Abstract
Background
Predicting whether an obese critically ill patient can be successfully extubated may be specially challenging. Several weaning tests have been described but no physiological study has evaluated the weaning test that would best reflect the post-extubation inspiratory effort.
Methods
This was a physiological randomized crossover study in a medical and surgical single-center Intensive Care Unit, in patients with body mass index (BMI) >35 kg/m2 who were mechanically ventilated for more than 24 h and underwent a weaning test. After randomization, 17 patients were explored using five settings : pressure support ventilation (PSV) 7 and positive end-expiratory pressure (PEEP) 7 cmH2O; PSV 0 and PEEP 7cmH2O; PSV 7 and PEEP 0 cmH2O; PSV 0 and PEEP 0 cmH2O; and a T piece, and after extubation. To further minimize interaction between each setting, a period of baseline ventilation was performed between each step of the study. We hypothesized that the post-extubation work of breathing (WOB) would be similar to the T-tube WOB.
Results
Respiratory variables and esophageal and gastric pressure were recorded. Inspiratory muscle effort was calculated as the esophageal and trans-diaphragmatic pressure time products and WOB. Sixteen obese patients (BMI 44 kg/m2 ± 8) were included and successfully extubated. Post-extubation inspiratory effort, calculated by WOB, was 1.56 J/L ± 0.50, not statistically different from the T piece (1.57 J/L ± 0.56) or PSV 0 and PEEP 0 cmH2O (1.58 J/L ± 0.57), whatever the index of inspiratory effort. The three tests that maintained pressure support statistically underestimated post-extubation inspiratory effort (WOB 0.69 J/L ± 0.31, 1.15 J/L ± 0.39 and 1.09 J/L ± 0.49, respectively, p < 0.001). Respiratory mechanics and arterial blood gases did not differ between the five tests and the post-extubation condition.
Conclusions
In obese patients, inspiratory effort measured during weaning tests with either a T-piece or a PSV 0 and PEEP 0 was not different to post-extubation inspiratory effort. In contrast, weaning tests with positive pressure overestimated post-extubation inspiratory effort.
Trial registration
Clinical trial.gov (reference NCT01616901), 2012, June 4th
Electronic supplementary material
The online version of this article (doi:10.1186/s13054-016-1457-4) contains supplementary material, which is available to authorized users.
Keywords: Weaning, Mechanical ventilation, Obese, Work of breathing, Acute respiratory failure
Background
Extubation is a critical decision in the Intensive Care Unit (ICU). Extubation failure may occur in up to 20 % [1] of patients and is associated with morbidity. Excessive and non-sustainable work of breathing (WOB) is likely a major reason for extubation failure [2–5]. Evaluation of how the critically ill patient is breathing with no assistance or a minimal level of assistance (the period known as the weaning test or the spontaneous breathing trial) [4] is therefore recommended before extubation [3, 4, 6, 7]. Different weaning tests are suggested for non-selected adult patients: a T-piece trial (oxygen supply without positive pressure), continuous positive airway pressure (CPAP) and low pressure support ventilation (PSV), with a low level of PSV, from 5 to 8 cmH2O, to compensate for the imposed workload due to the ventilator circuit [3, 4, 6, 7]. Although these weaning tests are not equivalent in term of the WOB [8, 9] and studies are underpowered to assess the risk of extubation failure, they are recommended to assess whether a patient is ready to be extubated [3, 6].
Predicting whether an obese critically ill patient can be successfully extubated may be specially challenging. Obesity decreases respiratory system compliance, inspiratory and expiratory lung volumes, functional residual capacity, upper airway mechanical function and neuromuscular strength [10]. Moreover, in obese patients, oxygen consumption is increased, with a high proportion of this consumption spent in the WOB [11–13]. Although the T piece, CPAP and low PSV levels have been used to reproduce post-extubation conditions in non-selected critically ill patients, the weaning test modality that would best reproduce post-extubation inspiratory effort (WOB and pressure time product indexes) in obese critically ill patients has never been evaluated and many clinicians are worried about using no support during the test [14, 15].
The aim of our study was thus to assess which weaning test would best reproduce post-extubation inspiratory effort in obese critically ill patients. We compared a T-piece trial to weaning tests with PSV 7 and positive end-expiratory pressure (PEEP) 7 cmH2O; PSV 0 and PEEP 7 cmH2O; PSV 7 and PEEP 0 cmH2O; PSV 0 and PEEP 0 cmH2O, in this particular population. We hypothesized that the T-tube or PSV 0 and PEEP 0 cmH2O would best approximate the post-extubation WOB.
Methods
Study
This was a physiological prospective randomized crossover study (Additional file 1: Table S1), approved by the Ethics Committee of the Saint-Eloi Teaching Hospital (2012 A-00294-39, Comité de Protection des Personnes Sud Méditerranée III, Montpellier, France), and registered on clinical trial.gov (reference NCT01616901, registered June 4th, 2012). All patients provided their written informed consent.
Patients
Upon admission, height and weight were measured using the bed scale and a tape measure. All morbidly obese patients, defined by a body mass index (total body weight in kg/height in m2) >35 kg/m2 [16], were considered eligible for inclusion in the study if they were mechanically ventilated for at least 24 h and were considered by the physician on duty to be ready for extubation. Patients were not included in the study if there was any contraindication to the insertion of an esophageal catheter.
Experimental procedure and study design
A 15-minute period corresponding to a baseline state was first recorded (using PSV and PEEP set by the clinician in charge of the patient before inclusion). Patients were then randomly assessed using computer-driven software with five settings: PSV 7 and PEEP 7 cmH2O; PSV 0 and PEEP 7 cmH2O; PSV 7 and PEEP 0 cmH2O; PSV 0 and PEEP 0 cmH2O or the T piece. Each setting lasted 15 minutes with a 10-minute period of return to baseline steady state between each setting (Fig. 1). Steady state was defined clinically as a period sufficient to ensure clinical stability in respiratory and hemodynamic variables assessed by a physical exam which took into account heart rate, respiratory rate, paradoxical breathing pattern, accessory muscle use, grunting at end expiration and nasal flaring [17], and as previously performed by our group [18, 19].
