Abstract
Fluorescence spectroscopy is a powerful method for monitoring protein folding in real-time with high resolution and sensitivity, but requires the site-specific introduction of labels into the protein. The ability to genetically incorporate unnatural amino acids allows for the efficient synthesis of fluorescently-labeled proteins with minimally perturbing fluorophores. Here, we describe recent uses of labeled proteins in dynamic structure determination experiments and advances in unnatural amino acid incorporation for dual site-specific fluorescent labeling. The advent of increasingly sophisticated bioorthogonal chemistry reactions and the diversity of unnatural amino acids available for incorporation will greatly enable protein folding and stability studies.
Graphical abstract
Introduction
Fluorescence spectroscopy can be used to study protein structural dynamics by harnessing two distance-dependent interactions between chromophores, Förster resonance energy transfer (FRET) and photoinduced electron transfer (PET). [1,2] FRET and PET studies of protein conformational change offer a combination of structural and temporal resolution that is difficult to achieve using other methods. FRET interactions depend on the geometries and spectral characteristics of donor and acceptor chromophores, allowing for distance measurements on the 1-10 nm scale. PET quenching interactions occur on shorter length scales (0.5-2 nm). Recent developments in double site-specific labeling of proteins have dramatically increased the ease with which one can introduce the probes necessary for FRET and PET experiments.
Fusions of intrinsically fluorescent proteins or of tag constructs such as HaloTag,[3] SNAP-tag,[4] CLIP-tag,[5] and TMP-tag[6] have been widely applied in FRET-based studies of protein folding, protein-protein interactions, and protease activity. [7] However, protein tags can be perturbing to the processes being studied, owing to the size of the label relative to the protein of interest. [8,9] Smaller label strategies include fluorogenic bisarsenical dyes that are selectively chelated by tetracysteine motifs and chemoenzymatic labeling using enzymatic recognition of short peptide sequences.[10-12] Such methods rely on insertion of a 5-15 amino acid sequence, which may still be disruptive to native protein structure and function.
The desire for non-perturbing, single residue labels has brought about a wide array of reagents used for protein conjugation, including classic Cys-selective reagents like maleimides.[13-15] However, such techniques are typically only suitable for systems lacking endogenous cysteine or which are amenable to mutation to obtain a single reactive position. A variety of strategies for introducing labels using unnatural amino acids (Uaas) have been developed, with the techniques pioneered by Schultz now established as the most broadly accessible methods for site-specific incorporation of Uaas.[16,17] Insertion of a Uaa is accomplished by a mutant aminoacyl tRNA synthetase capable of transferring the Uaa onto a cognate tRNA for ribosomal translation (Figure 1). To be site-specific, this tRNA must recognize an “unassigned” codon such as a stop codon or a four base codon. The Uaa may either be intrinsically fluorescent[18-21] or modified by a so-called bioorthogonal reaction that is selective in the presence of biological functional groups.[15] The FRET or PET quenching of intrinsic protein fluorescence (e.g. Trp) by incorporation of Uaas has also served as a useful strategy for biophysical characterization of proteins in vitro. [22]
Figure 1.
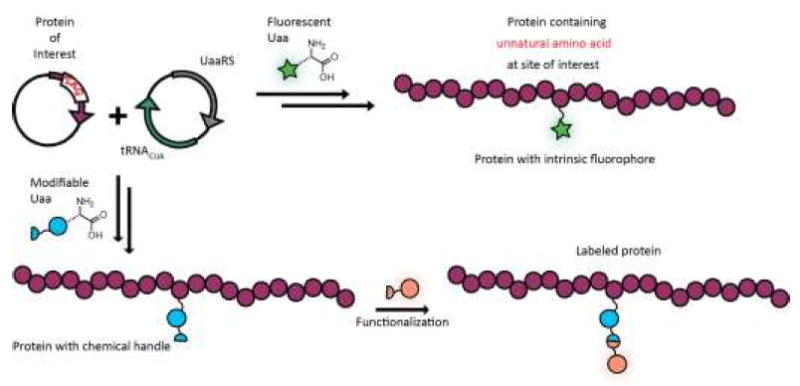
Complementary strategies for generating fluorescently labeled proteins using Uaa mutagenesis that rely on genetic encoding of the protein of interest with a nonsense codon and an orthogonal tRNA/synthetase pair to incorporate the Uaa cotranslationally. Top: Incorporation of a fluorescent Uaa leads directly to fluorescently labeled protein. Bottom: A complementary strategy based on Uaa incorporation and subsequent functionalization allows for modular fluorophore incorporation.
Several groups have reported useful strategies for dual incorporation of fluorescent probes through selective cysteine conjugation and Uaa incorporation. [23-26] Though readily applicable with a minimum of manipulation for Uaa incorporation, these strategies still have many of the limitations of standard labeling because they use Cys. Here, we focus on recent work in which Uaa mutagenesis has been employed in a sequence-independent manner to obtain site-specific dual incorporation of chromophores for studies of protein folding.* While these newly-developed methods have not yet seen significant application by the protein folding community, we highlight a few recent studies of protein folding and stability to illustrate their potential impact.
