Abstract
Fourteen cases with 18 grossly expansive lesions diagnosed over a period of 15 years as either “familial gigantiform cementoma” or “osseous dysplasia with jaw expansion” in an African population sample were reviewed. Eight lesions occurred in the anterior mandible, the maxilla was affected by four, three patients presented with more than one lesion and the most common associated pathologies were tooth displacement, conventional non expanding florid osseous dysplasia and simple bone cyst. No history of similar lesions in relatives of the diseased were recorded. The radiolucent fibrous component contained globular bone deposits and cellular osteoid with trabecular differentiation which matured into radiodense mineralized masses. Resorption of the cellular bone created cavities which are proposed to represent the early stage of simple bone cyst formation. It is recommended that “expansive osseous dysplasia” replace the out-dated term “familial gigantiform cementoma”. The differential diagnoses of expansive osseous dysplasias are discussed.
Keywords: Osseous dysplasia, Gigantiform cementoma, Fibro osseous lesion
Introduction
Osseous dysplasias (OD’s) are defined as idiopathic dysplastic processes located in the apical region of the tooth bearing areas of the jaws and are characterized by replacement of normal bone by fibrous tissue and metaplastic bone [1]. They are usually asymptomatic unless pain due to secondary infection or expansion attracts attention to the lesion. OD’s are rare in young patients and have a predilection for middle-aged black females [2]. They occur in three distinct clinical forms. Periapical OD’s involve the periapical areas of a few mandibular incisor teeth. A single lesion of a similar nature but located in the posterior mandible is designated focal OD. Florid OD’s involve more than one jaw quadrant and may show minimal expansion [2–4]. Familial gigantiform cementoma [4], a rare form of OD, [1] is a term coined after case reports appeared on a rare autosomal dominant inherited disease with grossly expansive “cemental” masses [5, 6]. Sporadic familial gigantiform cementomas without a familial history were subsequently reported [4, 7] and although more common than the familial type, the controversial term “familial gigantiform cementoma” remains unchanged in the modern literature [1]. Maturation of conventional OD’s occurs in three radiologic phases. Early lesions are predominantly radiolucent and after passing through a mixed radiolucent-radiodense stage, they ultimately become lobulated, radiodense and cease to enlarge further [1]. Microscopically OD’s lack a capsule and consist of a mixture of cellular fibrous connective tissue with woven- and lamellar bone trabecula and relatively acellular concentric mineralized deposits referred to as “cementum-like” material [1].
The goals of this study were to record the microscopic features of OD’s that manifest with gross expansion and provide guidelines that distinguish them from other expansive fibro-osseous proliferations. In order to avoid confusion the term “expansive osseous dysplasia” will be used throughout the manuscript to describe the lesions recorded.
Materials and Methods
The files of an Oral and Maxillofacial Pathology laboratory which serves a rural and peri-urban African population sample, were reviewed for cases confirmed microscopically as OD’s with gross expansion between 2000 and 2015. Conventional florid OD’s with minimal expansion were excluded.