Fig. 1.
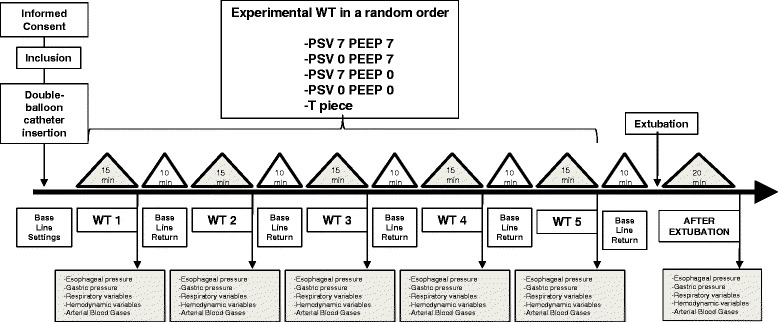
Study design. Eleven morbidly obese patients ventilated in pressure support ventilation (PSV) and positive end-expiratory pressure (PEEP), considered as baseline settings, were included to randomly perform the five weaning test modalities of the study before extubation: PSV 7 cmH2O + PEEP 7 cm H2O; PSV 0 cmH2O + PEEP 7 cmH2O; PSV 7 cmH2O + PEEP 0 cmH2O; PSV 0 cmH2O + PEEP 0 cmH2O or the T piece. All measurements were obtained after 15 minutes of each test. A 10-minute period of return to baseline state (with initial settings of ventilation parameters before the first weaning test) was performed between each test and before extubation. WT weaning test
After being explored with these five settings, and in the case of clinical success in the different weaning trials, patients were ventilated for 10 minutes using baseline state variables and then were extubated if the clinical state was judged adequate by the clinician in charge. A post-extubation measurement was performed 20 minutes after extubation using an oro-nasal oxygen mask with a flow of 5 L/minute (equivalent to inspired oxygen fraction (FiO2) of 0.4 [20]). According to our local protocol described in detail in a previous review [21], and after having achieved each step of the protocol, non-invasive ventilation was performed as a prophylactic routine measure in the immediate post-extubation period, for between 30 and 45 minutes every 4 to 6 h. Settings were adjusted to target the following: tidal volume (VT) 6–10 ml/kg of ideal body weight, respiratory rate (RR) 12–20 c/minute and pulse arterial oxygen saturation (SpO2) equal or above 95 %. Non-invasive ventilation was never performed before the end of the protocol.
Measurements
All patients were studied in a semi-recumbent position with the head of the bed elevated to an angle from 30 to 45 degrees, according to patient comfort. [22] Procedures are detailed in the additional material. Briefly, the respiratory mechanics measurements comprised flow, airway pressure, esophageal (Pes) and gastric (Pga) pressure swings. Trans-diaphragmatic swings (Pdi) were calculated by subtracting Pes from Pga. Minute ventilation (VE), tidal volume (VT), inspiratory (Ti), expiratory time (Te), total cycle duration (Ttot) and RR were calculated from the numerical integration of the flow signal.
The inspiratory WOB per breath performed by the patient was calculated from a Campbell diagram taking into account the presence of intrinsic PEEP. Eesophageal and trans-diaphragmatic pressure-time products (PTPes and PTPdi) were also measured as previously reported [23, 24]. Analyses of arterial blood gases were obtained at the end of each test.
Statistical analysis
All values are presented as mean ± SD. To assess differences between the weaning tests, we used the Friedman test and then pairwise comparisons with the Wilcoxon test if a significant difference appeared. Statistical analysis was performed by an independent statistician (NM) using R software© (R Foundation for Statistical Computing, Auckland, New Zealand).
Based on the literature review, we hypothesized that the post-extubation WOB would be similar to the T-tube WOB [25, 26] and would approximate 1.5 +/- 0.9 J/L in obese critically ill patients. We also hypothesized that WOB in PSV 7 cmH2O and PEEP 7 cmH2O would approximate 0.7 +/- 0.5 J/L [27]. Then, with an alpha risk at 0.05 and a power at 0.90, 12 patients would be needed. We decided to include 17 patients in order to make sure that 12 patients would complete the study. Significance was set at p < 0.01 after correction for the number of multiple comparisons, i.e., using the Bonferroni test.
Results
Patients
Between March and December 2012, 40 obese patients with body mass index ≥35 kg/m2 were admitted in our center. Among them, 17 met the inclusion criteria. Sixteen patients (13 women and 3 men) with mean body mass index of 44 kg/m2 (±8 kg/m2) were prospectively enrolled in the present study, as shown in Fig. 2. Characteristics of the subjects are detailed in Table 1. Mean duration of invasive mechanical ventilation before enrollment in the study was 6 days (±7 days). The five weaning tests were well-tolerated by all patients and all of them but one were successfully extubated.
Fig. 2.

Flow chart of the study. One patient fulfilled the inclusion criteria but was not included because of extubation during the weekend with no investigator available. BMI body mass index, SBT spontaneous breathing trial
Table 1.
