Abstract
Background
Physical activity (PA) during pregnancy has been shown to be associated with several positive effects for mother, fetus, and offspring. Heart rate variability (HRV) is a noninvasive and surrogate marker to determine fetal overall health and the development of fetal autonomic nervous system. In addition, it has been shown to be significantly influenced by maternal behavior. However, the influence of maternal PA on HRV has not yet been systematically reviewed. Therefore, the aim of this systematic review was to assess the influence of regular maternal PA on maternal, fetal or infant HRV.
Methods
A systematic literature search following a priori formulated criteria of studies that examined the influence of regular maternal PA (assessed for a minimum period of 6 weeks) on maternal, fetal or infant HRV was performed in the databases Pubmed and SPORTDiscus. Quality of each study was assessed using the standardized Quality Assessment Tool for Quantitative Studies (QATQS).
Results
Nine articles were included into the present systematic review: two intervention studies, one prospective longitudinal study, and six post-hoc analysis of subsets of the longitudinal study. Of these articles four referred to maternal HRV, five to fetal HRV, and one to infant HRV. The overall global rating for the standardized quality assessment of the articles was moderate to weak. The articles regarding the influence of maternal PA on maternal HRV indicated contrary results. Five of five articles regarding the influence of maternal PA on fetal HRV showed increases of fetal HRV on most parameters depending on maternal PA. The article referring to infant HRV (measured one month postnatal) showed an increased HRV.
Conclusions
Based on the current evidence available, our overall conclusion is that the hypothesis that maternal PA influences maternal HRV cannot be supported, but there is a trend that maternal PA might increase fetal and infant HRV (clinical conclusion). Therefore, we recommend that further, high quality studies addressing the influence of maternal PA on HRV should be performed (methodological conclusion).
Keywords: Pregnancy, Public health, Heart rate variability, Childbirth, Stress, Offspring
Background
During pregnancy, maternal physiological systems progressively adapt to accommodate the increasing demands of fetal growth and development [1]. This includes an increase in cardiovascular and respiratory output, an increase in renal function as well as endocrine and metabolic changes [2–4]. Due to these changes, pregnancy is a ‘stress test’ for the metabolic and physiologic system of women, and can lead to pregnancy-induced complications and adverse health effects. Pregnancy associated conditions such as gestational diabetes, hypertension, mental disorders and maternal obesity have been shown to alter fetal development and increase the risk of diseases in the offspring and the infant’s later life [5–8]. Furthermore, the risk for some of these maternal adverse health effects has been shown to be associated with increased sympathetic activity during gestation [9].
One method to monitor sympathetic and parasympathetic control of the autonomic nervous system (ANS) is heart rate variability (HRV). It describes beat-to-beat variation in cardiac R-R interval. Analyzing these R-R intervals in different frequency and time domains provides information on whether changes in heart rate (HR) are mediated primarily by the sympathetic or parasympathetic arm of the ANS [10, 11]. In adults, increased HRV has been associated with higher aerobic fitness and may therefore lead to possible maternal health benefits [12]. Furthermore, several studies indicated that exercise has positive effects on HRV in both healthy adults and children [13, 14]. With regard to the influence of overweight and obesity (OaO) on HRV, increased BMI has been associated with reduced HRV and increased sympathetic and decreased parasympathetic activity [15–18]. Part of this might be due to reduced physical activity (PA) levels in people with higher BMI, since in OaO adults who were regularly physically active HRV was higher compared to sedentary peers [19].
In healthy pregnant women, sympathetic control also increases with progressing gestation as consequence of physiological adaptations of the maternal body, resulting in an altered HRV compared to healthy non-pregnant women [20]. In the growing fetus, HRV is used as a surrogate marker to determine the development of fetal ANS and overall fetal health [11, 21, 22]. However, fetal HRV is strongly influenced by maternal behavior (e.g. cigarette smoking and alcohol consumption) [23, 24].
Throughout pregnancy, regular PA, which is defined as ‘any bodily movement produced by skeletal muscles that results in energy expenditure’ [25], has been shown to improve maternal cardiovascular and respiratory output, improve insulin resistance and metabolic control, reduce the risk of gestational diabetes mellitus as well as depressive symptoms [26–31]. In non-pregnant women, adult men as well as in children, PA has further been shown to affect cardiovascular autonomic control and HRV [32–35].
Although HRV is a noninvasive method to monitor control of ANS of the mother as well as overall fetal development, the influence of maternal PA on HRV has not yet been systematically reviewed. Therefore, within the present article we systematically reviewed studies that assessed the influence of PA during pregnancy on maternal, fetal or infant HRV. Based on the review, a more definitive conclusion on the influence of maternal PA on maternal, fetal and infant HRV can be obtained. In addition, recommendations for future studies in this field will be formulated.
Methods
Literature search
A systematic literature search of studies that examined the influence of regular maternal PA on maternal, fetal or infant HRV was performed in the databases Pubmed and SPORTDiscus. Therefore, a search term was created that combines ‘physical activity’, ‘pregnancy’, and ‘cardiovascular outcomes’. The final search term for Pubmed can be found in Appendix 1. For the search on SPORTDiscus, this search term was adapted following the SPORTDiscus search guidelines. Since the search term was very specific, no further limits were used to specify search. The search was completed on February 2nd 2016.
Inclusion criteria and selection process
In this systematic review, all studies had to fulfil the following inclusion criteria: (1) assessing the influence of PA during pregnancy on HRV in (a) mother, (b) fetus or (c) infant; (2) dealing with human subjects; (3) dealing with any kind of PA (e.g. aerobic exercise, resistance exercise, yoga, gymnastics, etc.); from a public health point of view, the effects of regular PA and not the effects of acute PA are of interest, consequently (4) PA levels should be assessed for a minimum period of 6 weeks; (5) abstracts had to be available in English, German or Dutch language; (6) only full papers published in peer-reviewed journals were considered. Since the aim of this systematic review is providing an overview of all studies assessing HRV dependent on PA in pregnancy, (7) all study designs were included (e.g. prospective studies, retrospective studies, intervention studies, observational studies etc.) in order to draw an overall conclusion.
Altogether, 124 articles were identified (Fig. 1). After removing three duplicates, 121 articles were screened by abstract and title for possible inclusion. Of these, 108 articles were excluded due to not passing all of the a priori formulated criteria. Finally, full texts of 13 articles were screened [34, 36–47]. Of these, 4 were excluded for specific reasons (Fig. 1) so that nine articles were included into the final systematic review.
Fig. 1.
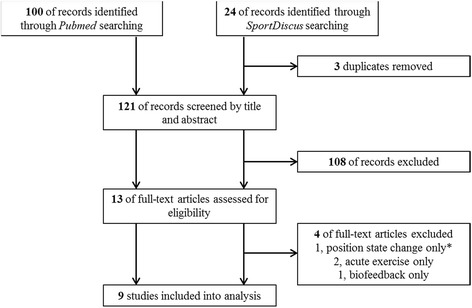
Flow chart of the literature search. *‘Position state change only’ means that the only measured activity was the change from sitting to standing position
Quality assessment and strength of evidence
For assessing the quality of the included articles, the standardized Quality Assessment Tool for Quantitative Studies (QATQS) [48], a well-established and validated tool for assessing the quality of studies in the field of public health research [49, 50], was used. It consists of six components: (1) the extent to which the participants are representative of the target population (selection bias), (2) study design, (3) control of confounding factors, (4) blinding of outcome assessors and participants, (5) reliability and validity of the data-collection tools, and (6) the number of and reasons for withdrawals and drop-outs. The component ‘blinding of outcome assessors and participants’ was considered not applicable for observational and intervention studies in this review. The reason for considering ‘blinding’ not applicable for intervention studies in this case is that in studies with PA intervention the assessors (i.e. researchers) and the participants are very likely to know the outcome of the randomization [51]. According to standardized criteria, each component (1-6) of each article was rated as ‘strong’, ‘moderate’ or ‘weak’. Finally, an overall rating was calculated on the basis of the individual ratings also following standardized criteria: the overall rating was ‘strong’, when there was no ‘weak’ component rating, ‘moderate’ when there was only one ‘weak component rating, and ‘weak’ when there were two or more ‘weak’ component ratings. A detailed description of, as well as the criteria for, the six component ratings can be found online [48].
Data extraction
The systematic literature search using the above described search terms as well as the a priori formulated inclusion criteria has been performed by PD and was checked by a second researcher (ST). Of the nine included articles, all relevant data were extracted by PD following a commonly used table format including study design, sample and subjects, methods, outcomes, statistics. Extracted data were checked by two researchers (EW, MvP). Quality assessment for each study using the QATQS has been performed by two researchers independently (PD, MS). When researches came to different results, these points were discussed within the research team to come to a consensus.
