Abstract
The seed-transmitted fungal symbiont, Epichloë festucae, colonizes grasses by infecting host tissues as they form on the shoot apical meristem (SAM) of the seedling. How this fungus accommodates the complexities of plant development to successfully colonize the leaves and inflorescences is unclear. Since adenosine 3′, 5′-cyclic monophosphate (cAMP)-dependent signaling is often essential for host colonization by fungal pathogens, we disrupted the cAMP cascade by insertional mutagenesis of the E. festucae adenylate cyclase gene (acyA). Consistent with deletions of this gene in other fungi, acyA mutants had a slow radial growth rate in culture, and hyphae were convoluted and hyper-branched suggesting that fungal apical dominance had been disrupted. Nitro blue tetrazolium (NBT) staining of hyphae showed that cAMP disruption mutants were impaired in their ability to synthesize superoxide, indicating that cAMP signaling regulates accumulation of reactive oxygen species (ROS). Despite significant defects in hyphal growth and ROS production, E. festucae ΔacyA mutants were infectious and capable of forming symbiotic associations with grasses. Plants infected with E. festucae ΔacyA were marginally less robust than the wild-type (WT), however hyphae were hyper-branched, and leaf tissues heavily colonized, indicating that the tight regulation of hyphal growth normally observed in maturing leaves requires functional cAMP signaling.
Keywords: Epichloë festucae, Lolium perenne, symbiosis, cAMP signaling, reactive oxygen species, hyphal branching
Introduction
Temperate grasses such as Lolium perenne (Poaceae, subfamily Pooideae) often host mutualistic endophytic fungi in the genus Epichloë of the family Clavicipitaceae (Christensen and Voisey, 2009; Card et al., 2014; Leuchmann et al., 2014; Simpson et al., 2014). This genus became prominent when these endophytes were proven to be agents of chronic circulatory and neurological disorders in livestock feeding on infected forage (Bacon et al., 1977; Ball and Prestidge, 1993; Leuchmann et al., 2014). Several classes of endophyte metabolites with mammalian and insect toxicity, as well as invertebrate deterrent effects, have been characterized (Schardl and Phillips, 1997; Schardl et al., 2007, 2013). Epichloë infection can also elevate the tolerance of grasses to certain abiotic stresses (Arachevaleta et al., 1989; Malinowski and Belesky, 2000; Vázquez-de-Aldana et al., 2013). Given these attributes, these fungi are often prevalent in native grass habitats, but are also essential for persistence of forage in managed pastoral farming systems where insect pressure is high, as in New Zealand, Australia, and the Americas (Johnson et al., 2013a).
Epichloë hyphae within host tissues are confined to the intercellular spaces and do not invade cells (Christensen et al., 2008). They are notable for their complex biotrophic lifecycle which is synchronized with growth and development of the host from seedling to mature plant. Colonization of the plant by the endophyte proceeds through discrete modes of hyphal growth that alternate between apical extension and branch formation of hyphae in the shoot apical meristem (SAM), intercalary hyphal growth along the length of the filament in expanding host structures, and a phase in mature plant tissues where the fungus stops growing but remains metabolically active (Christensen et al., 2008; Christensen and Voisey, 2009; Voisey, 2010; Eaton et al., 2011). Each phase of vegetative hyphal development is seemingly initiated in response to changes in host development. For example, the transition between plant cell division and extension in developing leaves correlates with repression of hyphal lateral branch formation and initiation of intercalary hyphal extension; and maturation (cessation of growth) of host leaves correlates with a transition from hyphal intercalary growth to little or no extension or tip (polar) growth. The developmental switch from polar to intercalary hyphal growth is a critical stage in host colonization, and is achieved through initiation of the full cell cycle within intercalary hyphal compartments, including mitosis, the laying down of new septa, and cell expansion, a mechanism of growth rarely observed in vegetative filamentous fungi (Christensen et al., 2008; Christensen and Voisey, 2009; Voisey, 2010). Growth of Epichloë hyphae in plants is therefore restricted to developing plant tissues, particularly those arising from the SAM, axillary meristems (from which new tillers form) and floral meristems; and plant structures undergoing cell expansion such as developing leaves and floral spikes. How Epichloë colonization processes are synchronized with host development is likely mediated through hyphal sensing of changes in host development that induce corresponding changes in fungal development. E. festucae genes in the stress-activated mitogen-activated protein kinase (sakA) (Eaton et al., 2010), pH-sensing (pacC) (Lukito et al., 2015) and striatin-interacting phosphatase and kinase complex (mobC) (Green et al., 2016) are required for regulation of hyphal growth in L. perenne, and their deletion induces aberrant hyphal distribution in plants and alters host growth and development. Production of reactive oxygen species (ROS) by E. festucae in culture and in planta is also vital for establishment of normal symbiotic associations between these organisms. Superoxide ions regulate many processes in fungal morphogenesis and growth, and deletion of E. festucae genes encoding proteins of the NADPH oxidase complex responsible for superoxide synthesis, including noxA, noxR, racA, and bemA also disrupts the phenotype of both the endophyte and the host during symbiosis (Takemoto et al., 2006, 2011; Tanaka et al., 2006, 2008).
The ubiquitous signaling molecule, adenosine 3′5′- cyclic AMP (cAMP) is an integral component of signaling in most organisms. Accumulation of cAMP in the cytoplasm is modulated through the activities of adenylate cyclase (AC) and phosphodiesterase enzymes that synthesize or degrade cAMP respectively. The main target of cAMP in fungi is cAMP-dependent protein kinase (PKA) which mediates many of the physiological effects (D'Souza and Heitman, 2001) by phosphorylating target proteins such as protein kinases, ion channels, and transcription factors. Recently however, other unidentified target(s) of cAMP have been detected, such as those shown to be involved in the regulation of the Fusarium fujikuroi secondary metabolite, fusarubin (Studt et al., 2013). To date fungi have been shown to possess a single adenylate cyclase gene that encodes a large membrane-bound enzyme that is stimulated by a variety of environmental signals, and acts down-stream of heterotrimeric G proteins (Ivey and Hoffman, 2005; Kamerewerd et al., 2008) and CO2 or (Bahn and Mühlschlegel, 2006; Mogensen et al., 2006). This versatility contrasts with mammalian cells that possess several adenylate cyclase enzymes, both cytosolic and plasma-membrane localized, each responding to specific stimuli (McDonough and Rodriguez, 2012). The implication of this is that the single fungal AC enzyme has many interaction partners and is highly interconnected with other pathways to mediate its effects.