Ligation/Uaa Labeling
Chemical protein synthesis allows for precise modifications to the resulting protein molecule. [27,28] A combination of Uaa mutagenesis in an expressed protein fragment, along with chemical synthesis of a modified N- or C-terminal sequence, can allow for direct incorporation of two specific modifications through expressed protein ligation (EPL). Our laboratory has focused on the semisynthetic incorporation of a backbone thioamide as a minimal protein alteration for fluorescence-based studies. [29] Thioamides serve as FRET or PET-based quenchers for a variety of fluorophores. [30,31] We have incorporated thioamides into full-length proteins through synthesis of either an N-terminal or C-terminal thiopeptide fragment, which is then ligated to the expressed protein fragment containing a fluorophore (Figure 2). We have applied our techniques to study the misfolding of the Parkinson’s Disease associated protein α-synuclein (αS) via quenching of tryptophan fluorescence[32] as well as the Uaas p-cy anophcnylalaninc[33] and acridon-2-ylalanine (unpublished results). The resulting constructs would necessarily contain a cysteine residue at the EPL site, but we have eliminated this restriction through ligation and alkylation to yield a native methionine[34] and through the development of thioamide-compatible desulfurization conditions (unpublished results). The combination of EPL and Uaa mutagenesis is the most general double labeling method, since the synthetic portion may contain groups that cannot be incorporated cotranslationally. However, it can be labor intensive and low-yielding, and efforts to improve efficiency are ongoing in our laboratory.
Figure 2.
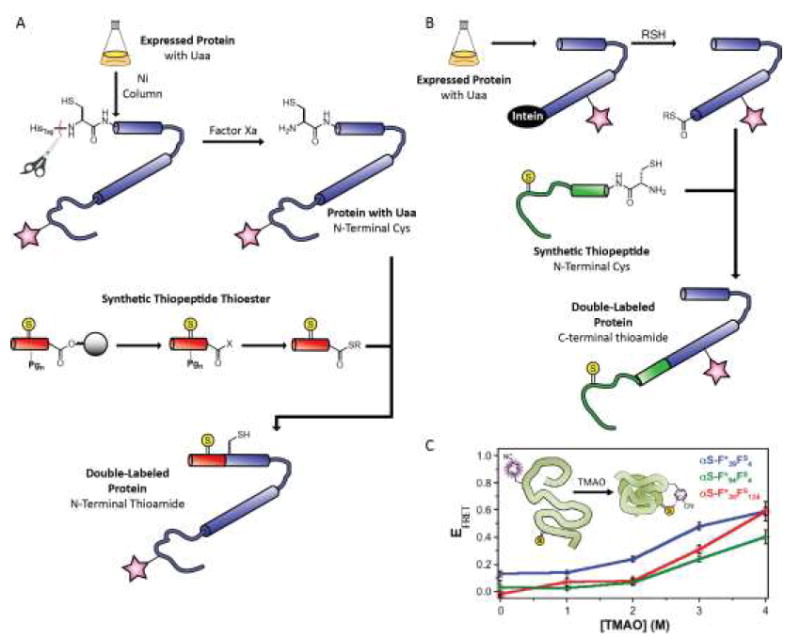
Double-labeled proteins produced through Uaa mutagenesis and expressed protein ligation. (A) Incorporation of N-terminal thioamides through semisynthesis. (B) Incorporation of C-terminal thioamides through expressed protein ligation, where the expressed fragment is fused to a C-terminal intein. (C) Measurements of protein compaction in the presence of trimethylamine-N-oxide (TMAO) using α-synuclein containing a cyanophenylalanine (F*) and a thioamide (Thio-Ala, As or Thio-Phe, Fs) FRET pair; subscript indicates position in the α-synuclein sequence.
