Abstract
Ameloblastoma is a rare, locally aggressive odontogenic neoplasm, accounting for fewer than 1 % of head and neck tumors. Recent literature suggests that the initial surgical approach and histologic growth patterns are the most important prognostic determinants in ameloblastoma. The aim of this study was to compare the clinical presentation, management, and outcomes of patients with ameloblastoma with data reported in the literature; the study spanned 2 decades at a single institution. The institution’s database was searched for all patients with pathologically confirmed ameloblastoma, diagnosed between 1990 and 2015. The data collected included sex, age, clinical and imaging findings, management, histologic pattern, clearance of surgical margins, length of follow-up, time to recurrence, and disease-related mortality. The potential risk factors of recurrence were evaluated using log-rank test, proportional hazard model, and Fisher exact test. Review of the database yielded 54 patients with pathologically confirmed ameloblastoma and follow-up. Recurrence was noted in 13 (24 %) patients. Surgical approach was associated with the risk of recurrence (6.1 % following radical resection vs. 52 % following limited surgery, p = 0.002). There were trends toward higher recurrence rate in the group with pathologically documented positive margins (p = 0.054) and in follicular ameloblastoma (p = 0.35). Transformation into ameloblastic carcinoma was identified in two patients. There was no disease-related mortality. Our study confirms the recent data regarding the importance of radical surgical resection in management of ameloblastoma. Surgical approach appears to be the strongest predictor of tumor clearance.
Keywords: Ameloblastoma, Ameloblastoma recurrence, Ameloblastoma pathology, Ameloblastoma histologic pattern, Ameloblastoma management, Ameloblastoma prognosis
Introduction
Ameloblastoma is an uncommon odontogenic epithelial neoplasm, comprising approximately 1 % of all cysts and tumors of the jaws and from 11 to 59 % of odontogenic tumors [1, 2].
Four distinct growth variants of ameloblastoma are recognized in the current 2005 WHO classification for head and neck tumors: (1) peripheral, in which tumor is extraosseous and shows continuity with the oral mucosal stratified squamous epithelium, (2) unicystic, in which a single cystic intraosseous growth pattern is observed grossly and radiographically, (3) solid/multicystic, in which invasive tumor permeates bone marrow spaces and may show multicystic foci, and (4) desmoplastic, an infiltrative intraosseous tumor dominated by the stromal component, radiographically reminiscent of a fibro-osseous lesion [3]. The intraosseous ameloblastomas are collectively termed “central ameloblastomas” [3]. Approximately 80 % of central ameloblastomas arise in the mandible, but involvement of the maxilla is rare [1]. Various histologic patterns are recognized in unicystic, solid/multicystic, and peripheral growth variants of ameloblastoma. The two main histologic patterns are plexiform and follicular. The follicular ameloblastoma is further subdivided into acanthomatous, granular, spindle cell, and basal cell types. The tumors are classified by the most predominant histologic pattern present [3, 4].
The benign histology and indolent behavior of ameloblastoma have led to a traditionally conservative surgical approach. However, while histologically benign, ameloblastoma, particularly the solid/multicystic variant, is characterized by locally aggressive spread with up to 90 % recurrence rate following conservative excision [1, 3, 4]. Prolonged tumor duration and multiple recurrences have been associated with metastases of the histologically benign-appearing ameloblastoma, so called “metastasizing ameloblastoma” [5]. Additionally, long-standing and recurrent ameloblastoma has been shown to occasionally transform into an aggressive ameloblastic carcinoma [2, 6–8].
Recent data from several centers suggests that the initial surgical management approach and histologic growth pattern are the most important prognostic determinants in ameloblastoma [2, 9–11]. Radical surgery can achieve recurrence rates as low as 0–4.5 % and wider resection may be required for ameloblastomas with more aggressive histologic patterns, such as follicular, granular cell, and acanthomatous variants [9, 11, 12]. Despite these findings, the debate on an ideal management that would achieve cure without unnecessary compromise of cosmesis and function, is ongoing. The continued controversy stems in part from the outcome data generated by the small retrospective case series without sufficient patient follow-up [13].
Thus, a thorough understanding of this tumor’s clinicopathological behavior is essential to avoid the local morbidity of recurrence and the potential malignant transformation or metastases from inadequately treated disease. The aim of this study was to compare the clinical presentation, surgical management, and outcomes of patients with ameloblastoma, spanning the course of 2 decades at a single institution, with the data reported in the literature.