Results
Study descriptions
Nine articles were included into the present systematic review of which three refer to maternal HRV, four to fetal HRV, one to both maternal and fetal HRV, and one to infant HRV. In summary, four articles provide results on maternal HRV, five on fetal HRV, and one on infant HRV. Of these, two articles were intervention studies [36, 43]. The intervention study performed by Stutzman et al. [43] assessed the effects of low intensity exercise in normal weight (NW) and overweight and obese (OaO) pregnant women on maternal HRV. The intervention study performed by Satyapriya et al. [36] assessed the effect of yoga specifically designed for the second and third trimester of pregnancy, compared to standard prenatal exercise (intensity not specified) on maternal HRV. One article was a prospective longitudinal study which was designed to determine the influence of self-reported maternal exercise on fetal cardiac ANS development [40]. To assess self-reported maternal exercise, the authors used the ‘Modifiable Physical Activity Questionnaire’ (MPAQ). Finally, the remaining six articles [37–39, 41, 42, 47] were post-hoc analyses of subsets of the before mentioned longitudinal study of May et al. [40]. Four of these post-hoc analyses referred to maternal or fetal HRV measurements performed in gestational week 36, one to maternal HRV measurements performed at 28, 32, and 36 weeks of gestation, and one to infant HRV measurements at one month of age. The different HRV parameters assessed in the nine studies as well as their physiological association are summarized and described in Table 1. The cohort and study characteristics are summarized in Table 2.
Table 1.
HRV parameters and their physiological association
| Parameter | Abbreviation | Physiological association |
|---|---|---|
| Time domain | ||
| Standard deviation of NN intervals | SDNN | Overall HRV, sympathetic and parasympathetic innervation |
| Root mean square of successive difference between NN intervals | RMSSD | Short-term HRV, primarily parasympathetic innervation |
| Frequency domain | ||
| Total power | TP | Total HRV (as the band encompasses all frequencies) |
| High frequency | HF | Parasympathetic control |
| Ratio high frequency and total power | HF/TP | Parasympathetic control |
| Intermediate frequency | IntF | Sympathetic and parasympathetic arm of the ANS |
| Low frequency | LF | Sympathetic and parasympathetic arm of the ANS |
| Very low frequency | VLF | Sympathetic control |
| Ratio very low frequency and high frequency | VLF/HF | Sympatho-vagal balance |
| Ratio very low frequency and low frequency | VLF/LF | Sympatho-vagal balance |
| Ratio low frequency and high frequency | LF/HF | Sympatho-vagal balance |
(ANS autonomic nervous system, NN normal-to-normal)
Table 2.
Cohort and study characteristics
| First author, year | Study design | Sample (pregnant women) | Intervention, exercise mode, PA determination | HRV outcomes (maternal/fetal/infant) | HRV measure technique | Time(s) of measurement | Data analysis |
|---|---|---|---|---|---|---|---|
| May, [47] | Post-hoc analysis of maternal magnetocardiograms (May et al. [40]) to assess maternal heart measures depending on maternal exercise behavior (exercise vs. control group) at different weeks of gestation |
N = 56 (gw36 N = 51); 20–39 years singleton, low-risk pregnancies |
MPAQ to retrospectively categorize women into exercise (≥30 min. aerobic exercise/ 3 times a week) and control groupa | maternal M: SDNN, RMSSD, VLF, LF, HF, LF/HF |
3 continuous, 18 min simultaneous fetal-maternal MCG were recorded for each subject using an investigational 83-channel dedicated fetal biomagnetometer | Gw28, gw32, and gw36 | Students t-test and Wilcoxon rank sum test to contrast variables between exercise and control groups |
| Van Leeuwen, [41] | Post-hoc analysis of fetal-maternal magnetocardiograms (May et al. [40]) to assess maternal and fetal heart measures depending on maternal exercise behavior (exercise vs. control group) |
N = 40; 20–35 years singleton, low-risk pregnancies |
MPAQ to retrospectively categorize women into exercise (≥ 30 min. of moderate to vigorous aerobic exercise/ 3 times a week; n = 21) and control group (n = 19)a | maternal, fetal M: SDNN, RMSSD F: SDNN, RMSSD |
A continuous, 18 min simultaneous fetal-maternal MCG was recorded for each subject using an investigational 83-channel dedicated fetal biomagnetometer | Gw36 | Comparison between control and exercise groups using Mann-Whitney-U test |
| May, Suminski, [37] | Post-hoc analysis of fetal magnetocardiograms (May et al. [40]) to assess fetal heart measures depending on the duration of maternal continuous and non-continuous leisure-time physical activity |
N = 40; 23–39 years singleton, low-risk pregnancies |
MPAQ to retrospectively classify whether the women perform continuous (e.g., walking, jogging) or non-continuous (e.g. weight lifting, yoga)a leisure-time physical activity | fetal F: SDNN, RMSSD, VLF, LF, HF |
A continuous, 18 min simultaneous fetal-maternal MCG was recorded for each subject using an investigational 83-channel dedicated fetal biomagnetometer | Gw36 | Pearson Product Monument correlation to assess relationships between duration (min) of maternal continuous and non-continuous LTPA and fetal heart measures; Multiple regression analyses to predict fetal heart measures |
| May, Scholtz, [39] | Post-hoc analysis of a subset of infants from a prospective longitudinal pregnancy study (May et al. [40]) to assess infant heart measures depending on maternal exercise behavior (exercise vs. control) | N = 43; 20–35 years, singleton, low-risk pregnancies | MPAQ to retrospectively categorize infants of women who were in the exercise (≥ 30 min. of moderate to vigorous aerobic exercise/ 3 times a week; n = 16) and control group (n = 27)a | infant I: SDNN, RMSSD, LF, HF |
A continuous, 18 min simultaneous fetal-maternal MCG was recorded for each subject using an investigational 83-channel dedicated fetal biomagnetometer | One month of age | Student´s t-tests to compare infant HRV measures between exercise and control group |
| Gustafson, [42] | Post-hoc analysis of fetal magnetocardiograms (May et al. [40]) to assess fetal heart measures depending on maternal exercise (exercise vs. control) | N = 30; 20–35 years, singleton, low-risk pregnancies | MPAQ to retrospectively categorize women into exercise (≥ 30 min. of moderate to vigorous aerobic exercise/ 3 times a week; n = 15) and control group (n = 15)a | fetal F: RMSSD, VLF, LF, LF/HF, VLF/LF, VLF/HF, INT |
A continuous, 18 min simultaneous fetal-maternal MCG was recorded for each subject using an investigational 83-channel dedicated fetal biomagnetometer | Gw36 | Mixed-effects models with post-hoc comparisons. Comparisons for exercise vs. control were adjusted for breathing patterns (breathing vs. apnea) |
| May, [38] | Post-hoc analysis of fetal magnetocardiograms (May et al. [40]) to assess effects of maternal exercise dose on fetal heart measures | N = 50; 23–39 years, singleton, low-risk pregnancies | MPAQ to retrospectively assess maternal physical activity behavior, specifically duration and intensitya | fetal F: SDNN, RMSSD, VLF, LF, HF, VLF/LF, VLF/HF, LF/HF |
A continuous, 18 min simultaneous fetal-maternal MCG was recorded for each subject using an investigational 83-channel dedicated fetal biomagnetometer | Gw36 | Spearman correlations were used to assess relationships between maternal LTPA (intensity, duration) during third trimester and fetal heart measures Multiple regression were additionally performed to assess how well LTPA intensity and duration predict fetal heart measures |
| Stutzman [43] | Prospective controlled interventional study to assess pre-to-post changes of exercise conditioning on HRV in normal and overweight women. HRV was measured in supine position and during low-intensity exercise | N = 22; healthy, singleton pregnancy; n = 10 NW (BMI < 25.0 kg/m2), n = 12 OaOb(BMI > 25.0 kg/m2) | Measurements: laboratory testing at gw20 ± 2 and gw36 Intervention: E: Instructed on a16 week progressive low-intensity walking exercisec, 5 days/week; n = 6 OaO, n = 5 NW C: Activity log only; n = 6 OaO, n = 5 NW →4 group design: a) walking/NW, b) non-walking/NW, c) walking/OaO, d) non-walking/OaO |