Dual Uaa Labeling
Dual Uaa incorporation into proteins has previously been achieved by cell-free translation systems in response to three and four base codons, allowing for incorporation of fluorophores for proof-of-principle studies in the calcium-binding protein calmodulin (CaM) and the enzyme dihydrofolate reductase. [35,36] Although cell-free systems are amenable to a variety of Uaas, protein yields can be limited, making cell-based expression systems desirable. Cotranslational insertion of two Uaas into a protein in cells requires the use of two mutually orthogonal Uaa synthetase/tRNA pairs which must also be orthogonal to the endogenous translational machinery. Derivatives of the M. jannashii tyrosyl tRNA synthetase and M. barkeri or M. mazei pyrrolysl tRNA synthetase fulfill these criteria for expression in E. coli. Each of these synthetase/tRNA pairs has been frequently employed for the incorporation of a single Uaa. [17]
In recent years, dual stop codon suppression using orthogonal synthetase/tRNA pairs has been used to produce doubly labeled proteins. Liu and co-workers utilized amber/ochre (UAG/UAA) suppression for the dual incorporation of p-azidophenylalanine (Azf) and 2-amino-8-oxononanoic acid (Oxo) into glutamine binding protein.[37] These residues were site-specifically labeled in a one-pot reaction using fluorescein-dibenzocyclooctyne (Azf) and hydroxylamine-coumarin (Oxo) for ensemble FRET studies. A study by Park and colleagues used amber/opal (UAG/UGA) suppression to incorporate N6-propargyloxycarbonyl-lysine (Pok) and p-acetylphenylalanine (AcF) into CaM. [38] The dual-functionalized CaM was labeled in one pot using Cy3-hydrazide (Acf) and Cy5-azide (Pok), and the resulting construct was analyzed by ensemble and single molecule FRET (smFRET) in the presence or absence of Ca2+ and M13 binding peptide. However, the efficiency of dual suppression techniques like these is limited by truncation at the UAG codon due to competition with release factors or incorporation of natural amino acids in response to the suppressed codon. [39,40] Efforts to solve the “truncation problem,” have included deletion of release factors or reassignment of all UAG stop codons in the bacterium. [41,42]
In an important step forward in dual Uaa incorporation, Chin and colleagues developed a platform utilizing orthogonal ribosomes, which are not responsible for synthesis of the proteome and thus can be manipulated in ways that would otherwise be lethal to the host cell. [43] They developed a ribosome which decodes both a UAG codon and a four base codon to guide insertion of two Uaas into a protein at specific, independent positions (Figure 3). [44] This orthogonal dual-incorporation system has recently been applied to generate CaM containing norbomyl-lysine (NorK) and tetrazinyl-phenylalanine (TetPhe). This protein was labeled in a one-pot reaction using a BODIPY-F1 tetrazine (NorK) and BODIPY-TMR cyclooctyne (TetPhe). [45] The resulting double-labeled protein was employed in FRET studies of conformational changes in CaM upon titration with Ca2+. Subsequent work utilizing the dual incorporation of propargyltyrosine for copper-catalyzed “click” labeling and a cyclopropene-functionalized Uaa for tetrazine-inverse electron demand Diels Alder labeling in CaM demonstrated the general applicability of this dual-Uaa incorporation system. [46]
Figure 3.
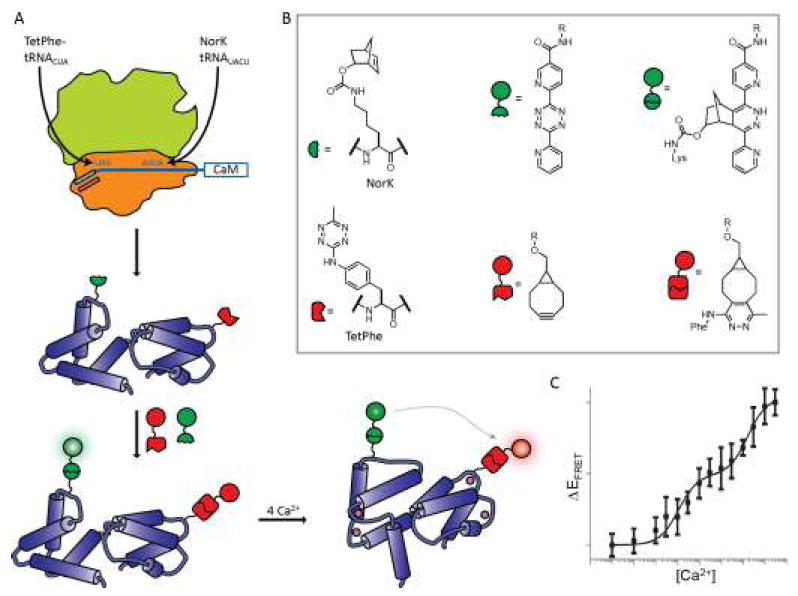
Ribosomal incorporation of two Uaas using an evolved orthogonal ribosome. (A) Schematic representation of dual incorporation of TetPhe in response to a stop codon (UAG) and of NorK in response to a four base codon (AGUA). CaM was then labeled and FRET observations made. (B) Unnatural amino acids NorK and TetPhe are incorporated and subsequently labeled with a BODIPY-fluorescein-derivatized tetrazine (NorK) or BODIPY-tetramethylrhodamine derivatized cyclooctyne. (C) FRET efficiency increases as Ca2+ is added to doubly labeled CaM.
FRET Studies in Biological Systems
In addition to highlighting advances in labeling, we also wish to highlight recent experiments in protein folding and stability that were carried out with older labeling techniques which could benefit from more facile or less perturbing labeling strategies.
The first type of experiment uses FRET to define the ternary structures of dynamic protein complexes from subunits previously characterized at higher resolution by crystallography or NMR. Seidel has established sophisticated methods taking advantage of fluorescence intensity, lifetime, polarization and wavelength information obtained from smFRET measurements to build models using maximal information on the donor and acceptor chromophores.[47] For example, they recently used FRET (as well as double electron-electron resonance spectroscopy) to build a model of the dimer of the immune defense protein human guanylate binding protein 1 in the presence of GTP.[48] FRET measurements using an Alexa488 and Alexa647 pair were used to drive molecular modeling of the dimer, in particular an α13-α13 helical interaction, that would not have been anticipated from docking crystal structures of the monomers (Figure 4A). For complexes with more than two subunits, one can make a series of pairwise FRET measurements with different components labeled, or take the more ambitious approach of Hugel and co-workers, which used a four-color FRET system to investigate the heat shock protein system HSP90, including cochaperone p23 and nucleotides. [49] Their design utilized Cys-maleimide labeling of HSP90 monomers and hydrazide labeling of AcF incorporated into p23, in conjunction with fluorescently-labeled ATP. The four-color FRET system allowed them to generate a model of the assembly of the HSP90 complex with p23, and its ATP-dependent conformational rearrangements. The ability to encode multiple Uaas would allow such studies to include intraprotein distance measurements as well as interprotein measurements.