Results
During the study period a total of more than 3000 OD’s were diagnosed radiographically in the patient cohort studied. Amongst these cases eighteen lesions in fourteen patients were confirmed as OD’s with gross expansion (Fig. 1). The radiological appearances of nine of these lesions were reported in 2011 [8]. Eight lesions in the present study were in the anterior mandible (Fig. 2), the maxilla was affected by four, three patients presented with more than one expansive lesion and the most common associated pathologies were conventional florid OD (which showed no clinical signs of expansion) (eight patients), tooth displacement (10 lesions) and simple bone cysts (5 lesions). Tables 1 and 2 reflect the gender, age, location, radiological features and associated pathologies of the eleven patients with solitary and three with multiple expansive OD’s respectively. None of the patients gave a history of similar lesions in relatives. No genetic data were available on the patients. The biopsies and excision specimen showed well demarcated fibrous proliferations containing a mixture of bone and globular mineralized deposits. Small foci of cortical perforation were seen only in large lesions, soft tissue invasion was limited and attachment to root surfaces of adjacent teeth and merger with cortical bone could not be demonstrated. Most lesions were unifocal with only one lesion demonstrating a multifocal confluent growth pattern (Fig. 3). The peripheral radiolucent zone of lesser mineralized areas corresponded with cellular fibro-osseous tissue seen microscopically. In more mature parts of the lesion, dense confluent mineralized deposits were present (Fig. 4). Two morphologic types of mineralised tissue, the ratios of which varied from case to case, were present. Hypocellular globular masses enlarged and merged through deposition of metaplastic bone on their surfaces. These masses frequently demonstrated Sharpey- like fibres which radiated into the connective tissue. Maturity of the globular masses was reached when bone apposition ceased and a distinct basophilic line appeared on its surface which demarcated it from the surrounding connective tissue (Fig. 5). The characteristic radiodense masses developed through a gradual process of enlargement- and merging. The cellularity of the connective tissue between these masses decreased with advancing maturation of the mineralized component. The second morphological type of mineralized tissue was cellular osteoid which failed to show osteoblastic rimming or mitotic activity and matured into more discrete bony trabecula (Fig. 6). These deposits were distributed haphazardly between the globular bone masses. Osteoclastic activity was associated only with the second bone type and not the globular bone deposits, unless inflammation due to secondary infection was present. Resorption of the cellular bone created fluid filled spaces which appeared to coalesce forming smooth surfaced voids (Fig. 7) which were macroscopically visible in the excision specimen. The confluent mineralized structures in mature lesions showed macroscopically a ginger root-appearance in the excision specimen (Fig. 8) which could be shelled out of the cortical bone plates. Confluence and lamellar transformation of both types of bone occurred in the mature parts of a lesion.
Fig. 1.
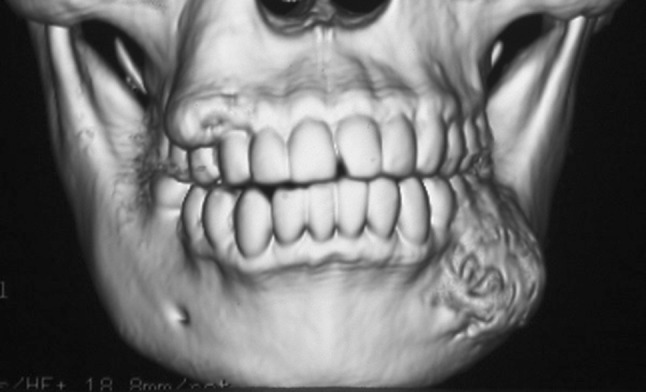
CBCT 3-D reconstruction of a lesion in the right mandible of Case 7 showing buccal expansion
Fig. 2.
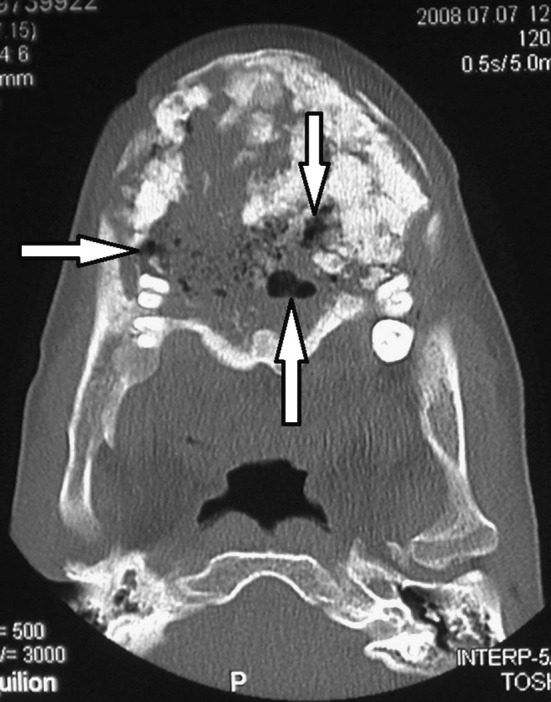
Axial view of an expansive osseous dysplasia in the anterior, left and right mandible of case 1. Note the foci of simple bone cyst formation (arrows)
Table 1.