maternal M: LF, HF, TP, HF/TP, LP/HP |
Beat-by-beat R-R intervals were obtained and recorded continuously during testing using three latex-free standard surface ECG electrodes and a Spacelab 514 cardiac monitor | Gw20 and gw36 | ANOVAs were used to obtain within and between group changes from gw20 to gw36 |
| May, [40] | Prospective, longitudinal, non-blinded study to assess fetal heart measures depending on self-reported maternal exercise and fetal state |
N = 61; 20–35 years singleton, low-risk pregnancies |
MPAQ to retrospectively categorize women into exercise (≥ 30 min. of moderate to vigorous aerobic exercise/ 3 times a week; n = 26) and control group (n = 35)a | fetal F: SDNN, RMSSD, VLF, LF, IntF, HF |
3 continuous, 18 min simultaneous fetal-maternal MCG were recorded for each subject using an investigational 83-channel dedicated fetal biomagnetometer | Gw28, gw32, and gw36 | Post-hoc comparisons to obtain changes in fetal heart outcomes between exercise and control groups depending on fetal state (active vs. passive) at gw28, gw32, and gw36 |
| Satyapriya [36] | Prospective, RCT to assess the influence of yoga on maternal HRV (two-group, pre-post-design). Measurements were changes in HRV pre and during exercise session as well as pre and post exercise session (acute effects) at gw20 and gw36 (effects over intervention time; not tested for significance) |
N = 90; 20–35 years; mean BMI: 25.1 (E) and 25.6 (C) |
Intervention: E: two modules of integrated yoga, specifically designed for the second and third trimester of pregnancy (n = 45) C: standard prenatal exercise (n = 45) |
Maternal M: LF, HF, LF/HF |
Electrocardiogram was recorded continuously for 5 min before, 10 min during and 5 min after intervention | Gw20 and gw36 Measuring pre, during, and post each session |
ANOVAs to obtain within and between group differences in maternal HRV between exercise and control group |
(C control group, E exercise group, ECG electrocardiograph, F fetal, gw gestational week, HF high frequency, HRV heart rate variability, I infant, IntF intermediate frequency, LF low frequency, M maternal, MCG magnetocardiogram, MPAQ Modifiable Physical Activity Questionnaire, NW normal weight, OaO overweight and obese, RMSSD root mean square of successive difference, SDNN standard deviation of normal-to-normal intervals, TP total power, VLF very low frequency)
aThe MPAQ was used to retrospectively assess all leisure-time physical activities (LTPA) performed during the last 9 months of pregnancy, plus 3 months before pregnancy (self-reported). Note that no exercise intervention was performed within the highlighted studies
bpre-pregnancy BMI; pre-pregnancy weights of the participants were obtained by self-report from the women and confirmed from medical records
cthe steady-state testing protocol involved a 3-min warm-up at 20 W, followed by a ramp increase in work rate with 30 s to a level corresponding to 40 % of the maximal heart rate reserve
The HRV outcomes depending on PA of the two intervention studies from pre to post, the longitudinal study, as well as of the six post-hoc analyses are presented in Tables 3, 4, and 5. It has to be stressed that for the longitudinal study as well as for the six post-hoc analyses the MPAQ had been used to assess leisure-time PA (LTPA) performed during the 9 months of pregnancy plus 3 months before pregnancy. On the basis of these self-reports, the sample(s) of pregnant women were characterized into exercise or control group (more or less maternal PA) or continuous or non-continuous LTPA group. LTPA had not been objectively measured using, for example, accelerometers in any of the included articles.
Table 3.
Outcome measurements of each study
| First author, year | Type of analyses | SDNN (ms) | RMSSD (ms) | TP (ms2) |
|---|---|---|---|---|
| May, [47] | Difference in maternal HRV between exercise and control group at gw28, gw32, and gw36 | maternal gw28: exercise: 53.9 ± 27.4 control: 38.7 ± 12.9 (p = 0.01) |
maternal gw28: exercise: 40.2 ± 34.1 control 25.9 ± 13.5 (p = 0.05)d |
--- |
| gw32: exercise: 53.0 ± 19.8 control: 38.1 ± 13.7 (p = 0.002) |
gw32: exercise: 32.7 ± 24.9 control: 22.9 ± 11.7 (n.s.) |
|||
| gw36: exercise: 55.8 ± 20.6 control: 44.6 ± 14.0 (p = 0.03) |
gw36: exercise: 32.4 ± 24.9 control: 21.3 ± 11.2 (p = 0.05)d |
|||
| Van Leeuwen, [41] | Examining the occurrence of fetal-maternal heart rate synchronization in which the mothers were either exercising regularly or were sedentary | maternal exercise: 57.6 ± 22.2 control: 44.9 ± 12.5 (n.s.) |
maternal exercise: 35.2 ± 26.9 control: 20.8 ± 9.1 (n.s.) |
--- |
| fetal exercise: 29.3 ± 9.6 control: 21.4 ± 6.2 (p = 0.01) |
fetal exercise: 8.6 ± 3.8 control: 6.2 ± 2.1 (p = 0.02) |
|||
| May, Suminski, [37] | Correlation between continuous and non-continuous maternal LTPA and fetal HRV | fetal continuous LTPA R = 0.38 (p < 0.05) |
fetal continuous LTPA R = 0.31 (n.s.) |
--- |
| non-continuous LTPA R = 0.33 (p < 0.05) |
non-continuous LTPA R = 0.04 (n.s.) |
|||
| May, Scholtz, [39] | Difference in infant HRV between exercise and control group | infant exercise: 38.8 ± 10.8 control: 34.8 ± 14.6 (n.s.) |
infanta
exercise: 1.03 ± 0.14 control: 0.87 ± 0.21 (p = 0.01) |
--- |
| Gustafson, [42] | Difference in fetal HRV between exercise and control group adjusted for fetal breathing | --- | fetala
estimate: 0.34; SE: 0.14 (n.s.) |
fetala
estimate: 1.06; SE: 0.36 (n.s.) |
| May, [38] | Correlation between maternal LTPA (intensity, duration) and fetal HRV Regression to predict fetal heart measures |
fetal intensityb R = 0.28 (p < 0.05) |
fetal intensityb R = 0.27 (n.s.) |
--- |
| durationc
R = 0.44 (p < 0.005) |
durationc
R = 0.39 (p < 0.01) |
|||
| positively predicted by intensity (p = 0.033) | positively predicted by duration (p = 0.005) | |||
| Stutzman [43] | Within and between group changes from pre (gw20) to post (gw36) for each of the four groups; results are only presented for the HRV outcomes measured in supine position | --- | --- | maternal exercise – NW: gw20: 777.1 ± 1072 gw36: 787.8 ± 1042 (n.s.) |
| exercise – OaO: gw20: 523.2 ± 621 gw36: 231.9 ± 203.5 (n.s.) | ||||
| control – NW: gw20: 746.3 ± 667 gw36: 461.3 ± 583 (n.s.) | ||||
| control – OaO: gw20: 652.1 ± 984 gw36: 326.1 ± 506 (sig.)* | ||||
| May, [40] | Changes in fetal HRV between exercise and control groups depending on fetal state (active vs. passive) at gw28, gw32, and gw36 | fetala,e
gw28: exercise: 3.1 ± 0.3 control 2.9 ± 0.2 (n.s.) |
fetala,e
gw28: exercise: 1.8 ± 0.3 control 1.6 ± 0.3 (n.s.) |
--- |
| gw32: exercise: 3.2 ± 0.3 control: 3.1 ± 0.3 (n.s.) |
gw32: exercise: 1.8 ± 0.3 control: 1.6 ± 0.3 (n.s.) |
|||
| gw36: exercise: 3.4 ± 0.3 control: 3.1 ± 0.3 (p = 0.03) |
gw36: exercise: 2.0 ± 0.5 control: 1.7 ± 0.3 (p = 0.03) |
|||
| Satyapriya [36] | Changes in maternal HRV pre and during exercise session and pre and post exercise session (acute effects) at gw20 and gw36 (effects over time) Important: differences between maternal HRV measurements at gw20 and gw36 (effects over time) were not reported to be tested for significance |
--- | --- | --- |
(F fetal, gw gestational week, HRV heart rate variability, LTPA leisure-time physical activity, M maternal, ms millisecond, NW normal weight; n.s not significant, OaO overweight and obese, RMSSD root mean square of successive difference, SE standard error, sig significant, SDNN standard deviation of normal-to-normal intervals, TP total power)
*p-values were not provided
adata were log transformed to fulfill Gaussian distribution
bkcal · min−1
cminutes during third trimester
dlevel of significance was defined as p ≤ 0.05 for all analyzes performed by Van Leeuwen et al. [41]
emeasurements are only presented for active fetal state. Note that sample size is reduced at all time points (GA weeks 28 (n = 39), 32 (n = 37), 36 (N = 29)); results for quiet fetal state are not presented in this table because sample size is very small in this group
Table 4.
