Abstract
Glioblastoma multiforme (GBM) is the most common and most lethal primary brain tumor with a 5 year overall survival rate of approximately 5%. Currently, no therapy is curative and all have significant side effects. Focal thermal ablative therapies are being investigated as a new therapeutic approach. Such therapies can be enhanced using nanotechnology. Carbon nanotube mediated thermal therapy (CNMTT) uses lasers that emit near infrared radiation to excite carbon nanotubes (CNTs) localized to the tumor to generate heat needed for thermal ablation. Clinical translation of CNMTT for GBM will require development of effective strategies to deliver CNTs to tumors, clear structure-activity and structure-toxicity evaluation, and an understanding of the effects of inherent and acquired thermotolerance on the efficacy of treatment. In our studies, we show that a dense coating of phospholipid-poly(ethylene glycol) on multiwalled CNTs (MWCNTS) allows for better diffusion through brain phantoms, while maintaining the ability to achieve ablative temperatures after laser exposure. Phospholipid-poly(ethylene glycol) coated MWCNTs do not induce a heat shock response (HSR) in GBM cell lines. Activation of the HSR in GBM cells via exposure to sub-ablative temperatures or short term treatment with an inhibitor of heat shock protein 90 (17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG)), induces a protective heat shock response that results in thermotolerance and protects against CNMTT. Finally, we evaluate the potential for CNMTT to treat GBM multicellular spheroids. These data provide pre-clinical insight into key parameters needed for translation of CNMTT including nanoparticle delivery, cytotoxicity, and efficacy for treatment of thermotolerant GBM.
Keywords: Cancer, nanotechnology, convection, ablation, hyperthermia, heat shock, brain tumor
Graphical abstract
INTRODUCTION
Glioblastoma multiforme (GBM) is the most common and most lethal primary brain tumor with a 5 year overall survival rate of less than 5% and a median survival of approximately 14 months1. Current treatment modalities for patients with GBM include surgical resection followed by a combination of ionizing radiation and chemotherapy with the alkylating agent temozolomide (TMZ). However, no standard treatment for GBM is curative and minimal advances in prognosis have been seen over the past decade1. Multiple challenges to effective GBM therapy remain, including: (i) tumor inaccessibility and risk of damage to the normal brain; (ii) insufficient and heterogeneous blood–brain barrier (BBB) permeability; and (iii) resistance to conventional chemo- and radiation therapies2.
Thermal ablative therapies such as laser induced thermal therapy are being investigated for treatment of GBM patients3, 4. LITT uses an interstitial fiber optic laser, inserted via a catheter into the tumor through a small hole drilled in the skull4, 5, to heat malignant tissue to temperatures in excess of 50 ºC, which leads to protein denaturation, aggregation and oxidation, and ultimately results in cell death6. Current clinical LITT systems allow real-time adjustments in the placement of lasers to direct treatment toward irregular margins, and magnetic resonance imaging enables monitoring of both temperature and laser position7. Despite these advances, an inability to achieve sufficiently high temperatures throughout the target lesion and lack of specificity of heat delivery still limit the clinical utility of LITT.
Nanoparticles, in combination with different sources of electromagnetic radiation, can improve the efficacy and cancer specificity of heat based therapies8. Of particular interest for thermal therapies is the development of nanoparticles that generate heat following exposure to near infrared light (NIR)9. Compared to other wavelengths of light, NIR is more penetrative in tissue 10 and NIR lasers are currently in clinical use for thermal ablation of GBM4, 5. Carbon nanotubes (CNTs), which consist of sheets of sp2 carbon rolled into single or multiwalled tubes, are highly efficient absorbers of NIR and generate substantial amounts of heat following NIR exposure9, 11. We and others have shown that CNT Mediated Thermal Therapy (CNMTT) allows far greater heat generation and localization within a tumor target than laser irradiation alone12 and is effective for treating bulk cancer and stem cell-like cancer populations11, 13, including GBM14 and GBM stem cells15. In general, multiwalled CNTs (MWCNTs) have proven more effective for CNMTT than single-walled CNTs (SWCNTs)9. Furthermore, CNTs are internalized by cells16, 17 and can incorporate multiple diagnostic and therapeutic functions into a single particle which may allow for image guided therapy18. The toxicity profile of CNTs is dependent upon the physicochemical characteristics of the specific particles and remains a subject of debate9, but proper functionalization of CNT surfaces can render CNTs safe for use in the blood19 or brain20, 21.
The unique constraints on the delivery of nanomaterials to the brain require careful consideration of CNT physicochemical characteristics. At present, CNTs do not efficiently cross the BBB22. However, advances in the use of catheter driven infusion strategies such as convection enhanced delivery (CED)23 may allow for tumor specific delivery of CNTs. CED bypasses the BBB and relies on pressure-driven infusion through an intracranial catheter to distribute macromolecules along a pressure gradient rather than relying only on diffusion. CED of nanomaterials is an area of current research24, 25, but CED has not been tested for the delivery of CNTs.
A second challenge to heat-based therapies is overcoming resistance due to elevated expression of the family of heat shock proteins (HSPs)11, which are a key component of the cytoprotective process which cells use to survive exposure to heat and other stressors26, 27. Many types of cancers including GBM express high levels of HSPs28–30, which enable GBM to adapt to the dramatic changes in physiology that accompany malignant transformation31 and contribute to tolerance to chemo- and radiotherapy30. Transcription of HSPs including HSP90, HSP70 and HSP27 is driven by the master regulator of the heat shock response, heat shock factor 1 (HSF1)27–29. In both normal and cancerous tissue, thermotolerance can be induced by sub-lethal heat conditioning and reduces the efficacy of subsequent heat-based treatments32. Chemotherapy also can induce a protective HSR33, but it is not known what role this may play in thermotolerance.
Clinical translation of CNMTT for GBM will require development of effective strategies to deliver CNTs to tumors, clear structure-activity and structure-toxicity evaluation, and an understanding of the effects of inherent and acquired thermotolerance on the efficacy of treatment. Little is known about the role of the heat shock response with regards to the efficacy of thermal therapy for GBM, and no studies have examined the use of CNMTT or other nanoparticle-based thermal therapies in the context of induced expression of HSPs. Furthermore, few studies have used three dimensional cell cultures such as multicellular tumor spheroids, which may more closely mimic tumors in vivo34, to evaluate nanoparticle-based thermal therapy. Therefore, the purpose of this study was: (i) to evaluate the feasibility of using slow infusion techniques similar to CED to deliver CNTs into brain extracellular matrix (ECM)-mimicking phantoms; (ii) to determine the efficacy of CNMTT in comparison to conventional heat delivery in GBM cell lines and multicellular tumor spheroids; and (iii) to determine if CNMTT could overcome the protective effects of induction of the heat shock response prior to initiation of CNMTT.
MATERIALS AND METHODS
Cell Culture
U87 glioblastoma, U373MG and Normal Human Astrocytes (NHA) were obtained from American Type Culture Collection (ATCC) while D54 glioblastoma cells were donated by Dr. Hui-Wen Lo (Wake Forest University, Department of Cancer Biology). All cell lines were maintained in normal growth media Dulbecco’s modified Eagle’s medium (DMEM) (Lonza) supplemented with 10% fetal bovine serum (FBS) (Sigma), 1% penicillin-streptomycin (Gibco), 1% L-glutamine (Gibco) at 37 ºC under 5% CO2 in a humidified incubator.
Reagents
10 mM KRIBB11 (N2-(1H-indazole-5-yl)-N6-methyl-3-nitropyridine-2,6-diamine) (EDM Millipore - Calbiochem) was prepared in dimethyl sulfoxide (DMSO) and stored at −20 ºC. 5 mM 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG)(Sigma) was prepared in DMSO and stored at −20 ºC. All reagents were diluted in normal growth media prior to use.
Preparation of MWCNT dispersions
Short multi-walled carbon nanotubes (MWCNTs) 8–15 nm in diameter (Nanostructured & Amorphous Materials, Inc.) were acid oxidized and purified as previously described 35. Preparations of 1% Pluronic (Sigma), 1% 1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine conjugated to Polyethylene Glycol (DSPE-PEG) or 2% DSPE-PEG (Nanocs, Inc.) were made by dissolving the surfactants at a concentration of 1% or 2% weight to volume in deionized water. Nanoparticle dispersions were prepared by hydrating 10 mg of unmodified or acid oxidized MWCNTs with 10 mL of degassed Milli-Q (type I) water (with or without surfactants) in a 20 mL glass vial, followed by 30 minutes of bath sonication (Branson 2510). Nanoparticle suspensions were rendered isotonic by the addition of one part in 10 of 10× phosphate-buffered saline (PBS) (Invitrogen, Carlsbad, CA, USA) prior to dilution in cell culture media.
Physiochemical Characterization of MWCNTs by Dynamic Light Scattering
Hydrodynamic diameter and zeta potential were measured using the Zetasizer Nano ZS90 (Malvern Instruments, Malvern, UK) at 25ºC, with automatic settings, adjusting for the refractive index and viscosity of the dispersant. The particles were diluted to 5 μg/mL, and 1 mL was added to a disposable, clear plastic cuvette (Sarstedt, Newton, NC, USA). Each measurement was taken in triplicate.
Brain Phantoms and MWCNT Infusion
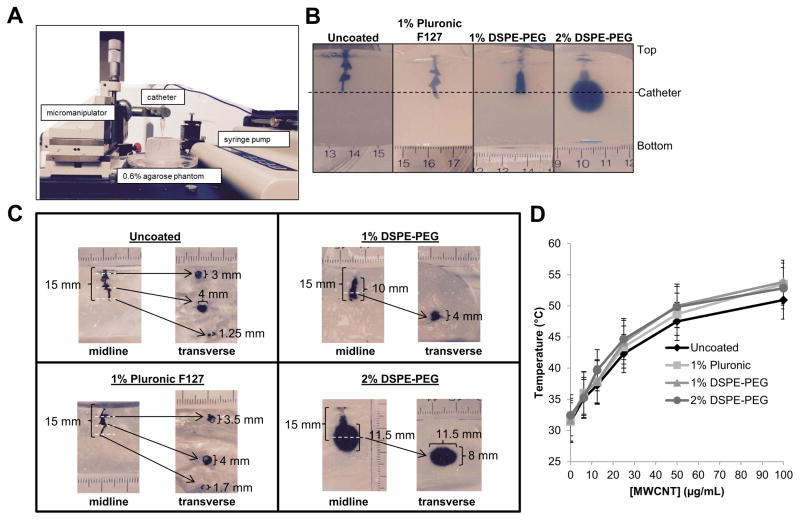
Agarose (Sigma) was dissolved in PBS at 1.2% weight to volume by boiling. After cooling to 43 ºC, the agarose solution was mixed with an equal volume of a 10% (weight to volume) bovine serum album (BSA) (Sigma) in PBS, which was 43 ºC. The resulting mixture (0.6% agarose and 5% BSA in PBS) was aliquoted into 20 ml glass vials and allowed to solidify at room temperature to form brain phantoms. The hydrogels were topped with PBS, capped, wrapped in parafilm, and then stored at 4 ºC. Phantoms were warmed to room temperature prior to use. For infusion studies, MWCNTs were diluted to 100 μg/mL in deionized water (uncoated MWCNTs) or PBS (Pluronic or PEG-coated). 500 μL MWCNT solutions were injected into brain phantoms at a rate of 2 μL/min using an infusion syringe pump (KD Scientific) via 26 gauge single-port catheter (Hospira Venisystems) placed 3 cm below the gel surface. A micromanipulator was used to precisely place the catheter within the phantom, allowing reproducibility from sample to sample.