Although highly conserved across the fungal kingdom, the components of the cAMP-PKA signaling pathway regulate functionally diverse processes including hyphal growth, secondary metabolite biosynthesis (García-Martínez et al., 2012), conidiation (Mukherjee et al., 2007), reaction to oxidative stress (Choi and Xu, 2010; Deveau et al., 2010), and virulence (Kohut et al., 2010; García-Martínez et al., 2012; McDonough and Rodriguez, 2012). Disruption of AC can have opposing effects in different fungi, even in closely related species (McDonough and Rodriguez, 2012). For example, in F. fujikuroi, deletion of the adenylate cyclase gene increases colony sensitivity to oxidative stress (García-Martínez et al., 2012) while in F. proliferatum and F. verticillioides the mutants are less sensitive than wild-type (Choi and Xu, 2010; Kohut et al., 2010). Similarly, disruption in cAMP signaling has no impact on virulence of F. fujikuroi on tomato (García-Martínez et al., 2012), while in F. proliferatum virulence on tomato and maize is reduced (Kohut et al., 2010). Generally however, AC is indispensable for virulence in many pathogenic fungi, or nearly so (Klimpel et al., 2002), including the pathogens of insects (Liu et al., 2012), fungi (Mukherjee et al., 2007), humans (Bahn and Sundstrom, 2001; Brakhage and Liebmann, 2005) and plants (Kulkarni and Dean, 2004; Martínez-Espinoza et al., 2004; Yamauchi et al., 2004; Mukherjee et al., 2007; Choi and Xu, 2010; Kohut et al., 2010; Bormann et al., 2014). Reduction in virulence in AC pathway deletion mutants is largely due to the pleiotropic effects of cAMP on the development and functionality of specialized infection structures (Adachi and Hamer, 1998; Yamauchi et al., 2004) or alterations in secondary metabolite biosynthesis (Brakhage and Liebmann, 2005; Sugui et al., 2007; Gallagher et al., 2012) and other virulence factors (Alspaugh et al., 2002).
The cAMP pathway is also important in fungal growth and development. Deletion of the adenylate cyclase gene reduces radial growth and produces a more compact colony in F. verticilloides, F. proliferatum and F. fujikuroi (Choi and Xu, 2010; Kohut et al., 2010; García-Martínez et al., 2012). Cyclic-AMP also regulates the transition from yeast to filamentous forms in Candida albicans (Xu et al., 2008), Ustilago maydis (Martínez-Espinoza et al., 2004), and Paracoccidioides brasiliensis (Chen et al., 2007) and can either increase (Kohut et al., 2010) or decrease (Choi and Xu, 2010) production of conidia, and delay conidial germination (Kohut et al., 2010).
Despite extensive investigation of the multiple processes influenced by cAMP signaling in pathogens, the role of this pathway in regulating colonization and symbiosis by mutualistic fungi is currently unknown. Here we describe the role of cAMP signaling in the establishment and maintenance of a symbiotic partnership between E. festucae and the temperate grass species Lolium perenne (perennial ryegrass). We report on disruption of the adenylate cyclase gene (herein designated acyA) and consequent defects in hyphal growth and morphology in colonies growing in axenic culture, and the requirement for functional cAMP signaling in E. festucae for accumulation of ROS. We also show that E. festucae cAMP signaling, unlike most pathogens, appears to modulate growth of the fungus in plants, limiting over-colonization and enabling the symbionts to grow synchronously with plants during development.
Materials and methods
Fungal strains
The adenylate cyclase gene (acyA) was originally cloned and sequenced from E. festucae var. lolii, previously Neotyphodium lolii (Leuchmann et al., 2014), strain Lp19, isolated from L. perenne. Gene disruption experiments were performed on the closely related strain E. festucae Fl1, isolated from Festuca trachyphylla (Hack.) Krajina. The strains used in this study are presented in Table 1.
Table 1.
E. festucae strains used in this study.
| Strain | Genotype | References |
|---|---|---|
| E. festucae var. lolii Lp19 | wild type | Christensen et al., 1993 |
| E. festucae Fl1 | wild type | Leuchtmann, 1994; Moon et al., 1999 |
| E. festucae Fl1 EGFP | pTef::EGFP::GA; hph | Christensen et al., 2008 |
| E. festuae Fl1 acyA19 | Ectopic insertion of acyA | This study |
| E. festuae Fl1 acyA49 | Ectopic insertion of acyA | This study |
| E. festuae Fl1 ΔacyA34 | Δacy::hph | This study |
| E. festuae Fl1 ΔacyA42 | Δacy::hph | This study |
| E. festuae Fl1 ΔacyA47 | Δacy::hph | This study |
| E. festuae Fl1 ΔacyA34/acyA | Δacy::hph; acyA, gen | This study |
| E. festuae Fl1 ΔacyA42/acyA | Δacy::hph; acyA, gen | This study |
| E. festuae Fl1 ΔacyA42/EGFP | Δacy; hph; pTef::EGFP::GA, gen | This study |
Fungal and plant growing conditions
Fungi were cultured on potato dextrose agar (PDA, Difco, Le Pont, De Claix, France) at 22°C in an 8 h light, 16 h dark cycle. L. perenne cv. Nui or Samson plants infected with E. festucae Fl1 wild-type or mutant strains were maintained in glasshouse conditions under ambient light and temperature.
Identification and sequencing of Epichloë acyA genes
We previously cloned and sequenced a 300 bp acyA PCR product from E. festucae var. lolii strain Lp19 (Johnson et al., 2007). The DNA fragment was used as a probe to screen an Lp19 genomic DNA lambda library (Fleetwood et al., 2007) to recover a larger fragment of the gene for functional analysis. A single lambda clone (designated 6163) with homology to the adenylate cyclase gene fragment was recovered (Genbank accession KR815911). Sequencing of the 6047 bp insert indicated the presence of 5819 bp of the adenylate cyclase open reading frame (ORF) plus 228 bp of the acyA 3' region. The first 1419 bp of the open reading frame was missing. This sequence was used to design the gene disruption vector. Later, de novo sequencing of the E. festucae Fl1 genome by Schardl et al. (2013) enabled a complete genomic acyA sequence (gene model Fl1M3.048730, http://www.endophyte.uky.edu/) to be recovered. BLASTn was used to recover the acyA gene from other haploid Epichloë species (Table 2).
Table 2.
Copy number of acyA in haploid and allopolyploid Epichloë species.
| Species | Parental species | Strain | No. of genomes | acyA copy number | Genome References |
|---|---|---|---|---|---|
| E. festucae | n/a | E2368 | 1 | 1 | Schardl et al., 2013 |
| E. festucae | n/a | Fl1 | 1 | 1 | Schardl et al., 2013 |
| E. brachyelytri | n/a | E4804 | 1 | 1 | Schardl et al., 2013 |
| E. glyceriae | n/a | E277 | 1 | 1 | Schardl et al., 2013 |
| E. amarillans | n/a | ATCC 200744 | 1 | 1 | Schardl et al., 2013 |
| E. typhina | n/a | ATCC 200736 | 1 | 1 | Schardl et al., 2013 |
| E. typhina | n/a | E5819 | 1 | 1 | Schardl et al., 2013 |
| Epichloë sp. | E. baconii/amarillans x E. bromicola | AR3046* | 2 | 2 | unpublished |
| E. uncinata | E. typhina x E. bromicola | AR1006* | 2 | 2 | unpublished |
allopolyploid strains, n/a is not applicable.