Outcome measurements of each study
| First author, year | Type of analyses | VLF (ms2) | LF (ms2) | HF (ms2) | IntF (ms2) |
|---|---|---|---|---|---|
| May, [47] | Difference in maternal HRV between exercise and control group at gw28, gw32, and gw36 | maternal gw28: exercise: 1082.6 ± 1135.6 control: 558.9 ± 392.0 (p = 0.03) |
maternal gw28: exercise: 436.8 ± 614.0 control: 263.7 ± 213.0 (n.s.) |
maternal gw28: exercise: 1402.3 ± 2990.0 contro:l 431.1 ± 395.4 (n.s.) |
--- |
| gw32: exercise: 1237.2 ± 942.5 control: 669.1 ± 513.1 (p = 0.008) |
gw32: exercise: 377.2 ± 473.7 control: 186.3 ± 135.9 (p = 0.05)d |
gw32: exercise: 911.2 ± 1334.8 control: 363.5 ± 365.0 (p = 0.048) |
|||
| gw36: exercise: 1529.4 ± 1325.8 control: 1097.2 ± 701.9 (n.s.) |
gw36: exercise: 368.6 ± 394.1 control: 201.2 ± 163.3 (n.s.) |
gw36: exercise: 805.6 ± 1165.5 control: 322.4 ± 387.4 (n.s.) |
|||
| Van Leeuwen, [41] | Difference in maternal and fetal HRV between exercise and control group | --- | --- | --- | --- |
| May, Suminski, [37] | Correlation between continuous and non-continuous maternal LTPA and fetal HRV | fetal continuous LTPA R = 0.45 (p < 0.005) |
fetal continuous LTPA R = 0.34 (p < 0.05) |
fetal continuous LTPA R = 0.4 (p < 0.05) |
--- |
| non-continuous LTPA R = 0.06 (n.s.) |
non-continuous LTPA R = 0.29 (n.s.) |
non-continuous LTPA R = 0.03 (n.s.) |
|||
| May, Scholtz, [39] | Difference in infant HRV between exercise and control group | --- | infanta
exercise: 2.38 ± 0.2 control: 2.06 ± 0.36 (p = 0.002) |
infanta
exercise: 1.72 ± 0.27 control: 1.38 ± 0.39 (p = 0.004) |
--- |
| Gustafson, [42] | Difference in fetal HRV between exercise and control group adjusted for fetal breathing | fetala
estimate: 1.25; SE: 0.45 (n.s.) |
fetala
estimate: 1.01 SE: 0.46 (n.s.) |
fetala
estimate: 0.81; SE: 0.25 (p = 0.03) |
fetala
estimate: 1.22; SE: 0.42 (p = 0.03) |
| May, [38] | Correlation between maternal LTPA (intensity, duration) and fetal HRV Regression to predict fetal heart measures |
fetal intensityb R = 0.29 (p < 0.05) |
fetal intensityb R = 0.20 (n.s.) |
fetal intensityb R = 0.24 (n.s.) |
fetal intensityb R = 0.27 (n.s.) |
| durationc
R = 0.34 (p < 0.05) |
durationc
R = 0.32 (p < 0.05) |
durationc
R = 0.38 (p < 0.01) |
durationc
R = 0.32 (p < 0.05) |
||
| positively predicted by duration (p = 0.007) | positively predicted by duration (p = 0.014) | positively predicted by duration (p = 0.003) | --- | ||
| Stutzman [43] | Within and between group changes from pre (gw20) to post (gw36) for each of the four groups; results are only presented for the HRV outcomes measured in supine position | --- | maternal exercise – NW: gw20: 194.4 ± 242 gw36: 116.2 ± 148 (n.s.) |
maternal exercise – NW: gw20: 278.9 ± 504 gw36: 290.8 ± 606 (n.s.) |
--- |
| exercise – OaO: gw20: 126.6 ± 143.7 gw36: 37.8 ± 15 (n.s.) |
exercise – OaO: gw20: 101.1 ± 99.7 gw36: 25.4 ± 23.8 (n.s.) |
||||
| control – NW: gw20: 184.2 ± 176 gw36: 83.4 ± 76 (sig.)* |
control – NW: gw20: 361.9 ± 370 gw36: 111.8 ± 161 (n.s.) |
||||
| control – OaO: gw20: 141.1 ± 181 gw36: 107.5 ± 202.1 (sig.)* |
control – OaO: gw20: 376.1 ± 723 gw36: 122.2 ± 265.7 (n.s.) |
||||
| May, [40] | Changes in fetal HRV between exercise and control groups depending on fetal state (active vs. passive) at gw28, gw32, and gw36 | fetala,e
gw28: exercise: 4.2 ± 0.5 control 3.9 ± 0.5 (n.s.) |
fetala,e
gw28: exercise: 4.2 ± 0.5 control 3.9 ± 0.5 (n.s.) |
fetala,e
gw28: exercise: 2.2 ± 0.5 control 1.8 ± 0.5 (n.s.) |
fetala,e
gw28: exercise: 2.3 ± 0.6 control 1.7 ± 0.9 (n.s.) |
| gw32: exercise: 4.6 ± 0.6 control: 4.1 ± 0.5 (n.s.) |
gw32: exercise: 4.6 ± 0.6 control: 4.1 ± 0.5 (n.s.) |
gw32: exercise: 2.2 ± 0.6 control: 1.9 ± 0.5 (n.s.) |
gw32: exercise: 1.9 ± 0.8 control: 1.3 ± 0.6 (n.s.) |
||
| gw36: exercise: 4.8 ± 0.6 control: 4.2 ± 0.6 (p = 0.04) |
gw36: exercise: 4.8 ± 0.6 control: 4.2 ± 0.6 (p = 0.03) |
gw36: exercise: 2.6 ± 0.8 control: 2.0 ± 0.4 (p = 0.01) |
gw36: exercise: 2.3 ± 0.9 control: 1.4 ± 0.7 (p = 0.02) |
||
| Satyapriya [36] | Changes in maternal HRV pre and during exercise session and pre and post exercise session (acute effects) at gw20 and gw36 (effects over time) Important: differences between maternal HRV measurements at gw20 and gw36 (effects over time) were not reported to be tested for significance |
maternal pre (gw20) and post (gw36) intervention |
maternal pre (gw20) and post (gw36) intervention |
--- | |
| gw20: exercise pre session: 74.8 ± 5.9 post session: 74.1 ± 6.6 (n.s.) control pre session: 73.3 ± 8.0 post session: 70.5 ± 11.6 (n.s.) |
gw20: exercise pre session: 25.2 ± 5.9 post session: 26.0 ± 6.6 (n.s.) control pre session: 26.7 ± 8.0 post session: 29.4 ± 11.6 (n.s.) |
||||
| gw36: exercise pre session: 76.3 ± 4.9 post session: 72.5 ± 6.0 (p = 0.001) control pre session: 74.2 ± 9.5 post session: 70.9 ± 7.4 (n.s.) |
gw36: exercise pre session: 23.2 ± 4.9 post session: 27.5 ± 6.0 (p = 0.001) control pre session: 25.0 ± 7.2 post session: 29.1 ± 7.4 (p = 0.006) |
(F fetal, gw gestational week, HF high frequency, HRV heart rate variability, IntF intermediate frequency, LF low frequency, LTPA leisure-time physical activity, M maternal, ms millisecond, NW normal weight, n.s not significant, OaO overweight and obese, SE standard error, sig significant, VLF very low frequency)
* p-values were not provided
adata were log transformed to fulfil Gaussian distribution
bkcal · min−1
cminutes during third trimester
dlevel of significance was defined as p ≤ 0.05 for all analyzes performed by Van Leeuwen et al. [41]
emeasurements are only presented for active fetal state. Note that sample size is reduced at all time points (GA weeks 28 (n = 39), 32 (n = 37), 36 (N = 29)); results for quiet fetal state are not presented in this table because sample size is very small in this group
Table 5.
