional factors for supporting oocyte maturation is useful and cost effective. Furthermore, the easy handling of oocytes using a simple IVM technique contributes to storing valuable animal strains, which experience reproductive difficulties, as genetic resources in biobanking. It was reported that rat oocytes collected from ovaries could be matured in MEM medium [12, 13], and these matured oocytes developed into offspring after fertilization [14]. However, no detailed studies addressing the influence of strain and medium components on oocyte maturation, fertilization, and subsequent development have been reported.
This study evaluated the maturation to second metaphase of meiosis (MII) of germinal vesicle (GV) oocytes collected from the ovaries of Wistar, F344, and BN rats using various culture media. Furthermore, the subsequent ability of these IVM oocytes to develop into offspring was studied.
Materials and Methods
Animals
Jcl:Wistar and BN/SsNSlc rats were purchased from CLEA Japan (Tokyo, Japan) and Japan SLC (Shizuoka, Japan), respectively. F344/Stm rats were supplied by the National BioResource Project-Rat, Kyoto University (Kyoto, Japan). All animals were housed in plastic cages in a specific pathogen-free barrier facility that was air-conditioned (temperature = 24 ± 2°C, humidity = 50 ± 10%) and light-controlled (lights on from 0700 h to 1900 h). All animal care and procedures performed in this study conformed to the Guidelines for Animal Experiments of Kyoto University and were approved by the Animal Research Committee of Kyoto University.
Collection and IVM of oocytes
Female Wistar, F344, and BN rats aged 8 to 12 weeks were induced to superovulate by intraperitoneal injection of PMSG (ASKA Pharmaceutical, Tokyo, Japan) at doses of 150, 150, and 300 IU/kg, respectively, at 1600–1700 h, followed by intraperitoneal injection of hCG (ASKA Pharmaceutical) at doses of 75, 75, and 300 IU/kg, respectively, 48 h later [15, 16]. The doses of PMSG and hCG injected to each strain were optimized by our preliminary experiments. Cumulus-oocyte complexes were collected from the oviducts at 16–18 h after hCG injection. Oocytes were freed from cumulus cells by treatment with 0.1% hyaluronidase in PB1 medium [17] for 5 min. Oocytes that reached MII were then recorded as matured.
Female Wistar, F344, and BN rats aged 8 to 13 weeks were induced to superovulate by intraperitoneal injection of PMSG at doses of 150, 150, and 300 IU/kg, respectively, at 1600–1700 h. Forty-eight hours after PMSG injection, ovaries were removed from the females and washed in PB1 medium. Antral follicles of the ovarian cortex were cut with a steel needle, and oocyte-granulosa cell complexes (OGCs) were collected in PB1 medium. After washing twice in PB1 medium, 10–20 OGCs were cultured in vitro in 50 μl drops of HTF, αMEM (Thermo Fisher Scientific, Waltham, MA, USA), a 1:1 mixture of HTF and αMEM (HTF + αMEM), or a 1:1 mixture of TYH and αMEM (TYH + αMEM ) [18]. All media were supplemented with 3 mg/ml bovine serum albumin. Oocytes were cultured for 16 h in 5% CO2 and humidified air at 37.5°C. The in vivo matured MII oocytes that were collected from oviducts of females at estrous stage without superovulation were used as control.
Parthenogenetic activation of oocytes after IVM
After culturing in vitro, artificial parthenogenetic activation was carried out with oocytes that reached MII. Oocytes were physically denuded with a glass capillary pipette. Oocytes were then cultured in HTF supplemented with 10 mM strontium chloride (SrCl2; Sigma-Aldrich, St. Louis, MO, USA) for 21 h in 5% CO2 and humidified air at 37.5°C [19]. After culturing, the oocytes that contained a distinct pronucleus were counted.