The acyA genes from allopolyploids AR3046 (E. baconii/amarillans × E. bromicola) and AR1006 (E. typhina × E. bromicola) were recovered by mapping their genome read pairs (unpublished) to 7 kb genomic scaffolds (containing the acyA gene) of extant strains of their parental species using the “aln” algorithm BWA version 0.7.9a-r786. For AR3046, 43,768,368 78 bp read pairs with an insert size of ~290 bp were individually mapped to E. baconii strain ATCC 200745 and E. bromicola strain AL0434 (Schardl et al., 2014). For AR1006, 21,293,733 100 bp read pairs with an insert size of ~160 bp were mapped to E. typhina strain ATCC 200736 and E. bromicola strain AL0434. The mapped reads were extracted using SAMtools (Li et al., 2009) version 0.1.19-44428cd and imported into Geneious V8.1 (Biomatters, http://www.geneious.com) (Kearse et al., 2012) and consensus sequences for each mapping file were generated based on a threshold of 95% identity.
Vector construction
The acyA disruption vector (pCRVacyhph) was constructed using the Multisite Gateway system (ThermoFisher Scientific, Walden, MA, USA) following the manufacturer's instructions. The entry vector pDONR221/hygromycin, containing the hph cassette from pAN7-1 (Punt et al., 1987), was constructed as previously described (Fleetwood et al., 2007). Two further Multisite Gateway entry vectors, pDONRP2R-P3/AC3′ and pDONRP4-P1R/AC5′ containing a 5′ (3002 bp) and 3′ (3064 bp) region respectively, flanking the integration site in acyA, were created. PCR products were amplified from E. festucae var. lolii Lp19 genomic DNA using primer pairs AC5′-attB4 and AC5′-attB1 (for the 5′ flank) and AC3′-attB2 and AC3′-attB3 (for the 3′ flank) using Platinum Pfx DNA polymerase (ThermoFisher Scientific) according to the manufacturer's instructions. Primer sequences are listed in Supplementary Table 1. The PCR products were purified using the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany), and the products quantified by fluorometric quantitation using the Qubit system (ThermoFisher Scientific). The PCR fragments were then recombined into Gateway donor vectors pDONRP4-P1R and pDONRP2R-P3 using Gateway BP Clonase II. The resulting vectors, pDONRP4-P1R/AC5′and pDONRP2R-P3/AC3,′ along with pDONR221/hygromycin, were then recombined into the destination vector pDESTR4-R3 using Gateway LR Clonase II Plus, to create pCRVacyhph.
Disruption of E. festucae acyA
Gene disruption experiments were originally designed to disrupt acyA in strain E. festucae var. lolii Lp19, however as this strain proved to be relatively intractable to homologous recombination the closely-related strain E. festucae Fl1 was used instead. Homologous recombinants were obtained by PEG-mediated transformation of protoplasts. Protoplasts were prepared using the method of Young et al. (1998), except that 10 mg/ml of Glucanex (InterSpex Products, San Mateo, CA, USA) was used to digest cell walls for 3 h at 30°C with shaking (100 rpm). E. festucae Fl1 was transformed using 5 μg of each plasmid by the method of Vollmer and Yanofsky (1986) with modifications (Itoh et al., 1994). Protoplasts were co-transformed with plasmid pCRVacyhph (see above) or pTEFEGFP (EGFP fused to the tef2 promoter from Aureobasidium pullulans) (Vanden Wymelenberg et al., 1997) plus either pPN1688 (Young et al., 2005) or pII99 (Namiki et al., 2001) for resistance against hygromycin B or geneticin (both Gibco, ThermoFisher Scientific) respectively. Transgenic colonies were selected on PDA containing 150 μg/mL or 200 μg/mL of hygromycin B or geneticin respectively, and regenerating colonies were purified to homogeneity by sub-culturing hyphal tips onto fresh selective media three times. A number of colonies were analyzed by Southern-blot hybridization to confirm that each contained a single integration of the hygromycin resistance cassette at the desired locus. Genomic DNA was extracted from putative recombinant strains using the method of Byrd et al. (1990) and 2 μg of DNA was digested to completion with HindIII. The DNA was subjected to standard agarose gel (0.8% w/v) electrophoresis, transferred to nylon (Hybond N+, GE Healthcare, Buckinghamshire, UK) and the membrane hybridized independently against two dideoxygenin-labeled probes following the manufacturer's instructions (Roche, Basel, Switzerland). Probe 1 (482 bp) was amplified by PCR using the TripleMaster polymerase system (Eppendorf, Hamburg, Germany) using primers ACseqrev4120/ACseqfor3657 and probe 2 (644 bp) was synthesized using primers AC SeqIntRev2/ACM13ForRev (see Supplementary Table 1 for primer sequences).
To complement the mutation, the full length wild-type acyA was PCR-amplified from E. festucae Fl1genomic DNA using Platinum Pfx Polymerase (ThermoFisher Scientific) and primers AcyAF and AcyAR. The 8371 bp fragment comprising 859 bp upstream of the putative start site, 7170 bp of the open reading frame and 342 bp of the 3′ un-translated region was purified according to the manufacturer's instructions (DNA Clean and Concentrator -5, Zymo Research, Irvine, CA, USA) and protoplasts of ΔacyA34 and ΔacyA42 disruption mutants transformed as described above. Ectopic integration of wild-type acyA was confirmed by PCR using primers ACKOF/R (data not shown).
Fungal growth on media supplemented with cAMP
Mycelial sections (approximately 1 mm2) were inoculated onto PDA plates supplemented with 2.5, 5.0 or 7.5 mM cAMP sodium salt (Sigma-Aldrich, St. Louis, MO, USA). Three control strains (E. festucae Fl1 wild-type plus two strains with ectopic insertions, acyA19 and acyA49) and three independent disruption mutants (ΔacyA34, ΔacyA42, and ΔacyA47) were inoculated in triplicate onto each medium supplemented with cAMP (including a no-cAMP control) in a randomized design. The radial measurement (mm) of the colony was taken at approximately 24 h intervals over 165 h. The first measurement was taken 20 h after the colonies were inoculated. The growth rate of each strain was obtained by least squares regression and the slope of the line used to represent the rate of radial growth in mm/h. Analysis of variance was used to estimate the effects of strain and cAMP concentration, and their interaction. Means for each strain and cAMP combination were obtained, together with the least significant difference (LSD—calculated at the 5% significance level) between means. The residual plot from the ANOVA was checked and displayed no evidence of heterogeneity of variance, thus the pooled LSD was used for comparing means at the same level of cAMP.
Infection of L. perenne with E. festucae Fl1
Mycelial sections were inoculated into incisions created in the SAM of sterile 5 days old L. perenne seedlings growing on 1.5% (w/v) water agar, according to the method of Latch and Christensen (1985). Strains inoculated included the wild-type, mutants ΔacyA34, ΔacyA42 and ΔacyA47, plus two independent complementation strains, ΔacyA34/acyA and ΔacyA42/acyA. Inoculated plants were then maintained at 22°C in the dark for 7 days, followed by 22°C in the light for 7 days, before being transplanted into potting mix and maintained under glasshouse conditions. After 12 weeks, six tillers from each plant were tested for endophyte infection by tissue print immuno-assay using an Epichloë-specific polyclonal anti-serum (Simpson et al., 2012). Plants were inoculated as described on two independent occasions.