Characteristics of the patients
| Patient | Sex | Age | SAPS II | Height | Weight | BMI | Underlying | Etiology of respiratory failure | ETT ID | MV before extubation | PSV at baseline | PEEP at baseline | Extubation failure | Outcome (D/S) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| number | (years) | (cm) | (kg) | (kg/m2) | diseases | (mm) | (days) | (cmH2O) | (cmH2O) | (Y/N) | ||||
| 1 | F | 83 | 109 | 150 | 80 | 35 | CHF | Small bowel ischemia | 7.5 | 7 | 8 | 6 | N | D |
| 2 | F | 85 | 68 | 163 | 115 | 43 | NIDDM | Pneumonia | 7.5 | 4 | 15 | 7 | N | S |
| 3 | M | 64 | 50 | 170 | 130 | 44 | NIDDM | Acute pancreatitis | 8 | 3 | 12 | 8 | N | S |
| 4 | F | 59 | 60 | 155 | 95 | 39 | None | Peritonitis | 7.5 | 3 | 12 | 8 | N | S |
| 5 | F | 49 | 66 | 160 | 174 | 67 | COPD, OSA | Septic shock | 7.5 | 6 | 10 | 10 | N | S |
| 6 | F | 25 | 29 | 172 | 145 | 49 | None | Asthma | 7.5 | 1 | 10 | 8 | N | S |
| 7 | F | 54 | 19 | 153 | 121 | 51 | Asthma, HTN | Post abdominal surgery | 7.5 | 1 | 10 | 8 | N | S |
| 8 | M | 37 | 54 | 180 | 130 | 40 | None | Acute pancreatitis | 8 | 14 | 8 | 10 | N | S |
| 9 | F | 78 | 90 | 155 | 87 | 36 | None | Bowel obstruction | 7.5 | 4 | 8 | 5 | N | S |
| 10 | F | 49 | 78 | 167 | 112 | 41 | Asthma, OSA | Peritonitis | 7.5 | 30 | 8 | 5 | N | S |
| 11 | F | 73 | 77 | 150 | 93 | 41 | CHF, AF | Septic shock | 7.5 | 4 | 12 | 6 | N | D |
| 12 | F | 50 | 45 | 162 | 94 | 36 | None | Necrotizing fasciitis | 7.5 | 2 | 9 | 7 | N | S |
| 13 | M | 63 | 64 | 175 | 180 | 56 | NIDDM, HTN | Small bowel bleeding | 7.5 | 1 | 8 | 10 | N | S |
| 14 | F | 43 | 48 | 155 | 105 | 43 | OSA, home ventilation | Pneumonia | 7.5 | 3 | 12 | 7 | N | S |
| 15 | F | 77 | 41 | 155 | 84 | 36 | NIDDM, HTN | Pancreatitis | 7.5 | 7 | 9 | 7 | Y | S |
| 16 | F | 50 | 64 | 164 | 124 | 46 | OSA, home ventilation | Post abdominal surgery | 7.5 | 8 | 14 | 8 | N | S |
| Mean | 59 | 60 | 162 | 117 | 44 | . | 6 | 10 | 7 | |||||
| SD | 17 | 22 | 9 | 30 | 8 | . | 7 | 2 | 2 |
Abbreviations: AF atrial fibrillation, BMI body mass index; CHF chronic heart failure, D deceased; ETT ID endotracheal tube internal diameter; F female; M male; HTN hypertension, mechanical ventilation; NIDDM non-insulin-dependent diabetes mellitus; OSA obstructive sleep apnoea, PEEP positive end-expiratory pressure; PSV pressure support ventilation; SAPS II Simplified Acute Physiology Score II [34]; S survived
The first patient was initially unable to complete the five weaning tests. She was re-challenged 72 h later, and succeeded the tests and extubation. Seven days after extubation, she developed cardio-respiratory distress and was re-intubated. Patient number 15 developed hypoxemic acute respiratory failure and was re-intubated 12 h after extubation. One patient had accidental nasogastric catheter removal after extubation, preventing the measurement of respiratory muscle work variables after extubation. This patient was excluded from the final analysis.
Respiratory variables and gas exchange
There was no statistical difference in any of the different respiratory variables (shown in Table 2) among the five weaning tests or at 20 minutes after extubation. In particular, differences in the RR/VT ratio were not statistically significant between the five weaning tests or at 20 minutes after extubation. There was no statistically significant difference in arterial blood gases or hemodynamic variables among the six steps of the study, as shown in Table 3.
Table 2.
Respiratory variables during the five different weaning tests and 20 minutes after extubation
| PSV | PSV | PSV | PSV | T piece | After extubation | |
|---|---|---|---|---|---|---|
| +7 cmH2O PEEP | 0 cmH2O PEEP | +7 cmH2O PEEP | 0 cmH2O PEEP | |||
| +7 cmH2O | +7 cmH2O | 0 cmH2O | 0 cmH2O | |||
| Ti, s | 0.90 ± 0.2 | 0.93 ± 0.23 | 0.82 ± 0.24 | 0.84 ± 0.28 | 0.81 ± 0.3 | 0.89 ± 0.43 |
| Ttot, s | 2.6 ± 0.8 | 2.4 ± 0.6 | 2.2 ± 0.6 | 2.1 ± 0.6 | 2.1 ± 0.6 | 2.2 ± 0.8 |
| Ti/Ttot, % | 35.7 ± 3.6 | 38.7 ± 4.2 | 37.8 ± 4.2 | 39.3 ± 4.4 | 38.7 ± 4.7 | 40.8 ± 4.3 |
| VT, L | 0.43 ± 0.12 | 0.41 ± 0.1 | 0.38 ± 0.1 | 0.37 ± 0.1 | 0.35 ± 0.1 | 0.36 ± 0.1 |
| RR, breaths/minute | 25 ± 6 | 26 ± 7 | 29 ± 6 | 30 ± 8 | 31 ± 7 | 30 ± 8 |
| RR/VT, minutes/mL | 64.5 ± 26.8 | 69.7 ± 25.0 | 83.1 ± 34.4 | 87.8 ± 36.4 | 94.7 ± 38.1 | 88.6 ± 34 |
| VE, L/minute | 10.3 ± 2.4 | 10.41 ± 2.9 | 10.8 ± 2.6 | 10.8 ± 3.3 | 10.5 ± 3.2 | 11.2 ± 4.4 |
| PEEPi, cmH2O | 1.1 ± 0.9 | 1.7 ± 1.2 | 2.5 ± 2.3 | 2.6 ± 2.2 | 2.4 ± 2.6 | 2.2 ± 2.3 |
There were no statistically significant differences between respiratory variables among the successive tests. Abbreviations: PSV pressure support ventilation; PEEP positive end-expiratory pressure; PEEPi intrinsic positive end-expiratory pressure; RR respiratory rate; Ti inspiratory time; Ttot total respiratory time; VE volume per minute; V T tidal volume
Table 3.