Age, gender, size, site and radiology of patients with single expansive OD’s
| Case | Gender | Age | Size cm | Site | Radiological appearance | Tooth displacement | SBC | FOD |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 26 | 18 | Ant, L & R mand | Mixed with lobular radiopacities, cortical perforation in small foci | Yes | Yes | Yes |
| 2 | F | 6 | 8 | L max | Narrow radiolucent border, lobulated radiodensity | Yes | No | No |
| 3 | F | 35 | 5 | Ant mand | Wide radiolucent border, lobulated radiodensities | Yes | No | Yes |
| 4 | M | Adult | 4 | Ant mand | Narrow radiolucent border, lobulated radiodensity | No | No | Yes |
| 5 | F | 69 | 4 | Ant mand | Wide radiolucent border, lobulated radiodensity | Yes | Yes | Yes |
| 6 | F | 34 | 6 | Ant mand | Mixed with lobular radiopacities, cortical perforation in small foci | Yes | No | No |
| 7 | F | 42 | 3 | R mand | Mixed with lobular radiopacities | No | No | No |
| 8 | F | 38 | 4 | L mand | Radiolucent, at operation multiple OD’s in wall of cystic space | No | Yes | No |
| 9 | F | 52 | 5 | Ant mand | Narrow radiolucent border, lobular radiodensities | No | No | No |
| 10 | F | 40 | 4.5 | L max | Wide lucent border, lobular radiopacities | Yes | No | Yes |
| 11 | F | 31 | 4 | L mand | Mixed with lobular radiopacities | No | No | Yes |
Table 2.
Age, gender, size, site and radiology of patients with multiple expansive OD’s
| Case | Gender | Age | Size cm | Site | Radiological appearance | Tooth displacement | SBC | FOD |
|---|---|---|---|---|---|---|---|---|
| 12 | F | 43 | 5 | L mand | Narrow radiolucent border, mixed radiolucent radiopaque with lobular masses | No | Yes | Yes |
| 4.5 | Ant mand | Narrow radiolucent border, Mixed radiolucent radiopaque with lobular masses | Yes | No | Yes | |||
| 4.5 | R mand | Mixed radiolucent radiopaque | No | Yes | Yes | |||
| 13 | M | 17 | 5 | R Max | Confluence of 3 lesions with lobular radiopaque masses | Yes | No | No |
| 7 | L Mand | Mixed radiolucent radiopaque with lobular radiodense masses, cortical perforation in small foci | Yes | No | No | |||
| 14 | F | 40 | 4 | L max | Mixed radiolucent with small radiodense masses | Yes | No | Yes |
| 7 | Ant mand | Mixed radiolucent radiopaque with lobular radiodense mass, cortical perforation in small foci | Yes | No | Yes |
Fig. 3.
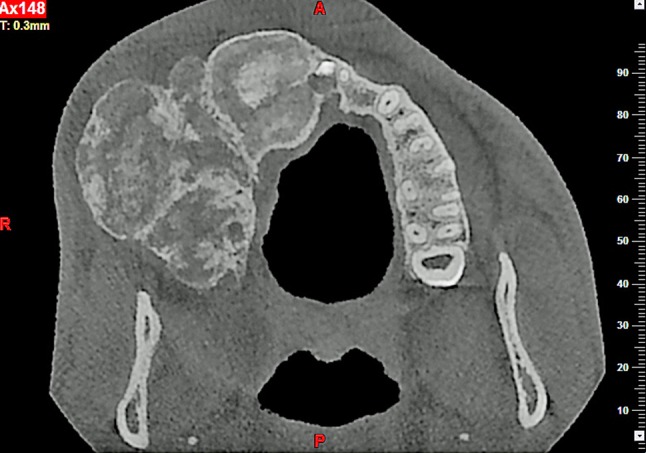
Case 13: Axial view of the multifocal confluent lesion in the right maxilla
Fig. 4.
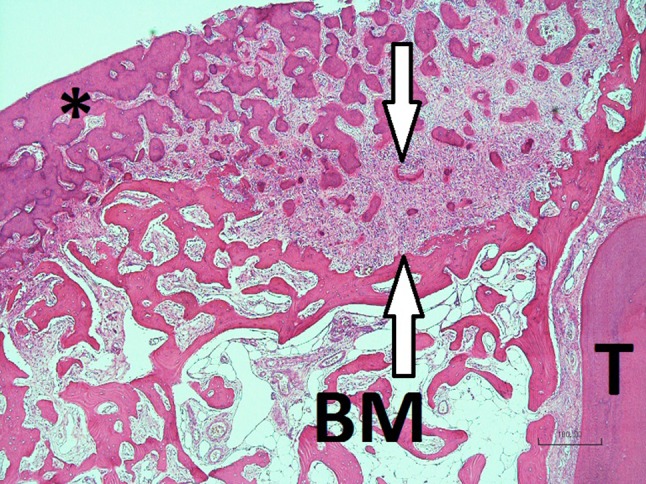
Micrograph showing the fibrous nature of the radiolucent peripheral border (between the white arrows, the more mature confluent mineralized mass (asterisk), mandibular trabecular bone and BM bone marrow and T tooth (Case 1, H&E stain, ×40)
Fig. 5.