Light microscopy
E. festucae Fl1 wild-type and acyA deletion mutants were grown in triplicate on potato dextrose broth (PDB, Difco, Le Pont, De Claix, France), either 1X or diluted 1:100 in water, containing 0.8% (w/v) agarose. The broths were inoculated and grown for 5 days at 22°C in continuous light. Alternatively, cultures were grown on the same medium on sterile microscope slides and incubated as described above. If grown in a culture dish, 0.5 cm2 of agar was cut from the outer edge of the colony, mounted onto a microscope slide, and a drop of water and a cover glass placed directly onto the specimen. If grown on a microscope slide, a drop of water was added to the mycelium and a cover glass applied. Cultures were imaged by bright field microscopy using a BX50 fluorescent microscope (Olympus, Tokyo, Japan) and a 40X UPLANFLN objective with a 0.75 numerical aperture. Images were taken using an Olympus Colorview III camera with AnalySISB image processing software. Endophytes in the epidermal layer of the host leaf sheath were stained with 0.15% (w/v) aniline blue and examined by bright field microscopy as described previously (Christensen et al., 2008). Leaf sheaths from two tillers per plant, and at least three infected plants per strain in each inoculation experiment, were examined.
Confocal laser scanning microscopy (CLSM)
To examine hyphae in the host shoot apex, semi-thin (0.5–1 mm) longitudinal or transverse sections were taken through the shoot apex and true stem of at least six independent L. perenne tillers infected with each strain and mounted on a microscope slide in water. CLSM images were captured on an inverted FluoView FV10i Confocal Laser Scanning Microscope (Olympus). For visualizing EGFP the excitation wavelength was 457 nm and detection wavelength was between 465 and 565 nm. Two dimensional images were taken using the 10x phase contrast objective, numerical aperture 0.4 (equivalent to UPLSAPO 10x). To examine hyphal morphology in the expansion zone of the developing leaf, young leaves of approximately 5 cm in length were dissected from tillers and cut in half along the midrib. A 1 cm section from the base of a halved blade was mounted in water on a microscope slide for imaging as described above. For each image, the optimal depth to show the most hyphae possible in a sample was selected, and the laser sensitivity then adjusted to immediately below saturation levels.
Transmission electron microscopy (TEM)
Endophyte-infected pseudostem material from two plants each infected with wild-type, ΔacyA34, ΔacyA42, or ΔacyA34/acyA, was dissected from L. perenne plants 0.5 cm above the tiller crown, and 0.5 mm transverse sections fixed in 2% (w/v) formaldehyde and 3% (w/v) glutaraldehyde in 0.1 M sodium/potassium phosphate (pH 7.2) for 2 h at room temperature. The samples were washed three times in 0.1 M sodium/potassium phosphate (pH 7.2) and post-fixed in 1% (w/v) osmium tetroxide in the same buffer for 1 h. After washing again as described above, the samples were subjected to a graded acetone/water series with 10 min each in 25, 50, 75, and 95% (v/v) acetone, followed by concentrated acetone for 2 h. The samples were embedded in Procure 812 resin (ProSciTech, Kirwan, Qld. Australia):acetone (50:50) overnight under constant stirring and then in 100% resin overnight. The resin was refreshed for a further 8 h and then mounted in 100% resin at 60°C for 48 h. Ultra-thin sections were collected onto a copper grid and stained with saturated uranyl acetate in 50% (v/v) ethanol for 4 min and then in lead citrate for a further 4 min. Specimens were examined at 46 000X magnification on a Philips CM10 (Philips Electron Optics, Eindhoven, The Netherlands) transmission electron microscope. Micrographs of at least 12 hyphae in developing leaves and in the second mature leaf sheath were captured. The thickness of hyphal cell walls was measured at eight equidistant positions around the circumference of each hypha using open source ImageJ 1.45 s software (Wayne Rasband, National Institutes of Health, USA). The cell wall thickness measurements were analyzed using one way ANOVA to compare the strains. The least significant differences (P = 0.05 level) between means were calculated.
Detection of ROS
Superoxide radicals in E. festucae in axenic culture were stained using NBT (Sigma-Aldrich) as described by Tanaka et al. (2006). Three replicates for each strain were included in the analysis and the experiment was repeated twice. Strains were grown on PDA for 1 week at 22°C under 8 h light and 16 h dark, and mycelia stained for 5 h at 22°C in continuous light by incubation in 20 μL of 0.05% (w/v) NBT dissolved in 0.05 M sodium phosphate pH 7.5. The reaction was stopped by removing the stain and adding 40 μL of absolute ethanol. The ethanol was removed after 5 min, and mycelia were analyzed at 400X magnification under bright field illumination using an Olympus BX50 compound microscope with a 40X UPLANFLN objective and a 0.75 numerical aperture. Images were taken using an Olympus Colorview III camera with AnalySISB image processing software.
Quantitation of hyphal biomass
Genomic DNA was extracted from freeze-dried pseudostems (leaf and blade tissue between the crown and the first ligule of a tiller) dissected from three tillers of each plant using the DNAeasy Plant Kit (Qiagen). The plants were infected with either the wild-type or ΔacyA42 mutant strain. There were three replicate plants per strain. Hyphal biomass (expressed as endophyte concentration) was determined by quantitative PCR of the single copy E. festucae NRPS-1 gene (EFM3.005350, http://www.endophyte.uky.edu/) from 1 ng of genomic DNA using a MyiQ™ cycler (Bio-Rad Laboratories, Hercules, California, USA), with primers 1-1F, and 1-1R (Supplementary Table 1) as described (Rasmussen et al., 2007; Liu et al., 2011). Hyphal biomass between strains was compared using one way ANOVA. The least significant differences (P = 0.05 level) between means was calculated.
Results
The E. festucae acyA gene
A partial acyA gene (1419–5816 bp) was initially recovered from E. festucae var. lolii (Lp19). Subsequent to functional characterization of this gene in E. festucae Fl1 (reported here), the Fl1 genome became available and the full length acyA gene recovered (gene model Fl1M3.048730, http://www.endophyte.uky.edu/). Analysis of the partial and full length conceptual AcyA proteins from the Lp19 and Fl1 strains respectively, confirmed the presence of the key motifs consistent with fungal Class III adenylate cyclases, including the domains for adenylate cyclase G-alpha binding (IPR013716), ras association (IPR000159), leucine rich repeats (IPR001611, IPR003591, IPR025875), protein phosphatase 2C-like (IPR001932), and the adenylate cyclase (IPR001054) catalytic core (Figure 1A). The E. festucae var. lolii acyA sequence contains a 66 bp indel that is not present in the E. festucae Fl1 acyA. Consistent with other fungal species, Southern-blot hybridization confirmed that E. festucae Fl1 contains a single acyA gene (Figure 1C) and BLASTn analysis of the genomes of other Epichloë species (Schardl et al., 2013) also suggests the presence of a single acyA gene in the haploid strains examined (Table 2).