Flow Cytometry
GBM or NHA cells were plated in 100 mm tissue culture plates (BD Falcon) and allowed to recover for 1–4 days. Plates were treated with 2% DSPE-PEG MWCNTs at the specified concentrations for 24 hours. Cells were washed with PBS and co-stained with Annexin V (APC) and propidium iodide (BD Pharmingen) per the manufacturer’s protocol. Briefly, cells were trypsinized, pelleted, washed twice with cold PBS, and then suspended in 1X Annexin V binding buffer at a concentration of 1×106 cells/ml. 1×105 cells were then mixed with Annexin V and incubated for 15 minutes at room temperature in the dark. Next, 400 ul of 1X Binding Buffer was added, mixed, and samples were analyzed on the Accuri6 Flow Cytometer (BD Biosciences). Analysis of data was performed using FCS Express version 3 (De Novo Software).
MTT-based Viability
Cells were treated and subsequently aliquoted into 96 well plates at a density of 20,000 cells per well. After 24 or 48 h, media was removed and replaced with 10% MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) reagent (5mg/mL thiozyl blue tetrazolium) in normal growth media and incubated for 15–60 minutes at 37 ºC. After incubation, media was removed and 200 μL of DMSO was added per well to dissolve formazan crystals. Absorbance was measured at 595 nm and 650 nm using spectrophotometer microplate reader (Molecular Devices, USA).
CellTiter-Glo® Assay
Cells were grown on 96 well plates at a density of 20,000 cells/well or 1×105 cells/well for NHAs. After indicated recovery time, media was removed and replaced with a 1:1 mixture of normal growth media and CellTiter-Glo® (Promega), which incubated at room temperature for 10 minutes in the dark. The resulting solution was mixed, transferred to Thermo Scientific™ Microtiter™ Microlite™ White Strip Plates, and total luminescence was measured via the Tecan GENios microplate reader.
Water bath heating
Cells were suspended in normal growth media at a concentration of 200,000 cells/mL in a sterile plastic vial and were heated in a circulating water bath (Thermo-Fisher) at specified temperatures for 15 minutes. After heating, cells were plated in 96-well plates at a density of 20,000 cells/well. After 2 day recovery in normal growth conditions, MTT assay (see above) was performed to determine viability.
Photothermal heating
To determine the heating efficacy of MWCNTs, various types of MWCNTs at a concentration of 100 μg/ml were suspended in dye free DMEM (Gibco) then transferred to triplicate wells on a 48-well tissue culture plate (Falcon). Next, samples in each well were exposed to a 970 nm diode laser (K-laser, USA) for 0–60 seconds using a 3 watt continuous wave beam with an area of 1 cm2. Pre- and post-NIR temperatures of wells were determined via thermocouple (Fluke). Temperature change as a function of laser exposure time was used to determine the duration of laser exposure time needed to achieve the necessary temperature ranges for our heating model. For all cell culture experiments, specific temperatures were verified in parallel, cell free wells receiving equivalent treatments. Cells were trypsinized, washed in PBS, then suspended in 300 μl of a 100 μg/mL DSPE-PEG-MWCNT solution in dye free DMEM then transferred to sterile wells of a 48-well tissue culture plate at a cell density of 1–2 × 105 cells per well, and exposed to NIR as above. Triplicate samples were prepared for each condition. 10 minutes after NIR exposure, cells were then pelleted, washed in DMEM, and re-plated into triplicate wells of 96-well tissue culture plates with a density of 20,000 cells per well for subsequent viability analysis using the MTT assay after a 2 day recovery. For treatment of cell monolayers, adherent U87 cells (20,000 cells/well in a 48 well plate) were incubated with 0, 5 or 10 μg/mL 2% DSPE-PEG MWCNTs overnight. Cells were washed with PBS to remove excess MWCNTs, and 500 μL of dye free DMEM was added to the wells. Wells were exposed to NIR as above for 0–180 s. After heating, dye free DMEM was replaced with fresh, complete media. Cell viability was assessed by CellTiter-Glo® Promega 24 h after laser exposure. All experiments were repeated 2–3 times to verify results.
Treatment of GBM Spheroids
U87 cells were added to low adherence, round bottom wells in a 96 well plate at a density of 4,000 cells/well and allowed to recover for 4 days without perturbation. For one set of experiments, spheroids were transferred to low adherence, 6 well plates and incubated for 24 h with 100 μg/mL 2% DSPE-PEG MWCNT in growth media. The spheroids were washed with PBS to remove excess MWCNTs, and groups of 4–6 spheroids per treatment condition were transferred to 48-well tissue culture plate and exposed to NIR as above for 0–300 s. For other experiments, groups of 4 spheroids were transferred to 48 well plates and suspended in 500 μL of dye free DMEM alone or containing 20 μg/mL 2% DSPE-PEG MWCNT and exposed to NIR as above for 0–90 s without removing the MWCNTs. Spheroids were then washed in PBS and transferred to 6 well plates in growth media with 4–6 spheroids per well. Media was changed every 2–3 days. Spheroid size was monitored over time and photographed using Invitrogen™ EVOS™ FL Auto Imaging System (Thermo Fisher). Spheroid area was quantified using Image J software (https://imagej.nih.gov/ij/). Briefly, regions of interest corresponding to individual spheroids were identified and total area in each region (in pixels) was calculated.
Western Blot Analysis
Lysates were collected using triton lysis buffer (20 mM Tris-HCl, 5mM EDTA, 1% Triton-X 100, pH 8.3) supplemented with 1% Halt™ Protease & Phosphatase Inhibitor Cocktail (78440, Thermo Scientific). Protein concentration was determined for each sample using a Bicinchoninic acid (BCA) protein assay kit (Thermo-Fisher/Pierce). Next, 10–30 μg of protein lysate were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% Ready Gel® Tris-HCl gels (161–156, Bio-Rad) and transferred to nitrocellulose (Bio-Rad). Membranes were blocked with 5% milk solution in tris-buffered saline (TBS) with 1% Tween 20 (P1379, Sigma-Aldrich) for 30 minutes. Blots were probed with antibodies diluted in 5% BSA or 5% milk. Primary phospho-HSF1 (S326) antibody (1:1000) was purchased from Abcam. Antibodies were purchased from Cell Signaling Technology and diluted to the following concentrations: HSF1 (1:1000), HSP90 (1:5000), HSP70 (D69) (1:1000), HSP27 (G31) (1:1000), β-actin (13E5) (1:5000), GAPDH (1:2000). HRP-conjugated secondary antibodies were diluted 1:1000 (for pHSF1, HSF1, HSP70 and HSP27) and 1:10,000 (for HSP90, β-actin, and GAPDH) in 5% milk. Membranes were developed using SuperSignal® West Pico Chemiluminescent Substrate (Thermo Scientific). Densitometry was performed in Image J. Membranes were stripped using Restore™ PLUS Western Blot Stripping Buffer (46430, Thermo Scientific) according to the manufacturers recommendations and re-probed as described above.
Induction of HSR
For quantification of the effect of MWCNTs on HSP expression, adherent U87 cells were treated overnight (~18 hr) with increasing concentrations (0–100 μg/mL) of 2% DSPE-PEG MWCNT in normal growth media. After incubation, nanotube solution was removed and normal growth media warmed to 43 ºC was added. Cells were then placed in an incubator set at 43 ºC, 5% CO2 for one hour, then whole cell lysates were collected according to methods described above. To induce a protective HSR, adherent U87 cells treated with growth media warmed to 43 ºC then placed in a 43 ºC incubator for one hour as above. Afterward, the cells were allowed to recover for 5 h. Cell lysates were then extracted for immunoblot studies, or cells were used for photothermal and water bath heating experiments as described in the text. To induce a HSR via 17-DMAG, cells were treated with 1 μM 17-DMAG for 5 h. To inhibit a 17-DMAG-induced HSR, cells were pretreated with 10 μM KRIBB11 30 min prior to 17-DMAG treatment and remained on during the entire duration of 17-DMAG treatment.
RESULTS
Rationale design of MWCNTs for convection enhanced delivery
In order for CNMTT to achieve sufficient ablative temperatures within the entire target lesion, MWCNTs must be readily delivered throughout the tumor volume. Aggregation of MWCNTs in biologic settings limits their diffusion and must be addressed19. Modifications to MWCNTs surfaces that may increase their colloidal stability include acid oxidation and surfactant coating, which also influence biodistribution, toxicity9 and may affect particle diffusion and optical-thermal properties. To determine an optimal particle that would yield sufficient diffusion while still maintaining heating properties, we investigated the effects of different types of surface functionalization on toxicity, colloidal stability, diffusion, and heating of MWCNTs.
For these studies, MWCNTs with an initial size of 8–15 nm in diameter and 0.5–2 μm in length were acid oxidized and base washed to remove contaminants, shorten the MWCNTs, and introduce carboxyl groups to their surface. As we previously reported, these MWCNTs were generally 100–200 nm in length and retained diameters of 8–15 nm35. MWCNTs were then dispersed by sonication in water (uncoated) or an aqueous solution of 2% weight/volume (w/vol) distearylphosphatidylethanolamine-polyethylene glycol5000 (DSPE-PEG). Acid oxidized MWCNTs were dispersed by sonication in water or various surfactants (1% w/vol Pluronic F-127, 1% w/vol DSPE-PEG, or 2% w/vol DSPE-PEG) and we assessed the effects of the different coatings on particle size and dispersion stability. The hydrodynamic diameter and ζ-potential of the various dispersions were measured by dynamic light scattering immediately following suspension in water or PBS. In water, all four preparations exhibited monomodal size distributions with similar mean (Z-average) hydrodynamic diameters ranging from 150–190 nm (Table 1). The differences likely were due to variations in placement of each sample within the bath sonicator, which is known to affect CNT size36. An increase in the hydrodynamic diameter of a particle can be indicative of increased aggregate formation and decreased stability in solution. Notably, all three types of surfactant coated MWCNTs did not aggregate in phosphate buffered saline (PBS) but the uncoated MWCNTs did as indicated by an increase in hydrodynamic diameter from 151 nm in water to 533 nm in PBS (Table 1). Initial measurements were taken within 5 minutes following dilution in water or PBS. Subsequent DLS measurements indicated that all coated MWCNTs remained in stable suspension without aggregation for months (not shown). In contrast, uncoated MWCNTs precipitated after overnight storage in PBS. The ζ-potential indicates the separation of charge between the surface of a particle and the surrounding media, influences the stability of particles in dispersion, and dictates the interaction of the nanoparticles with charged molecules. Among the three different coatings, 2% DSPE-PEG provided the most charge shielding as indicated by the 23 mV shift in ζ-potential compared to uncoated MWCNTs (-27.9 mV in 2% DSPE-PEG vs. −51.6 mV for uncoated MWCNTs). More neutral particles may be desirable for in vivo use since they are less likely to interact with other charged species.