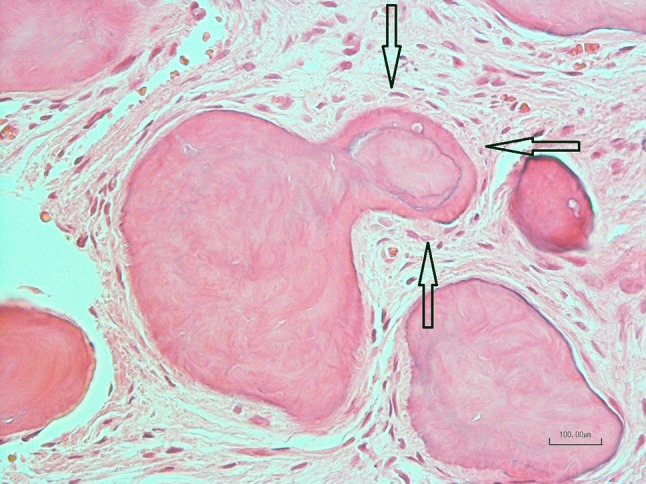
Globular mineralized deposit with metaplastic bone deposits on the surface (arrows) in moderately cellular fibrous connective tissue. Note the basophilic line on the surface of the deposits (anterior mandibular lesion, Case 12, H&E stain, ×200)
Fig. 6.
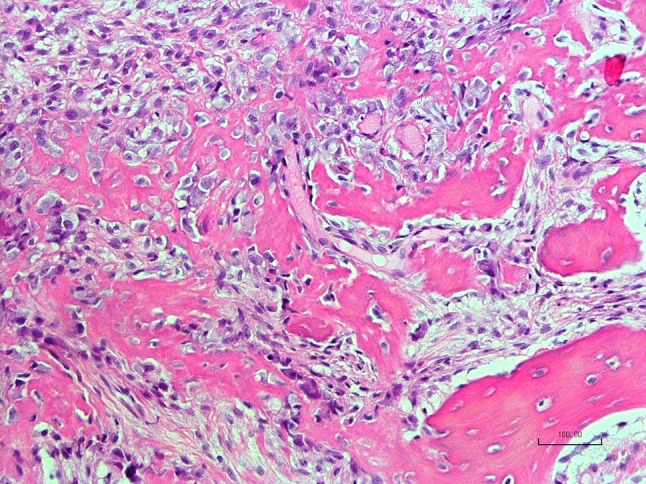
Delicate cellular osteoid showing maturation into trabecular bone (anterior mandibular lesion, Case 12, H&E stain, ×200)
Fig. 7.
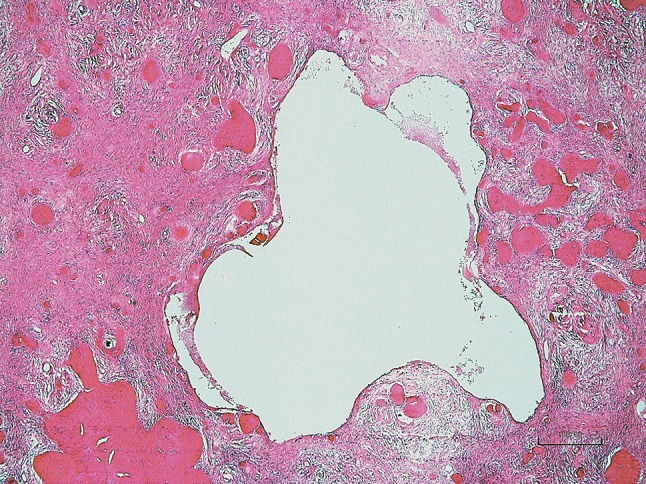
Large cavity which is macroscopically recognisable on cut surface of a specimen (Case 3, H&E stain, ×100)
Fig. 8.