Figure 1.
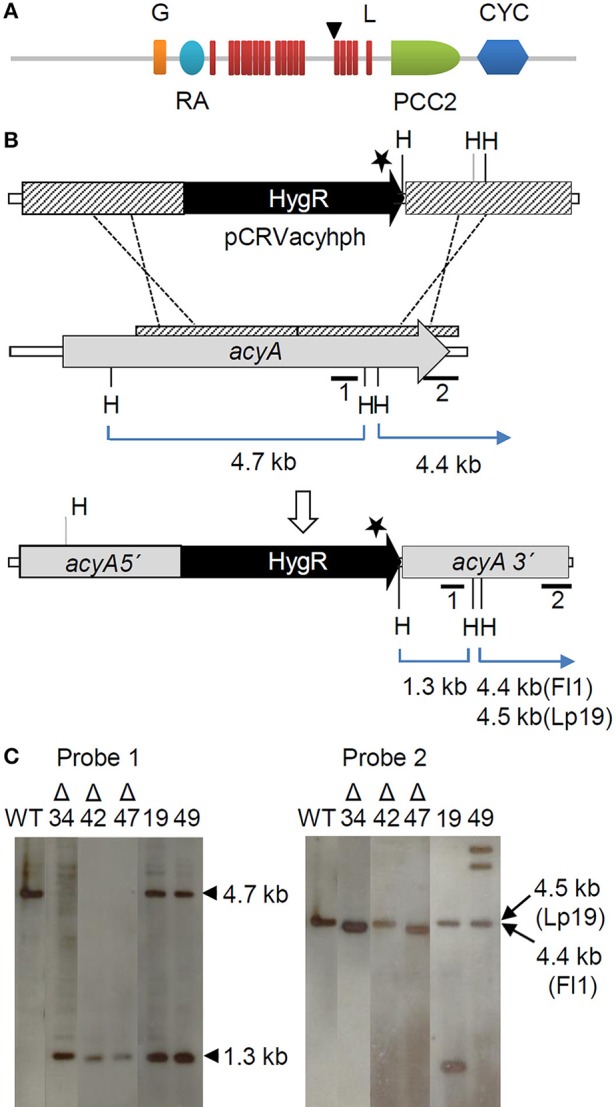
Disruption of acyA in E. festucae Fl1. (A) Domain architecture of AcyA in relation to the insertion site of the hygromycin resistance cassette (HygR). The protein contains the domains for G-alpha binding (G), ras association (RA), leucine rich repeats (L), protein phosphatase 2C (PP2C), and catalytic cyclic nucleotide biosynthesis (CYC) (Baker and Kelly, 2004). The HygR cassette was inserted (arrow) upstream of the phosphatase 2C (PPC2) and catalytic (CyC) domains. (B) Diagram of the acyA disruption locus in E. festucae Fl1. In vector pCRVacyhph, the HygR cassette is flanked by approximately 3 kb of DNA from the partial acyA gene (hatched) cloned from E. festucae var. lolii Lp19 (KR815911). The stop codon in the hygromycin resistance gene is indicated (⋆). The regions of homology between the Lp19 flanking regions and the corresponding Fl1 acyA sequence (hatched) is shown immediately above the Fl1 acyA gene, gene model Fl1M3.048730 (http://www.endophyte.uky.edu). Probes 1 and 2 (solid bars) were used in Southern-blot hybridization experiments (C) to confirm that the endogenous acyA gene had been disrupted and to ascertain the number of acyA copies in each strain respectively. (C) Southern-blot hybridization to confirm acyA insertion mutants. Genomic DNA was isolated from the wild-type (WT) plus a number of putative insertional mutants, and digested with HindIII. Probe 1 bound to the predicted 4.7 kb fragment in the wild-type acyA also in ectopic integrants acyA19 and acyA49 and to the 1.3 kb HindIII fragment of the disrupted acyA gene in ectopic integrants acyA19 & acyA49 and insertional mutants ΔacyA34 (Δ34), ΔacyA42 (Δ42) and ΔacyA47 (Δ47). Probe 2 was used to determine copy number, and bound to either a 4.4 kb or 4.5 kb HindIII fragment depending on whether the recombination locus was before or after the 66 bp indel in the Lp19 acyA.
Since genome hybridization has been a relatively common occurrence in the Epichloë genus, we investigated whether two recently-sequenced allopolyploid strains had retained both copies of the acyA gene from their progenitors, with the attendant prospects for neofunctionalisation (as is observed in mammals). Reads from the genomes of strains AR1006 (E. uncinata) and AR3046 (Epichloë sp.) were mapped independently to the haploid genomes of extant strains related to the original progenitors. E. uncinata (AR1006) is a hybrid between E. bromicola and E. typhina (Craven et al., 2001; Moon et al., 2004), and AR3046 is a hybrid between E. bromicola and the E. baconii/E. amarillans clade (unpublished). Two acyA genes were recovered from each allopolyploid genome examined (Table 2), consistent with a gene originating from each of the species that contributed to the allopolyploid. The acyA homeologs from AR3046 (KT732649 and KT732650) and AR1006 (KT732647 and KT732648) were then mapped back to the genomes of the predicted progenitor species, which confirmed that each acyA gene mapped with higher identity to one or the other of the species that contributed the genes to the allopolyploid (Supplementary Table 2A). The acyA genes from AR3046 encoded conceptual full-length proteins indicating that both genes had the potential to be functional, however in AR1006 one of the genes (KT732647) encoded a full length protein while the other, KT732648, encoded a conceptual protein truncated after amino acid position 686. This gene has also lost a triplet 232–234 bp from the start codon and sustained a number of other deletions relative to the presumed parent E. typhina (Supplementary Table 2B). Alignment of acyA genes within each allopolyploid strain indicated they had no more identity between each other than was found in the comparisons between genes from other species (Supplementary Table 2C) confirming that the genes came together through a genome hybridization event and was not the result of gene duplication.
Disruption of E. festucae Fl1 acyA
An acyA gene disruption vector (pCRVacyhph) was constructed using flanking regions designed from the partial E. festucae var. lolii Lp19 sequence, and gene disruption experiments were performed in strain Fl1 as the frequency of homologous recombination in Lp19 is extremely low. Insertion of the hygromycin cassette introduced a stop codon into the acyA open reading frame upstream of the catalytic core domain (Figures 1A,B). Three colonies (ΔacyA34, ΔacyA42, and ΔacyA47), each with a single gene disruption event and no ectopic insertions, were identified by PCR and confirmed by Southern-blot hybridization (Figure 1C). The recombination breakpoints of the mutant strains differed with respect to the presence or absence of the 66 bp indel (Figure 1C), presumably due to differences in the recombination site during homologous recombination in the disruption mutants. E. festucae ΔacyA34 contained the insertion, while ΔacyA42 and ΔacyA47 did not. The integrity of each gene immediately flanking the disrupted acyA was checked by PCR to ensure that the disruption event was confined to the acyA gene. Two primer pairs spanning the intergenic region and the flanking gene 5′ and 3′ of the acyA were used. No evidence of untargeted rearrangements were detected (Supplementary Figure 1). Two further colonies (acyA19 and acyA49) retained the intact acyA gene plus an ectopic insertion of the gene replacement vector and were used as transformation controls.