Outcome measurements of each study
| First author, year | Type of analyses | LF/HF (ms2) | HF/TP (ms2) | VLF/LF (ms2) | VLF/HF (ms2) |
|---|---|---|---|---|---|
| May, [47] | Difference in maternal HRV between exercise and control group at gw28, gw32, and gw36 | maternal gw28: exercise: 0.6 ± 0.4 control: 0.85 ± 0.6 (n.s.) |
--- | --- | --- |
| gw32: exercise: 1.05 ± 0.9 control: 0.77 ± 0.5 (n.s.) | |||||
| gw36: exercise: 1.07 ± 1.5 control: 1.2 ± 1.1 (n.s.) | |||||
| Van Leeuwen, [41] | Difference in maternal and fetal HRV between exercise and control group | --- | --- | --- | --- |
| May, Suminski, [37] | Correlation between continuous and non-continuous maternal LTPA and fetal HRV | --- | --- | --- | --- |
| May, Scholtz, [39] | Difference in infant HRV between exercise and control group | infant exercise: 5.02 ± 2.16 control: 5.23 ± 2.24 (n.s.) |
--- | --- | --- |
| Gustafson, [42] | Difference in fetal HRV between exercise and control group adjusted for fetal breathing | fetala
estimate: 0.20; SE: 0.37 (n.s.) |
--- | fetala
estimate: 0.24; SE: 0.41 (n.s.) |
fetala
estimate: 0.59; SE: 0.46 (n.s.) |
| May, [38] | Correlation between maternal LTPA (intensity, duration) and fetal HRV Regression to predict fetal heart measures |
--- | --- | --- | |
| Stutzman [43] | Within and between group changes from pre (gw20) to post (gw36) for each of the four groups; results are only presented for the HRV outcomes measured in supine position | maternal exercise – NW: gw20: 2.02 ± 1.6 gw36: 3.51 ± 3.16 (n.s.) |
maternal exercise – NW: gw20: 0.217 ± 0.15 gw36: 0.187 ± 0.22 (n.s.) |
--- | --- |
| exercise – OaO: gw20: 1.66 ± 1.1 gw36: 2.08 ± 1.3 (n.s.) |
exercise – OaO: gw20: 0.207 ± 0.12 gw36: 0.120 ± 0.06 (n.s.) |
||||
| control – NW: gw20: 0.97 ± 0.92 gw36: 2.26 ± 2.1 (n.s.) |
control – NW: gw20: 0.396 ± 0.16 gw36: 0.175 ± 0.09 (n.s.) |
||||
| control – OaO: gw20: 1.3 ± 1.7 gw36: 2.18 ± 1.3 (n.s.) |
control – OaO: gw20: 0.365 ± 0.19 gw36: 0.188 ± 0.19 (n.s.) |
||||
| May, [40] | Changes in fetal HRV between exercise and control groups depending on fetal state (active vs. passive) at gw28, gw32, and gw36 | --- | --- | --- | --- |
| Satyapriya [36] | Changes in maternal HRV pre and during exercise session and pre and post exercise session (acute effects) at gw20 and gw36 (effects over time) Important: differences between maternal HRV measurements at gw20 and gw36 (effects over time) were not reported to be tested for significance |
maternal pre (gw20) and post (gw36) intervention |
--- | --- | --- |
| gw20: exercise pre session: 3.5 ± 0.2 post session: 3.1 ± 1.0 (n.s.) control pre session: 2.9 ± 0.8 post session: 2.8 ± 1.2 (n.s.) | |||||
| gw36: exercise pre session: 3.5 ± 0.8 post session: 2.8 ± 0.8 (p = 0.001) control pre session: 3.2 ± 0.9 post session: 2.6 ± 0.9 (p = 0.001) |
(F fetal, gw gestational week, HF high frequency, HRV heart rate variability, LF low frequency, LTPA leisure-time physical activity, M maternal, ms millisecond, NW normal weight, n.s not significant, OaO overweight and obese, sig significant, SE standard error, TP total power, VLF very low frequency)
adata were log transformed to fulfil Gaussian distribution
bmeasurements are only presented for active fetal state. Note that sample size is reduced at all time points (GA weeks 28 (n = 39), 32 (n = 37), 36 (N = 29)); results for quiet fetal state are not presented in this table because sample size is very small in this group
Study outcomes on HRV
Maternal outcomes
Four articles presented results concerning the influence of regular maternal PA on maternal HRV [36, 41, 43, 47]. May et al. [47] showed that the standard deviation of normal-to-normal intervals (SDNN), the root mean square of successive difference (RMSSD), very low frequency (VLF) HRV parameter, low frequency (LF) HRV parameter as well as high frequency (HF) HRV parameter were significantly increased in women who consistently exercised for a minimum of 30 min on three or more days (exercise group) at least at one time point of measurement in pregnancy compared to women of the control group who exercised less (detailed information see Tables 3, 4, and 5). Van Leeuwen et al. [41] showed no significant differences in maternal HRV parameters but showed a trend of increased maternal RMSSD (p = 0.07) in women who exercised for a minimum of 30 min on three or more days a week (exercise group) compared to women of the control group who exercised less during pregnancy.
Stutzman et al. [43] showed that total power frequency (TP) HRV parameter was significantly decreased from pre-to-post intervention only in the OaO control group. LF was also significantly decreased over time in both control groups (NW and OaO) but not in the two exercise groups (NW and OaO). HF and the ratio of LF and HF (LF/HF), as well as the ratio of HF and TP (HF/TP) showed no significant changes from pre-to-post intervention in any of the four groups.
Satyapriya et al. [36] tested maternal HRV of an exercise group which performed yoga and a control group which performed standard prenatal exercise. They assessed changes pre and post the exercise session (acute effects of PA) at gestational week 20 and gestational week 36 (intervention effect over time). At gestational week 20, no significant changes from pre-to-post exercise session were found for any HRV parameter (LF, HF, LF/HF) neither in the yoga nor in the prenatal exercise group. At gestational week 36, LF was significantly decreased from pre-to-post exercise session in the yoga group but not in the prenatal exercise group. Both groups showed a significant increase of HF from pre-to-post exercise session at gestational week 36 and a significant decrease of LF/HF from pre-to-post exercise session at gestational week 36. In summary, significant acute effects of yoga and standard prenatal exercise on HRV were only measured at gestational week 36 and not at gestational week 20 (Tables 3, 4, and 5).
Fetal outcomes
Five articles presented results concerning the influence of regular maternal PA on fetal HRV [37, 38, 40–42].
Van Leeuwen et al. [41] showed that the SDNN and the RMSSD were significantly increased in fetuses of women who exercised for a minimum of 30 min on three or more days a week (exercise group) compared fetuses of women in the control group. May, Suminski et al. [37] assessed whether fetal HRV was correlated with continuous or non-continuous maternal LTPA. The SDNN significantly correlated with both continuous and non-continuous maternal LTPA. VLF, LF, and HF were significantly correlated with continuous but not with non-continuous maternal LTPA. RMSSD did not significantly correlate with continuous nor did it correlate with non-continuous maternal LTPA. Gustafson et al. [42] estimated the difference of fetal HRV exercise in periods of fetal breathing and periods of no-fetal breathing of women who exercised for a minimum of 30 min on three or more days a week (exercise group) compared to the control group who did less exercise. RMSSD, TP, VLF, and LF were not significantly different between the exercise and control group, regardless of fetal breathing or not, but showed trends of significance (p = 0.08, p = 0.06, p = 0.06, and p = 0.07 respectively). The intermediate frequency (IntF) as well as the HF were significantly increased in fetuses of the exercise group compared to fetuses of the control group. May et al. [38] assessed whether fetal HRV is correlated with intensity (kilocalories per minute) and duration (minutes of PA during third trimester) of maternal PA performed in the third trimester of pregnancy. SDNN and VLF significantly correlated with both intensity as well as duration. The RMSSD, LF, HF, and IntF significantly correlated with duration but not with intensity. Finally, May et al. [40] assessed the difference of fetal HRV of fetuses in women who exercised for a minimum of 30 min on three or more days a week (exercise group) compared fetuses in women of the control group who did less exercise, depending on fetal state at three different time points (weeks of gestation 28, 32, and 36) in pregnancy. In active fetal state, at gestational week 36 all assessed parameters of HRV (SDNN, RMSSD, VLF, LF, IntF, HF) were significantly higher in fetuses of women in the exercise group compared to fetuses of women in the control group. At gestational week 28 and gestational week 32 differences were not significant for any of these parameters (Tables 3, 4, and 5).
Infant outcomes
Only one article referred to the influence of regular maternal PA on infant HRV [39]. The authors showed that RMSSD, LF and HF were significant higher in infants born of women who performed a minimum of 30 min of exercise three times a week (exercise group) compared to women who did less exercise (control group). SDNN and LF/HF were not significantly different between these groups (Tables 3, 4, and 5).
Quality assessment
Using the standardized QATQS, the overall rating of the two articles referring to the intervention studies was strong [36, 43], the articles of May, Scholtz et al. [39] and May et al. [38] was moderate, and the articles of May et al. [47], Van Leeuwen et al. [41], May, Suminski et al. [37], Gustafson et al. [42], and May et al. [40] was weak (Table 6). The longitudinal study by May et al. [40] and related articles were rated weak for ‘representativeness’ since the authors provided inconsistent reporting of the sample size without providing reasons for drop-outs. In consequence, the readers of the articles are not able to retrace whether the samples of the articles are representative of the target population or not. In the intervention study performed by Satyapriya et al. [36], trainers and participants could not be blinded, but the team who did the assessments and statistics was blinded.
Table 6.