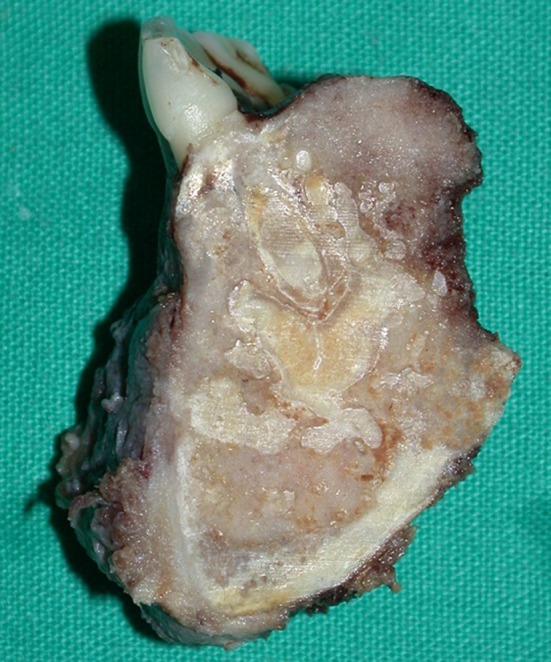
Macroscopic view of a section through the excision specimen of Case 4. Note the cortical expansion and the lobulated ginger root-like bone deposits in the lesion
Discussion
OD is a common fibro-osseous lesion particularly in black Africans and East Asians. The lesions are generally asymptomatic and most are diagnosed on radiographs taken for non-related clinical indications. OD’s are biologically distinct from neoplastic fibro-osseous lesions due to their limited growth potential and maturation which are key radiographic- and microscopic diagnostic features [1]. OD’s with gross expansion, referred to in the 2005 WHO classification as ‘familial gigantiform cementoma’ are rare [1]. Expansive OD’s accounted for less than 1 % of all OD’s in our sample. No history of similar lesions in relatives could be obtained and we therefore postulate that the familial subtype is rare in our population. This is supported by another South African study [9] which recorded twenty-eight cases all of which were sporadic and without a family history. The predominance of females in our sample corresponded with the literature [7, 9] and the average age of 34.6 years was slightly younger than for the conventional OD’s [10, 11]. The occurrence of conventional non expanding OD’s affecting young patients was extremely rare in our sample. If an OD occurs in a young patient it may indicate a future expansive growth potential as three of our patients were younger than 30 years. The most common site of involvement of our expansive OD’s was the anterior mandible with only four lesions affecting the maxilla. Three patients presented with multiple lesions and five lesions showed simple bone cysts, supporting the well documented association between the two pathologies [12]. Eight patients had conventional florid OD in other parts of the jaw bones without clinical- or radiological signs of expansion.
The lesions in our study were well demarcated and even in the mature lesions, a thin fibrous band separated the mineralized mass from the surrounding bone. One case showed confluent growth of multifocal lesions (Case 13). It is theoretically possible for nerves and other anatomical structures to become assimilated in such growths, and the presence thereof within a lesion should not raise the prospect of a diagnosis of an invasive tumor. The study failed to demonstrate involvement of the periodontal ligaments and roots of adjacent teeth. This supports a recommendation made by Waldron and Giansanti in 1973 [13] to abolish the use of the term “cementum” in terminology applied to jaw tumors not attached to tooth root surfaces. The radiolucent parts of the lesions corresponded microscopically with areas in which cellular fibrous tissue dominated. In the fibrous component early trabecular- and globular mineralized deposits were present which followed distinct patterns of maturation. This occurred through enlargement and confluence of the globular- and trabecular mineralized deposits into large masses which obliterated the spaces occupied by the fibrous connective tissue. The fibrous tissue progressively became less cellular and more collagenous, possibly as a result of the hypoxia resulting from the increased mineralized tissue content which impinged on the blood supply. In our sample the globular masses frequently showed Sharpey-like fibres radiating from the surface and as soon as bone apposition stopped, the mass becomes framed by a distinct basophilic line. When this stage is reached, the potential for enlargement of the globular mass diminished as no active osteoblasts could be demonstrated on the framed bone surfaces. In non-infected cases, osteoclast-induced resorption of the globular masses did not occur, probably due to the lack of a supportive metabolic environment. In the excision specimen of mature OD’s the characteristic curvilinear mineralized masses resemble “ginger roots” [11]. From a diagnostic point of view, maturation of the mineralized component distinguishes OD’s from other