Regulation of radial growth by cAMP signaling in E. festucae in axenic culture
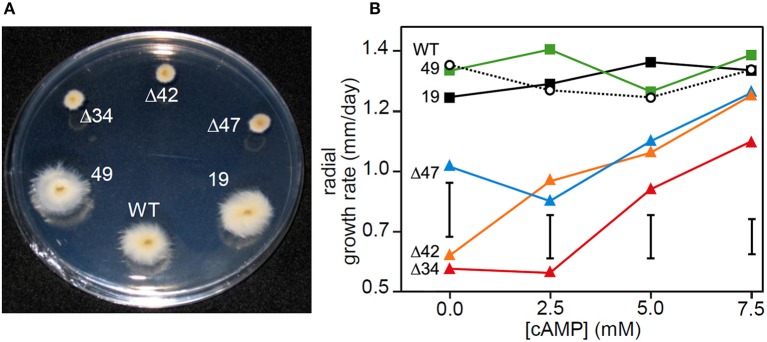
The three independent E. festucae mutants, ΔacyA42, ΔacyA34, and ΔacyA47, grew more slowly in axenic culture compared with the wild-type, or control strains acyA19 and acyA49 (Figure 2A). The mutant colonies were also highly compact compared to the controls. The radial growth rates of ΔacyA42, ΔacyA34, and ΔacyA47 increased in a dose-dependent manner in response to supplementation of the media with exogenous cAMP (Figure 2B). The growth rates of E. festucae ΔacyA42 and ΔacyA47 were statistically indistinguishable from wild-type when the medium was supplemented with 7.5 mM cAMP. E. festucae ΔacyA34 grew more slowly than the other strains under all conditions; however its growth rate on PDA containing 7.5 mM cAMP was almost 2 fold higher than when growing on PDA alone. The growth rates of the control strains (wild-type, acyA19 and acyA49) were not altered by cAMP supplementation (Figure 2B) indicating that endogenous cAMP does not limit growth of strains with functional AC enzymes.
Figure 2.
Growth of E. festucae acyA disruption mutants in axenic culture. (A) Growth of wild-type, disruption mutants ΔacyA34 (Δ34), ΔacyA42 (Δ42), ΔacyA47 (Δ47), and colonies with an intact acyA gene plus an ectopic insertion(s) of the transformation vector, acyA19, acyA49 on PDA (22°C for 7 days). (B) The effect of exogenous cAMP on the radial growth rate of the same strains. The mean growth rate of each strain (with three clonal replicates) at each cAMP concentration is shown. Analysis of variance was used to compare the growth rate of strains between and within each concentration of cAMP. The vertical bar represents the least significant differences (LSD) between means at the 5% significance level when comparing strains at the same concentration of cAMP.
E. festucae ΔacyA47 produced a faster-growing sector (named ΔacyA47var) on PDA supplemented with 150 μg/mL hygromycin. Southern-blot hybridization confirmed that the sector was identical to ΔacyA47 at the disruption locus (data not shown). Strains ΔacyA34 and ΔacyA42 did not produce overt spontaneous growth revertants, however if repeatedly sub-cultured onto fresh media, gradually grew faster until their growth rates were similar to wild-type (data not shown). PCR (using primers ACKO F and ACKO R) was used to check the fidelity of the integration locus during this period (data not shown). We saw no evidence of a loss of the integrated DNA over time in these colonies suggesting that the changes in phenotype were due to mutations or epigenetic changes at other loci.
Regulation of hyphal branching in E. festucae growing in axenic culture
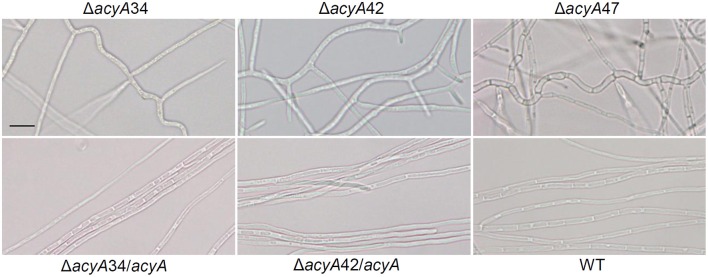
We next examined the hyphal morphology of acyA disruption mutants in culture by bright field microscopy. In wild-type cultures, hyphae were long and straight, relatively sparsely branched and produced lateral branches at the proximal end of compartments immediately adjacent to septa several compartments behind the tip (Figure 3). Conversely, the hyphae of E. festucae ΔacyA34, ΔacyA42, and ΔacyA47 were highly convoluted, sometimes forming thick aggregates or cables, and produced multiple lateral branches. Excessive lateral branches accounted for the compact nature of the mutant colonies. The morphology of strains complemented with the wild-type acyA gene were similar to the wild-type strain (Figure 3) confirming the role of cAMP in suppressing lateral branches. This phenotype was reproducible, however similarly to the observations on effects of AC disruption on colony growth rate, hyphal morphology in mutant colonies was not stable over time and reverted to the wild-type form if the cultures were maintained continuously in axenic culture.
Figure 3.
Effects of acyA disruption on E. festucae hyphal morphology in axenic culture. Bright field images of E. festucae Fl1 hyphae growing on water agar at 22°C for 7 days (bar, 10 μM). Images were taken approximately 3 mm behind the colony margin. Hyphae of all independent mutants (ΔacyA34, ΔacyA42, and ΔacyA47) were convoluted, irregular in diameter (ΔacyA47) and heavily branched compared to the wild-type. Ectopic integration of the wild-type acyA gene into ΔacyA34 and ΔacyA42 resulted in a reversion to the wild-type phenotype to greater or lesser extents dependent on the individual strain (ΔacyA34/acyA, ΔacyA42/acyA). Gross morphology at the hyphal tips appeared similar in mutants and wild-type.