Table 1.
Physicochemical characterization of MWCNTs dispersed in surfactants
| Dispersant | Uncoated | 1% Pluronic | 1% DSPE-PEG | 2% DSPE-PEG | |
|---|---|---|---|---|---|
| Hydrodynamic diameter (D)/Polydispersity index (PI) | H2O | D=151 ± 26 nm PI=0.31 ± 0.09 |
D=189 ± 2.3 nm PI=0.32 ± 0.03 |
D=179 ± 2.4 nm PI=0.31 ± 0.04 |
D=176 ± 27 nm PI=0.31 ± 0.09 |
| PBS | D=533 ± 102 nm PI=0.24 ± 0.03 |
D=184 ± 0.8 nm PI=0.36 ± 0.02 |
D=165 ± 2.6 nm PI=0.27± 0.01 |
D=155 ± 20 nm PI=0.29 ± 0.09 |
|
| Zeta Potential | H2O | −51.6 ± 0.8 mV | −45.3 ± 1.0 mV | −35.0 ± 1.9 mV | −27.9 ± 0.4 mV |
D: hydrodynamic diameter; PI: Polydispersity index
We then investigated the convection of the different MWCNT suspensions in ECM. To do this, 500 μl of 100 μg/ml dispersions of surfactant coated or uncoated MWCNTs were infused via a catheter at a rate of 2 μl/min into 0.6% w/vol agarose gels (Fig. 1A). Similar hydrogels are commonly used as brain ECM mimicking phantoms for CED studies37. The resulting volume of distribution of the MWCNTs into brain phantoms was qualitatively (Fig. 1B) and quantitatively (Fig. 1C) monitored. Substantial backflow up the catheter track was observed following infusion of uncoated MWCNTs. 1% DSPE-PEG coating of MWCNTs further increased the diffusion of nanotubes in brain phantoms compared to uncoated and Pluronic F127 coated MWCNTs, and increasing the concentration of DSPE-PEG from 1% to 2% in the MWCNT dispersion solution yielded the largest distribution volume. Notably, 2% DSPE-PEG MWCNTs diffused 4–5 mm away from the infusion site in all directions, producing a near spherical distribution. In contrast, all other MWCNT preparations diffused no more than 2 mm below at laterally to the catheter, with most diffusing back along the catheter track.
Figure 1. Assessment of structure-activity relationships for MWCNTs modified by acid oxidation and coating with various surfactants.
A) Acid oxidized MWCNTs dispersed in surfactants were infused at a rate of 0.2 μL/min via a catheter inserted 15 mm below the surface of a 0.6% agarose brain-mimicking hydrogels using a micromanipulator to place the catheter and an infusion pump to regulate flow as shown. B) Representative images illustrating the effect of the coatings on the distribution of the MWCNTs in the hydrogels after infusion of MWCNT dispersions are shown as follows: 1) Uncoated; 2) 1% Pluronic; 3) 1% DSPE-PEG; 4) 2% DSPE-PEG. The depth at which the catheter was placed is indicated by the dashed line. C) Hydrogels infused with MWCNTs were bisected alone the midline and photographed as shown on the left in each panel. The two halves were place back together and transverse sections were made at the points indicated by the white dashed lines. An image of each transverse section is shown on the right of each panel. D) Increasing concentrations of MWCNTs dispersed in surfactants were exposed to a 970 nm diode laser at 3 W/cm2 for 30 seconds. Temperatures of the MWCNT suspensions were determined via thermocouple immediately after laser exposure.
Engineering of MWCNTs to overcome delivery constraints must be balanced with maintaining the capacity to generate heat in response to NIR. Therefore, the effect of different coatings on the heating properties of MWCNTs was investigated. Increasing concentrations of uncoated, 1% Pluronic, 1% DSPE-PEG, or 2% DSPE-PEG MWCNTs were exposed to a 970 nm diode laser (3 W/cm2; 30 s) and temperatures of the solutions were determined via thermocouple. All MWCNT suspensions exhibited similar heating curves with a dose dependent increase in temperatures with increasing MWCNT concentration (Fig. 1D). These findings suggest that 2% DSPE-PEG MWCNTs exhibited the best combination of stability in physiologic solution, increased diffusion, and maintained sufficient heating properties to achieve ablative temperatures at modest concentrations and laser power/duration.
Evaluation of cytotoxic and heat shock responses in GBM cells following exposure to MWCNTs
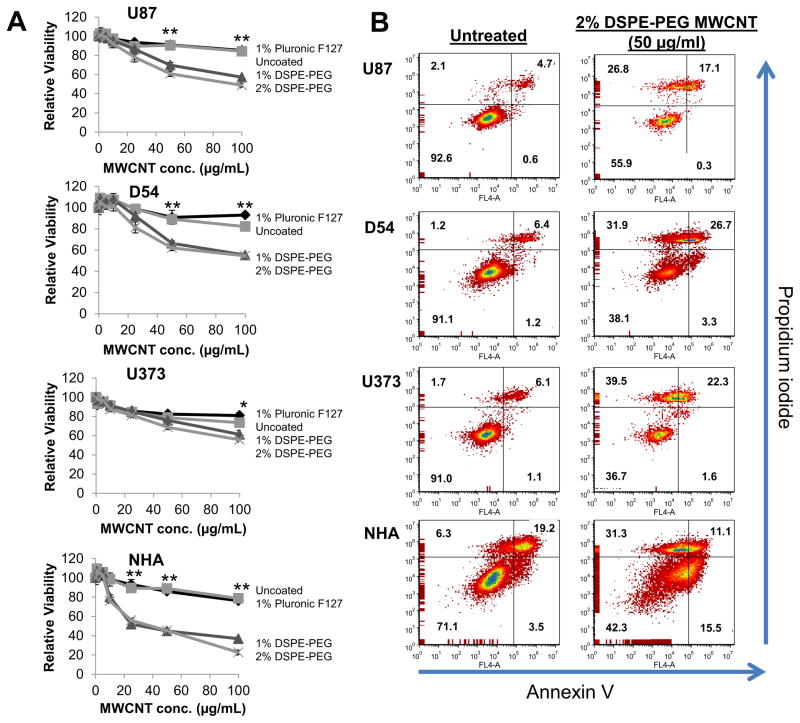
The cytotoxicity of MWCNTs in various coatings (uncoated; 1% w/vol Pluronic F-127, 1% w/vol DSPE-PEG, or 2% w/vol DSPE-PEG) was assessed in three GBM cell lines (U87, U373, and D54) and normal human astrocytes (NHA) following 24 or 48 h exposures. As a measure of cell viability, ATP was quantified using the CellTiter-Glo assay. To avoid interference of MWCNTs with luminescence measurements, cell lysates were centrifuged to pellet MWCNTs and luminescence was quantified only in the supernatants. As shown in figure 2A, 1% and 2% DSPE-PEG MWCNTs exhibited similar cytotoxicity profiles after 24 h treatment and were significantly more cytotoxic than uncoated or Pluronic F127 coated MWCNTs to all cell lines. Similar results were observed after 48 h treatment (Supplemental Figure S1). Despite their cytotoxicity, only 2% DSPE-PEG MWCNTs exhibited a suitable diffusion profile for in vivo delivery by CED, and therefore we focused on these tubes for subsequent studies.
Figure 2. Evaluation of the cytotoxicity induced by MWCNTs modified by acid oxidation and coating with various surfactants.
A) U87, U373, and D54 GBM cells or NHA cells were treated for 24 h with increasing doses of acid oxidized MWCNTs dispersed in the indicated surfactants. After 24 h, cells were lysed, pelleted and the supernatant was analyzed for ATP content as a measure of cell viability using the CellTiter-Glo assay. Samples were prepared and measured in sextuplicate and are displayed as the mean ± standard deviation of each measurement. Significant differences in viability between cells treated with 1% or 2% DSPE-PEG coated MWCNTs and uncoated or Pluronic F-127 coated MWCNTs (determined by ANOVA followed by Student’s T-Test when appropriate) are indicated by (*; p<0.05) or (**; p<0.01). B) U87, U373, and D54 GBM cells or NHA cells were treated for 24 h with 2% DSPE-PEG MWCNTs (50 μg/ml). Cells were co-stained with propidium iodide and Annexin V and then evaluated by flow cytometry. the percentages of cells characterized as viable (lower-left quadrant), early apoptotic (lower-right quadrant), late-apoptotic (upper-right quadrant), and necrotic (upper-left quadrant) are shown within each quadrant. At least 10,000 cells were counted for each measurement and the experiments were repeated 2–3 times for each cell line with similar results.
To determine if the reduced viability of GBM and NHA treated with 2% DSPE-PEG MWCNTs was due to cell death or growth inhibition, cells treated with 2% DSPE-PEG MWCNTs were co-stained with propidium iodine (PI) and Annexin V (AnnV) and staining was quantified by flow cytometry (Fig. 2B). This analysis allows discrimination between viable cells (PI- and AnnV-; lower left quadrant), early apoptotic cells (PI- and AnnV+; lower right quadrant), late apoptotic/necrotic cells (PI+ and AnnV+; upper right quadrant), and necrotic cells (PI+ and AnnV-; upper leftquadrant). The percentage of cells exhibiting each phenotype is shown quantitatively in figure 2B. U373, D54, and NHAs exhibited similar cytotoxicity profiles in response to 24 hour treatment with 2% DSPE-PEG MWCNTs, while U87 cells were less sensitive. From these data, it is apparent that 2% DSPE-PEG MWCNTs induce both apoptosis and necrotic cell death. The lack of a difference in the cytotoxicity of MWCNTs toward GBM cells as compared to NHAs is of concern for in vivo applications. However, it is important to note that in contrast to the GBM cell lines, which are immortalized, the NHAs used for this study were not transformed and have a limited lifetime on plastic. The flow cytometry data indicate that almost 30% of the starting population of NHAs is undergoing apoptosis or necrosis prior to MWCNT exposure, and thus in vitro measurements may overestimate the sensitivity of these cells to nanotube exposure. Nonetheless, the risk of nanotube cytotoxicity toward normal tissue emphasizes the importance of using local infusion techniques like CED to confine MWCNTs to the tumor volume.