fibro-osseous lesions such as fibrous dysplasias and ossifying fibromas where the microscopic appearance is more uniform and lobular radiodense masses fail to develop. Fibrous dysplasia occurs at a younger age and in a different clinical setting, with merging between the uniform metaplastic bone and the surrounding normal bone a prominent microscopic feature. The only maturation observed in craniofacial fibrous dysplasia is parallel ordering of bone trabecula and lamellar transformation [13]. Ossifying fibromas, which are not intimately associated with the roots of teeth [11], cause characteristic downward bowing of the inferior cortex of the mandible [14], are single and not associated with simple bone cysts or florid OD. Although both trabecular- and psammomatoid mineralized tissue may be present in both expansive OD’s and ossifying fibromas, maturation into large mineralized masses do not occur in ossifying fibromas. The fibrous component in ossifying fibromas frequently has a vague storiform appearance and aneurysmal bone cyst formation is common in the juvenile variety [15, 16], which are both not features of expansive OD’s. The images presented in a case report of “multiple ossifying fibromas” [17] closely resemble those of expansive OD’s with radiological evidence of the formation of globular radiodense masses in one of the lesions.
The second bone pattern manifested as randomly distributed hypercellular delicate trabecular configurations. These attract osteoclasts probably through elaboration of RANKL, a potent paracrine stimulus for osteoclastogenesis sourced from osteoblasts [18] which are multiple in these areas. Resorption creates voids which coalesce forming small smooth cavities which can be seen macroscopically on cut surfaces of the specimen. It is proposed that this process represents the early stage of simple bone cyst formation, which is a commonly reported association with OD’s. The delicate atypical cellular osteoid may erroneously be interpreted as the lace-like osteoid in osteosarcoma or trabecular type juvenile ossifying fibroma, particularly in a small biopsy sample where the mature globular deposits are not included in the specimen. Features which should raise the suspicion of an endosteal osteosarcoma include large areas of perforation of the cortical plates, infiltration of the surrounding soft tissue, failure to demonstrate maturation of the mineralized tissue into lobular bone masses and brisk mitotic activity [19]. MDM2 and CDK4 are sensitive markers for the diagnosis of low grade osteosarcomas [20] and may be helpful in differentiating the lace-like neoplastic osteoid from the cellular delicate osteoid and bone trabecula seen in expansive OD’s. Trabecular juvenile ossifying fibromas are true neoplasms with an uncontrolled growth potential, affect younger patients and fail to demonstrate lobular maturation of mineralized tissue. Opacification of large ossifying fibromas is the result of an increase of tumor bulk and does not occur through a lobular enlargement of the individual bone deposits which encroach on the fibrous tissue component. Some large trabecular juvenile ossifying fibromas show increasing collagenisation with a decrease of mineralized tissue content [16], a feature not present in mature expansive OD’s. In a recent report on a “rapidly maturing juvenile ossifying fibroma” [21] the transformation of the lesion into a radiopaque mass is a feature highly suggestive of an expansive OD [1, 10] and mitigates against the diagnosis of a juvenile ossifying fibroma. The axial CT scan of a patient with multiple ossifying fibromas [17] shows lobulated ginger-root like mineralized masses in one of the lesions, a radiological feature diagnostic for OD [1, 8]. Although multifocal expansive OD’s are common, multiple ossifying fibromas in the same patient is an extremely rare occurrence. A final diagnosis should not be made on microscopy alone and the application of stringent radiological criteria is pivotal in distinguishing ossifying fibromas from expansive OD.