IVM oocyte fertilization
The developmental ability of oocytes that matured in vitro in various culture media was estimated by fertilization with sperm using intracytoplasmic sperm injection (ICSI) [16, 20]. Sperm that were cryopreserved in Tris-EDTA (TE) buffer (10 mM Tris and 1 mM ethylenediaminetetraacetic acid; Thermo Fisher Scientific/Ambion, Waltham, MA, USA) were used in this study [21]. Sperm were thawed and then sonicated for 5 sec using an ultrasonic cell disrupter (VP-050, TAITEC, Saitama, Japan) to separate the sperm head from the tail. A small volume (1–2 μl) of the sperm suspension was thoroughly mixed with a 5 μl drop of HEPES-mR1ECM [20] supplemented with 12% (w/v) polyvinylpyrrolidone (PVP, MW = 360.000; ICN Pharmaceuticals, Costa Mesa, CA, USA). A single, normally shaped sperm head was hung on the tip of an injection pipette. The sperm head was then immediately injected into the oocytes in HEPES-mR1ECM. After sperm injection, the oocytes were cultured in modified Krebs-Ringer bicarbonate medium [22] in 5% CO2 and humidified air at 37.5°C.
Embryo transfer
After 20 h of culturing, pronuclear- and 2-cell-stage embryos were transferred into the oviducts of female Jcl:Wistar rats that had been mated with vasectomized male rats of the same strain on the day before the transfer. Three or four females were used in each experiment. The numbers of implantation sites and offspring were assessed after 19 to 21 days of gestation.
Statistical analysis
The resulting data were analyzed using Tukey-Kramer method and chi-square test followed by multiple comparison using Ryan’s method.
Results
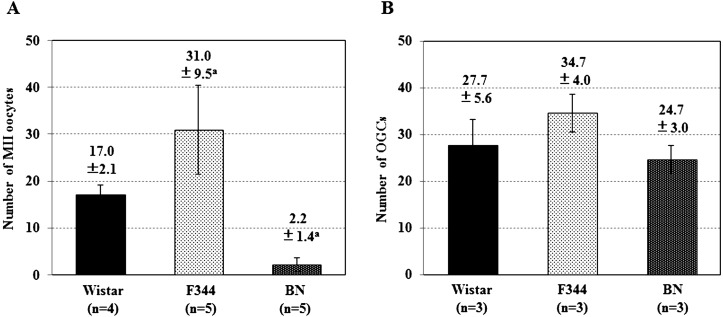
The number of MII oocytes that ovulated by injection of PMSG and hCG are shown in Fig. 1A. The number of MII oocytes collected from Wistar and F344 rats were 17.0 and 31.0, respectively. The number of oocytes collected from BN rats (2.2) was significantly lower than that of F344 rats. However, similar numbers of OGCs were obtained from PMSG-injected Wistar (27.7), F344 (34.7), and BN (24.7) rats (Fig. 1B).
Fig. 1.
(A) Number of MII oocytes collected from oviducts of females that were superovulated by injection of PMSG and hCG. (B) Number of OGCs collected from ovaries of females 48 h after injection of PMSG. All data shown represent the mean ± SE. a Significantly different at P < 0.05 (Tukey-Kramer method).
Table 1 shows the developmental ability of Wistar oocytes collected from ovaries of PMSG-injected females after culturing in vitro in various media. Five females were used in each experiment. The percentage of oocytes that were pronuclear upon activation with SrCl2 was significantly higher after they were cultured in αMEM (78%) and TYH + αMEM (75%) compared with those that were cultured in HTF (34%) and HTF + αMEM (54%). There was no significant difference in the percentage of activated oocytes among the oocytes cultured in αMEM or TYH + αMEM and those that matured in vivo (91%).