Methods
Following the institutional review board (IRB) approval, the electronic database of the Hospital of University of Pennsylvania was searched for all patients with pathologically confirmed ameloblastoma, diagnosed between 1990 and 2015. The data collected included sex, age at the time of diagnosis, clinical presentation, tumor location, imaging findings, initial and subsequent management, length of follow-up, presence of recurrence, time to recurrence, disease-related mortality, histopathologic diagnosis, predominant histologic growth pattern, and clearance of surgical margins. The available histopathology slides on identified tumors were reviewed for concordance with original diagnosis and for assessment of histologic pattern (TM). The patients without essential clinical information (age, sex, tumor location, surgical management, or follow-up of <1 year) or those with discrepant pathologic diagnosis were excluded from the study.
Descriptive analysis of patient characteristics was performed using mean, standard deviation (SD), median and range for continuous variables and percentage for categorical variables. The risk of time to recurrence was estimated using Kaplan–Meier method. The potential risk factors of recurrence were evaluated using log-rank test and univariate proportional hazard model for calculating hazard ratio and 95 % confidence intervals (95 % CI). For the analysis of association between pathology pattern and recurrence, Fisher exact test was used because there was no recurrence in several categories of pathology patterns. All statistical analyses were performed in SAS V9.4 (SAS Institute Inc, Cary, NC) and two-sided p < 0.05 was considered to be statistically significant.
Results
Review of medical records database yielded 113 patients with pathologically confirmed ameloblastoma. Of these, 54 patients met the study’s inclusion criteria and were selected for further review. Patient demographics, tumor characteristics, surgical management, and recurrence rates are summarized in Table 1.
Table 1.
Univariate analysis for the predictors of ameloblastoma recurrence (N = 54)
| Parameters | N | Recurrence (%) | Hazard ratio (95 % CI) | P valuea | |
|---|---|---|---|---|---|
| Age, years (continuous) | 1.02 (0.98, 1.06) | 0.28 | |||
| Gender | Male | 39 | 9 (23.1 %) | Reference | |
| Female | 15 | 4 (26.7 %) | 0.98 (0.29, 3.25) | 0.97 | |
| Location | Maxillary | 19 | 7 (36.8 %) | Reference | |
| Mandibular | 35 | 6 (17.1 %) | 0.55 (0.18, 1.63) | 0.26 | |
| Surgical approach | Radical resection | 33 | 2 (6.1 %) | Reference | |
| Limited resection | 21 | 11 (52.4 %) | 11.1 (2.44, 50.2) | 0.002 | |
| Margins | 0.16 | ||||
| Positive | 19 | 8 (42.1 %) | Reference | ||
| Clear | 27 | 4 (14.8 %) | 0.31 (0.09, 1.02) | 0.054 | |
| N/A | 8 | 1 (12.5 %) | 0.67 (0.08, 5.49) | 0.71 | |
| Pathology pattern | 0.35*, 0.48* | ||||
| Unicystic | 4 | 0 (0.0 %) | |||
| Desmoplastic | 6 | 0 (0.0 %) | |||
| Solid/Multicystic (overall) | 31 | 8 (25.8 %) | |||
| Follicular (overall) | 23 | 7 (30.4 %) | |||
| Follicular (conventional) | 14 | 4 (28.6 %) | |||
| Basal cell | 4 | 1 (25.0 %) | |||
| Acanthomatous | 3 | 1 (33.3 %) | |||
| Granular | 2 | 1 (50.0 %) | |||
| Plexiform | 8 | 1 (12.5 %) | |||
| N/A | 13 | 5 (38.5 %) |
N Number of patients, CI confidence interval, N/A not available
* Fisher exact p value was used. Because there was no recurrence in a group of patients, the time to recurrence analysis could not be performed
* p = 0.35 calculated by including all follicular ameloblastoma subtypes into a single group (N = 23)
* p = 0.48 calculated by assessing recurrence by individual subtype
aFrom the univariate proportional hazard model for time to recurrence analysis
Patient Demographics, Clinical Presentation, and Their Association with Recurrence
Thirty-nine patients were males (M:F = 2.6:1). The patients presented at a median age of 56 years (mean 53 years, range 13–88 years). Nineteen (35 %) tumors were localized to the maxilla and the remainder involved the mandible (65 %). None of the tumors were peripheral in location. Information on clinical presentation was available on 25 patients (17 with mandibular and 8 with maxillary ameloblastomas). The most common presenting symptom was regional swelling or mass (14/25, 56 %) followed by pain (4/25, 16 %). Patients with maxillary ameloblastoma additionally described symptoms of nasal obstruction (4/8, 50 %). Three mandibular ameloblastomas were identified incidentally on a routine dental exam (3/17, 18 %). Imaging data was available on 30 patients (2 with unicystic ameloblastoma, 2 with desmoplastic ameloblastoma, and 28 with solid-multicystic ameloblastoma). The appearance of unicystic ameloblastoma on computed tomography (CT) was that of a unicystic, lytic, and expansile mass. The solid/multicystic and desmoplastic ameloblastomas were described as unilocular or multiloculated, lytic, and sclerotic masses with occasional soft tissue component. Additionally, all 4 maxillary ameloblastomas diagnosed in the last 5 years were evaluated pre-operatively with concurrent magnetic resonance imaging (MRI). Disease recurrence monitoring was most frequently performed on an annual basis with serial clinical examinations and imaging studies (CT for maxillary and mandibular ameloblastomas and CT or MRI for maxillary ameloblastomas).