Multiple fibro-osseous proliferations occur in several parathyroid hormone related diseases and should be excluded when considering a case of expansive OD with a multifocal distribution. The expansive jaw lesions in chronic kidney disease occur in a different metabolic setting and although they contain osteoclast-like giant cells in a fibrous stroma with bone formation, bone maturation never occurs within these lesions [22]. Similar lesions may be present in patients suffering the hyperparathyroidism-jaw tumor syndrome of primary hyperparathyroidism [23] and should be differentiated from the former through the exclusion of chronic kidney disease. Central giant cell lesions arising in association with the Noonan syndrome, neurofibromatosis type 1 and the LEOPARD syndrome present in young patients and are characterized by associated birth defects and multiple giant cells in a fibrous setting with less bone formation than in the expansive OD’s [24].
Contrary to the conventional OD’s, which are not be managed surgically unless secondarily infected, the expansive OD’s should be removed early due to their disfiguring growth potential. Before expansion occurs the radiological differentiation between conventional non expansive OD’s and those with an expansive potential remains an enigma. A slightly younger age onset (and particularly when presenting in a young individual) than for conventional OD’s, signs of tooth displacement and a disproportionately large radiolucent component may however be pointers to an expansive growth potential before a lesion manifests clinically with cortical expansion. The term “familial gigantiform cementoma” is inappropriate and requires revision. “Gigantiform” refers only to a late stage when the lesion reaches massive dimensional proportions. We also suggest that “cementoma” be abandoned from diagnostic- and descriptive terminology applied to pathology unrelated to mineralized proliferations on the surface of the root of a tooth. A more detailed critique of the terminology applied to fibro-osseous lesions appeared under the authorship of EJR and CEN in 2012 [25].
Conclusions
We recommend that the term “familial gigantiform cementoma” be abolished and replaced by “expansive OD’s” which should be sub classified into familial- and non-familial types. Expansive OD’s share the following features with non-expansive conventional OD: Radiological evidence of either a unifocal- or multifocal lesion with evidence of maturation into dense lobular mineralized masses, frequent association with simple bone cysts and microscopic features of a well-defined fibro-osseous lesion in which metaplastic mineralized tissues resembling lobular hypocellular masses and delicate cellular osteoid and bone trabecula are present. Enlargement and confluence of the former reflect on radiographs as ginger root-like lobular radiopacities. In mature lesions the fibrous component becomes less cellular. Resorption of the cellular bone trabecula initiates the elaboration of cavities which is postulated to play a role in the early development of a simple bone cyst. Criteria for differentiating between expansive- and conventional OD’s are a slightly younger age onset (and in particular a more common occurrence in young individuals), tooth displacement, cortical perforation and limited soft tissue invasion (which are evident in large lesions only) for the former. Surgical removal of an expansive OD is indicated due to its disfiguring growth potential unlike conventional OD’s which should not be managed surgically unless secondarily infected.
References
- 1.Slootweg PJ. Osseous dysplasias. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. p. 323. [Google Scholar]
- 2.Brannon RB, Fowler CB. Benign fibro-osseous lesions: a review of the current concepts. Adv Anat Pathol. 2001;8:126–143. doi: 10.1097/00125480-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Summerlin DJ, Tomich CE. Focal cement-osseous dysplasia: a clinicopathologic study of 221 cases. Oral Surg Oral Med Oral Pathol. 1994;78:611–620. doi: 10.1016/0030-4220(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 4.Young SK, Markovitz NR, Sullivan S, Seale TW, Hirschi R. Familial gigantiform cementoma: classification and presentation of a large pedigree. Oral Surg Oral Med Oral Pathol. 1989;68:740–747. doi: 10.1016/0030-4220(89)90165-5. [DOI] [PubMed] [Google Scholar]