Table 1. Developmental ability of Wistar oocytes matured in vitro in various culture media.
| Culture media | No. of oocytes collected from ovaries |
No. (%) of oocytes matured to MII |
No. of MII oocytes activated |
No. (%) of oocytes after activation |
||
| Pronuclear stage | MII stage (non-activated) |
Degenerated and fragmented |
||||
| In vivo | - | - | 32 | 29 (91) c | 3 (9) | 0 (0) |
| HTF | 109 | 100 (92) a | 100 | 34 (34) d | 50 (50) | 16 (16) |
| αMEM | 137 | 110 (80) b | 110 | 86 (78) e | 17 (15) | 7 (6) |
| HTF + αMEM | 183 | 169 (92) a | 169 | 92 (54) f | 63 (37) | 14 (8) |
| TYH + αMEM | 168 | 142 (85) | 142 | 107 (75) g | 27 (19) | 8 (6) |
The percentages of oocytes that matured to MII and those that formed a pronucleus after activation were statistically analyzed. P < 0.05: a vs. b; c vs. d and f; d vs. e, f, and g; e vs. f; f vs. g (chi-square test followed by multiple comparison using Ryan’s method).
The oocytes collected from ovaries of PMSG-injected female F344 rats were cultured in vitro in αMEM and TYH + αMEM (Table 2). Three females were used in each experiment. The percentage of activated oocytes was not significantly different among the oocytes that were cultured in αMEM (74%) or TYH + αMEM (78%) and those that matured in vivo (68%). Table 3 shows the developmental ability of BN oocytes collected from ovaries of PMSG-injected females after culturing in vitro in αMEM and TYH + αMEM. Three females were used in each experiment. The results also showed that there was no significant difference in the percentage of oocytes that were pronuclear among the oocytes cultured in αMEM (30%) or TYH + αMEM (23%) and those that matured in vivo (46%).
Table 2. Developmental ability of F344 oocytes matured in vitro in various culture media.
| Culture media | No. of oocytes collected from ovaries |
No. (%) of oocytes matured to MII |
No. of MII oocytes activated |
No. (%) of oocytes after activation |
||
| Pronuclear stage | MII stage (non-activated) |
Degenerated and fragmented |
||||
| In vivo | - | - | 47 | 32 (68) | 1 (2) | 14 (30) |
| αMEM | 97 | 69 (71) | 69 | 51 (74) | 9 (13) | 9 (13) |
| TYH + αMEM | 102 | 64 (63) | 64 | 50 (78) | 6 (9) | 8 (13) |
Table 3. Developmental ability of BN oocytes matured in vitro in various culture media.
| Culture media | No. of oocytes collected from ovaries |
No. (%) of oocytes matured to MII |
No. of MII oocytes activated |
No. (%) of oocytes after activation |
||
| Pronuclear stage | MII stage (non-activated) |
Degenerated and fragmented |
||||
| In vivo | - | - | 28 | 13 (46) | 2 (7) | 13 (46) |
| αMEM | 128 | 92 (72) | 92 | 28 (30) | 63 (68) | 1 (1) |
| TYH + αMEM | 141 | 94 (67) | 94 | 22 (23) | 69 (73) | 3 (3) |
The oocytes from each strain that were cultured in vitro were fertilized with sperm from the same strain. The fertilized oocytes were then transferred to foster mothers. Offspring were obtained from fertilized Wistar oocytes that matured in all media types (Table 4). The percentage of fertilized oocytes matured in αMEM that developed into offspring (10%) was high. Additionally, there was no significant difference in the development into offspring of oocytes that matured in various media and of those that matured in vivo (9%). Offspring were also obtained from fertilized F344 oocytes after maturing in different media (Table 5). There was no significant difference in the development into offspring of fertilized oocytes after maturing in αMEM (10%) and of those that matured in vivo (23%). However, the development into offspring of fertilized oocytes after maturing in TYH + αMEM (6%) was significantly lower than those that matured in vivo or in αMEM. Furthermore, as shown in Table 6, only 2% of BN oocytes matured in αMEM and TYH + αMEM developed into offspring.
Table 4. Development into offspring of Wistar IVM oocytes after fertilization with sperm.
| Culture media | No. of fertilized oocytes transferred |
No. (%) of embryos implanted |
No. (%) of embryos that developed into offspring |
| In vivo | 54 | 16 (30) | 5 (9) |
| HTF | 73 | 12 (16) | 1 (1) |
| αMEM | 72 | 20 (28) a | 7 (10) |
| HTF + αMEM | 80 | 21 (26) | 4 (5) |
| TYH + αMEM | 116 | 13 (11) a | 4 (3) |
a Significantly different at P < 0.05 (chi-square test followed by multiple comparison using Ryan’s method).