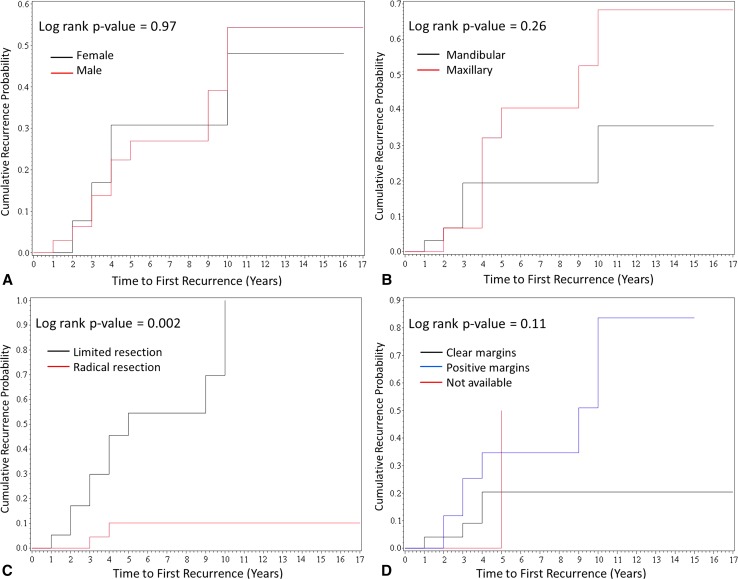
Recurrence was noted in 13 (24 %) of 54 patients during the average of 6.5 years follow-up (median 4.0 years, range 1–30 years). The average time to first recurrence was 4.6 years (median 4.0 years, range 1–10 years). Six of 13 patients (46 %) had more than 1 recurrence. There was no significant association between recurrence and patient age (p = 0.28) or sex (p = 0.97) (Table 1; Fig. 1a). Although there was no significant association between tumor location and recurrence (p = 0.26) (Table 1; Fig. 1b), patients with maxillary ameloblastoma tended to develop more frequent recurrences (average 2.3 per patient, range 1–4), when compared to mandibular counterparts (average 1.3 per patient, range 1–2, p = 0.11). Additionally, patients with maxillary ameloblastoma had more locally aggressive disease, requiring radical maxillectomy with skull base resection (8/19, 42 %), ethmoidectomy and turbinectomy (2/19, 11 %), orbital soft tissue resection, and orbital exenteration (2/19, 11 %).
Fig. 1.
Kaplan–Meier curves demonstrating time to first recurrence in 54 patients by gender (a), location (b), initial surgical approach (c), and microscopic involvement of margins (d)
Initial Surgical Management and its Association with Recurrence
Radical resection as initial surgical approach (segmental resection, maxillectomy, or mandibulectomy) was performed in 33 (61 %) of 54 patients, while the remainder (21/54, 39 %) were managed with limited surgery [excision with a narrow margin (17/54, 31.5 %), enucleation (2/54, 3.7 %), or curettage (2/54, 3.7 %)]. The initial surgical approach was strongly associated with the risk of recurrence (Table 1; Fig. 1c). Two tumors (6.1 %) recurred in the cohort of 33 patients managed with initial radical surgery as compared to 11 (52 %) tumors in 21 patients managed with limited surgery (p = 0.002). Of 11 recurrent tumors in the patients managed by limited surgery, 10 were excised with a narrow margin.