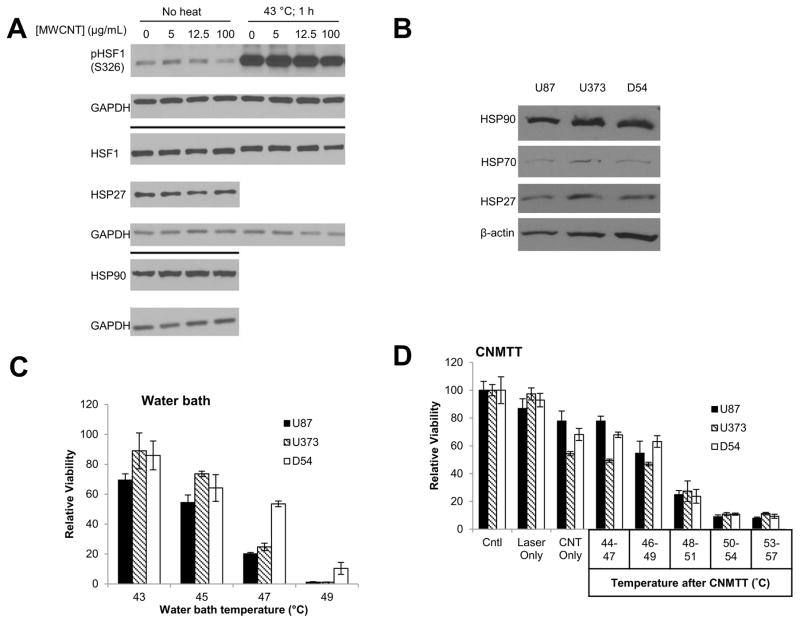
In addition to cytotoxicity, some types of nanoparticles, including CNTs, have been reported to induce the HSR38, 39, while others report that CNTs can reduce the expression of some HSPs40. We therefore investigated the HSR after overnight treatment of U87 with increasing concentrations of 2% DSPE-PEG MWCNTs. Extracted cell lysates were immunoblotted and no change in phosphorylation indicative of HSF1 activation was detected, nor were there changes in expression of total HSF1, HSP27 or HSP90 after 2% DSPE-PEG MWCNT treatment (Fig. 3A). HSP70 was not detected under these conditions (data not shown). Additionally, prior exposure of U87 cells to 2% DSPE-PEG MWCNTs did not alter the ability of cells to phosphorylate HSF-1 following incubation at 43 ºC for 1 h. Taken together, these data suggest that 2% DSPE-PEG MWCNTs do not affect the HSR in GBM cells.
Figure 3. Quantification of heat shock protein expression and response of GBM cells to thermal therapies.
A) Adherent U87 cells were treated with increasing concentrations of 2% DSPE-PEG MWCNTs overnight. To determine if the nanotubes influenced activation of the heat shock response, half of the cells were subsequently exposed to 43 ºC for 1 h. Whole cell lysates were collected and probed for proteins involved in the heat shock response via western blot analysis. GAPDH was used as a loading control. B) Basal expression of heat shock proteins in U87, U373, and D54 GBM cells was assessed by western blot analysis and normalized to β-actin. C) GBM cells were heated to increasing temperatures (43–49 ºC) in a circulating water bath to mimic conventional heating methods. Cells were allowed to recover for two days and cell viability was determined by MTT assay. D) GBM cells were heated to increasing temperatures (44–57 ºC) using CNMTT. Cells were allowed to recover for two days and cell viability was determined by MTT assay. For (C) and (D), data are expressed as the mean of triplicate samples and are normalized to the unheated control for each cell line. Significant decreases in viability with each increase in temperature (p<0.05) were detected for all cell lines. The results shown are representative of at least 3 independent experiments.
Determination of the relative sensitivity of GBM cell lines to water bath heating and CNMTT
Initially, we quantified basal protein levels of HSPs in three GBM cell lines (U87, U373, and D54) by immunoblotting (Fig. 3B). We found that expression of HSP90, HSP70, and HSP27 was similar across all cell lines. Next, we determined the relative sensitivity of the three GBM cell lines to a model of conventional heating (incubation for 15 min between 43 and 49 ºC in a circulating water bath) or following CNMTT (2% DSPE-PEG MWCNT (100 μg/ml) combined with NIR laser exposure (3 W/cm2 for 20–40 s depending upon target temperature)) as previously described11 (Fig. 3C, D). Cells were treated in suspension and the entire volume of MWCNT-containing media was heated to the indicated temperature. We and others previously demonstrated that heating of nanoparticles dispersed across a tumor volume under conditions typically used clinically for thermal ablation produces an overall temperature rise that is far greater than the localized temperature increase near each particle12, 41. Therefore, these experimental conditions are designed to mimic the bulk heating of a tumor, rather than selective heating of individual tumor cells. Due to variations in the temperatures achieved in triplicate wells following CNMTT, a temperature range is shown. Sub-ablative temperatures are used to mimic the exposure of tumor cells at the margin of the treatment area which might not be exposed to lethal ablative (>50 ºC) temperatures. As expected, cell viability decreased with increasing temperature. U87 and U373 showed similar responses to water bath heating, while D54 cells were more tolerant (Fig. 3C). All cell lines exhibited similar sensitivity to CNMTT (Fig. 3D).
Evaluation of the efficacy of water bath heating and CNMTT following induction of a heat shock response in GBM
Recently, we showed that CNMTT can overcome basal thermotolerance due to high expression of HSP90 in stem cell-like breast cancer11, indicating that CNMTT may be effective for treatment of inherently heat resistant cancers. However, it is unknown if CNMTT could overcome the protective effects of an induced heat shock response. HSP90 is overexpressed in many GBMs, and HSP90 inhibitors are being tested for GBM treatment 42, 43. HSP90 inhibitors may induce compensatory expression of other HSPs through an HSF1 mediated feedback mechanism. HSP90 acts as a chaperone for HSF1and upon HSP90 inhibition, HSF1 is released from its complex with HSP90, ultimately leading to transcription of other HSF1 targets44, 45.
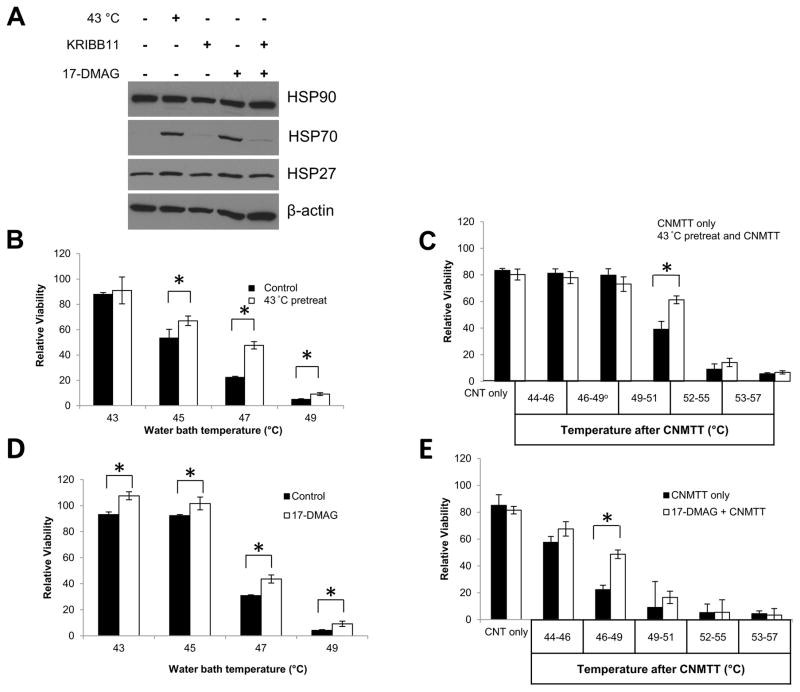
To induce a transient HSR, U87 cells were heated at 43 ºC for 60 min then allowed to recover for 4 h. Western Blot analysis confirmed that the HSR was activated, as indicated by an increase in of HSP70 (Fig. 4A). We also tested to see if treatment of U87 cells with HSP90 inhibitor 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG) resulted in activation of the HSR. Five hours after treatment of cells with 17-DMAG, HSP70 was induced at levels similar to those found following 43 ºC exposure (Fig. 4A). Treatment with KRIBB11, an HSF1 inhibitor, was sufficient to mitigate HSP70 induction by 43 ºC or 17-DMAG, confirming that HSP70 induction was HSF1-mediated. Treatment with 17-DMAG under these conditions was non-cytotoxic to the cells (Supplemental Fig. S2). We further investigated if the HSR induced by 43 ºC or 17-DMAG exposure induced thermotolerance. Cells pre-exposed to 43 ºC for 1 hr, which increased HSP70 expression, were more tolerant to subsequent conventional water bath heating and CNMTT than cells receiving no pre-treatment (Fig. 4B,C). Similarly, pretreatment with 17-DMAG, which also increased HSP70 expression, increased thermotolerance of U87 cells to both water bath and CNMTT (Fig. 4D,E).
Figure 4. Determination of the effect of sublethal heat or chemically induced heat shock protein expression on thermotolerance of GBM cells.
To induce the HSR, U87 cells were placed in a 43 ºC incubator for 1 hour and recovered at 37 ºC for 4 hours, or treated with a combination of 1 μM 17-DMAG and 10 μM KRIBB11 for 5 hours. KRIBB11 treatment occurred 30 minutes prior to 17-DMAG treatment to allow sufficient time for the inhibitor to take effect. A) Whole cell lysates were collected from all treatment groups and probed for HSP90, HSP70, or HSP27 via western blot analysis. β-actin was used as a loading control. The HSR was induced in U87 cells by placement in a 43 ºC incubator for 1 h and recovery at 37 ºC for 4 h as above. Cells were subsequently heated to increasing temperatures in B) a circulating water bath to mimic conventional heating methods or C) by CNMTT. The HSR was induced in U87 cells by treatment with 1 μM of 17-DMAG for 5 h as above. Cells were subsequently heated to increasing temperatures in D) a circulating water bath E) or by CNMTT. Cells were allowed to recover for two days after each treatment and cell viability was determined by MTT assay. Data are expressed as the mean of triplicate samples normalized to untreated (no heat and no 17-DMAG) controls. Significant differences (p<0.05) between treatment groups determined by Student’s T-Test are indicated by (*). The results shown are representative of 2–3 independent experiments per condition tested.