Arterial blood gases and hemodynamic variables during the five different weaning tests and at 20 minutes after extubation
| PSV | PSV | PSV | PSV | T piece | After extubation | |
|---|---|---|---|---|---|---|
| +7 cmH2O PEEP | 0 cmH2O PEEP | +7 cmH2O PEEP | 0 cmH2O PEEP | |||
| +7 cmH2O | +7 cmH2O | 0 cmH2O | 0 cmH2O | |||
| Ph | 7.45 ± 0.06 | 7.44 ± 0.06 | 7.44 ± 0.06 | 7.44 ± 0.06 | 7.43 ± 0.06 | 7.42 ± 0.06 |
| PaCO2, mmHg | 41 ± 11 | 42 ± 11 | 43 ± 12 | 43 ± 12 | 44 ± 13 | 44 ± 10 |
| PaO2/FIO2 | 277 ± 76 | 257 ± 81 | 252 ± 73 | 230 ± 65 | 217 ± 65 | 224 ± 51 |
| SBP, mmHg | 148 ± 22 | 148 ± 26 | 148 ± 26 | 146 ± 30 | 150 ± 18 | 147 ± 24 |
| DBP, mmHg | 72 ± 12 | 71 ± 12 | 73 ± 12 | 72 ± 15 | 69 ± 13 | 70 ± 15 |
| HR, beats/minute | 96 ± 14 | 97 ± 16 | 98 ± 16 | 100 ± 16 | 99 ± 14 | 101 ± 15 |
There were no statistically significant differences between respiratory variables among the successive tests. Abbreviations: DBP diastolic blood pressure, HR heart rate, ND not done, PEEP positive end-expiratory pressure, PSV pressure support ventilation, SBP systolic blood pressure
Inspiratory effort
Figures 3, 4, and 5 show the individual and mean values of the main variables studied, and representative tracings of Pes, Pga and Pdi can be seen in Fig. 6. There was a significant difference in all respiratory effort variables (swings of Pes and Pdi, PTPes and PTPdi, WOB in J/L and in J/min) between the weaning tests and after the extubation period (p < 0.001) (Table 4). Weaning tests performed with positive pressure constantly overestimated post-extubation inspiratory effort. Inspiratory effort measured with either the T tube or PSV 0 + PEEP 0 cmH2O was not different to post-extubation inspiratory effort. We then identified both PSV 0 + PEEP 0 cmH2O and the T-piece trial as the weaning tests that reproduce post-extubation inspiratory effort and the WOB (Additional files 2, 3, 4, 5, 6 and 7).
Fig. 3.

Esophageal (a) and trans-diaphragmatic (b) swings. Individual and mean changes in esophageal and trans-diaphragmatic swings during the five weaning tests and 20 minutes after extubation. All the tests show that the weaning tests that best reproduce respiratory muscle work after extubation were pressure support ventilation (PSV) 0 cmH2O + positive end-expiratory pressure (PEEP) 0 cmH2O and the T piece, with no statistically significant difference between the two. *p < 0.001 when compared with after extubation. Pdi transdiaphragmatic pressure, pes esophageal pressure
Fig. 4.

Esophageal (a) and trans-diaphragmatic (b) pressure time products. Individual and mean changes in esophageal and trans-diaphragmatic pressure time products during the five weaning tests and 20 minutes after extubation. All the tests show that the weaning tests that best reproduce respiratory muscle work after extubation were pressure support ventilation (PSV) 0 cmH2O+ positive end-expiratory pressure (PEEP) 0 cmH2O and the T piece, with no statistically significant difference between the two. *p < 0.001 when compared with after extubation. PTPdi trans-diaphragmatic pressure-time product, PTPes trans-esophageal pressure-time product
Fig. 5.
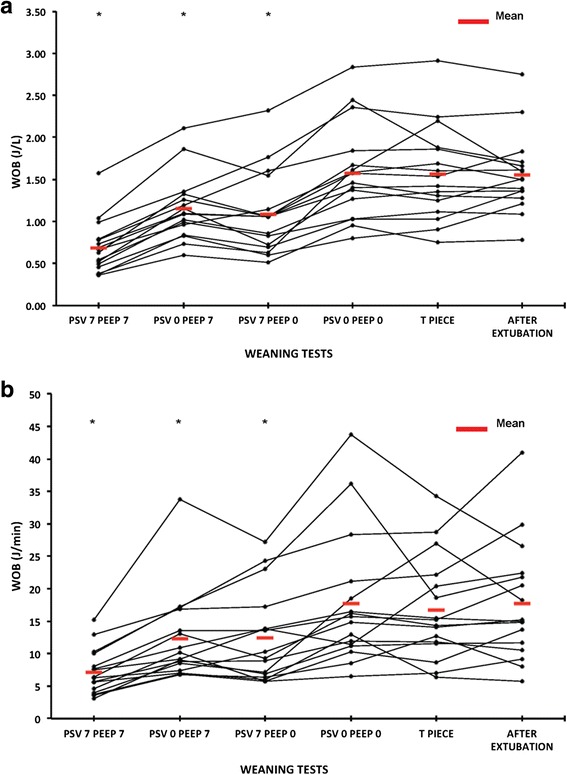
Work of breathing (WOB) in J/L (a) and in J/minute (b). Individual and mean changes in the WOB during the five weaning tests and 20 minutes after extubation. All the tests show that the weaning tests that best reproduced respiratory muscle work after extubation were pressure support ventilation (PSV) 0 cmH2O cmH2O + positive end-expiratory pressure (PEEP) 0 cmH2O and the T piece, with no statistically significant difference between the two. *p < 0.001 when compared with after extubation
Fig. 6.