Pathology Data and its Association with Recurrence
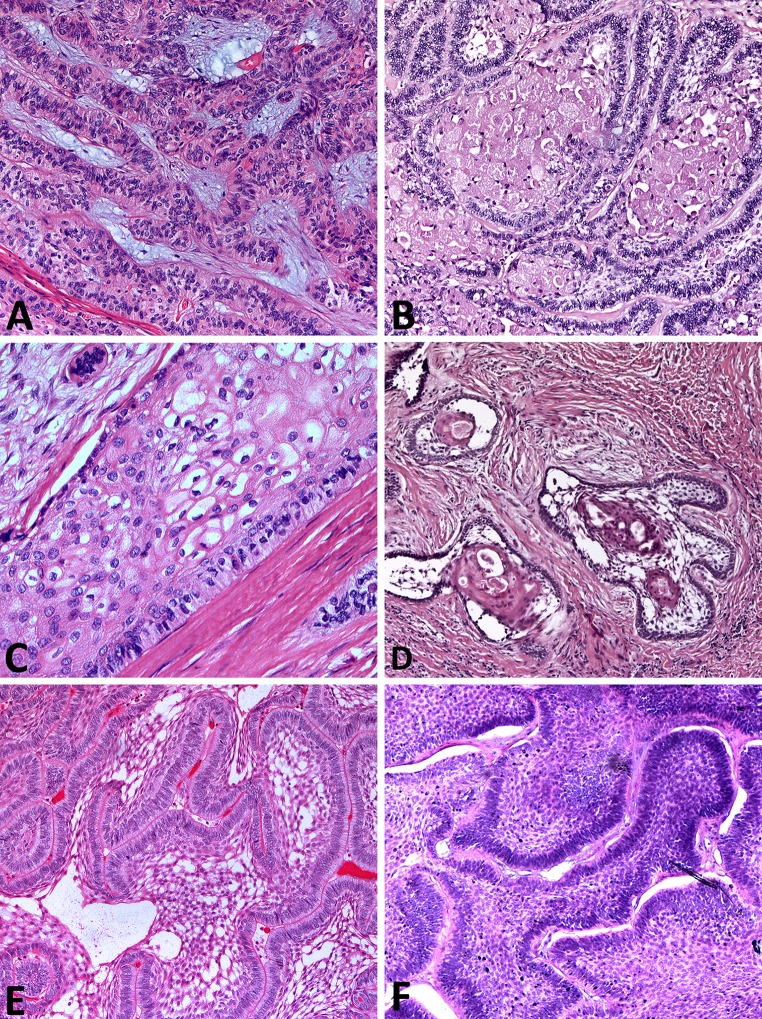
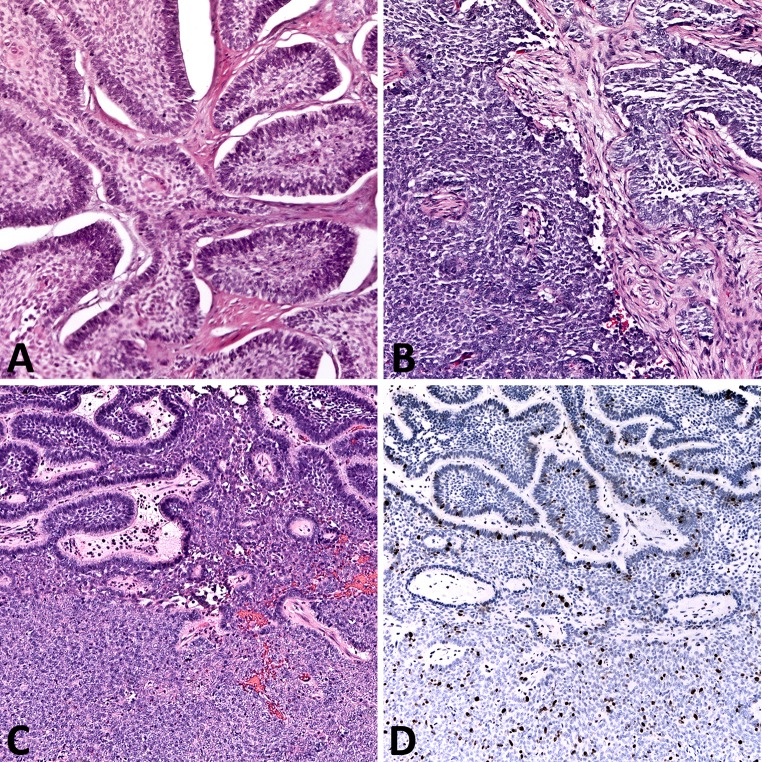
Histopathology slides were available for review on 41 patients (Fig. 2; Table 1). There were no discrepant histopathologic diagnoses on re-review of the available material. Histologic growth pattern designation was rendered in accordance with 2005 WHO classification for head and neck tumors for 15 ameloblastomas with unavailable histologic pattern data [3]. Solid/multicystic ameloblastoma was the most common variant (31/41, 76 %), with follicular histologic pattern most commonly encountered (23/41, 56 %). There was no significant association between histologic pattern and tumor recurrence (p = 0.48), although there were trends toward higher recurrence rate in follicular ameloblastoma and lower recurrence rate in unicystic and desmoplastic ameloblastomas (Table 1). There was a trend toward higher recurrence rate in ameloblastomas with pathologically documented positive margins (defined as tumor present at the inked margin of oriented specimen), but it reached borderline statistical significance (p = 0.054) (Table 1; Fig. 1d). Intraoperative frozen sections were performed on 4 ameloblastomas with confirmed margin clearance on subsequent permanent sections in all 4 tumors. However, one maxillary follicular ameloblastoma recurred despite intraoperatively confirmed negative margins. Malignant transformation into ameloblastic carcinoma was identified in two patients with maxillary ameloblastoma. One patient had multiple recurrences prior to emergence of malignant tumor 6 years following the original surgery (Fig. 3a, b). The second patient had a long-standing history of symptomatic maxillary mass, with a focus of ameloblastic carcinoma in a background of benign ameloblastoma identified in the radical resection specimen (Fig. 3c, d). Both patients are disease-free with post-operative follow-up of 2 and 1 years, respectively.
Fig. 2.
Histologic patterns in solid/multicystic ameloblastoma. a Plexiform ameloblastoma demonstrates interweaving fascicles of neoplastic cells with hyperchromatic columnar nuclei, polarized away from the basement membrane (“reversed polarity”, “piano-key” arrangement). b Islands of granular ameloblastoma demonstrate central stellate reticulum-like cells with abundant eosinophilic granular cytoplasm. c Acanthomatous ameloblastoma shows squamous-type differentiation of the central stellate reticulum-like