- 5.Cannon JS, Keller EE, Dahlin DC. Gigantiform cementoma: report of two cases (mother and son) J Oral Surg. 1980;38:65–70. [PubMed] [Google Scholar]
- 6.Punniamoorthy A. Gigantiform cementoma: review of the literature and a case report. Br J Oral Surg. 1980;18:221–229. doi: 10.1016/0007-117X(80)90066-9. [DOI] [PubMed] [Google Scholar]
- 7.Abdelsayed RA, Eversole LR, Singh BS, Scarborough FE. Gigantiform cementoma: clinicopathologic presentation of 3 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:438–444. doi: 10.1067/moe.2001.113108. [DOI] [PubMed] [Google Scholar]
- 8.Noffke CE, Raubenheimer EJ. Expansive osseous dysplasia: report of 9 lesions in an African population sample and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:e35–e41. doi: 10.1016/j.tripleo.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Thompson SH, Altini M. Gigantiform cementoma of the jaws. Head Neck. 1989;11:538–544. doi: 10.1002/hed.2880110612. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Hiranuma H, Kishino M, Jikko A, Sakuda M. Cemento-osseous dysplasia of the jaws in 54 Japanese patients: a radiographic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:107–114. doi: 10.1016/S1079-2104(99)70303-3. [DOI] [PubMed] [Google Scholar]
- 11.Su L, Wheathers DR, Waldron CA. Distinguishing features of focal cement-osseous dysplasia and cement-ossifying fibromas. II: a clinical and radiologic spectrum of 316 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:107–114. doi: 10.1016/S1079-2104(99)70303-3. [DOI] [PubMed] [Google Scholar]
- 12.Waldron CA. Fibro-osseous lesions of the jaws. J Oral Maxillofac Surg. 1885;43:249–262. doi: 10.1016/0278-2391(85)90283-6. [DOI] [PubMed] [Google Scholar]
- 13.Waldron CA, Giansanti JS. Benign fibro-osseous lesions of the jaws. Part II: benign fibro-osseous lesions of periodontal ligament origin. Oral Surg. 1973;35:340–346. doi: 10.1016/0030-4220(73)90072-8. [DOI] [PubMed] [Google Scholar]
- 14.Jundt G. Fibrous dysplasia. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 319–320. [Google Scholar]
- 15.Alawi F. Benign fibro-osseous disease of the maxillofacial bones: a review and differential diagnosis. Am J Pathol. 2002;118(suppl 1):S50–S70. doi: 10.1309/NUXA-JUT9-HA09-WKMV. [DOI] [PubMed] [Google Scholar]
- 16.Van Heerden WFP, Raubenheimer EJ, Weir RG, Kreidler J. Giant ossifying fibroma: a clinicopathologic study of 8 tumors. J Oral Pathol Med. 1989;18:506–509. doi: 10.1111/j.1600-0714.1989.tb01352.x. [DOI] [PubMed] [Google Scholar]
- 17.Hwang E-H, Kim H-W, Kim K-D, Lee S-R. Case report. Multiple ossifying fibroma: report of an 18-year follow-up. Dentomaxillofac Radiol. 2001;30:230–234. doi: 10.1038/sj.dmfr.4600608. [DOI] [PubMed] [Google Scholar]
- 18.Abu-Amer Y. NFkB signalling and bone resorption. Osteoporos Int. 2013 doi: 10.1007/s00198-013-2313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raubenheimer EJ, Noffke CE. Low grade introosseous osteosarcoma of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:82–85. doi: 10.1016/S1079-2104(98)90154-8. [DOI] [PubMed] [Google Scholar]
- 20.Dujardin F, Binh MB, Bouvier C, Gomez-Brouchet A, et al. MDM2 and CKD4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of bone. Mod Pathol. 2011;24:624–637. doi: 10.1038/modpathol.2010.229. [DOI] [PubMed] [Google Scholar]
- 21.Lohe VK, Degwekar SS, Bhowate RR, Kadu RP, Motwani MB, Indukar AD, Dangore SB. Case report. Rapidly maturing juvenile ossifying fibroma: a case report. Dentomaxillofac Radiol. 2011;40:195–198. doi: 10.1259/dmfr/67780763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raubenheimer EJ, Noffke CEE, Mohamed A. Expansive jaw lesions in chronic kidney disease: review of the literature and a report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:340–345. doi: 10.1016/j.oooo.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Guerrouani A, Rzin A, El Khatib K. Hyperparathyroidism-jaw tumour syndrome detected by aggressive generalized osteitis fibrosa cystica. Clin Cases Min Bone Metab. 2013;10:65–67. doi: 10.11138/ccmbm/2013.10.1.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flanagan AM, Speight PM. Giant cell lesions of the craniofacial bones. Head Neck Pathol. 2014;8:445–453. doi: 10.1007/s12105-014-0589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noffke CE, Raubenheimer EJ, MacDonald D. Fibro-osseous disease: harmonizing terminology with biology. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:388–392. doi: 10.1016/j.oooo.2012.06.002. [DOI] [PubMed] [Google Scholar]