Regulation of ROS in E. festucae growing in axenic culture
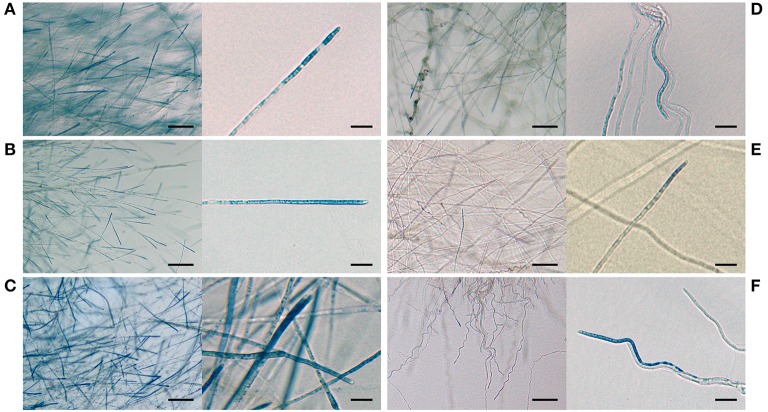
Synthesis of ROS is essential for mutualism in the E. festucae Fl1/L. perenne interaction, and disruption results in hyper-colonization of host tissues, stunting and premature leaf senescence (Tanaka et al., 2006, 2008). In order to determine whether the morphology of the E. festucae ΔacyA disruption mutants was linked to changes in ROS production, strains were grown in culture on microscope slides and stained with NBT. NBT forms a blue precipitate on exposure to superoxide ions, and blue deposits were typically observed in the hyphal apices of the E. festucae Fl1 wild-type (Figure 4) and occasionally in some compartments behind the tip (data not shown). NBT staining of E. festucae acyA disruption mutants revealed a substantial reduction in superoxide radicals relative to the wild-type strain (Figure 4). The localization of superoxide radicals was similar between the wild-type and mutants, however superoxide ions were not detectable in the majority of mutant hyphae (Figure 4). Strains complemented with the wild-type acyA gene were able to produce ROS at similar or higher levels than the wild-type confirming that cAMP signaling directly or indirectly regulates accumulation of superoxide ions in E. festucae.
Figure 4.
Influence of acyA disruption on production of ROS in E. festucae Fl1 hyphae in axenic culture. Bright field images of E. festucae Fl1 growing on PDA at 22°C for 7 days and stained with NBT. Included is a low (scale bar = 50 μm) and high (scale bar = 5 μm) resolution image of the wild-type (A) plus mutants ΔacyA34 (D), Δ42 (E), and Δ47 (F), and complementation strains ΔacyA34/acyA (B) and ΔacyA42/acyA (C).
Regulation of E. festucae growth by cAMP during colonization of L. perenne
To determine the role of fungal cAMP signaling in E. festucae during mutualistic interactions with L. perenne, mycelia of strains carrying the disrupted gene were inoculated though a small incision into young seedlings. In each inoculation experiment, between 25 and 50% of inoculated plants (n = 25) became infected with wild-type, ΔacyA34, ΔacyA42, and the complementation strains, however despite repeated attempts (n > 75 plants), no plants infected with the original E. festucae ΔacyA47 strain were obtained. It is unclear why this strain was apparently incapable of host colonization since the acyA locus was identical to ΔacyA34 and ΔacyA42. Inoculation of plants with E. festucae ΔacyA47var produced infected plants with similar frequency to the other strains. This strain was not included in further experiments due to its unstable phenotype.
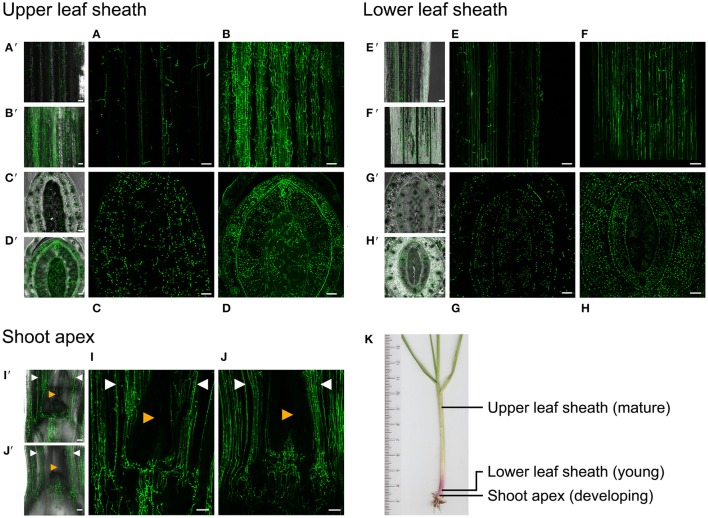
We next investigated whether a functional fungal cAMP signaling pathway is required for a mutualistic interaction between the symbionts and the host grass. Three to five independent plants for each strain were examined. Consistent differences in the growth and phenotype of plants infected with wild-type, ΔacyA, or Δacy/acyA complemented strains were not observed, although plants infected with mutant strains did express a variable marginally-stunted phenotype (Supplementary Figure 2). After 3 months, hyphae within the mature leaf sheaths of infected plants were stained with aniline blue and analyzed by bright field microscopy. Wild-type hyphae in this tissue were long and straight, seldom branched, and oriented in the direction of leaf growth. In contrast, hyphae of the two independent acyA mutants were highly branched and convoluted (Figure 5). The phenotypes of ΔacyA34 and 42 in all the plants examined (at least three plants per strain per experiment) were highly consistent. The complementation strains ΔacyA34/acyA and ΔacyA42/acyA both resembled the wild type (Figure 5). To further investigate the role of cAMP signaling in endophyte colonization of the host at different developmental stages, we transformed ΔacyA42 with vector pTEFEGFP (for constitutive EGFP expression in E. festucae) and inoculated EGFP-expressing strains into L. perenne seedlings for examination by confocal microscopy. Wild-type E. festucae Fl1 transformed with the same plasmid was used as a control. An EGFP-expressing acyA-complemented strain was not included in this experiment as it was not technically feasible to conduct a third transformation on this mutant strain. We first examined the hyphae in longitudinal sections taken through the shoot apex at the base of the tiller. In host tissues immediately below the meristem, and in the youngest developing leaves, the mycelial density of ΔacyA42/EGFP was similar to wild-type controls (Figures 6I,J), however in young leaf sheaths above the shoot meristem (lower leaf sheath), hyphae of the mutant strain appeared more numerous than wild-type (Figures 6E–H). A key feature of E. festucae-L. perenne mutualism is that once host tissues are mature and have stopped expanding, colonizing endophytes also cease growing (Tan et al., 2001). To determine whether E. festucae ΔacyA42/EGFP was still capable of responding to host developmental cues, and to stop growing, epidermal peels from mature (upper) leaf sheaths were examined. Contrary to the uniform appearance of wild-type hyphae in this tissue, disruption of the cAMP signaling pathway resulted in a dense and heavily-branched mycelium (Figures 6A–D). To confirm the microscopy observations, the biomass of the wild-type and ΔacyA42 strains in the pseudostem of these plants was determined by quantitative PCR of a single copy E. festucae gene. Hyphal biomass (expressed as endophyte concentration) was an average of 2081 (SE 222.7) gene copies per ng of plant and endophyte genomic DNA for wild-type, and 3949 (SE 226.1) for the ΔacyA42 mutant, 1.9 fold higher than wild-type (Figure 7). The means of the technical replicates for each biological replicate per strain were compared using the Student's T-test. The T-test indicated that the mean hyphal biomass of the mutant strain was significantly greater than the wild-type (P = 0.004).
Figure 5.