cells, while maintaining the reverse polarization of the nuclei in columnar cells lining the nests. d Desmoplastic ameloblastoma shows islands of odontogenic epithelium in abundant desmoplastic stroma. e Follicular ameloblastoma islands demonstrate peripheral columnar cells exhibiting reversal of polarity and central stellate-reticulum like cells with cystic degeneration. f Basal cell type ameloblastoma islands contain basaloid cells with scant cytoplasm and peripheral palisading, reminiscent of basal cell carcinoma. [H&E stain; original magnification ×25 (a, b, d, e, f); original magnification ×50 (c)]
Fig. 3.
Histopathology of the tumors in 2 patients with secondary ameloblastic carcinoma. a Patient 1. Initial specimen demonstrates benign-appearing nests of basal cell type ameloblastoma. b Recurrent tumor 6 years later shows dedifferentiation into ameloblastic carcinoma. c Patient 2. Nests of follicular ameloblastoma (top) transition into a sheet-like proliferation of pleomorphic, mitotically active cells of ameloblastic carcinoma (bottom). d Corresponding ki-67 immunostain highlights the low proliferative activity in ameloblastoma component and high proliferative activity in the emergent ameloblastic carcinoma. [H&E stain (a–c); antibody, ki-67 (d); original magnification ×25]
Disease-Related Mortality
None of the patients succumbed to their disease.
Discussion
Ameloblastoma is an uncommon benign, slowly growing neoplasm of odontogenic origin. The largest combined data on the demographics and clinical presentation of patients with ameloblastoma comes from the work by Reichart et al. [1], who analyzed the available literature on 3677 patients with this tumor. According to their analysis, ameloblastoma most commonly presents as swelling over the affected region (mandible in 80 % of cases) at an average age of 36 years with equal sex distribution. The current series has greater proportion of patients with maxillary ameloblastoma, which may reflect a tertiary, referral nature of our center. Maxillary ameloblastoma has been shown to occur at an older age with male predilection, which may explain the demographics in our patient population [2, 4]. Additionally, the patients from developed countries have been noted to present at an older age, which may contribute further to an older patient age in the current series [1]. The clinical presenting symptoms of swelling, pain, and nasal obstruction in our patients are similar to those reported previously [1, 2, 11].