Figure 4.
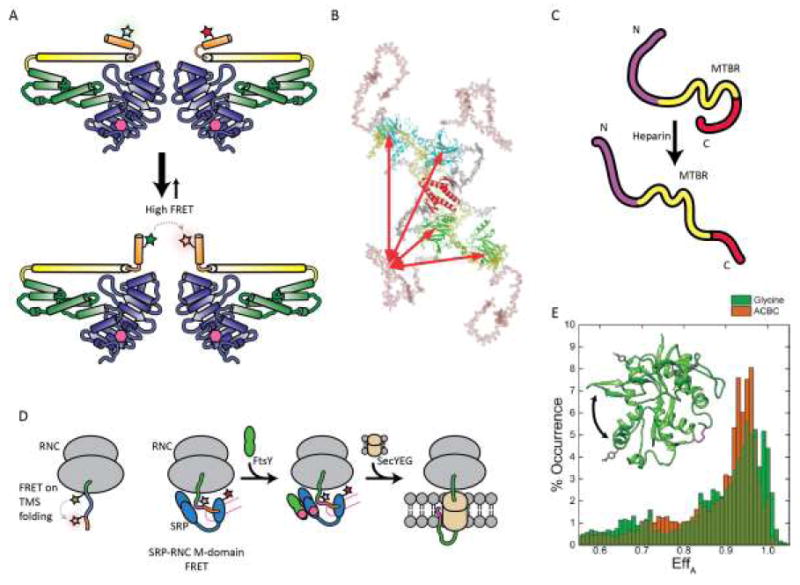
FRET based studies of protein folding and interactions. (A) FRET and double electron-electron resonance based observations of close spatial proximity between two α13 helices in the human glutamate binding protein 1 homodimer. (B) Schematic model of full length p53 conformations; DNA binding domain is shown in green or cyan, tetramerization domain shown in gray, N-terminal domain in salmon, and C-terminus in yellow. (C) Model for tau conformational changes in the presence of heparin based on smFRET and coarse-grained modeling. (D) FRET -pair systems for studying transmembrane sequence (TMS) folding in ribosomal nascent chain complex (RNC) and RNC-signal recognition particle (SRP) interaction during cotranslational translocation to membranes. (E) Comparison of smFRET histograms of NMDA glycine binding domain (GluNl) in the presence of full agonist glycine (green) or partial agonist 1 -amino-1-cyclobutane carboxylic acid (ACBC; orange). Inset: crystal structure of the GluNl agonist-binding domain showing AcF side chains (grey) and native Cys (magenta, disulfide; yellow, free Cys) in stick form; arrow indicates domain closure. (B) Reproduced from ref. [50] , Copyright 2009, National Academy of Sciences. (E) Adapted from ref. [59], Copyright 2015, the American Society for Biochemistry and Molecular Biology.
A second area in which FRET is particularly useful is the study of intrinsically disordered proteins (IDPs), or disordered protein regions. These proteins are insufficiently ordered for study by crystallization or NMR, but FRET can still be used to determine inter-residue separation because it can be sampled on short timescales and/or on the single molecule level where no conformational averaging takes place. To identify the conformational ensemble of disordered regions of the tumor suppressor protein p53, Fersht and coworkers used extensive mutation to enable labeling at single engineered Cys sites in either the N-terminal domain (NTD) or DNA-binding domain for smFRET and time-resolved FRET studies.[50] The combined observations indicated an extended NTD conformation with a ~20-residue polyproline-II type helix, and intramolecular interactions between the NTD and DNA binding domain but not between the NTD and C-terminus (Figure 4B). Of particular interest are amyloidogenic IDPs, such as αS and tau, which have been implicated in a number of neurodegenerative diseases. Rhoades and co-workers have undertaken de novo structure determination studies of αS and tau using large numbers of pairwise FRET measurements to define distance constraints for coarse-grained computational modeling. [51,52] A recent study of tau provided some structural understanding of the well-established effect of heparin in accelerating amyloid fibril formation. Heparin binding leads to a loss of long-range interactions between the N- and C-termini and between each terminus and the microtubule binding region (Figure 4C).