Quality assessment of the included articles according to the EPHPP tool
| First author, year | Representativeness | Design | Confounders | Blindingb | Methods | Drop-outs | Global ratinga |
|---|---|---|---|---|---|---|---|
| May, [47] | Weak | Moderate | Strong | NA | Strong | Weak | Weak |
| Van Leeuwen, [41] | Weak | Moderate | Weak | NA | Strong | Weak | Weak |
| May, Suminski, [37] | Weak | Moderate | Strong | NA | Strong | Weak | Weak |
| May, Scholtz, [39] | Weak | Moderate | Strong | NA | Strong | Strong | Moderate |
| Gustafson, [42] | Weak | Moderate | Weak | NA | Strong | Weak | Weak |
| May, [38] | Weak | Moderate | Strong | NA | Strong | Strong | Moderate |
| Stutzman [43] | Moderate | Strong | Strong | NA | Strong | Strong | Strong |
| May, [40] | Weak | Moderate | Weak | NA | Strong | Weak | Weak |
| Satyapriya [36] | Moderate | Strong | Strong | NA | Moderate | Moderate | Strong |
(EPHPP Effective Public Health Practice Project, NA not applicable)
aStrong, no weak component rating; moderate, one weak component rating; weak, two or more weak component ratings
bThe component ‘blinding of outcome assessors and participants’ has been considered not applicable for observational and interventional studies. The reason for considering blinding not applicable for intervention studies in this case is that in studies with physical activity intervention the assessors (i.e. researchers) and the participants are very likely to know the outcome of the randomization [51]
Discussion
This systematic review of scientific literature on the influence of maternal PA on maternal, fetal or infant HRV identified nine articles. A summary of all findings is given in Table 7. Overall, the four articles that included maternal HRV as an outcome showed inconsistent results [36, 41, 43, 47]. Of the five articles that referred to the influence of maternal PA on fetal HRV, all the articles showed increases in fetal HRV depending on maternal PA on most parameters [37, 38, 40–42].
Table 7.
Summary of study results concerning the influence of maternal PA on HRV
| Finding | First author, year | SDNN | RMSSD | TP | HF | HF/TP | IntF | LF | VLF | VLF/HF | VLF/LF | LF/HF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal | May, [47] | ↑ | ↑a | - | ↑a | - | - | ↑a | ↑a | - | - | n.s. |
| Van Leeuwen, [41] | n.s. | n.s. | - | - | - | - | - | - | - | - | - | |
| Stutzman [43] | - | - | (↑) | n.s. | n.s. | - | (↑) | - | - | - | n.s. | |
| Satyapriya [36] | - | - | - | ↑ | - | - | ↓ | - | - | - | ↓ | |
| Fetal | Van Leeuwen, [41] | ↑ | ↑ | - | - | - | - | - | - | - | - | - |
| May, Suminski, [37] | ↑ | n.s. | - | ↑ | - | - | ↑ | ↑ | - | - | - | |
| Gustafson, [42] | - | n.s. | n.s. | ↑ | - | ↑ | n.s. | n.s. | n.s. | n.s. | n.s. | |
| May, [38] | ↑ | ↑ | - | ↑ | - | ↑ | ↑ | ↑ | - | - | - | |
| May, [40] | ↑a | ↑a | - | ↑a | - | ↑a | ↑a | ↑a | - | - | - | |
| Infant | May, Scholtz, [39] | n.s. | ↑ | - | ↑ | - | - | ↑ | - | - | - | - |
(HF high frequency, HRV heart rate variability, IntF intermediate frequency, LF low frequency, n.s. RMSSD root mean square of successive difference, SDNN standard deviation of normal-to-normal intervals, TP total power, VLF very low frequency)
↑, increase; ↓, decrease; -, variable not assessed
(↑), HRV parameters were decreased from gw20 to gw36 in all 4 groups but these decreases were only significant in the control groups. In consequence, there was a relative increase of maternal HRV parameters in the exercise groups compared to the control groups namely that the decrease of HRV was smaller in the exercise groups. Therefore, we used this symbol (↑) to illustrate relative increase
asignificant changes in HRV between exercise and control group have been found but not at all time-points of measuring (gw28, gw32, gw36)
The inconsistency of findings with regard to maternal HRV might be due to differences in PA intensity, timing in pregnancy, or due to differences in weight status of the participants. One article showed trends of increased HRV (moderate intensity exercise, small sample size) [41], one showed decreased HRV (OaO pregnant women) [43], another showed increased HRV (moderate intensity exercise) [47], and one article showed no changes on HRV (low intensity exercise group) [36]., Both, increased BMI and progressing pregnancy cause increased sympathetic control resulting in altered HRV outcomes [15–20]. It is expected that based on both of these scenarios the OaO pregnant women will have a different exercise HRV adaptation and therefore, the study performed by Stutzman et al. [43] found a decreased HRV compared to the other studies that investigated normal weight pregnant women. Additionally, moderate intensity PA was found to be associated with a significant increase [47] or a trend of increased HRV [41] whereas low intensity PA was not associated with changes in HRV [36]. This, however may lead to the conclusion that regularly performed maternal PA of moderate intensity may be more effective to improve maternal HRV than low intensity PA. To verify this hypothesis, more studies, including different intensities of maternal PA and its influence on maternal HRV, should be performed.
Results of the five articles on fetal HRV were more consistent, but the association of maternal PA with fetal HRV might change over time. The significant increases of different fetal HRV parameters presented in the article of May et al. [40] were only significant at gestational week 36 but not at weeks of gestation 28 and 32. The only article that referred to the influence of maternal PA on infant HRV reported significant increases in most of the HRV parameters [39]. Therefore, the overall consistency of the findings is high reflecting that maternal PA may increase fetal HRV, especially in late pregnancy. Nevertheless, since quality of four of five articles assessing fetal HRV was weak, and were based on only one study, level of evidence has to be rated as low.
The overall global rating for the standardized quality assessment of the articles is moderate to weak (Table 6). Only the two intervention studies were of strong quality [36, 43]. However, these articles only reported data on maternal and not on fetal or infant HRV. Furthermore, they reported inconsistent results showing decreases as well as increases of maternal HRV depending on the type of maternal exercise. In addition, Satyapriya et al. [36] compared maternal HRV between women of an exercise group that performed yoga and women of a control group that performed standard prenatal exercise. Since both groups, the exercise as well as the control group, were physically active, it is unclear whether the changes in HRV depends on PA alone or on for example pregnancy induced cardiovascular adaptations. Therefore, future intervention studies in pregnant women assessing HRV measurements depending on maternal PA should include a physically non-active control group.
Seven articles originated from the same study and sample carried out by May et al. [40]. Although the authors highlight in each article that respective data are post-hoc analyses of a subset of the original longitudinal study, it is unclear why the number of participants differs among these articles (Table 1). In the original article the authors report that 61 women were enrolled into the study and state that not all women participated in all three visits due to non-compliance or delivery prior to 36 weeks of gestation. However, the reasons for the different sample sizes reported in the studies, as well as for participant drop-out, were unclear. For studies assessing PA in participants, especially in pregnant women, it is of utter importance to clarify why participants were excluded or dropped out of a study to ensure that the reason for drop-out was not potential complications as a result of PA. Only one article [40] took fetal state (active vs. quiet) into account when assessing the influence of maternal PA on fetal HRV depending on fetal state (active vs. quiet). Since fetal heart measurements are mediated by fetal behavior [52, 53], future studies should consider this aspect. Without taking fetal state into account, interpretations of fetal HRV data are impossible. Furthermore, the authors used the MPAQ to retrospectively assess self-reported LTPA performed during the nine months of pregnancy plus three months before pregnancy. Since objective measurements of PA delivers more valid results than self-reported (subjective) measurements [54, 55], future studies assessing PA in pregnancy should use objective devices such as accelerometers, or use an exercise intervention (randomized design).
Conclusions
Based on the current evidence available and the inconsistency of the study results, our overall conclusion is that the hypothesis that maternal PA influences maternal HRV cannot be supported. Nonetheless, some studies showed a trend that maternal HRV may be improved, especially after regularly performed maternal PA of moderate intensity.
Given that most of the articles referring to fetal HRV showed positive effects, we also conclude that there is a trend that maternal PA might increase fetal HRV. Only one study assessed infant HRV, and therefore no conclusion can be drawn as yet. As a consequence, we are not able to provide a clinical conclusion at this time but moderate intensity maternal PA might be more beneficial to improve HRV compared to low intensity maternal PA. Since HRV is a noninvasive and surrogate marker to determine fetal overall health and the development of fetal ANS, more evidence is needed to clarify whether this highly relevant marker during pregnancy can be influenced by maternal PA. Therefore, we recommend that future studies on the influence of maternal PA on HRV should consider exercise intensity, maternal overweight and obesity, progression of gestation as well as fetal state. Additionally, future studies should include well-designed intervention studies with a physically inactive control group or longitudinal studies objectively measuring maternal PA.
Acknowledgements
None.
Funding
None.
Availability of data and materials
The whole data set is available at the University of Graz. Contact: pavel.dietz@uni-graz.at
Authors’ contributions
PD and MvP contributed substantially to concept and design of the review, acquisition of data as well as to analysis and interpretation of data. ST contributed substantially to acquisition of data as well as to analysis and interpretation of data. EW, MS, and WR contributed substantially to data analysis and interpretation. All authors drafted the article and approved the final version.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable for systematic reviews.