The relationship between tumor location and recurrence risk has been, thus far, controversial. Multiple studies point to a conclusion that maxillary ameloblastomas are inherently more difficult to manage, with extensive disease at presentation, multiple recurrences, and locally aggressive behavior [2, 14, 15]. However, the cumulative data comparing recurrence rates of mandibular and maxillary ameloblastomas do not show significant difference between the two sites [1, 9]. The current study reconciles these seemingly conflicting observations. While we similarly did not find a significant difference in proportion of recurrent maxillary and mandibular ameloblastomas, maxillary tumors demonstrated propensity for multiple recurrences and regionally aggressive behavior, emphasizing the need for a more aggressive initial surgical intervention.
Radiographic studies play a cardinal role in pre-operative and post-operative management of patients with ameloblastoma. Dental X-rays (pantomography) typically demonstrate a lytic lesion with scalloped margins or a “soap bubble” appearance, which can be associated with resorption of tooth roots and impacted teeth [1, 4]. Pantomography can help detect asymptomatic ameloblastomas, a finding which was noted in 3 of our patients. CT has emerged as the most useful diagnostic imaging modality, demonstrating expansile, lytic, unilocular or multilocular cystic lesions with or without soft tissue extension. In addition to CT, MRI has been recommended in evaluation of patients with maxillary ameloblastoma, because of its potential to more precisely evaluate the soft tissue extent of the lesion [4]. The increased reliance on CT and MRI in both pre-operative and post-operative patient care is reflected in management of patients in our series.
At the present time, ameloblastoma is regarded as a surgically managed disease. Multiple studies have consistently indicated that the initial surgical approach most strongly correlates with tumor recurrence [1, 9–13]. While curettage and enucleation with or without adjuvant Carnoy’s solution and marsupialization may be acceptable therapies for some unicystic ameloblastomas, most experts currently believe that these treatment modalities have no role in management of solid/multicystic ameloblastoma [4, 10]. This shift in paradigm is reflected in the surgical approach to management of ameloblastoma in our series. There continues to be a debate on the extent of recommended surgical margin, however. Histopathologic study of 82 ameloblastoma resections showed that the tumor extends with a range of 2–8 mm beyond its radiographic margins [16]. Thus, many experts endorse that solid/multicystic ameloblastomas should be excised with at least 1–2 cm margin, which typically results in a segmental resection, maxillectomy or mandibulectomy [4, 9–11]. In a recent study of 305 patients with ameloblastoma and long-term follow-up, Hong et al. [9] found that patients managed by marginal resection demonstrated significantly higher recurrence rate when compared to those who underwent segmental resection or maxillectomy (11.6 vs. 4.5 %, p = 0.004). However, the latest meta-analysis of solid-multicystic ameloblastomas showed no significant difference in recurrence rates of tumors managed with a primary marginal resection and segmental resection (11.9 vs. 12.1 %), while there was a significantly higher recurrence rate in enucleated and curetted tumors (40 %) [13]. Our study supports observations of Hong et al. demonstrating significantly lower recurrence rates (6.1 %) in patients managed with primary segmental resection, maxillectomy or mandibulectomy when compared to a marginal excision, enucleation and curettage (52 %). Thus, it appears that adherence to the recommended 1–2 cm margin is prudent.
While most surgeons rely on preoperative imaging to correlate the tumor boundaries with identifiable surgical landmarks, the use of intraoperative imaging has been advocated by some experts as a useful adjunct for assessing tumor clearance, particularly when a narrow margin is desired or intraoperative frozen section is not available [4, 16, 17]. Analogously, intraoperative consultation has been advocated in a setting where intraoperative imaging is not available, when a narrow margin is desired, or when a soft tissue component is noted [4, 16]. Intra-operative frozen sections on jaw bones demonstrate 62–98 % accuracy, defined as agreement with the permanent section diagnosis [18–20]. However, there is scant data on long-term outcomes of patients with ameloblastoma and intraoperatively cleared margins. Despite the high accuracy of frozen sections in our series, one tumor recurred, suggestive of inadequate sampling. Similarly, we found only borderline association between the risk of recurrence and assessment of surgical margins on permanent sections. This may be related to a small sample size, a sampling error, or may potentially reflect the use of adjuvant therapies, such as Carnoy’s solution, in lesions with grossly or microscopically positive margins.