FRET studies are ideally suited for monitoring co-translational protein folding, which requires temporally defined studies. A 2004 study by Johnson and co-workers utilized FRET pairs incorporated at amber and Lys codons using semi-synthetic tRNAs to examine co-translational folding of transmembrane sequences in nascent polypeptides. [53] Importantly, judicious experimental design, including placement of a single Lys C-terminal to the amber codon, was required to accomplish this. This allowed observation of helical folding while still in the ribosomal exit tunnel, a landmark finding. Recently, Shan has built on this work with FRET studies of the interaction of the ribosome-nascent chain complex (RNC) with membrane insertion machinery, such as the bacterial signal recognition particle (SRP) and SRP receptor FtsY.[54] Synthetase-based co-translational incorporation of the intrinsically fluorescent Uaa, 7-hydroxycoumaryl ethylglycine, allowed for a minimum of chemical manipulation of the RNC complex. A FRET partner, BODIPY-F1, was introduced in the SRP M-domain or N-domain through site-selective cysteine labeling (Figure 4D).[55] These and other FRET experiments demonstrated a step-wise mechanism of cargo transfer: engagement of the SRP M-domain with the RNC, formation of an early targeting complex including FtsY, followed by formation of a closed targeting complex and cargo transfer. [56,57]
Finally, FRET is very useful for studying dynamic proteins in which some states have defined structures, but many complex rearrangements are necessary for function, such as ion channels. A 2013 study utilized co-translational incorporation of the fluorescent Uaa 3-(6-acetylnapthalen-2-ylamine)-2-aminopropionic acid and cysteine labeling with a rhodamine acceptor to investigate voltage-gated potassium channel channel dynamics. [58] FRET measurements made in conjunction with electrophysiology allowed for a time-resolved description of channel gating. An smFRET study of cleft closure in the N-methyl-D-aspartate receptor glycine binding domain (NMDA-GBD) was enabled by dual incorporation of AcF and stochastic labeling with hydrazide-functionalized Alexa Fluor 555 and Alexa Fluor 647 (Figure 4E).[59] The use of AcF was essential because NMDA-GBD has three disulfide bridges and removal of an endogenous free Cys proved deleterious to recombinant expression in E. coli. FRET measurements showed different levels of domain closure in antagonist-, partial-agonist- and agonist-bound states of the NMDA-GBD. While this study used dual incorporation of the same amino acid, the ability to site-specifically introduce two different Uaas using Liu or Chin’s methods would have been highly beneficial.
Conclusion
There is strong interest in the biophysics community in the ability to incorporate fluorophores directly, or through selective chemical modification, for the efficient synthesis of proteins containing FRET pairs. As briefly illustrated here, such labeled proteins can be used in tracking conformational changes, modeling structures of dynamic ternary complexes, or even generating low resolution de novo structures of disordered proteins. New developments in labeling using Uaa mutagenesis in combination with EPL or bioorthogonal reactions enable facile dual Uaa incorporation or use of minimally-perturbing probes like thioamides. These strategies should enable researchers to generate libraries of precisely labeled constructs for mapping dynamic protein structures to understand these important systems that are refractory to traditional structural techniques.
Footnotes
Additional excellent developments in protein double labeling have been made by Edward Lemke and coworkers. We have limited our discussion of these articles here, as they are summarized in the chapter by Nikic and Lemke in this volume.
References
- 1.Orevi T, Lemer E, Rahamim G, Amir D, Haas E. Ensemble and Single-Molecule Detected Time-Resolved FRET Methods in Studies of Protein Conformations and Dynamics. Fluorescence Spectroscopy and Microscopy. :113–169. doi: 10.1007/978-1-62703-649-8_7. [DOI] [PubMed] [Google Scholar]; Methods in Molecular Biology. 1076 [Google Scholar]
- 2.Lakowicz JR. Principles of Fluorescence Spectroscopy. 3. Springer; US: 2006. [Google Scholar]
- 3.Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, et al. HaloTag: A Novel Protein Labeling Technology for Cell Imaging and Protein Analysis. ACS Chemical Biology. 2008;3:373–382. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- 4.Keppler A, Kindermann M, Gendreizig S, Pick H, Vogel H, Johnsson K. Labeling of fusion proteins of 06-alkylguanine-DNA alkyltransferase with small molecules in vivo and in vitro. Methods. 2004;32:437–444. doi: 10.1016/j.ymeth.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Gautier A, Juillerat A, Heinis C, Corrêa IR, Jr, Kindermann M, Beaufils F, Johnsson K. An Engineered Protein Tag for Multiprotein Labeling in Living Cells. Chemistry & Biology. 2008;15:128–136. doi: 10.1016/j.chembiol.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher SS, Sable JE, Sheetz MP, Cornish VW. An In Vivo Covalent TMP-Tag Based on Proximity-Induced Reactivity. ACS Chemical Biology. 2009;4:547–556. doi: 10.1021/cb900062k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson HJ, Campbell RE. Genetically encoded FRET-based biosensors for multiparameter fluorescence imaging. Current Opinion in Biotechnology. 2009;20:19–27. doi: 10.1016/j.copbio.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Piston DW, Kremers G-J. Fluorescent protein FRET: the good, the bad and the ugly. Trends in Biochemical Sciences. 2007;32:407–414. doi: 10.1016/j.tibs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Speight LC, Samanta M, Petersson EJ. Minimalist Approaches to Protein Labelling: Getting the Most Fluorescent Bang for Your Steric Buck. Australian Journal of Chemistry. 2014;67:686–700. [Google Scholar]
- 10.Griffin BA, Adams SR, Tsien RY. Specific Covalent Labeling of Recombinant Protein Molecules Inside Live Cells. Science. 1998;281:269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- 11.Scheck RA, Schepartz A. Surveying Protein Structure and Function Using Bis-Arsenical Small Molecules. Accounts of Chemical Research. 2011;44:654–665. doi: 10.1021/ar2001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rashidian M, Dozier JK, Distefano MD. Enzymatic Labeling of Proteins: Techniques and Approaches. Bioconjugate Chemistry. 2013;24:1277–1294. doi: 10.1021/bc400102w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutureira O, Bemardes GJL. Advances in Chemical Protein Modification. Chemical Reviews. 2015;115:2174–2195. doi: 10.1021/cr500399p. [DOI] [PubMed] [Google Scholar]
- 14.Stephanopoulos N, Francis MB. Choosing an effective protein bioconjugation strategy. Nat Chem Biol. 2011;7:876–884. doi: 10.1038/nchembio.720. [DOI] [PubMed] [Google Scholar]
- 15.Spicer CD, Davis BG. Selective chemical protein modification. Nat Commun. 2014;5 doi: 10.1038/ncomms5740. [DOI] [PubMed] [Google Scholar]
- 16.Lang K, Chin JW. Cellular Incorporation of Unnatural Amino Acids and Bioorthogonal Labeling of Proteins. Chemical Reviews. 2014;114:4764–4806. doi: 10.1021/cr400355w.. •This comprehensive review discusses strategies for the introduction and chemical functionalization of Uaas in proteins expressed recombinantly.