Table 5. Development into offspring of F344 IVM oocytes after fertilization with sperm.
| Culture media | No. of fertilized oocytes transferred |
No. (%) of embryos implanted |
No. (%) of embryos that developed into offspring |
| In vivo | 52 | 20 (38) | 12 (23) a |
| αMEM | 96 | 21 (22) | 10 (10) |
| TYH + αMEM | 67 | 15 (22) | 4 (6) a |
a Significantly different at P < 0.05 (chi-square test followed by multiple comparison using Ryan’s method).
Table 6. Development into offspring of BN IVM oocytes after fertilization with sperm.
| Culture media | No. of fertilized oocytes transferred |
No. (%) of embryos implanted |
No. (%) of embryos that developed into offspring |
| In vivo | ND | ND | ND |
| αMEM | 110 | 14 (13) | 2 (2) |
| TYH + αMEM | 61 | 10 (16) | 1 (2) |
ND: No data. The number of oocytes that ovulated after superovulation was too low for fertilization.
Discussion
This study evaluated the developmental ability of immature rat oocytes under simple in vitro culture conditions. It has been reported that superovulation in Wistar and F344 rats can be induced by injecting PMSG and hCG [1, 15]. On the contrary, it is empirically known that the number of BN oocytes that ovulated by induction of superovulation is extremely low. Indeed, the number of MII oocytes that ovulated after injection of PMSG and hCG was significantly different between the F344 and BN rats (Fig. 1A). However, a similar number of OGCs was obtained from the ovaries after injection of PMSG among Wistar, F344, and BN rats (Fig. 1B). The results of this study indicate that a large number of follicles, including oocytes, were also present in BN rat ovaries [5, 6]. The development of these follicles was stimulated by injection of PMSG. In this study, the sensitivity of BN rats to hCG injection at 48 h after PMSG injection was extremely low. Although it has been reported that the effect of superovulation by PMSG and hCG injection was not dependent on the estrous cycle and age of rats [2, 3], a detailed study on such dependence to different factors is required in BN rats. Kon et al. [3] injected hCG to females at 55 h after PMSG injection by changing time interval of hCG injection. Synchronizing the hCG injection to the endogenous luteinizing hormone surge might improve the ovulation of oocytes [23, 24].
OGCs collected from ovaries of Wistar rats after injection of PMSG were cultured in vitro in HTF, αMEM, HTF + αMEM, and TYH + αMEM. A high proportion of oocytes matured to MII in all media. The percentage of oocytes that were parthenogenetically activated was high in αMEM and TYH + αMEM (Table 1), which suggests that αMEM and the addition of αMEM to other culture media strongly support the in vitro maturation of oocytes. Furthermore, the percentage of oocytes that were parthenogenetically activated after IVM showed no significant difference compared with the oocytes that matured in vivo. However, our result shows that the components of HTF were insufficient for IVM of immature rat oocytes. Similar results were also obtained in the F344 rats (Table 2). van de Sandt et al. studied the fertilization and subsequent development to offspring of mouse oocytes that matured in various culture media and found that the media used for IVM of mouse oocytes affect the subsequent development of the oocytes [25]. Our present study also showed that the maturation of rat oocytes was affected by the components of culture medium.