Four growth variants of ameloblastoma are recognized: solid/multicystic, which comprises approximately 90 % of the tumors, desmoplastic, unicystic and, the least common, peripheral [1, 3]. Additionally, the histologic patterns in ameloblastoma include plexiform, follicular and, less frequently, acanthomatous, granular cell, basal cell, and spindle cell [4]. The distribution of the tumor subtypes and histologic patterns in our study is similar to what has been reported in the literature. Several studies point to higher recurrence rates of follicular ameloblastomas when compared with plexiform, peripheral, and unicystic counterparts [1, 9]. The current study similarly demonstrates a trend toward higher recurrence in follicular ameloblastoma, while there were no recurrences of desmoplastic and unicystic variants, supporting prior observations. However, the relatively small patient number in our case series is insufficient for a definitive conclusion.
Ameloblastoma is, in essence, a lifelong disease. Although most tumors recur within 5 years of original diagnosis, late recurrences are not uncommon, and were seen in 23 % of the patients in the current study. While neglected and recurrent ameloblastoma can cause significant morbidity, mortality is extremely rare and is seen typically in a setting of maxillary ameloblastoma extending into the cranium [2, 14, 15]. The rare metastasizing ameloblastoma and ameloblastic carcinoma, each described in up to 2 % of patients with ameloblastoma, can also infrequently lead to mortality [2, 4, 5, 8, 21].
The role of radiotherapy and chemotherapy as adjuvant or sole treatment modalities in management of locally aggressive ameloblastoma, metastasizing ameloblastoma, and secondary ameloblastic carcinoma has been debated, though recent studies have shown promising outcomes with novel therapies, such as Gamma Knife stereotactic radiosurgery and carbon ion therapy [4, 8]. Additionally, several investigators identified a high incidence of mutually exclusive mutations in mitogen-activated protein kinase (MAPK) pathway genes (FGFR, BRAF V600E, and RAS family) and sonic hedgehog (SHH) signaling pathway gene SMO in ameloblastomas, providing potential avenues for targeted therapies with RTK, MEK, BRAF, and SMO inhibitors [22].
In summary, ameloblastoma is a locally aggressive neoplasm, which can be associated with substantial local morbidity and, rarely, mortality. Despite the promising emerging therapies, management of ameloblastoma at present remains primarily surgical. Our data indicate that a radical surgical approach, such as segmental resection, maxillectomy or mandibulectomy, is the strongest predictor of recurrence-free survival in patients with ameloblastoma. Ameloblastoma’s propensity for delayed recurrence emphasizes the need of life-long monitoring.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study was approved by the Hospital of University of Pennsylvania Ethics Committee (IRB). For this type of study formal consent was not required.
References
- 1.Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer Part B Oral Oncol. 1995;31B:86–99. doi: 10.1016/0964-1955(94)00037-5. [DOI] [PubMed] [Google Scholar]
- 2.Zwahlen RA, Grätz KW. Maxillary ameloblastomas: a review of the literature and of a 15-year database. J Craniomaxillofac Surg. 2002;30:273–279. doi: 10.1016/S1010-5182(02)90317-3. [DOI] [PubMed] [Google Scholar]
- 3.Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization classification of tumors. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 4.McClary AC, West RB, McClary AC, Pollack JR, Fischbein NJ, Holsinger CF, Sunwoo J, Colevas AD, Sirjani D. Ameloblastoma: a clinical review and trends in management. Eur Arch Otorhinolaryngol. 2015 doi: 10.1007/s00405-015-3631-8. [DOI] [PubMed] [Google Scholar]
- 5.Luo DY, Feng CJ, Guo JB. Pulmonary metastases from an ameloblastoma: case report and review of the literature. J Craniomaxillofac Surg. 2012;40:e470–e474. doi: 10.1016/j.jcms.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Kyriazis AP, Karkazis GC, Kyriazis AA. Maxillary ameloblastoma with intracerebral extension. Report of a case. Oral Surg Oral Med Oral Pathol. 1971;32:582–587. doi: 10.1016/0030-4220(71)90323-9. [DOI] [PubMed] [Google Scholar]