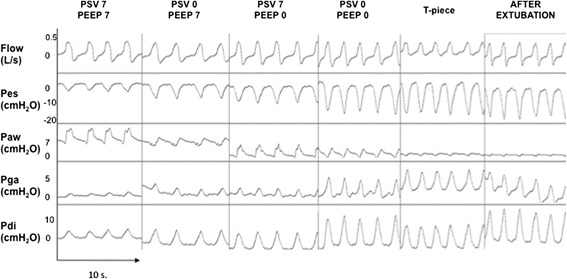
Ventilatory pattern during the five weaning tests and twenty minutes after extubation. One patient is presented with the acquisition of flow (L/s), esophageal (Pes, cmH2O), airway (Paw, cmH2O), gastric (Pga, cmH2O) and trans-diaphragmatic (Pdi, cmH2O) pressure signals. PSV pressure support ventilation, PEEP positive end-expiratory pressure
Table 4.
Inspiratory muscle effort during the five different weaning tests and 20 minutes after extubation
| PSV | PSV | PSV | PSV | T piece | After extubation | |
|---|---|---|---|---|---|---|
| +7 cmH2O PEEP | 0 cmH2O PEEP | +7 cmH2O PEEP | 0 cmH2O PEEP | |||
| +7 cmH2O | +7 cmH2O | 0 cmH2O | 0 cmH2O | |||
| Swing Pes, cmH2O | 7.2 ± 5.0* | 13.4 ± 5.5* | 12.3 ± 6.3* | 19.1 ± 7.7 | 19.8 ± 7 | 19.1 ± 5.4 |
| Swing Pdi, cmH2O | 8.4 ± 5.5* | 15.4 ± 5.7* | 14.2 ± 6.4* | 21.2 ± 8.1 | 21.7 ± 7.0 | 20.9 ± 5.5 |
| PTP es, cmH2O.s/minute | 141 ± 54* | 259 ± 84* | 231 ± 82* | 346 ± 97 | 332.9 ± 85.9 | 365 ± 87 |
| PTP di, cmH2O.s/minute | 157 ± 80* | 318 ± 113* | 302 ± 111* | 451 ± 151 | 439 ± 152 | 465 ± 117 |
| WOB, J/L | 0.69 ± 0.31* | 1.15 ± 0.39* | 1.09 ± 0.49* | 1.58 ± 0.57 | 1.57 ± 0.56 | 1.56 ± 0.5 |
| WOB, J/minute | 7.15 ± 3.5* | 12.2 ± 6.8* | 12.4 ± 7.1* | 17.7 ± 10.2 | 16.8 ± 8.0 | 17.8 ± 9.1 |
Abbreviations: Pdi trans-diaphragmatic pressure, PEEP positive end-expiratory pressure, Pes esophageal pressure, PTPdi trans-diaphragmatic pressure time product, PTPes esophageal pressure time product, PSV pressure support ventilation, WOB work of breathing. *p < 0.001 when compared with after extubation
Discussion
To our knowledge, this is the first physiological study that specifically investigates the inspiratory effort during weaning of mechanical ventilation in a population of critically ill morbidly obese patients. The main result of this study is that for obese patients, the T piece and PSV 0 + PEEP 0 cmH2O weaning tests are the tests that best predict post-extubation inspiratory effort and WOB.
Because of a lack of consensus on the best test to use before extubation in this population, we aimed to determine which one reflects the breathing effort after extubation. Some authors described extubation of obese patients after a 30-minute period of CPAP 5 cmH2O [14], others after a trial of FiO2 100 % combined with a CPAP of 10 cmH2O. [15] An ongoing multicenter observational study in France (FREEREA study), will provide some epidemiological data about weaning and extubation in this particular population. The preliminary results (unpublished) show that among 64 critically ill morbidly obese patients extubated, 22 (34 %) were extubated after a T tube, 28 (44 %) after a low PSV trial, 12 (19 %) with no spontaneous breathing trial and 2 (3 %) after a different weaning trial. These data justify our study as there is wide heterogeneity of extubation practice in this population, with a high proportion of patients being extubated from a substantial level of support.
Our study presents limitations. First, we investigated the inspiratory effort indexes twenty minutes after extubation and the study was not designed to explore long-term consequences of several weaning tests on oxygenation, end-expiratory lung volume or outcome. Because outcome was not a study endpoint, we cannot make any final recommendation about which weaning test is associated with the highest rate of weaning success. Ideally, a weaning test would perfectly predict the ability of the patient to breathe alone and without being ventilatory assisted by simulating the post-extubation respiratory constraint [26]. Second, post-extubation intermittent non-invasive ventilation is routinely used in our unit for high-risk patients [14, 21] to rest the inspiratory muscles and improve lung aeration. It may have contributed to our low rate of re-intubation (6 %).
The present study focused on morbidly obese patients and found results consistent with the studies published by Straus et al. [25] and Cabello et al. [8], which included non-obese patients. We report that the T piece and PSV 0 + PEEP 0 cmH2O weaning tests were the two tests that best approximated the WOB after extubation. We also found that the PSV 7 + PEEP 0 cmH2O test leads to a major underestimation of the WOB after extubation in obese patients with significantly less inspiratory effort in comparison with both the T piece test and 20 minutes after extubation. Straus et al demonstrated that post-extubation WOB was well-approximated by the WOB during a T-piece test and that the endotracheal tube was responsible for about 11 % of the total work of breathing. [25] More recently, Cabello et al. compared a spontaneous breathing trial on a T-piece with low PSV (7 cm H2O) with or without PEEP in a subpopulation of patients with heart failure who were difficult to wean. [8] The authors concluded that performing the weaning test while maintaining a positive pressure in the circuit underestimates the post-extubation WOB and unmasks a possible effect on left ventricular function, and suggested the T piece as the weaning test of choice in these patients.