- 17.Dumas A, Lercher L, Spicer CD, Davis BG. Designing logical codon reassignment - Expanding the chemistry in biology. Chemical Science. 2015;6:50–69. doi: 10.1039/c4sc01534g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Summerer D, Chen S, Wu N, Deiters A, Chin JW, Schultz PG. A genetically encoded fluorescent amino acid. Proceedings of the National Academy of Sciences. 2006;103:9785–9789. doi: 10.1073/pnas.0603965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HS, Guo J, Lemke EA, Dimla RD, Schultz PG. Genetic Incorporation of a Small, Environmentally Sensitive, Fluorescent Probe into Proteins in Saccharomyces cerevisiae. Journal of the American Chemical Society. 2009;131:12921–12923. doi: 10.1021/ja904896s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatterjee A, Guo J, Lee HS, Schultz PG. A Genetically Encoded Fluorescent Probe in Mammalian Cells. Journal of the American Chemical Society. 2013;135:12540–12543. doi: 10.1021/ja4059553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speight LC, Muthusamy AK, Goldberg JM, Warner JB, Wissner RF, Willi TS, Woodman BF, Mehl RA, Petersson EJ. Efficient Synthesis and In Vivo Incorporation of Acridon-2-ylalanine, a Fluorescent Amino Acid for Lifetime and Forster Resonance Energy Transfer/Luminescence Resonance Energy Transfer Studies. Journal of the American Chemical Society. 2013;135:18806–18814. doi: 10.1021/ja403247j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsao M-L, Summerer D, Ryu Y, Schultz PG. The Genetic Incorporation of a Distance Probe into Proteins in Escherichia coli. Journal of the American Chemical Society. 2006;128:4572–4573. doi: 10.1021/ja058262u. [DOI] [PubMed] [Google Scholar]
- 23.Brustad EM, Lemke EA, Schultz PG, Deniz AA. A General and Efficient Method for the Site-Specific Dual-Labeling of Proteins for Single Molecule Fluorescence Resonance Energy Transfer. Journal of the American Chemical Society. 2008;130:17664–17665. doi: 10.1021/ja807430h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen DP, Elliott T, Holt M, Muir TW, Chin JW. Genetically Encoded 1,2-Aminothiols Facilitate Rapid and Site-Specific Protein Labeling via a Bio-orthogonal Cyanobenzothiazole Condensation. Journal of the American Chemical Society. 2011;133:11418–11421. doi: 10.1021/ja203111c. [DOI] [PubMed] [Google Scholar]
- 25.Milles S, Tyagi S, Banterle N, Koehler C, VanDelinder V, Plass T, Neal AP, Lemke EA. Click Strategies for Single-Molecule Protein Fluorescence. Journal of the American Chemical Society. 2012;134:5187–5195. doi: 10.1021/ja210587q. [DOI] [PubMed] [Google Scholar]
- 26.Tyagi S, Lemke EA. Chapter 9 - Genetically Encoded Click Chemistry for Single-Molecule FRET of Proteins. In: Conn PM, editor. Methods in Cell Biology. Vol. 113. Academic Press; 2013. pp. 169–187. [DOI] [PubMed] [Google Scholar]
- 27.Muralidharan V, Muir TW. Protein ligation: an enabling technology for the biophysical analysis of proteins. Nat Meth. 2006;3:429–438. doi: 10.1038/nmeth886. [DOI] [PubMed] [Google Scholar]
- 28.Kent SBH. Total chemical synthesis of proteins. Chemical Society Reviews. 2009;38:338–351. doi: 10.1039/b700141j. [DOI] [PubMed] [Google Scholar]
- 29.Wang YJ, Szantai-Kis DM, Petersson EJ. Semi-synthesis of thioamide containing proteins. Organic & Biomolecular Chemistry. 2015 doi: 10.1039/c5ob00224a. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg JM, Batjargal S, Chen BS, Petersson EJ. Thioamide Quenching of Fluorescent Probes through Photoinduced Electron Transfer: Mechanistic Studies and Applications. Journal of the American Chemical Society. 2013;135:18651–18658. doi: 10.1021/ja409709x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldberg JM, Batjargal S, Petersson EJ. Thioamides as Fluorescence Quenching Probes: Minimalist Chromophores To Monitor Protein Dynamics. Journal of the American Chemical Society. 2010;132:14718–14720. doi: 10.1021/ja1044924. [DOI] [PubMed] [Google Scholar]
- 32.Batjargal S, Wang YJ, Goldberg JM, Wissner RF, Petersson EJ. Native Chemical Ligation of Thioamide-Containing Peptides: Development and Application to the Synthesis of Labeled a-Synuclein for Misfolding Studies. Journal of the American Chemical Society. 2012;134:9172–9182. doi: 10.1021/ja2113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wissner RF, Batjargal S, Fadzen CM, Petersson EJ. Labeling Proteins with Fluorophore/Thioamide Förster Resonant Energy Transfer Pairs by Combining Unnatural Amino Acid Mutagenesis and Native Chemical Ligation. Journal of the American Chemical Society. 2013;135:6529–6540. doi: 10.1021/ja4005943.. •• The utility of EPL for FRET pair incorporation into full length proteins was demonstrated by semisynthesis of the amyloidogenic protein a-synuclein. We expect these techniques to be widely applicable in situations where one desires to use a FRET donor or acceptor that cannot be cotranslationally incorporated.