A previous report indicated that the chemotherapeutic agent temozolomide (TMZ) significantly increased HSP levels in GBM cells that survived initial treatment46. Because most GBM patients will receive TMZ as part of their therapy, we pretreated U87 cells with TMZ (50 μM) for 48 h. We did not detect an increase HSP expression following TMZ treatment, though a modest decrease in HSP27 was noted in U87 cells treated with DMSO, the vehicle for TMZ [data not shown]. Surviving cells were then subjected to water bath heating or CNMTT. Following heating by water bath or CNMTT, cells surviving TMZ responded similarly to untreated controls with no significant differences detected between groups (p > 0.1 for all comparisons as indicated by ANOVA) (Supplemental Fig. S2). These results indicate that TMZ treatment does not influence the efficacy of subsequent thermotherapy, but a sublethal heat or chemically-induced HSR can render U87 cells resistant to subsequent thermal therapies.
Treatment of three dimensional GBM spheroid cultures by CNMTT
To this point our studies focused on the ability of MWCNTs to generate heat within a macroscale volume. However, several reports exist that describe the capacity for MWCNTs and other NIR absorptive nanoparticles to significantly increase the efficacy of photothermal therapy versus NIR alone at the cellular level without generating macroscale ablative temperatures, a phenomenon known as selective photothermolysis9. To recapitulate this phenomenon in cell monolayers, adherent U87 cells were sequentially treated with 2% DSPE-PEG MWCNTs for 24 h, washed to remove extracellular MWCNTs, and then exposed to NIR. To minimize cytotoxicity due to MWCNTs, only low doses (5 or 10 μg/ml) were used. Consistent with previous reports using this model14, both 5 and 10 μg/ml MWCNT-treatment significantly increased cell death compared to NIR alone (Supplemental Fig. S3). However, cell monolayers fail to recapitulate the more complex interactions between cells organized into a tissue, which is known to affect the response of cancer cells to therapy34 including photothermal treatment47. In addition, cell monolayers lack the ability to mimic barriers to nanoparticle delivery such as diffusion through extracellular space. We therefore evaluated the efficacy of CNMTT for the treatment of U87 cells grown in a three dimensional format as multicellular tumor spheroids.
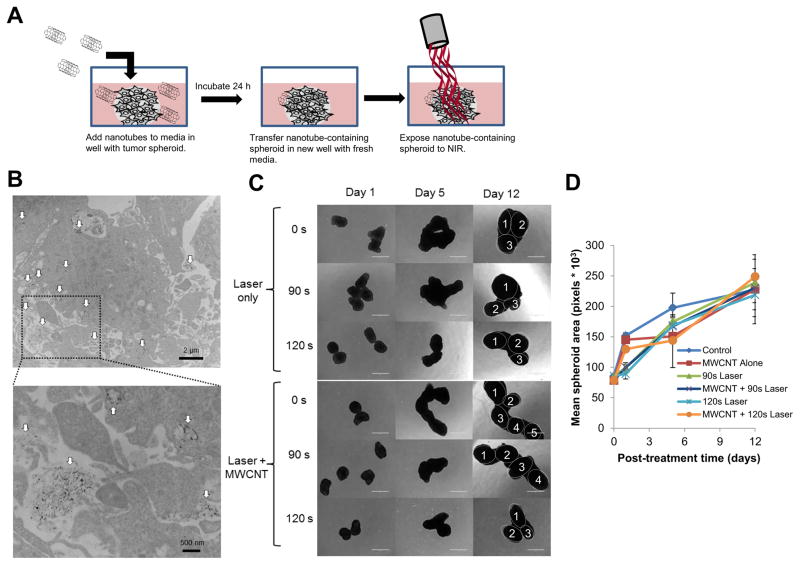
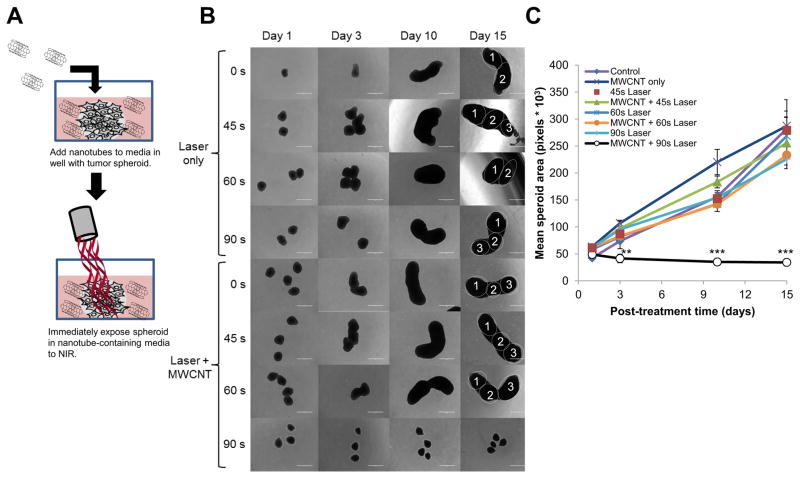
Tumor spheroids were incubated with 2% DSPE-PEG MWCNTs for 24 h to allow time for the nanotubes to diffuse through the spheroids and be taken up by the cancer cells. After washing to remove excess MWCNTs, some spheroids were fixed and sectioned for imaging by transmission electron microscopy (TEM) and others were exposed to NIR and spheroid growth was monitored over time (shown schematically in figure 5A). TEM images (Fig. 5B) confirmed that the nanotubes penetrated throughout the interior of the spheroids, and were detected at depths of over 100 μm. This is the first time the penetration of MWCNTs into a tumor spheroid has been observed by TEM. Although intracellular nanotubes were abundant, the majority of the particles appeared to remain in the extracellular space. Exposure to NIR for 90–120 s, with or with nanotubes under conditions that resulted in increased cell death for nanotube treated cells in a monolayer, failed to inhibit the growth of GBM spheroids (Fig. 5C) and no significant differences in spheroid growth were found following any treatment (Fig. 5D). This result shows that internalization of nanotubes can result in increased efficacy of photothermal therapy in a cell monolayer, but under similar irradiation conditions, does not increase efficacy of NIR treatment in a three dimensional spheroid model. Increasing the duration of laser exposure to 180 s or greater raised the temperature of the surrounding media to more than 60 ºC. Under this longer irradiation, the bulk heating of the media dominated by the laser was sufficient to cause cell death, and the presence of MWCNTs within the GBM spheroids did not increase treatment efficacy compared to NIR alone (Supplemental Fig. S4). However, in a clinical setting using currently available lasers it is likely that nanoparticles distributed throughout a tumor will significantly increase the bulk heating of the tumor and not confine treatment to individual tumor cells12. Therefore, we assessed the effect of heating MWCNTs dispersed throughout the media on the growth of GBM spheroids (shown schematically in figure 6A). Following NIR exposure (3 W/cm2; 90 s), laser energy alone was not sufficient to stall growth of GBM spheroids and only reached temperatures of 42–43 ºC (Fig. 6B). In contrast, when the spheroids were similarly irradiated in the presence of 20 μg/mL 2% DSPE-PEG MWCNT, the media temperature increased to 55–59 ºC and the GBM spheroids failed to grow. Growth of tumor spheroids following this treatment is shown quantitatively in figure 6C.
Figure 5. Imaging the diffusion of 2% DSPE-PEG MWCNTs into three dimensional GBM tumor spheroids and treatment of nanotube containing spheroids by CNMTT.
Briefly, U87 cells were grown as three dimensional tumor spheroids and incubated overnight with 2% DSPE-PEG MWCNTs. The spheroids were washed, and then transferred to new wells for use in electron microscopy or photothermal treatment studies. A) Schematic illustrating the experimental design. U87 spheroids incubated overnight with 2% DSPE-PEG MWCNTs were washed, fixed, embedded, sectioned, overlaid onto copper-coated formvar grids and imaged by TEM. In the electron micrographs, B) MWCNTs (indicated by the white arrows) can be seen penetrating throughout the spheroids (upper panel). A higher powered image (lower panel) of the highlighted area indicates that the MWCNTs are both in intracellular compartments and in the extracellular space of spheroids. Groups of 4–6 spheroids alone or spheroids incubated overnight with 2% DSPE-PEG MWCNTs then washed to remove excess nanoparticles were exposed to laser emitted NIR energy (3 W/cm2) for 0–120 s. Spheroid growth over time was monitored and C) representative photomicrographs are shown. Individual spheroids fused into a single cluster are identified in the day 15 images. D) Mean surface area per spheroid was quantified in pixels using Image J software. For area measurements, images of spheroids in addition to those shown in B) were used. No significant differences in spheroid grow were detected between treatment groups.
Figure 6. Treatment of GBM spheroids by CNMTT in the presence of extracellular 2% DSPE-PEG MWCNTs.
Briefly, U87 cells were grown as three dimensional tumor spheroids. Groups of 4–6 spheroids were transferred to new wells containing 2% DSPE-PEG MWCNTs (20 μg/ml) in the media, and were exposed to laser emitted NIR energy (3 W/cm2) for 0–90 s. After treatment, the spheroids were washed, and then transferred to new wells with growth media only. A) Schematic illustrating the experimental design. Spheroid growth over time was monitored and B) representative photomicrographs are shown. Individual spheroids fused into a single cluster are identified in the day 15 images. C) Mean surface area per spheroid was quantified in pixels using Image J software. For area measurements, images of spheroids in addition to those shown in B) were used. Significant differences in spheroid grow were detected between spheroids treated with MWCNTs and laser for 90 s as compared to all other treatment groups (determined by ANOVA followed by Student’s T-Test when appropriate) are indicated by (**; p<0.01) or (***; p<0.001).
DISCUSSION
Despite numerous proof-of-principle studies demonstrating the efficacy of CNMTT for treatment of GBM and other tumors in vitro and in animal models9, CNMTT needs to show a clear advantage over other forms of thermal therapy prior to clinical translation. The key to success will be to localize CNTs throughout the tumor volume, which will enable the promised improvements in the rate, quantity and confinement of heat deposition. The efficacy of CNMTT for treatment of GBM also must be balanced with the toxicity risk. Additionally, it is necessary to understand the potential mechanisms of resistance to CNMTT and to determine the possible impact of resistance on this treatment. A major challenge to treatment of brain tumors is inaccessibility and risk of damage to the normal brain. Local infusion of therapeutics into the brain is possible using catheter based approaches to drug delivery, but this requires that nanoparticles be specifically designed for this purpose. In our studies, we show that acid oxidation and a dense coating of DSPE-PEG on MWCNTs allows for better diffusion through brain phantoms while maintaining the ability to achieve ablative temperatures after laser exposure. The innovation of this work lies in the application of a rational development process that allows us to generate MWCNTs with specific physicochemical characteristics designed to maximize intratumoral dissemination and heat generation while minimizing cytotoxicity. The assay cascade established here may serve as a model to accelerate the development of nanotherapeutics for brain tumors. We also show that CNMTT can be far more effective than NIR alone in inhibiting growth of GBM spheroids, a model for tumor treatment which may be more predictive of clinical outcome than monolayer studies34, 47. Activation of the protective HSR following exposure to sub-ablative temperatures or HSP90 inhibitor, 17-DMAG, renders U87 cells thermotolerant to both water bath heating and CNMTT. These data provide pre-clinical insight into key parameters needed for translation of CNMTT including nanoparticle delivery, cytotoxicity, and efficacy for treatment of thermotolerant GBM.