In a landmark physiological study, Brochard et al. demonstrated that breathing through the T piece overestimates the WOB by 27 ± 18 % compared to the post-extubation period [26]. Contrary to the present study, Brochard et al. included a high proportion of patients with chronic obstructive pulmonary disease, and used ventilators with higher ventilatory circuit-resistive load [28] and lower pressurization performance, especially in terms of inspiratory-trigger-imposed WOB [29, 30].
As compared to the literature on non-obese patients, WOB values evaluated in the present study were higher [26, 31]. In morbidly obese patients, an elevation of pharyngeal collapsibility and upper airway resistance related to fatty deposits on pharynx and oral soft tissue and associated with local inflammation can increase the WOB [32]. Weaning trials performed with positive pressure underestimated post-extubation WOB by 33 % (0.5 J/L) up to 50 % (0.8 J/L) according to the ventilator setting. An increase of 0.5–0.8 J/L represents a significant additional workload, as WOB in healthy subjects during quiet breathing is about 0.35–0.5 J/L [33, 34]. Furthermore, WOB ≥0.8 J/L has been reported as being associated with weaning failure [35]. Extubating an obese patient after having performed a weaning test without positive pressure could lead to early onset atelectasis if the patient was unable to control for end-expiratory lung volume without PEEP.
Conclusions
For the first time the present study reports new insights into respiratory physiology in morbidly obese critically ill candidates to be weaned from the ventilator. These data may be useful for clinicians managing these challenging patients and help make difficult decisions about extubation. We report that either a T piece or a PSV 0 and PEEP 0 cmH2O test are the trials that predict post-extubation work of breathing in morbidly obese patients. The consequences on mid-term oxygenation and lung aeration, and on the weaning success rate of such weaning tests were, however, not studied.
Additional files
Supplementary material. (DOCX 44 kb)
Figure S1. difference in esophageal pressure between each test and the post-extubation period. Dashed line represents the absence of difference between the test and the post-extubation period. (JPG 44 kb)
Figure S2. difference in the trans-diaphragmatic pressure between each test and the post-extubation period. Dashed line represents the absence of difference between the test and the post-extubation period. (JPG 48 kb)
Figure S3. difference in the esophageal pressure time product between each test and the post-extubation period. Dashed line represents the absence of difference between the test and the post-extubation period. (JPG 49 kb)
Figure S4. difference in the trans-diaphragmatic pressure time product between each test and the post-extubation period. Dashed line represents the absence of difference between the test and the post-extubation period. (JPG 47 kb)
Figure S5. difference in the work of breathing expressed in J/l between each test and the post-extubation period. Dashed line represents the absence of difference between the test and the post-extubation period. (JPG 44 kb)
Figure S6. difference in the work of breathing expressed in J/min between each test and the post-extubation period. Dashed line represents the absence of difference between the test and the post-extubation period. (JPG 44 kb)
Acknowledgements
We thank Albert Prades, Research Nurse, MSc, Department of Critical Care Medicine and Anesthesiology, Saint Eloi Teaching Hospital, Montpellier, France for his help conducting research in this topic.
Funding
Departmental resources funded the present study.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Authors’ contributions
MM and BJ contributed equally to this work. MM performed the study, analyzed the data and wrote the manuscript. BJ participated in the study design and wrote the manuscript. FG analyzed the data and made critical manuscript revisions. RV analyzed the data and made critical manuscript revisions. YC helped in designing the study, enrolled patients, analyzed the data and made critical manuscript revisions. NM and AJ performed the statistical analysis and made critical manuscript revisions. SM helped in designing the study and extensively corrected the manuscript. GC helped in designing the study and extensively corrected the manuscript. SJ designed the study, analyzed the data and made critical manuscript revisions. LB analyzed the data and made critical manuscript revisions. All authors read and approved the final manuscript.
Competing interests
Martin Mahul has nothing to disclose; Boris Jung reports personal fees from Merck (Whitehouse station, NJ) and Astellas (Tokyo, Japan) unrelated to the present study; Fabrice Gallia has nothing to disclose; Nicolas Molinari has nothing to disclose; Audrey De Jong has nothing to disclose; Yannaël Coisel has nothing to disclose; Rosanna Vaschetto has nothing to disclose; Stefan Matecki has nothing to disclose; Gerald Chanques has nothing to disclose; Laurent Brochard reports a research contract with Draeger, General Electric and Covidien and honorarium from Draeger unrelated to the present study; Samir Jaber reports personal fees from Maquet, Draeger, Hamilton Medical, Fisher Paykel and Abbott unrelated to the present study.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the Montpellier Teaching Hospital (2012 A-00294-39, Comité de Protection des Personnes Sud Méditerranée III, Montpellier, France), and registered on clinical trial.gov (reference NCT01616901). All patients provided their written informed consent.
Abbreviations
- BMI
Body mass index
- CPAP
Continuous positive airway pressure
- FiO2
Inspired oxygen fraction
- ICU
Intensive Care Unit
- Pdi
Trans-diaphragmatic pressure
- PEEP
Positive end-expiration pressure
- Pes
Esophageal pressure
- Pga
Gastric pressure
- PSV
Pressure support ventilation
- PTPdi
Trans-diaphragmatic pressure-time product
- PTPes
Trans-esophageal pressure-time product
- RR
Respiratory rate
- SBT
Spontaneous breathing trial
- SpO2
Pulse arterial oxygen saturation
- Te
Expiratory time
- Ti
Inspiratory time
- Ttot
Total cycle duration
- VE
Minute ventilation
- VT
Tidal volume
- WOB
Work of breathing
Contributor Information
Martin Mahul, Email: m-mahul@chu-montpellier.fr.