Ethical approval and consent to participate
Not applicable for systematic reviews.
Abbreviations
- ANS
autonomic nervous system
- C
control group
- E
exercise group
- ECG
electrocardiograph
- EPHPP
Effective Public Health Practice Project
- F
fetal
- gw
gestational week
- HF
high frequency
- HRV
heart rate variability
- I
infant
- IntF
intermediate frequency
- LF
low frequency
- LTPA
leisure-time physical activity
- M
maternal
- MCG
magnetocardiogram
- MPAQ
Modifiable Physical Activity Questionnaire
- NA
not applicable
- NN
normal-to-normal
- NW
normal weight
- OaO
overweight and obese
- RMSSD
root mean square of successive difference
- SDNN
standard deviation of normal-to-normal intervals
- SE
standard error
- TP
total power
- VLF
very low frequency
Appendix 1 Complete search term for Pubmed
("Motor Activity"[Mesh:NoExp] OR "Exercise"[Mesh] OR "Sports"[Mesh] OR "Physical Exertion"[Mesh] OR "Early Ambulation"[Mesh] OR "Exercise Therapy"[Mesh] OR "Exercise Movement Techniques"[Mesh] OR “Motor Activit*”[tiab] OR “Physical Activit*”[tiab] OR “Locomotor Activit*”[tiab] OR Exercis*[tiab] OR “Physical Exercis*”[tiab] OR “Isometric Exercis*”[tiab] OR “Aerobic Exercis*”[tiab] OR Training[tiab] OR Stretching[tiab] OR “Physical Condition*”[tiab] OR “Physical Fitness”[tiab] OR “Physical Endurance”[tiab] OR “Movement Therap*”[tiab] OR “Fitness Training”[tiab] OR Plyometric[tiab] OR Stretch-Shortening[tiab] OR Weight-Lifting[tiab] OR Weight-Bearing[tiab] OR Running[tiab] OR Jogging[tiab] OR Walk*[tiab] OR Bicycle[tiab] OR Cycle[tiab] OR Bicycling[tiab] OR Cycling[tiab] OR Rowing[tiab] OR Swim*[tiab] OR Ambulation[tiab] OR Mobil*[tiab] OR Pilates[tiab] OR Yoga[tiab]) AND (Pregnancy[Mesh] OR Gravidity[Mesh] OR “Pregnant Women”[Mesh] OR Pregnan*[tiab] OR Gravid*[tiab] OR Gestation*[tiab] OR “Pregnant Wom#n”[tiab] OR Childbearing[tiab] OR Matern*[tiab] OR Fetal[tiab]) AND (“Heart Rate Variability”[Mesh] OR “Heart Rate Variability”[tw] OR “Heart Outcome”[tiab] OR “Heart Measurement”[tiab] OR “Cardiovascular Outcome”[tiab] OR “Cardiovascular Measurement”[tiab] OR “Cardiovascular Adaptation”[tiab] OR “Autonomic Nervous System”[tiab] OR “Parasympathetic Nervous System”[tiab] OR “Sympathetic Nervous System”[tiab] OR “Vagus Nerve”[tiab]).
References
- 1.Weissgerber TL, Wolfe LA. Physiological adaptation in early human pregnancy: adaptation to balance maternal-fetal demands. Appl Physiol Nutr Metab. 2006;31:1–11. doi: 10.1139/h05-003. [DOI] [PubMed] [Google Scholar]
- 2.Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998;54:2056–63. doi: 10.1046/j.1523-1755.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 3.Capeless EL, Clapp JF. Cardiovascular changes in early phase of pregnancy. Am J Obstet Gynecol. 1989;161:1449–53. doi: 10.1016/0002-9378(89)90902-2. [DOI] [PubMed] [Google Scholar]
- 4.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–8. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 5.Johansson S, Norman M, Legnevall L, Dalmaz Y, Lagercrantz H, Vanpée M. Increased catecholamines and heart rate in children with low birth weight: perinatal contributions to sympathoadrenal overactivity. J Intern Med. 2007;261:480–7. doi: 10.1111/j.1365-2796.2007.01776.x. [DOI] [PubMed] [Google Scholar]
- 6.Wadhwa PD, Culhane JF, Rauh V, Barve SS. Stress and preterm birth: neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern Child Health J. 2001;5:119–25. doi: 10.1023/A:1011353216619. [DOI] [PubMed] [Google Scholar]
- 7.Satpathy HK, Fleming A, Frey D, Barsoom M, Satpathy C, Khandalavala J. Maternal obesity and pregnancy. Postgrad Med. 2008;120:9. doi: 10.3810/pgm.2008.09.1920. [DOI] [PubMed] [Google Scholar]
- 8.Phillips DIW. External influences on the fetus and their long-term consequences. Lupus. 2006;15:794–800. doi: 10.1177/0961203306069354. [DOI] [PubMed] [Google Scholar]
- 9.Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia -- a state of sympathetic overactivity. N Engl J Med. 1996;335:1480–5. doi: 10.1056/NEJM199611143352002. [DOI] [PubMed] [Google Scholar]
- 10.National Institute of Child Health and Human Development Research Planning Workshop Electronic fetal heart rate monitoring: research guidelines for interpretation. National Institute of Child Health and Human Development Research Planning Workshop. Am J Obstet Gynecol. 1997;177:1385–90. doi: 10.1016/S0002-9378(97)70079-6. [DOI] [PubMed] [Google Scholar]
- 11.Malik M. Heart rate variability. Curr Opin Cardiol. 1998;13:36–44. doi: 10.1097/00001573-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Raczak G, Daniłowicz-Szymanowicz L, Kobuszewska-Chwirot M, Ratkowski W, Figura-Chmielewska M, Szwoch M. Long-term exercise training improves autonomic nervous system profile in professional runners. Kardiol Pol. 2006;64:135–40. [PubMed] [Google Scholar]
- 13.Mandigout S, Melin A, Fauchier L, N'Guyen LD, Courteix D, Obert P. Physical training increases heart rate variability in healthy prepubertal children. Eur J Clin Invest. 2002;32:479–87. doi: 10.1046/j.1365-2362.2002.01017.x. [DOI] [PubMed] [Google Scholar]
- 14.Sandercock GRH, Bromley PD, Brodie DA. Effects of exercise on heart rate variability: inferences from meta-analysis. Med Sci Sports Exerc. 2005;37:433–9. doi: 10.1249/01.MSS.0000155388.39002.9D. [DOI] [PubMed] [Google Scholar]
- 15.Felber Dietrich D, Schindler C, Schwartz J, Barthelemy J-C, Tschopp J-M, Roche F, et al. Heart rate variability in an ageing population and its association with lifestyle and cardiovascular risk factors: results of the SAPALDIA study. Europace. 2006;8:521–9. doi: 10.1093/europace/eul063. [DOI] [PubMed] [Google Scholar]
- 16.Arone LJ, Mackintosh R, Rosenbaum M, Leibel RL, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol. 1995;269:R222–5. doi: 10.1152/ajpregu.1995.269.1.R222. [DOI] [PubMed] [Google Scholar]
- 17.Arrone LJ, Mackintosh R, Rosenbaum M, Leibel RL, Hirsch J. Cardiac autonomic nervous system activity in obese and never-obese young men. Obes Res. 1997;5:354–9. doi: 10.1002/j.1550-8528.1997.tb00564.x. [DOI] [PubMed] [Google Scholar]
- 18.Spraul M, Ravussin E, Fontvieille AM, Rising R, Larson DE, Anderson EA. Reduced sympathetic nervous activity. A potential mechanism predisposing to body weight gain. J Clin Invest. 1993;92:1730–5. doi: 10.1172/JCI116760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felber Dietrich D, Ackermann-Liebrich U, Schindler C, Barthelemy J-C, Brandli O, Gold DR, et al. Effect of physical activity on heart rate variability in normal weight, overweight and obese subjects: results from the SAPALDIA study. Eur J Appl Physiol. 2008;104:557–65. doi: 10.1007/s00421-008-0800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein PK, Hagley MT, Cole PL, Domitrovich PP, Kleiger RE, Rottman JN. Changes in 24-hour heart rate variability during normal pregnancy. Am J Obstet Gynecol. 1999;180:978–85. doi: 10.1016/S0002-9378(99)70670-8. [DOI] [PubMed] [Google Scholar]
- 21.Henje Blom E, Olsson EM, Serlachius E, Ericson M, Ingvar M. Heart rate variability (HRV) in adolescent females with anxiety disorders and major depressive disorder. Acta Paediatr. 2010;99:604–11. doi: 10.1111/j.1651-2227.2009.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiPietro JA, Bornstein MH, Hahn C-S, Costigan K, Achy-Brou A. Fetal heart rate and variability: stability and prediction to developmental outcomes in early childhood. Child Dev. 2007;78:1788–98. doi: 10.1111/j.1467-8624.2007.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graça LM, Cardoso CG, Clode N, Calhaz-Jorge C. Acute effects of maternal cigarette smoking on fetal heart rate and fetal body movements felt by the mother. J Perinat Med. 1991;19:385–90. doi: 10.1515/jpme.1991.19.5.385. [DOI] [PubMed] [Google Scholar]