Delivery of nanoparticles to tumors larger than 1 cm in diameter represents a major, under-investigated need for biomedical use of nanomaterials9, and is a central challenge to treatment of GBM. We postulated that a homogeneous distribution of CNTs could be achieved using CED. At present, clinical applications of CED for delivery of chemotherapeutics to GBM tumors are limited, but advances in catheters and image-guided drug delivery are beginning to facilitate its wider use48. Positive indications for use of CED for CNTs include: (i) CNTs can be synthesized with diameters (<30 nm) far smaller than the estimated pore size in the extracellular matrix (ECM) of many tumors49–51; (ii) CNTs tend to align parallel to the direction of fluid flow52; and (iii) as we show, their diffusion can be enhanced by selectively tuning their surfaces to the physical properties of porous media like the ECM. The slow infusion parameters used in our studies were in line with CED techniques that are currently used in the clinic. In agreement with recent studies showing that a dense PEG coating improves the passive diffusion of large polymeric nanoparticles in brain tissue53, we also find that dense PEG coating greatly enhances the transport of MWCNTs in brain phantoms following CED. Our results are consistent with a recent report that indicates that the intercellular diffusion rate for nanotubes is anomalously high for their size and is comparable to molecules with molecular weights 10000-fold lower54. This is likely due to the high aspect ratio of the nanotubes which allows them to align along their narrow axis and pass through small pores that may restrict the passage of globular macromolecules of similar mass.
Use of CNTs for treatment of GBM requires bypassing the body’s natural defenses, and toxicity is a key concern. Each change in particle design has the potential to generate novel toxicological properties. Therefore, we investigated the cytotoxic responses of NHAs and GBM cells following exposure to acid oxidized MWCNTs dispersed in various coating/surfactant solutions. Our results indicate that both 1% and 2% DSPE-PEG MWCNTs are cytotoxic at modest concentrations toward NHA and GBM cell lines. Precisely why the DSPE-PEG coating increased the cytotoxicity of the MWCNTs relative to uncoated or Pluronic F127 coating is unknown. This is of significant concern for clinical translation of CNMTT, especially in comparison to the use of less toxic NIR absorptive gold nanoparticles for similar applications55. However, when cells were grown as three dimensional spheroids rather than as monolayers, MWCNTs appeared to have no effect on growth and thus their toxicity may be highly context dependent. It is notable that despite the increased toxicity we find in vitro for DSPE-PEG coated MWCNTs relative to uncoated or Pluronic F127 coated MWCNTs, both acid-oxidation and DSPE-PEG-coating have been shown to mitigate acute CNT toxicity in vivo19, 35. A growing body of evidence suggests the existence of multiple in vivo biodegradation mechanisms for CNTs56, 57, and that CNTs can safely be used in the brain20, 58–60. Once introduced to the brain, MWCNTs that are not taken up by cancer cells may be cleared by microglia17, 61. Upward of 70–80% of MWCNTs injected into glioma bearing mice have been shown to be taken up by microglia18. Several reports indicate that CNTs can be enzymatically digested by various cellular peroxidases62–64 or other reactive oxygen species mediated pathways57, 65. Importantly, even partial enzymatic degradation of MWCNTs can substantially reduce their toxicity63, which may reduce the long-term exposure risk due to MWCNTs remaining in the brain after photothermal therapy. In addition, increased malignant cell binding and uptake of MWCNTs can be achieved with the addition of cancer cell-specific ligands which can increase the efficacy of CNMTT66, 67 and be used to target the brain tumor initiating cells thought to be responsible for disease recurrence67.
Despite toxicity concerns, CNMTT offers several advantages over gold nanoparticle-based therapy. Some estimates indicate that CNTs can ablate tumors at 10-fold-lower doses and 3-fold-less power than is needed for gold nanorods68, though direct comparisons are difficult to make. CNTs can be used to generate ablative temperatures in tumors without deforming after NIR exposure, allowing multiple heat cycles69. In contrast, gold nanoparticles become deformed following heating, which reduces the efficacy of subsequent heating cycles70. The broad electromagnetic absorbance spectrum of CNTs provides great versatility to tailor size, shape, and surface properties to optimize the tissue distribution of CNTs without a significant loss in thermal conversion efficiency9. This is also a significant advantage over plasmonically heated gold nanoparticles for which the excitation spectra are highly dependent upon the size and shape of the particles71.
In the absence of nanomaterials, reaching ablative temperatures is achievable in the region closest to the laser, but the ability to achieve ablative temperatures is lessened farther from the source 4. For laser induced thermal therapy, extended heating times are needed to increase the treatment area, but greater heat diffusion and convective cooling due to blood flow leads to heterogeneous delivery of heat, decreased treatment efficacy, and damage to surrounding tissue12. Using both theoretical and experimental data for heating MWCNTs embedded in brain phantoms, we previously showed that currently available clinical lasers and nanoparticle delivery strategies do not permit the cellular level of heat confinement that would be needed to achieve cell level selectivity of photothermal therapy.12 It is our belief that in the near term, nanomaterials will offer the greatest benefit as compared to laser heating alone by permitting more rapid heat deposition, which in turn will lead to more spatially confined heat delivery due to reduced thermal diffusion. This will lead to less collateral damage to surrounding tissue. Thus, the temporal confinement of heat offered by CNMTT provides “selectivity” for tumor treatment by increasing the localization of heat to the volume of tumor into which the nanomaterial has been infused. At the margin of the treatment area, GBM cells may be exposed to sub-ablative temperatures which are not always sufficient to induce tumor cell death, and can lead to activation of HSR pathways in the surviving tumor cells within this zone, decreasing the efficacy of subsequent therapy. We find that sub-lethal heating of U87 cells results in thermotolerance and protects against both CNMTT and water bath heating. The potential for induction of thermotolerance at the margin of tumors due to incomplete treatment should be considered when planning treatment schedules.
HSP90 inhibitors are being explored for cancer treatment72, and therefore understanding their potential interaction with thermal therapies could lead to novel combinatory treatments. In this study, we investigated the changes in efficacy of thermal therapies after treatment with the HSP90 inhibitor, 17-DMAG. We find that all methods of heating become less effective after treatment with a non-toxic dose of 17-DMAG due to a compensatory HSR mediated by transcriptional activity of HSF1. The mechanisms of the HSR and thermotolerance are multifactorial and the response of cancer cells to thermal therapy is dependent upon factors including temperature, duration of heat exposure, HSF1 activity and HSP expression, and cell type29, 32. Further investigation will be needed to identify any underlying differences between CNMTT and conventional heating that could be exploited to enhance GBM treatment with HSP90 inhibitors.
CONCLUSIONS
Taken together, these data offer pre-clinical insight into key parameters needed for translation of CNMTT including nanoparticle delivery, cytotoxicity, and treatment efficacy for GBM. We provide an optimal MWCNT design which greatly improves diffusion in brain-ECM-mimicking hydrogels and offer a greater understanding of how heat resistant cells and tumor cells that have received prior treatment respond to CNMTT. The greatest benefit of nanomaterials for laser ablation therapy in the near term likely will not be as a result of selective killing of individual cancer cells, but rather due to their capacity to efficiently and rapidly convert NIR into heat. As we previously showed, the more rapid deposition of heat following equivalent NIR exposure that is enabled by MWCNTs leads to higher peak temperature and a more homogenous distribution of ablative temperatures throughout the tumor lesion and minimizes collateral damage to surrounding normal cells and tissues due to thermal diffusion12. Moreover, while it may be possible to localize nanoparticle heating to individual cells under some conditions73–75, the necessary lasers are not currently available for routine clinical use. Thus, as demonstrated by our studies using tumor spheroids, NIR irradiation of nanoparticles distributed throughout the tumor volume (rather than confined only to tumor cell nodules) allows for more rapid heating of the tumor volume than NIR alone. This in turn will reduce thermal diffusion and confine heat to the tumor for more effective tumor ablation than NIR alone. These results may assist in the development of new strategies for treatment of non-resectable brain tumors and tumors that are resistant to current therapeutic modalities.
Supplementary Material
Acknowledgments
FUNDING SOURCES
This work was supported in part by grant NCI R00CA154006 (R.N.S.), by NCI CCSG P30CA012197 and by start-up funds from the Wake Forest School of Medicine Department of Cancer Biology. CDF was supported in part by training grant NCI T32CA079448.
We are grateful for the assistance of Ken Grant and Paula Graham of the Wake Forest University Comprehensive Cancer Center (WFUCCC) Cellular Imaging Shared Resource, and Yelena Karpova of the WFUCCC Cell Viral Vector Core Laboratory. The authors would also like to thank Nicole Levi-Polyachenko and Hui-Wen Lo for the use of essential equipment and reagents, Jessica Swanner for assistance in data collection and analysis, and Richard Carpenter for helpful discussion.
Footnotes
AUTHOR CONTRIBUTIONS
The manuscript was written through contributions of all authors. B.N.E., B.W.B. and C.D.F. developed the methodology, carried out the experiments, performed data analysis, and wrote the manuscript. R.S. conceived the research, performed data analysis, and wrote the manuscript. All authors reviewed and have given approval to the final version of the manuscript.