Boris Jung, Email: b-jung@chu-montpellier.fr.
Fabrice Galia, Email: f-galia@chu-montpellier.fr.
Nicolas Molinari, Email: n-molinari@chu-montpellier.fr.
Audrey de Jong, Email: a-de_jong@chu-montpellier.fr.
Yannaël Coisel, Email: yannacoi@free.fr.
Rosanna Vaschetto, Email: rosanna.vaschetto@med.unipmn.it.
Stefan Matecki, Email: s-matecki@chu-montpellier.fr.
Gérald Chanques, Email: g-chanques@chu-montpellier.fr.
Laurent Brochard, Email: BrochardL@smh.ca.
Samir Jaber, Phone: (33) 4 67 33 72 71, Email: s-jaber@chu-montpellier.fr.
References
- 1.Sellares J, Ferrer M, Cano E, Loureiro H, Valencia M, Torres A. Predictors of prolonged weaning and survival during ventilator weaning in a respiratory ICU. Intensive Care Med. 2011;37:775–84. doi: 10.1007/s00134-011-2179-3. [DOI] [PubMed] [Google Scholar]
- 2.Brochard L, Rauss A, Benito S, Conti G, Mancebo J, Rekik N, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med. 1994;150:896–903. doi: 10.1164/ajrccm.150.4.7921460. [DOI] [PubMed] [Google Scholar]
- 3.Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–56. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 4.Thille AW, Richard J-CM, Brochard L. The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187:1294–302. doi: 10.1164/rccm.201208-1523CI. [DOI] [PubMed] [Google Scholar]
- 5.Jubran A, Grant BJB, Laghi F, Parthasarathy S, Tobin MJ. Weaning prediction: esophageal pressure monitoring complements readiness testing. Am J Respir Crit Care Med. 2005;171:1252–9. doi: 10.1164/rccm.200503-356OC. [DOI] [PubMed] [Google Scholar]
- 6.MacIntyre NR, Cook DJ, Ely EW, Jr, Epstein SK, Fink JB, Heffner JE, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120:375S–95. doi: 10.1378/chest.120.6_suppl.375S. [DOI] [PubMed] [Google Scholar]
- 7.McConville JF, Kress JP. Weaning patients from the ventilator. New England J Med. 2012;367:2233–9. doi: 10.1056/NEJMra1203367. [DOI] [PubMed] [Google Scholar]
- 8.Cabello B, Thille AW, Roche-Campo F, Brochard L, Gómez FJ, Mancebo J. Physiological comparison of three spontaneous breathing trials in difficult-to-wean patients. Intensive Care Med. 2010;36:1171–9. doi: 10.1007/s00134-010-1870-0. [DOI] [PubMed] [Google Scholar]
- 9.Tobin MJ. Extubation and the myth of “minimal ventilator settings”. Am J Respir Crit Care Med. 2012;185:349–50. doi: 10.1164/rccm.201201-0050ED. [DOI] [PubMed] [Google Scholar]
- 10.Jones RL, Nzekwu M-MU. The effects of body mass index on lung volumes. Chest. 2006;130:827–33. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 11.Kress JP, Pohlman AS, Alverdy J, Hall JB. The impact of morbid obesity on oxygen cost of breathing (VO(2RESP)) at rest. Am J Respir Crit Care Med. 1999;160:883–6. doi: 10.1164/ajrccm.160.3.9902058. [DOI] [PubMed] [Google Scholar]
- 12.Littleton SW. Impact of obesity on respiratory function. Respirology. 2012;17:43–9. doi: 10.1111/j.1440-1843.2011.02096.x. [DOI] [PubMed] [Google Scholar]
- 13.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol. 2010;108:206–11. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- 14.El-Solh AA, Aquilina A, Pineda L, Dhanvantri V, Grant B, Bouquin P. Noninvasive ventilation for prevention of post-extubation respiratory failure in obese patients. Eur Respir J. 2006;28:588–95. doi: 10.1183/09031936.06.00150705. [DOI] [PubMed] [Google Scholar]
- 15.Zoremba M, Kalmus G, Begemann D, Eberhart L, Zoremba N, Wulf H, et al. Short term non-invasive ventilation post-surgery improves arterial blood-gases in obese subjects compared to supplemental oxygen delivery - a randomized controlled trial. BMC Anesthesiol. 2011;11:10. doi: 10.1186/1471-2253-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–the evidence report. national institutes of health. Obes Res. 1998;6 Suppl 2:51S–209S. [PubMed]
- 17.Persichini R, Gay F, Schmidt M, Mayaux J, Demoule A, Morélot-Panzini C, et al. Diagnostic accuracy of respiratory distress observation scales as surrogates of dyspnea self-report in intensive care unit patients. Anesthesiology. 2015;123:830–7. doi: 10.1097/ALN.0000000000000805. [DOI] [PubMed] [Google Scholar]
- 18.Coisel Y, Chanques G, Jung B, Constantin J-M, Capdevila X, Matecki S, et al. Neurally adjusted ventilatory assist in critically ill postoperative patients: a crossover randomized study. Anesthesiology. 2010;113:925–35. doi: 10.1097/ALN.0b013e3181ee2ef1. [DOI] [PubMed] [Google Scholar]
- 19.Clavieras N, Wysocki M, Coisel Y, Galia F, Conseil M, Chanques G, et al. Prospective randomized crossover study of a new closed-loop control system versus pressure support during weaning from mechanical ventilation. Anesthesiology. 2013;119:631–41. doi: 10.1097/ALN.0b013e3182952608. [DOI] [PubMed] [Google Scholar]
- 20.Baillard C, Fosse J-P, Sebbane M, Chanques G, Vincent F, Courouble P, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med. 2006;174:171–7. doi: 10.1164/rccm.200509-1507OC. [DOI] [PubMed] [Google Scholar]
- 21.Jaber S, Chanques G, Jung B. Postoperative noninvasive ventilation. Anesthesiology. 2010;112:453–61. doi: 10.1097/ALN.0b013e3181c5e5f2. [DOI] [PubMed] [Google Scholar]