The development into offspring of Wistar oocytes after culturing in vitro in αMEM was high and was not significantly different compared with that of the oocytes that matured in vivo (Table 4). Although the oocytes that were cultured in vitro in αMEM and TYH + αMEM were successfully parthenogenetically activated, the percentage of development into offspring of oocytes cultured in TYH + αMEM was lower than that of oocytes cultured in αMEM (Tables 1 and 4). Similar results were also obtained in the F344 rats (Tables 2 and 5). These results indicate that αMEM strongly promotes the maturation of rat oocytes. In the maturation of oocytes using TYH + αMEM, germinal vesicle breakdown, polar body formation, and parthenogenetic activation occurred normally (Tables 1 and 2). However, the development into offspring of the oocytes that underwent IVM after fertilization was not successful (Tables 4 and 5). This may be caused by the incomplete cytoplasmic maturation of the oocyte [26]. Miki et al. reported that mouse cumulus-free oocytes could mature in vitro in αMEM and TYH + αMEM and that the development of oocytes cultured in TYH + αMEM was slightly greater than those cultured in αMEM [18]. It is possible that various factors, such as differences in species and the interaction between granulosa and cumulus cells, caused this discrepancy in results. In this study, rat GV oocytes were cultured with the enclosing granulosa and cumulus cells. Cumulus cells are beneficial for maturation of oocyte cytoplasm, fertilization, and subsequent development [14]. It is thought that the addition of growth factors and hormones to the culture medium are required for maturation of mammalian oocyte. However, Vanderhyden and Armstrong [9] reported that the addition of follicle-stimulating hormone at low concentrations (1 to 10 ng/ml) to the maturation media decreased the fertility of rat oocytes. Although suitable culturing conditions for IVM may vary for each species, IVM of oocytes without the addition of growth factors and hormones is useful and cost effective. Furthermore, the easy handling of oocytes using a simple IVM technique contributes to storing valuable animal strains, which experience reproductive difficulties, as genetic resources in biobanking.
The results of this study showed that the development into offspring of in vitro matured BN oocytes was low (Table 6). It was thought that BN oocytes could not be fully matured in the culture media used in this study [26]. However, the number of oocytes collected from ovaries after injection of PMSG was significantly higher than the number of oocytes that ovulated by induction of superovulation (Fig. 1). BN rats are a valuable strain, and the sequence of over 90% of its genome was reported [27]. Further improvement of the IVM technique could lead to efficient production of BN rats and various other animals that experience reproductive difficulties.
Acknowledgments
We are thankful to the National BioResource Project - Rat (http://www.anim.med.kyoto-u.ac.jp/NBR/) for providing rat strains (F344/Stm). This work was supported in part by a Grant-in-Aid for Scientific Research from JSPS.
References
- 1.Mukumoto S, Mori K, Ishikawa H. Efficient induction of superovulation in adult rats by PMSG and hCG. Exp Anim 1995; 44: 111–118. [DOI] [PubMed] [Google Scholar]
- 2.Sotomaru Y, Kamisako T, Hioki K. Estrous stage- and animal age-independent superovulation in the BrlHan:WIST@Jcl(GALAS) rat. Exp Anim 2005; 54: 137–141. [DOI] [PubMed] [Google Scholar]