References
- 1.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–50. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 2.Lima FR, Kahn SA, Soletti RC, Biasoli D, Alves T, da Fonseca AC, Garcia C, Romao L, Brito J, Holanda-Afonso R, Faria J, Borges H, Moura-Neto V. Glioblastoma: Therapeutic challenges, what lies ahead. Biochim Biophys Acta. 2012;1826(12):338–49. doi: 10.1016/j.bbcan.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Sloan AE, Ahluwalia MS, Valerio-Pascua J, Manjila S, Torchia MG, Jones SE, Sunshine JL, Phillips M, Griswold MA, Clampitt M, Brewer C, Jochum J, McGraw MV, Diorio D, Ditz G, Barnett GH. Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma: clinical article. J Neurosurg. 2013;118(6):1202–19. doi: 10.3171/2013.1.JNS1291. [DOI] [PubMed] [Google Scholar]
- 4.Mohammadi AM, Hawasli AH, Rodriguez A, Schroeder JL, Laxton AW, Elson P, Tatter SB, Barnett GH, Leuthardt EC. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer Med. 2014;3(4):971–9. doi: 10.1002/cam4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpentier A, Chauvet D, Reina V, Beccaria K, Leclerq D, McNichols RJ, Gowda A, Cornu P, Delattre JY. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg Med. 2012;44(5):361–8. doi: 10.1002/lsm.22025. [DOI] [PubMed] [Google Scholar]
- 6.Bettaieb A, Wrzal PK, Averill-Bates DA. Hyperthermia: Cancer Treatment and Beyond. In: Rangel Letícia, Rangel L., editors. Cancer Treatment - Conventional and Innovative Approaches [Online] InTech; 2013. [Google Scholar]
- 7.Hawasli AH, Kim AH, Dunn GP, Tran DD, Leuthardt EC. Stereotactic laser ablation of high-grade gliomas. Neurosurg Focus. 2014;37(6):E1. doi: 10.3171/2014.9.FOCUS14471. [DOI] [PubMed] [Google Scholar]
- 8.Verma J, Lal S, Van Noorden CJ. Nanoparticles for hyperthermic therapy: synthesis strategies and applications in glioblastoma. Int J Nanomedicine. 2014;9:2863–77. doi: 10.2147/IJN.S57501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh R, Torti SV. Carbon nanotubes in hyperthermia therapy. Adv Drug Deliv Rev. 2013;65(15):2045–60. doi: 10.1016/j.addr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pansare VJ, Hejazi S, Faenza WJ, Prud’homme RK. Review of Long-Wavelength Optical and NIR Imaging Materials: Contrast Agents, Fluorophores, and Multifunctional Nano Carriers. Chemistry of Materials. 2012;24(5):812–827. doi: 10.1021/cm2028367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke AR, Singh RN, Carroll DL, Wood JC, D’Agostino RB, Jr, Ajayan PM, Torti FM, Torti SV. The resistance of breast cancer stem cells to conventional hyperthermia and their sensitivity to nanoparticle-mediated photothermal therapy. Biomaterials. 2012;33(10):2961–70. doi: 10.1016/j.biomaterials.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie B, Singh R, Torti FM, Keblinski P, Torti S. Heat localization for targeted tumor treatment with nanoscale near-infrared radiation absorbers. Phys Med Biol. 2012;57(18):5765–75. doi: 10.1088/0031-9155/57/18/5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke A, Ding X, Singh R, Kraft RA, Levi-Polyachenko N, Rylander MN, Szot C, Buchanan C, Whitney J, Fisher J, Hatcher HC, D’Agostino R, Jr, Kock ND, Ajayan PM, Carroll DL, Akman S, Torti FM, Torti SV. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc Natl Acad Sci U S A. 2009;106(31):12897–902. doi: 10.1073/pnas.0905195106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos T, Fang X, Chen MT, Wang W, Ferreira R, Jhaveri N, Gundersen M, Zhou C, Pagnini P, Hofman FM, Chen TC. Sequential administration of carbon nanotubes and near-infrared radiation for the treatment of gliomas. Front Oncol. 2014;4:180. doi: 10.3389/fonc.2014.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang CH, Chiou SH, Chou CP, Chen YC, Huang YJ, Peng CA. Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibody. Nanomed: Nanotech Biol Med. 2011;7(1):69–79. doi: 10.1016/j.nano.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Pantarotto D, Singh R, McCarthy D, Erhardt M, Briand JP, Prato M, Kostarelos K, Bianco A. Functionalized carbon nanotubes for plasmid DNA gene delivery. Angewandte Chemie-Internat Ed. 2004;43(39):5242–5246. doi: 10.1002/anie.200460437. [DOI] [PubMed] [Google Scholar]
- 17.VanHandel M, Alizadeh D, Zhang LY, Kateb B, Bronikowski M, Manohara H, Badie B. Selective uptake of multi-walled carbon nanotubes by tumor macrophages in a murine glioma model. J Neuroimmunol. 2009;208(1–2):3–9. doi: 10.1016/j.jneuroim.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Burke AR, Singh RN, Carroll DL, Owen JD, Kock ND, D’Agostino R, Jr, Torti FM, Torti SV. Determinants of the thrombogenic potential of multiwalled carbon nanotubes. Biomaterials. 2011;32(26):5970–8. doi: 10.1016/j.biomaterials.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kostarelos K, Bianco A, Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat Nanotech. 2009;4(10):627–633. doi: 10.1038/nnano.2009.241. [DOI] [PubMed] [Google Scholar]
- 20.Bardi G, Nunes A, Gherardini L, Bates K, Al-Jamal KT, Gaillard C, Prato M, Bianco A, Pizzorusso T, Kostarelos K. Functionalized carbon nanotubes in the brain: cellular internalization and neuroinflammatory responses. PLoS One. 2013;8(11):e80964. doi: 10.1371/journal.pone.0080964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bussy C, Al-Jamal KT, Boczkowski J, Lanone S, Prato M, Bianco A, Kostarelos K. Microglia Determine Brain Region-Specific Neurotoxic Responses to Chemically Functionalized Carbon Nanotubes. ACS Nano. 2015;9(8):7815–30. doi: 10.1021/acsnano.5b02358. [DOI] [PubMed] [Google Scholar]
- 22.Shityakov S, Salvador E, Pastorin G, Forster C. Blood-brain barrier transport studies, aggregation, and molecular dynamics simulation of multiwalled carbon nanotube functionalized with fluorescein isothiocyanate. Int J Nanomedicine. 2015;10:1703–13. doi: 10.2147/IJN.S68429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta AI, Linninger A, Lesniak MS, Engelhard HH. Current status of intratumoral therapy for glioblastoma. J Neurooncol. 2015;125(1):1–7. doi: 10.1007/s11060-015-1875-1. [DOI] [PubMed] [Google Scholar]
- 24.Biddlestone-Thorpe L, Marchi N, Guo K, Ghosh C, Janigro D, Valerie K, Yang H. Nanomaterial-mediated CNS delivery of diagnostic and therapeutic agents. Adv Drug Del Rev. 2012;64(7):605–613. doi: 10.1016/j.addr.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernal GM, LaRiviere MJ, Mansour N, Pytel P, Cahill KE, Voce DJ, Kang S, Spretz R, Welp U, Noriega SE, Nunez L, Larsen G, Weichselbaum RR, Yamini B. Convection-enhanced delivery and in vivo imaging of polymeric nanoparticles for the treatment of malignant glioma. Nanomedicine. 2014;10(1):149–57. doi: 10.1016/j.nano.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jego G, Hazoume A, Seigneuric R, Garrido C. Targeting heat shock proteins in cancer. Cancer Lett. 2013;332(2):275–85. doi: 10.1016/j.canlet.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Tomar MS, Acharya A. HSF1-mediated regulation of tumor cell apoptosis: a novel target for cancer therapeutics. Future Oncol. 2013;9(10):1573–86. doi: 10.2217/fon.13.106. [DOI] [PubMed] [Google Scholar]
- 28.Santagata S, Hu R, Lin NU, Mendillo ML, Collins LC, Hankinson SE, Schnitt SJ, Whitesell L, Tamimi RM, Lindquist S, Ince TA. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc Natl Acad Sci U S A. 2011;108:18378–83. doi: 10.1073/pnas.1115031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendillo ML, Santagata S, Koeva M, Bell GW, Hu R, Tamimi RM, Fraenkel E, Ince TA, Whitesell L, Lindquist S. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell. 2012;150:549–62. doi: 10.1016/j.cell.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hermisson M, Strik H, Rieger J, Dichgans J, Meyermann R, Weller M. Expression and functional activity of heat shock proteins in human glioblastoma multiforme. Neurology. 2000;54(6):1357–65. doi: 10.1212/wnl.54.6.1357. [DOI] [PubMed] [Google Scholar]
- 31.Santagata S, Xu YM, Wijeratne EMK, Kontnik R, Rooney C, Perley CC, Kwon H, Clardy J, Kesari S, Whitesell L, Lindquist S, Gunatilaka AAL. Using the Heat-Shock Response To Discover Anticancer Compounds that Target Protein Homeostasis. ACS Chem Biol. 2012;7(2):339–348. doi: 10.1021/cb200353m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai B, Gong A, Jing Z, Aldape KD, Kang SH, Sawaya R, Huang S. Forkhead box M1 is regulated by heat shock factor 1 and promotes glioma cells survival under heat shock stress. J Biol Chem. 2013;288(3):1634–42. doi: 10.1074/jbc.M112.379362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai S, Liu ZX, Yao J, Patel N, Chen JQ, Wu Y, Ahn EEY, Fodstad O, Tan M. Heat Shock Factor 1 (HSF1) Controls Chemoresistance and Autophagy through Transcriptional Regulation of Autophagy-related Protein 7 (ATG7) J Biol Chem. 2013;288(13):9165–9176. doi: 10.1074/jbc.M112.422071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park MC, Jeong H, Son SH, Kim YH, Han D, Goughnour PC, Kang T, Kwon NH, Moon HE, Paek SH, Hwang D, Seol HJ, Nam DH, Kim S. Novel morphological and genetic analysis of cancer cells in a 3D microenvironment identifies STAT3 as a regulator of tumor permeability barrier function. Cancer Res. 2016;76(5):1044–54. doi: 10.1158/0008-5472.CAN-14-2611. [DOI] [PubMed] [Google Scholar]