- 22.Deye N, Lellouche F, Maggiore SM, Taillé S, Demoule A, L’Her E, et al. The semi-seated position slightly reduces the effort to breathe during difficult weaning. Intensive Care Med. 2013;39:85–92. doi: 10.1007/s00134-012-2727-5. [DOI] [PubMed] [Google Scholar]
- 23.Jaber S, Carlucci A, Boussarsar M, Fodil R, Pigeot J, Maggiore S, et al. Helium-oxygen in the postextubation period decreases inspiratory effort. Am J Respir Crit Care Med. 2001;164:633–7. doi: 10.1164/ajrccm.164.4.2008027. [DOI] [PubMed] [Google Scholar]
- 24.Sassoon CS, Light RW, Lodia R, Sieck GC, Mahutte CK. Pressure-time product during continuous positive airway pressure, pressure support ventilation, and T-piece during weaning from mechanical ventilation. Am Rev Respir Dis. 1991;143:469–75. doi: 10.1164/ajrccm/143.3.469. [DOI] [PubMed] [Google Scholar]
- 25.Straus C, Louis B, Isabey D, Lemaire F, Harf A, Brochard L. Contribution of the endotracheal tube and the upper airway to breathing workload. Am J Respir Crit Care Med. 1998;157:23–30. doi: 10.1164/ajrccm.157.1.96-10057. [DOI] [PubMed] [Google Scholar]
- 26.Brochard L, Rua F, Lorino H, Lemaire F, Harf A. Inspiratory pressure support compensates for the additional work of breathing caused by the endotracheal tube. Anesthesiology. 1991;75:739–45. doi: 10.1097/00000542-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Mehta S, Nelson DL, Klinger JR, Buczko GB, Levy MM. Prediction of post-extubation work of breathing. Crit Care Med. 2000;28:1341–6. doi: 10.1097/00003246-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Lyazidi A, Thille AW, Carteaux G, Galia F, Brochard L, Richard J-CM. Bench test evaluation of volume delivered by modern ICU ventilators during volume-controlled ventilation. Intensive Care Med. 2010;36:2074–80. doi: 10.1007/s00134-010-2044-9. [DOI] [PubMed] [Google Scholar]
- 29.Jaber S, Tassaux D, Sebbane M, Pouzeratte Y, Battisti A, Capdevila X, et al. Performance characteristics of five new anesthesia ventilators and four intensive care ventilators in pressure-support mode: a comparative bench study. Anesthesiology. 2006;105:944–52. doi: 10.1097/00000542-200611000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Thille AW, Lyazidi A, Richard J-CM, Galia F, Brochard L. A bench study of intensive-care-unit ventilators: new versus old and turbine-based versus compressed gas-based ventilators. Intensive Care Med. 2009;35:1368–76. doi: 10.1007/s00134-009-1467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nathan SD, Ishaaya AM, Koerner SK, Belman MJ. Prediction of minimal pressure support during weaning from mechanical ventilation. Chest. 1993;103:1215–9. doi: 10.1378/chest.103.4.1215. [DOI] [PubMed] [Google Scholar]
- 32.Zerah F, Harf A, Perlemuter L, Lorino H, Lorino AM, Atlan G. Effects of obesity on respiratory resistance. Chest. 1993;103:1470–6. doi: 10.1378/chest.103.5.1470. [DOI] [PubMed] [Google Scholar]
- 33.Vaschetto R, De Jong A, Conseil M, Galia F, Mahul M, Coisel Y, et al. Comparative evaluation of three interfaces for non-invasive ventilation: a randomized cross-over design physiologic study on healthy volunteers. Crit Care. 2014;18:R2. doi: 10.1186/cc13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mancebo J, Isabey D, Lorino H, Lofaso F, Lemaire F, Brochard L. Comparative effects of pressure support ventilation and intermittent positive pressure breathing (IPPB) in non-intubated healthy subjects. Eur Respir J. 1995;8:1901–9. doi: 10.1183/09031936.95.08111901. [DOI] [PubMed] [Google Scholar]
- 35.Kirton OC, DeHaven CB, Morgan JP, Windsor J, Civetta JM. Elevated imposed work of breathing masquerading as ventilator weaning intolerance. Chest. 1995;108:1021–5. doi: 10.1378/chest.108.4.1021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material. (DOCX 44 kb)
Figure S1. difference in esophageal pressure between each test and the post-extubation period. Dashed line represents the absence of difference between the test and the post-extubation period. (JPG 44 kb)
Figure S2. difference in the trans-diaphragmatic pressure between each test and the post-extubation period. Dashed line represents the absence of difference between the test and the post-extubation period. (JPG 48 kb)
Figure S3. difference in the esophageal pressure time product between each test and the post-extubation period. Dashed line represents the absence of difference between the test and the post-extubation period. (JPG 49 kb)
Figure S4. difference in the trans-diaphragmatic pressure time product between each test and the post-extubation period. Dashed line represents the absence of difference between the test and the post-extubation period. (JPG 47 kb)
Figure S5. difference in the work of breathing expressed in J/l between each test and the post-extubation period. Dashed line represents the absence of difference between the test and the post-extubation period. (JPG 44 kb)
Figure S6. difference in the work of breathing expressed in J/min between each test and the post-extubation period. Dashed line represents the absence of difference between the test and the post-extubation period. (JPG 44 kb)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