- 3.Kon H, Tohei A, Hokao R, Shinoda M. Estrous cycle stage-independent treatment of PMSG and hCG can induce superovulation in adult Wistar-Imamichi rats. Exp Anim 2005; 54: 185–187. [DOI] [PubMed] [Google Scholar]
- 4.Corbin TJ, McCabe JG. Strain variation of immature female rats in response to various superovulatory hormone preparations and routes of administration. Contemp Top Lab Anim Sci 2002; 41: 18–23. [PubMed] [Google Scholar]
- 5.Miyano T. Bringing up small oocytes to eggs in pigs and cows. Theriogenology 2003; 59: 61–72. [DOI] [PubMed] [Google Scholar]
- 6.Miyano T. JSAR Outstanding Research Award. In vitro growth of mammalian oocytes. J Reprod Dev 2005; 51: 169–176. [DOI] [PubMed] [Google Scholar]
- 7.Eppig JJ, O’Brien M, Wigglesworth K. Mammalian oocyte growth and development in vitro. Mol Reprod Dev 1996; 44: 260–273. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder AC, Eppig JJ. The developmental capacity of mouse oocytes that matured spontaneously in vitro is normal. Dev Biol 1984; 102: 493–497. [DOI] [PubMed] [Google Scholar]
- 9.Vanderhyden BC, Armstrong DT. Effects of gonadotropins and granulosa cell secretions on the maturation and fertilization of rat oocytes in vitro. Mol Reprod Dev 1990; 26: 337–346. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong DT, Zhang X, Vanderhyden BC, Khamsi F. Hormonal actions during oocyte maturation influence fertilization and early embryonic development. Ann N Y Acad Sci 1991; 626: 137–158. [DOI] [PubMed] [Google Scholar]
- 11.Vanderhyden BC. Species differences in the regulation of cumulus expansion by an oocyte-secreted factor(s). J Reprod Fertil 1993; 98: 219–227. [DOI] [PubMed] [Google Scholar]
- 12.Daniel SA, Armstrong DT, Gore-Langton RE. Growth and development of rat oocytes in vitro. Gamete Res 1989; 24: 109–121. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Dorland M, Taverne MA, Van Der Weijden GC, Bevers MM, Van Den Hurk R. In vitro culture of rat pre-antral follicles with emphasis on follicular interactions. Mol Reprod Dev 2000; 55: 65–74. [DOI] [PubMed] [Google Scholar]
- 14.Vanderhyden BC, Armstrong DT. Role of cumulus cells and serum on the in vitro maturation, fertilization, and subsequent development of rat oocytes. Biol Reprod 1989; 40: 720–728. [DOI] [PubMed] [Google Scholar]
- 15.Taketsuru H, Kaneko T. Efficient collection and cryopreservation of embryos in F344 strain inbred rats. Cryobiology 2013; 67: 230–234. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko T. Simple sperm preservation by freeze-drying for conserving animal strains. In: Shondra M (ed.), Methods in Molecular Biology 1239. New York: Springer; 2015: 317–329. [DOI] [PubMed]
- 17.Whittingham DG. Embryo banks in the future of developmental genetics. Genetics 1974; 78: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miki H, Ogonuki N, Inoue K, Baba T, Ogura A. Improvement of cumulus-free oocyte maturation in vitro and its application to microinsemination with primary spermatocytes in mice. J Reprod Dev 2006; 52: 239–248. [DOI] [PubMed] [Google Scholar]
- 19.Krivokharchenko A, Popova E, Zaitseva I, Vil’ianovich L, Ganten D, Bader M. Development of parthenogenetic rat embryos. Biol Reprod 2003; 68: 829–836. [DOI] [PubMed] [Google Scholar]
- 20.Hirabayashi M, Kato M, Aoto T, Sekimoto A, Ueda M, Miyoshi I, Kasai N, Hochi S. Offspring derived from intracytoplasmic injection of transgenic rat sperm. Transgenic Res 2002; 11: 221–228. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko T, Kimura S, Nakagata N. Offspring derived from oocytes injected with rat sperm, frozen or freeze-dried without cryoprotection. Theriogenology 2007; 68: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 22.Toyoda Y, Chang MC. Fertilization of rat eggs in vitro by epididymal spermatozoa and the development of eggs following transfer. J Reprod Fertil 1974; 36: 9–22. [DOI] [PubMed] [Google Scholar]