- 34.Wissner RF, Wagner AM, Warner JB, Petersson EJ. Efficient, Traceless Semi-Synthesis of α-Synuclein Labeled with a Fluoro-phore/Thioamide FRET Pair. Synlett. 2013;24:2454–2458. doi: 10.1055/s-0033-1339853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kajihara D, Abe R, Iijima I, Komiyama C, Sisido M, Hohsaka T. FRET analysis of protein conformational change through position-specific incorporation of fluorescent amino acids. Nature Methods. 2006;3:923–929. doi: 10.1038/nmeth945. [DOI] [PubMed] [Google Scholar]
- 36.Chen S, Fahmi NE, Wang L, Bhattacharya C, Benkovic SJ, Hecht SM. Detection of Dihydrofolate Reductase Conformational Change by FRET Using Two Fluorescent Amino Acids. Journal of the American Chemical Society. 2013;135:12924–12927. doi: 10.1021/ja403007r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu B, Wang Z, Huang Y, Liu WR. Catalyst-Free and Site-Specific One-Pot Dual-Labeling of a Protein Directed by Two Genetically Incorporated Noncanonical Amino Acids. ChemBioChem. 2012;13:1405–1408. doi: 10.1002/cbic.201200281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J, Seo M-H, Lee S, Cho K, Yang A, Woo K, Kim H-S, Park H-S. Simple and Efficient Strategy for Site-Specific Dual Labeling of Proteins for Single-Molecule Fluorescence Resonance Energy Transfer Analysis. Analytical Chemistry. 2013;85:1468–1474. doi: 10.1021/ac303089v. [DOI] [PubMed] [Google Scholar]
- 39.Mukai T, Hayashi A, Iraha F, Sato A, Ohtake K, Yokoyama S, Sakamoto K. Codon reassignment in the Escherichia coli genetic code. Nucleic Acids Research. 2010;38:8188–8195. doi: 10.1093/nar/gkq707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aerni HR, Shifman MA, Rogulina S, O’Donoghue P, Rinehart J. Revealing the amino acid composition of proteins within an expanded genetic code. Nucleic Acids Research. 2015;43:e8. doi: 10.1093/nar/gku1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lajoie MJ, Rovner AJ, Goodman DB, Aerni H-R, Haimovich AD, Kuznetsov G, Mercer JA, Wang HH, Carr PA, Mosberg JA, et al. Genomically Recoded Organisms Expand Biological Functions. Science. 2013;342:357–360. doi: 10.1126/science.1241459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson DBF, Xu J, Shen Z, Takimoto JK, Schultz MD, Schmitz RJ, Xiang Z, Ecker JR, Briggs SP, Wang L. RF1 knockout allows ribosomal incorporation of unnatural amino acids at multiple sites. Nature Chemical Biology. 2011;7:779–786. doi: 10.1038/nchembio.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang K, Neumann H, Peak-Chew SY, Chin JW. Evolved orthogonal ribosomes enhance the efficiency of synthetic genetic code expansion. Nat Biotech. 2007;25:770–777. doi: 10.1038/nbt1314. [DOI] [PubMed] [Google Scholar]
- 44.Neumann H, Wang K, Davis L, Garcia-Alai M, Chin JW. Encoding multiple unnatural amino acids via evolution of a quadruplet-decoding ribosome. Nature. 2010;464:441–444. doi: 10.1038/nature08817. [DOI] [PubMed] [Google Scholar]
- 45.Wang K, Sachdeva A, Cox DJ, Wilf NW, Lang K, Wallace S, Mehl RA, Chin JW. Optimized orthogonal translation of unnatural amino acids enables spontaneous protein double labelling and FRET. Nature Chemistry. 2014 doi: 10.1038/nchem.1919.. •• Building on prior work engineering orthogonal ribosomes that recognize 4-base codons, the authors present simultaneous dual incorporation of two Uaas into calmodulin and subsequent simultaneous labeling. We expect wider application of dual Uaa incorporation by similar methods to greatly enable future FRET studies of protein folding and stability
- 46.Schmied WH, Elsasser SJ, Uttamapinant C, Chin JW. Efficient Multisite Unnatural Amino Acid Incorporation in Mammalian Cells via Optimized Pyrrolysyl tRNA Synthetase/tRNA Expression and Engineered eRFl. Journal of the American Chemical Society. 2014;136:15577–15583. doi: 10.1021/ja5069728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalinin S, Peulen T, Sindbert S, Rothwell PJ, Berger S, Restle T, Goody RS, Gohlke H, Seidel CAM. A toolkit and benchmark study for FRET-restrained high-precision structural modeling. Nature Methods. 2012;9:1218–1225. doi: 10.1038/nmeth.2222. [DOI] [PubMed] [Google Scholar]