- 7.Daramola JO, Abioye AA, Ajagbe HA, Aghadiuno PU. Maxillary malignant ameloblastoma with intraorbital extension: report of case. J Oral Surg. 1980;38:203–206. [PubMed] [Google Scholar]
- 8.Hayashi N, Iwata J, Masaoka N, Ueno H, Ohtsuki Y, Moriki T. Ameloblastoma of the mandible metastasizing to the orbit with malignant transformation. A histopathological and immunohistochemical study. Virchows Arch. 1997;430:501–507. doi: 10.1007/s004280050061. [DOI] [PubMed] [Google Scholar]
- 9.Hong J, Yun PY, Chung IH, Myoung H, Suh JD, Seo BM, Lee JH, Choung PH. Long-term follow up on recurrence of 305 ameloblastoma cases. Int J Oral Maxillofac Surg. 2007;36:283–288. doi: 10.1016/j.ijom.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Pogrel MA, Montes DM. Is there a role for enucleation in the management of ameloblastoma? Int J Oral Maxillofac Surg. 2009;38:807–812. doi: 10.1016/j.ijom.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Sham E, Leong J, Maher R, Schenberg M, Leung M, Mansour AK. Mandibular ameloblastoma: clinical experience and literature review. ANZ J Surg. 2009;79:739–744. doi: 10.1111/j.1445-2197.2009.05061.x. [DOI] [PubMed] [Google Scholar]
- 12.Becelli R, Carboni A, Cerulli G, Perugini M, Iannetti G. Mandibular ameloblastoma: analysis of surgical treatment carried out in 60 patients between 1977 and 1998. J Craniofac Surg. 2002;13:395–400. doi: 10.1097/00001665-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 13.de Almeida R AC, Andrade ES, Barbalho JC, Vajgel A, Vasconcelos BC. Recurrence rate following treatment for primary multicystic ameloblastoma: systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2016;45:359–367. doi: 10.1016/j.ijom.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Bredenkamp JK, Zimmerman MC, Mickel RA. Maxillary ameloblastoma. A potentially lethal neoplasm. Arch Otolaryngol Head Neck Surg. 1989;115:99–104. doi: 10.1001/archotol.1989.01860250101036. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Sudo S, Fukuya Y, Sakuma H. Maxillary ameloblastoma with intracranial invasion—case report. Neurol Med Chir (Tokyo) 1994;34:704–707. doi: 10.2176/nmc.34.704. [DOI] [PubMed] [Google Scholar]
- 16.Carlson ER, Marx RE. The ameloblastoma: primary, curative surgical management. J Oral Maxillofac Surg. 2006;64:484–494. doi: 10.1016/j.joms.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 17.De Silva I, Rozen WM, Ramakrishnan A, Mirkazemi M, Baillieu C, Ptasznik R, Leong J. Achieving adequate margins in ameloblastoma resection: the role for intra-operative specimen imaging. Clinical report and systematic review. PLoS ONE. 2012;7:e47897. doi: 10.1371/journal.pone.0047897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winther C, Graem N. Accuracy of frozen section diagnosis: a retrospective analysis of 4785 cases. APMIS. 2011;119:259–262. doi: 10.1111/j.1600-0463.2011.02725.x. [DOI] [PubMed] [Google Scholar]
- 19.Forrest LA, Schuller DE, Karanfilov B, Lucas JG. Update on intraoperative analysis of mandibular margins. Am J Otolaryngol. 1997;18:396–399. doi: 10.1016/S0196-0709(97)90060-0. [DOI] [PubMed] [Google Scholar]
- 20.Guthrie D, Peacock ZS, Sadow P, Dodson TB, August M. Preoperative incisional and intraoperative frozen section biopsy techniques have comparable accuracy in the diagnosis of benign intraosseous jaw pathology. J Oral Maxillofac Surg. 2012;70:2566–2572. doi: 10.1016/j.joms.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Du H, Li P, Zhang J, Tian W, Tang W. Ameloblastic carcinoma: an analysis of 12 cases with a review of the literature. Oncol Lett. 2014;8:914–920. doi: 10.3892/ol.2014.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heikinheimo K, Kurppa KJ, Elenius K. Novel targets for the treatment of ameloblastoma. J Dent Res. 2015;94:237–240. doi: 10.1177/0022034514560373. [DOI] [PMC free article] [PubMed] [Google Scholar]