- 24.Halmesmäki E, Ylikorkala O. The effect of maternal ethanol intoxication on fetal cardiotocography: a report of four cases. Br J Obstet Gynaecol. 1986;93:203–5. doi: 10.1111/j.1471-0528.1986.tb07893.x. [DOI] [PubMed] [Google Scholar]
- 25.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 26.Clapp JF. The effects of maternal exercise on fetal oxygenation and feto-placental growth. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl):80–5. doi: 10.1016/S0301-2115(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 27.Clapp JF, Kiess W. Effects of pregnancy and exercise on concentrations of the metabolic markers tumor necrosis factor alpha and leptin. Am J Obstet Gynecol. 2000;182:300–6. doi: 10.1016/S0002-9378(00)70215-8. [DOI] [PubMed] [Google Scholar]
- 28.Clapp JF. Effects of Diet and Exercise on Insulin Resistance during Pregnancy. Metab Syndr Relat Disord. 2006;4:84–90. doi: 10.1089/met.2006.4.84. [DOI] [PubMed] [Google Scholar]
- 29.Dye TD, Knox KL, Artal R, Aubry RH, Wojtowycz MA. Physical activity, obesity, and diabetes in pregnancy. Am J Epidemiol. 1997;146:961–5. doi: 10.1093/oxfordjournals.aje.a009223. [DOI] [PubMed] [Google Scholar]
- 30.Bung P, Artal R. Gestational diabetes and exercise: a survey. Semin Perinatol. 1996;20:328–33. doi: 10.1016/S0146-0005(96)80025-5. [DOI] [PubMed] [Google Scholar]
- 31.Downs DS, DiNallo JM, Kirner TL. Determinants of pregnancy and postpartum depression: prospective influences of depressive symptoms, body image satisfaction, and exercise behavior. Ann Behav Med. 2008;36:54–63. doi: 10.1007/s12160-008-9044-9. [DOI] [PubMed] [Google Scholar]
- 32.Carter JB, Banister EW, Blaber AP. Effect of Endurance Exercise on Autonomic Control of Heart Rate. Sports Med. 2003;33:33–46. doi: 10.2165/00007256-200333010-00003. [DOI] [PubMed] [Google Scholar]
- 33.Okazaki K, Iwasaki K-i, Prasad A, Palmer MD, Martini ER, Fu Q, et al. Dose-response relationship of endurance training for autonomic circulatory control in healthy seniors. J Appl Physiol. 2005;99:1041–9. doi: 10.1152/japplphysiol.00085.2005. [DOI] [PubMed] [Google Scholar]
- 34.D'Silva LA, Davies RE, Emery SJ, Lewis MJ. Influence of somatic state on cardiovascular measurements in pregnancy. Physiol Meas. 2014;35:15–29. doi: 10.1088/0967-3334/35/1/15. [DOI] [PubMed] [Google Scholar]
- 35.Routledge FS, Campbell TS, McFetridge-Durdle JA, Bacon SL. Improvements in heart rate variability with exercise therapy. Can J Cardiol. 2010;26:303–12. doi: 10.1016/S0828-282X(10)70395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satyapriya M, Nagendra HR, Nagarathna R, Padmalatha V. Effect of integrated yoga on stress and heart rate variability in pregnant women. Int J Gynaecol Obstet. 2009;104:218–22. doi: 10.1016/j.ijgo.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 37.May LE, Suminski RR, Berry A, Langaker MD, Gustafson KM. Maternal physical activity mode and fetal heart outcome. Early Hum Dev. 2014;90:365–9. doi: 10.1016/j.earlhumdev.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 38.May LE, Suminski RR, Langaker MD, Yeh H-W, Gustafson KM. Regular maternal exercise dose and fetal heart outcome. Med Sci Sports Exerc. 2012;44:1252–8. doi: 10.1249/MSS.0b013e318247b324. [DOI] [PubMed] [Google Scholar]
- 39.May LE, Scholtz SA, Suminski R, Gustafson KM. Aerobic exercise during pregnancy influences infant heart rate variability at one month of age. Early Hum Dev. 2014;90:33–8. doi: 10.1016/j.earlhumdev.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 40.May LE, Glaros A, Yeh H-W, Clapp JF, Gustafson KM. Aerobic exercise during pregnancy influences fetal cardiac autonomic control of heart rate and heart rate variability. Early Hum Dev. 2010;86:213–7. doi: 10.1016/j.earlhumdev.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 41.van Leeuwen P, Gustafson KM, Cysarz D, Geue D, May LE, Grönemeyer D. Aerobic exercise during pregnancy and presence of fetal-maternal heart rate synchronization. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gustafson KM, May LE, Yeh H-W, Million SK, Allen JJB. Fetal cardiac autonomic control during breathing and non-breathing epochs: the effect of maternal exercise. Early Hum Dev. 2012;88:539–46. doi: 10.1016/j.earlhumdev.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stutzman SS, Brown CA, Hains SMJ, Godwin M, Smith GN, Parlow JL, Kisilevsky BS. The effects of exercise conditioning in normal and overweight pregnant women on blood pressure and heart rate variability. Biol Res Nurs. 2010;12:137–48. doi: 10.1177/1099800410375979. [DOI] [PubMed] [Google Scholar]
- 44.Avery ND, Wolfe LA, Amara CE, Davies GA, McGrath MJ. Effects of human pregnancy on cardiac autonomic function above and below the ventilatory threshold. J Appl Physiol. 2001;90:321–8. doi: 10.1152/jappl.2001.90.1.321. [DOI] [PubMed] [Google Scholar]
- 45.Carpenter RE, D'Silva LA, Emery SJ, Uzun O, Rassi D, Lewis MJ. Changes in heart rate variability and QT variability during the first trimester of pregnancy. Physiol Meas. 2015;36:531–45. doi: 10.1088/0967-3334/36/3/531. [DOI] [PubMed] [Google Scholar]
- 46.Siepmann M, Hennig U-D, Siepmann T, Nitzsche K, Mück-Weymann M, Petrowski K, Weidner K. The effects of heart rate variability biofeedback in patients with preterm labour. Appl Psychophysiol Biofeedback. 2014;39:27–35. doi: 10.1007/s10484-013-9238-1. [DOI] [PubMed] [Google Scholar]
- 47.May LE, Knowlton J, Hanson J, Suminski R, Paynter C, Fang X, Gustafson KM. Effects of Exercise During Pregnancy on Maternal Heart Rate and Heart Rate Variability. PM R. 2016;8:611–7. doi: 10.1016/j.pmrj.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Effective Public Health Practice Project. Quality Assessment Tool for Quantitative Studies. 2008. http://www.ephpp.ca/tools.html. Accessed 30 Aug 2016.
- 49.Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract. 2012;18:12–8. doi: 10.1111/j.1365-2753.2010.01516.x. [DOI] [PubMed] [Google Scholar]
- 50.Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs. 2004;1:176–84. doi: 10.1111/j.1524-475X.2004.04006.x. [DOI] [PubMed] [Google Scholar]
- 51.Oja P, Titze S, Bauman A, de Geus B, Krenn P, Reger-Nash B, Kohlberger T. Health benefits of cycling: a systematic review. Scand J Med Sci Sports. 2011;21:496–509. doi: 10.1111/j.1600-0838.2011.01299.x. [DOI] [PubMed] [Google Scholar]
- 52.Davidson SR, Rankin JH, Martin CB, Reid DL. Fetal heart rate variability and behavioral state: analysis by power spectrum. Am J Obstet Gynecol. 1992;167:717–22. doi: 10.1016/S0002-9378(11)91577-4. [DOI] [PubMed] [Google Scholar]
- 53.Einspieler C. Fetal Behaviour: A Neurodevelopmental Approach. London: Mac Keith Press; 2012. [Google Scholar]
- 54.Rousham EK, Clarke PE, Gross H. Significant changes in physical activity among pregnant women in the UK as assessed by accelerometry and self-reported activity. Eur J Clin Nutr. 2006;60:393–400. doi: 10.1038/sj.ejcn.1602329. [DOI] [PubMed] [Google Scholar]
- 55.Brett KE, Wilson S, Ferraro ZM, Adamo KB. Self-report Pregnancy Physical Activity Questionnaire overestimates physical activity. Can J Public Health. 2015;106:302. doi: 10.17269/cjph.106.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The whole data set is available at the University of Graz. Contact: pavel.dietz@uni-graz.at