- 48.Vöpel T, Hengstenberg CS, Peulen T-O, Ajaj Y, Seidel CAM, Herrmann C, Klare JP. Triphosphate Induced Dimerization of Human Guanylate Binding Protein 1 Involves Association of the C-Terminal Helices: A Joint Double Electron-Electron Resonance and FRET Study. Biochemistry. 2014;53:4590–4600. doi: 10.1021/bi500524u. [DOI] [PubMed] [Google Scholar]
- 49.Ratzke C, Hellenkamp B, Hugel T. Four-colour FRET reveals directionality in the Hsp90 multicomponent machinery. Nature Communications. 2014;5 doi: 10.1038/ncomms5192.. • Hugel and co-workers construct a four-color FRET system based on both Cys labeling and Uaa incorporation. Their four color FRET systems enabled them to demonstrate a coupling between HSP90 ATP hydrolysis and cochaperone association
- 50.Huang F, Rajagopalan S, Settanni G, Marsh RJ, Armoogum DA, Nicolaou N, Bain AJ, Lerner E, Haas E, Ying L, et al. Multiple conformations of full-length p53 detected with single-molecule fluorescence resonance energy transfer. Proceedings of the National Academy of Sciences. 2009;106:20758–20763. doi: 10.1073/pnas.0909644106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nath A, Sammalkorpi M, DeWitt David C, Trexler Adam J, Elbaum-Garfinkle S, O’Hem Corey S, Rhoades E. The Conformational Ensembles of α-Synuclein and Tau: Combining Single-Molecule FRET and Simulations. Biophysical Journal. 2012;103:1940–1949. doi: 10.1016/j.bpj.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elbaum-Garfinkle S, Rhoades E. Identification of an Aggregation-Prone Structure of Tau. Journal of the American Chemical’Society. 2012;134:16607–16613. doi: 10.1021/ja305206m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woolhead CA, McCormick PJ, Johnson AE. Nascent Membrane and Secretory Proteins Differ in FRET-Detected Folding Far inside the Ribosome and in Their Exposure to Ribosomal Proteins. Cell. 2004;116:725–736. doi: 10.1016/s0092-8674(04)00169-2. [DOI] [PubMed] [Google Scholar]
- 54.Shen K, Arslan S, Akopian D, Ha T, Shan S-o. Activated GTPase movement on an RNA scaffold drives co-translational protein targeting. Nature. 2012;492:271–275. doi: 10.1038/nature11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saraogi I, Akopian D, Shan S-o. Regulation of cargo recognition, commitment, and unloading drives cotranslational protein targeting. The Journal of Cell Biology. 2014;205:693–706. doi: 10.1083/jcb.201311028.. • Incorporation of a coumarin-functionalized Uaa in the ribosomal nascent-chain complex proved key in deciphering the recruitment and trafficking of RNC-SRP. Of note is that the authors were able to use different signal sequences in order to propose a mechanism for SRP-mediated rejection of undesired substrates.
- 56.Akopian D, Dalai K, Shen K, Duong F, Shan S-o. SecYEG activates GTPases to drive the completion of cotranslational protein targeting. The Journal of Cell Biology. 2013;200:397–405. doi: 10.1083/jcb.201208045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saraogi I, Shan S-o. Co-translational protein targeting to the bacterial membrane. Biochimica et Biophysicci Acta (BBA) - Molecular Cell Research. 2014;1843:1433–1441. doi: 10.1016/j.bbamcr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalstrup T, Blunck R. Dynamics of internal pore opening in KV channels probed by a fluorescent unnatural amino acid. Proceedings of the National Academy of Sciences. 2013;110:8272–8277. doi: 10.1073/pnas.1220398110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dolino DM, Cooper D, Ramaswamy S, Jaurich H, Landes CF, Jayaraman V. Structural Dynamics of the Glycine-binding Domain of the N-Methyl-d-Aspartate Receptor. Journal of Biological Chemistry. 2015;290:797–804. doi: 10.1074/jbc.M114.605436.. • The use of Uaa incorporation for labeling proved necessary for this FRET-study of NMDA receptor interactions; future study of similar systems could be considerably aided by dual-Uaa incorporation methods.