- 23.Ishibashi I. Number of ovulation, fertilization and early development of ova in adult rats following treatment with various doses of PMSG and hCG. Jpn J Anim Reprod 1983; 29: 1–7. [Google Scholar]
- 24.Kon H, Hokao R, Shinoda M. Fertilizability of Superovulated Eggs by Estrous Stage-independent PMSG/hCG Treatment in Adult Wistar-Imamichi Rats. Exp Anim 2014; 63: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Sandt JJ, Schroeder AC, Eppig JJ. Culture media for mouse oocyte maturation affect subsequent embryonic development. Mol Reprod Dev 1990; 25: 164–171. [DOI] [PubMed] [Google Scholar]
- 26.Thibault C. Hammond Memorial Lecture. Are follicular maturation and oocyte maturation independent processes? J Reprod Fertil 1977; 51: 1–15. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs RA, Weinstock GM, Metzker ML, Muzny DM, Sodergren EJ, Scherer S, Scott G, Steffen D, Worley KC, Burch PE, Okwuonu G, Hines S, Lewis L, DeRamo C, Delgado O, Dugan-Rocha S, Miner G, Morgan M, Hawes A, Gill R, Celera, Holt RA, Adams MD, Amanatides PG, Baden-Tillson H, Barnstead M, Chin S, Evans CA, Ferriera S, Fosler C, Glodek A, Gu Z, Jennings D, Kraft CL, Nguyen T, Pfannkoch CM, Sitter C, Sutton GG, Venter JC, Woodage T, Smith D, Lee HM, Gustafson E, Cahill P, Kana A, Doucette-Stamm L, Weinstock K, Fechtel K, Weiss RB, Dunn DM, Green ED, Blakesley RW, Bouffard GG, De Jong PJ, Osoegawa K, Zhu B, Marra M, Schein J, Bosdet I, Fjell C, Jones S, Krzywinski M, Mathewson C, Siddiqui A, Wye N, McPherson J, Zhao S, Fraser CM, Shetty J, Shatsman S, Geer K, Chen Y, Abramzon S, Nierman WC, Havlak PH, Chen R, Durbin KJ, Egan A, Ren Y, Song XZ, Li B, Liu Y, Qin X, Cawley S, Worley KC, Cooney AJ, D’Souza LM, Martin K, Wu JQ, Gonzalez-Garay ML, Jackson AR, Kalafus KJ, McLeod MP, Milosavljevic A, Virk D, Volkov A, Wheeler DA, Zhang Z, Bailey JA, Eichler EE, Tuzun E, Birney E, Mongin E, Ureta-Vidal A, Woodwark C, Zdobnov E, Bork P, Suyama M, Torrents D, Alexandersson M, Trask BJ, Young JM, Huang H, Wang H, Xing H, Daniels S, Gietzen D, Schmidt J, Stevens K, Vitt U, Wingrove J, Camara F, Mar Albà M, Abril JF, Guigo R, Smit A, Dubchak I, Rubin EM, Couronne O, Poliakov A, Hübner N, Ganten D, Goesele C, Hummel O, Kreitler T, Lee YA, Monti J, Schulz H, Zimdahl H, Himmelbauer H, Lehrach H, Jacob HJ, Bromberg S, Gullings-Handley J, Jensen-Seaman MI, Kwitek AE, Lazar J, Pasko D, Tonellato PJ, Twigger S, Ponting CP, Duarte JM, Rice S, Goodstadt L, Beatson SA, Emes RD, Winter EE, Webber C, Brandt P, Nyakatura G, Adetobi M, Chiaromonte F, Elnitski L, Eswara P, Hardison RC, Hou M, Kolbe D, Makova K, Miller W, Nekrutenko A, Riemer C, Schwartz S, Taylor J, Yang S, Zhang Y, Lindpaintner K, Andrews TD, Caccamo M, Clamp M, Clarke L, Curwen V, Durbin R, Eyras E, Searle SM, Cooper GM, Batzoglou S, Brudno M, Sidow A, Stone EA, Venter JC, Payseur BA, Bourque G, López-Otín C, Puente XS, Chakrabarti K, Chatterji S, Dewey C, Pachter L, Bray N, Yap VB, Caspi A, Tesler G, Pevzner PA, Haussler D, Roskin KM, Baertsch R, Clawson H, Furey TS, Hinrichs AS, Karolchik D, Kent WJ, Rosenbloom KR, Trumbower H, Weirauch M, Cooper DN, Stenson PD, Ma B, Brent M, Arumugam M, Shteynberg D, Copley RR, Taylor MS, Riethman H, Mudunuri U, Peterson J, Guyer M, Felsenfeld A, Old S, Mockrin S, Collins F, Rat Genome Sequencing Project Consortium. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 2004; 428: 493–521. [DOI] [PubMed] [Google Scholar]