- 35.Fahrenholtz CD, Hadimani M, King SB, Torti SV, Singh R. Targeting breast cancer with sugar-coated carbon nanotubes. Nanomedicine. 2015;10(16):2481–97. doi: 10.2217/NNM.15.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Z, Tabakman SM, Chen Z, Dai H. Preparation of carbon nanotube bioconjugates for biomedical applications. Nat Protoc. 2009;4:1372–82. doi: 10.1038/nprot.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neeves KB, Lo CT, Foley CP, Saltzman WM, Olbricht WL. Fabrication and characterization of microfluidic probes for convection enhanced drug delivery. J Controlled Release. 2006;111(3):252–262. doi: 10.1016/j.jconrel.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Cicchetti R, Divizia M, Valentini F, Argentin G. Effects of single-wall carbon nanotubes in human cells of the oral cavity: Geno-cytotoxic risk. Toxicology In Vitro. 2011;25(8):1811–1819. doi: 10.1016/j.tiv.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Siddiqi NJ, Abdelhalim MAK, El-Ansary AK, Alhomida AS, Ong WY. Identification of potential biomarkers of gold nanoparticle toxicity in rat brains. J Neuroinflam. 2012;9:123. doi: 10.1186/1742-2094-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ju L, Zhang GL, Zhang X, Jia ZY, Gao XJ, Jiang Y, Yan CL, Duerksen-Hughes PJ, Chen FF, Li HJ, Zhu XQ, Yang J. Proteomic Analysis of Cellular Response Induced by Multi-Walled Carbon Nanotubes Exposure in A549 Cells. PLoS One. 2014;9(1):e84974. doi: 10.1371/journal.pone.0084974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keblinski P, Cahill DG, Bodapati A, Sullivan CR, Taton TA. Limits of localized heating by electromagnetically excited nanoparticles. J Appl Phys. 2006;100(5):054305. [Google Scholar]
- 42.Sauvageot CME, Weatherbee JL, Kesari S, Winters SE, Barnes J, Dellagatta J, Ramakrishna NR, Stiles CD, Kung ALJ, Kieran MW, Wen PYC. Efficacy of the HSP90 inhibitor 17-AAG in human glioma cell lines and tumorigenic glioma stem cells. Neuro Oncol. 2009;11(2):109–121. doi: 10.1215/15228517-2008-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu H, Woolfenden S, Bronson RT, Jaffer ZM, Barluenga S, Winssinger N, Rubenstein AE, Chen R, Charest A. The novel Hsp90 inhibitor NXD30001 induces tumor regression in a genetically engineered mouse model of glioblastoma multiforme. Mol Cancer Ther. 2010;9(9):2618–26. doi: 10.1158/1535-7163.MCT-10-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuballa P, Baumann AL, Mayer K, Bar U, Burtscher H, Brinkmann U. Induction of heat shock protein HSPA6 (HSP70B) upon HSP90 inhibition in cancer cell lines. FEBS Lett. 2015;589(13):1450–8. doi: 10.1016/j.febslet.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Chen J, Yu J, Yang G, Temple E, Harbinski F, Gao H, Wilson C, Pagliarini R, Zhou W. Identification of mixed lineage leukemia 1(MLL1) protein as a coactivator of heat shock factor 1(HSF1) protein in response to heat shock protein 90 (HSP90) inhibition. J Biol Chem. 2014;289(27):18914–27. doi: 10.1074/jbc.M114.574053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro GN, Cayado-Gutierrez N, Zoppino FCM, Fanelli MA, Cuello-Carrion FD, Sottile M, Nadin SB, Ciocca DR. Effects of temozolomide (TMZ) on the expression and interaction of heat shock proteins (HSPs) and DNA repair proteins in human malignant glioma cells. Cell Stress & Chaperones. 2015;20(2):253–265. doi: 10.1007/s12192-014-0537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang P, Huang H, Huang J, Chen H, Wang J, Qiu K, Zhao D, Ji L, Chao H. Noncovalent Ruthenium(II) Complexes-Single-Walled Carbon Nanotube Composites for Bimodal Photothermal and Photodynamic Therapy with Near-Infrared Irradiation. ACS Appl Mater Interfaces. 2015;7(41):23278–90. doi: 10.1021/acsami.5b07510. [DOI] [PubMed] [Google Scholar]
- 48.Debinski W, Tatter SB. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother. 2009;9(10):1519–27. doi: 10.1586/ern.09.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jain KK. Use of nanoparticles for drug delivery in glioblastoma multiforme. Expert Rev of Neurother. 2007;7(4):363–72. doi: 10.1586/14737175.7.4.363. [DOI] [PubMed] [Google Scholar]
- 50.Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev. 2008;88(4):1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci U S A. 2006;103(14):5567–5572. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan ZH, Advani SG. Characterization of orientation state of carbon nanotubes in shear flow. Polymer. 2005;46(14):5232–5240. [Google Scholar]
- 53.Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu QG, Swaminathan G, Xiang D, Eberhart C, Hanes J. A Dense Poly(Ethylene Glycol) Coating Improves Penetration of Large Polymeric Nanoparticles Within Brain Tissue. Sci Trans Med. 2012;4(149):149ra119. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang YC, Bahng JH, Che QT, Han JS, Kotov NA. Anomalously Fast Diffusion of Targeted Carbon Nanotubes in Cellular Spheroids. ACS Nano. 2015;9(8):8231–8238. doi: 10.1021/acsnano.5b02595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thakor AS, Gambhir SS. Nanooncology: the future of cancer diagnosis and therapy. CA Cancer J Clin. 2013;63(6):395–418. doi: 10.3322/caac.21199. [DOI] [PubMed] [Google Scholar]
- 56.Kotchey GP, Zhao Y, Kagan VE, Star A. Peroxidase-mediated biodegradation of carbon nanotubes in vitro and in vivo. Adv Drug Del Rev. 2013;65(15):1921–32. doi: 10.1016/j.addr.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elgrabli D, Dachraoui W, Menard-Moyon C, Liu XJ, Begin D, Begin-Colin S, Bianco A, Gazeau F, Alloyeau D. Carbon Nanotube Degradation in Macrophages: Live Nanoscale Monitoring and Understanding of Biological Pathway. ACS Nano. 2015;9(10):10113–10124. doi: 10.1021/acsnano.5b03708. [DOI] [PubMed] [Google Scholar]
- 58.Wang JT, Al-Jamal KT. Functionalized carbon nanotubes: revolution in brain delivery. Nanomedicine. 2015;10(17):2639–42. doi: 10.2217/nnm.15.114. [DOI] [PubMed] [Google Scholar]
- 59.Nunes A, Bussy C, Gherardini L, Meneghetti M, Herrero MA, Bianco A, Prato M, Pizzorusso T, Al-Jamal KT, Kostarelos K. In vivo degradation of functionalized carbon nanotubes after stereotactic administration in the brain cortex. Nanomedicine. 2012;7(10):1485–94. doi: 10.2217/nnm.12.33. [DOI] [PubMed] [Google Scholar]
- 60.Zhao DC, Alizadeh D, Zhang LY, Liu W, Farrukh O, Manuel E, Diamond DJ, Badie B. Carbon Nanotubes Enhance CpG Uptake and Potentiate Antiglioma Immunity. Clin Cancer Res. 2011;17(4):771–782. doi: 10.1158/1078-0432.CCR-10-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bussy C, Hadad C, Prato M, Bianco A, Kostarelos K. Intracellular degradation of chemically functionalized carbon nanotubes using a long-term primary microglial culture model. Nanoscale. 2016;8(1):590–601. doi: 10.1039/c5nr06625e. [DOI] [PubMed] [Google Scholar]
- 62.Allen BL, Kichambare PD, Gou P, Vlasova II, Kapralov AA, Konduru N, Kagan VE, Star A. Biodegradation of Single-Walled Carbon Nanotubes through Enzymatic Catalysis. Nano Lett. 2008;8(11):3899–3903. doi: 10.1021/nl802315h. [DOI] [PubMed] [Google Scholar]
- 63.Kagan VE, Konduru NV, Feng WH, Allen BL, Conroy J, Volkov Y, Vlasova II, Belikova NA, Yanamala N, Kapralov A, Tyurina YY, Shi JW, Kisin ER, Murray AR, Franks J, Stolz D, Gou PP, Klein-Seetharaman J, Fadeel B, Star A, Shvedova AA. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat Nanotech. 2010;5(5):354–359. doi: 10.1038/nnano.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andon FT, Kapralov AA, Yanamala N, Feng WH, Baygan A, Chambers BJ, Hultenby K, Ye F, Toprak MS, Brandner BD, Fornara A, Klein-Seetharaman J, Kotchey GP, Star A, Shvedova AA, Fadeel B, Kagan VE. Biodegradation of Single-Walled Carbon Nanotubes by Eosinophil Peroxidase. Small. 2013;9(16):2721–2729. doi: 10.1002/smll.201202508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kagan VE, Kapralov AA, St Croix CM, Watkins SC, Kisin ER, Kotchey GP, Balasubramanian K, Vlasova II, Yu J, Kim K, Seo W, MallampaIli RK, Star A, Shvedova AA. Lung Macrophages “Digest” Carbon Nanotubes Using a Superoxide/Peroxynitrite Oxidative Pathway. ACS Nano. 2014;8(6):5610–5621. doi: 10.1021/nn406484b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marches R, Mikoryak C, Wang RH, Pantano P, Draper RK, Vitetta ES. The importance of cellular internalization of antibody-targeted carbon nanotubes in the photothermal ablation of breast cancer cells. Nanotechnology. 2011;22(9):095101. doi: 10.1088/0957-4484/22/9/095101. [DOI] [PubMed] [Google Scholar]
- 67.Wang CH, Chiou SH, Chou CP, Chen YC, Huang YJ, Peng CA. Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibody. Nanomedicine. 2011;7(1):69–79. doi: 10.1016/j.nano.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 68.Robinson JT, Welsher K, Tabakman SM, Sherlock SP, Wang H, Luong R, Dai H. High Performance In Vivo Near-IR (>1 mm) Imaging and Photothermal Cancer Therapy with Carbon Nanotubes. Nano Research. 2010;3(11):779–793. doi: 10.1007/s12274-010-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ding X, Singh R, Burke A, Hatcher H, Olson J, Kraft RA, Schmid M, Carroll D, Bourland JD, Akman S, Torti FM, Torti SV. Development of iron-containing multiwalled carbon nanotubes for MR-guided laser-induced thermotherapy. Nanomedicine. 2011;6(8):1341–52. doi: 10.2217/nnm.11.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hleb EY, Lapotko DO. Photothermal properties of gold nanoparticles under exposure to high optical energies. Nanotechnology. 2008;19(35):355702. doi: 10.1088/0957-4484/19/35/355702. [DOI] [PubMed] [Google Scholar]
- 71.Sailor MJ, Park JH. Hybrid Nanoparticles for Detection and Treatment of Cancer. Adv Mat. 2012;24(28):3779–3802. doi: 10.1002/adma.201200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jhaveri K, Ochiana SO, Dunphy MPS, Gerecitano JF, Corben AD, Peter RI, Janjigian YY, Gomes-DaGama EM, Koren J, Modi S, Chiosis G. Heat shock protein 90 inhibitors in the treatment of cancer: current status and future directions. Expert Opin Investig Drugs. 2014;23(5):611–628. doi: 10.1517/13543784.2014.902442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Biris AS, Boldor D, Palmer J, Monroe WT, Mahmood M, Dervishi E, Xu Y, Li Z, Galanzha EI, Zharov VP. Nanophotothermolysis of multiple scattered cancer cells with carbon nanotubes guided by time-resolved infrared thermal imaging. J Biomed Optics. 2009;14(2):021007. doi: 10.1117/1.3119135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zharov VP, Galitovskaya EN, Johnson C, Kelly T. Synergistic enhancement of selective nanophotothermolysis with gold nanoclusters: Potential for cancer therapy. Laser Surg Med. 2005;37(3):219–226. doi: 10.1002/lsm.20223. [DOI] [PubMed] [Google Scholar]
- 75.Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220(4596):524–7. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.