Morphology of E. festucae ΔacyA in L. perenne. Aniline blue-stained hyphae (arrows) in the mature second leaf sheath of L. perenne infected with the wild-type and strains ΔacyA34 and ΔacyA42. The morphology of the mutant strains after complementation (ΔacyA34/acyA and ΔacyA42/acyA) with the wild-type gene is also shown. All images are at the same magnification. The scale bar is 5 μm.
Figure 6.
Disruption of E. festucae acyA deregulates hyphal branching during host colonization. (A–J). Confocal laser scanning micrographs (CLSM) of E. festucae hyphae at three stages of host development. L. perenne leaves infected with the wild-type and disruption mutant ΔacyA42 are shown. Both strains are transformed with plasmid pTEFEGFP and constitutively express EGFP. (A′–J′), CSLM overlaid on corresponding phase contrast images of the same field of view. (K) L. perenne tiller showing the positions at which the tissue sections were taken. A/B, CLSM of a longitudinal section of the mature upper leaf sheath of the pseudostem 3.5 cm above the crown (base) of the tiller showing colonization by WT and ΔacyA42 hyphae respectively. (C,D) CLSM of a transverse section though the tiller at the same position as (A,B). The youngest developing leaf is in the center of the section and is enclosed by progressively older leaf sheaths. (E,F) CLSM of a longitudinal section of a young developing leaf sheath immediately above the crown of the tiller. (G,H) CLSM of a transverse section through the pseudostem at the same position as (E,F). (I,J) CSLM of a longitudinal section through the crown showing the position of hyphae relative to the shoot apex. The shoot apex (red arrow) is a small dome of overlapping leaf primordia visible only in the phase contract images (I′, J′). Hyphae are either not detectable in this tissue by confocal microscopy or are not present. Hyphae in the youngest leaves emerging from the shoot apex are marked by white arrows.
Figure 7.
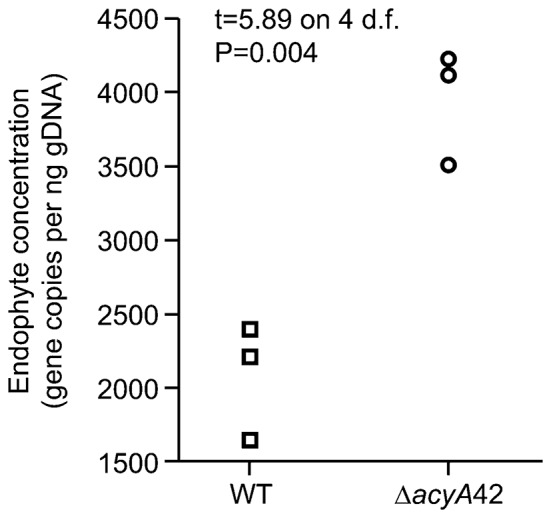
Effects of acyA disruption on biomass of E. festucae in host grasses. Comparison of hyphal biomass in the pseudostem (the region between the crown and the first ligule of a tiller) of plants infected with either the wild-type or ΔacyA42 mutant strains. Hyphal biomass (expressed as endophyte concentration) was determined by quantitative PCR of a single copy gene from 1 ng of genomic DNA. Hyphal biomass between strains was compared using the Students' T-test. The means of the two treatments were significantly different (P = 0.004).
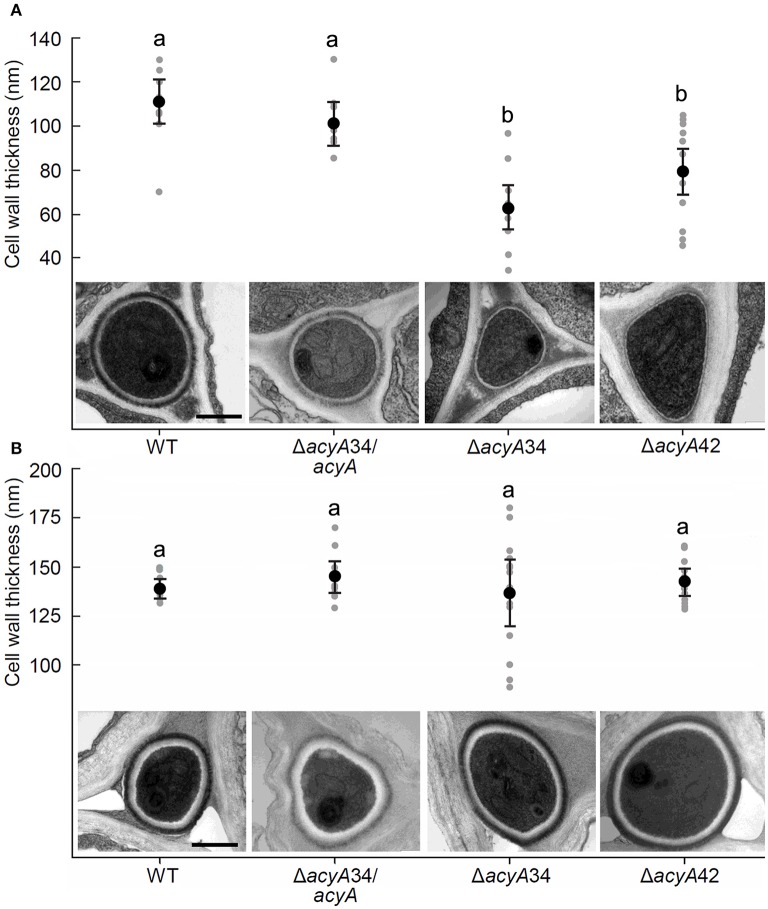
The impact of acyA disruption on hyphal ultrastructure in planta was investigated by TEM of infected L. perenne tillers. Tillers are comprised of bundles of leaves ranging in development from the most immature (in the middle of the tiller) to the fully mature outer leaf sheath. Transverse sections through the base of tillers were fixed and embedded. Ultra-thin sections of tillers infected with wild-type, ΔacyA34, ΔacyA42, and the ΔacyA34/acyA complementation strains were stained with osmium tetroxide and examined by TEM. The thickness of the cell wall was measured at eight equidistant positions around 12 hyphae from the immature leaf blade (Figure 8A) and the second fully mature leaf sheath (Figure 8B) of each tiller. In immature leaves, where both the plant and endophyte grow by intercalary growth, mean cell wall thickness of ΔacyA34 and ΔacyA42 was 70.3 and 62.8 nm respectively, significantly thinner (P ≤ 0.05) than the wild-type which had an average thickness of 111.3 nm (Figure 8A). The phenotype was nearly fully restored in the ΔacyA34/acyA complemented strain which had a mean cell wall thickness of 101.04 nm, indicating that cAMP signaling positively regulates cell wall biogenesis during intercalary growth. Conversely, in the fully developed leaf sheath where plant and fungal cells are no longer growing, there were no significant differences in cell wall thickness between any of the strains (Figure 8B). Cell walls of wild-type E. festucae hyphae in the leaf sheath were thicker than those in the immature leaf (139 nm vs. 111.3 nm respectively, P ≤ 0.001) confirming previous reports of thinner cell walls in E. festucae Fl1 hyphae in developing vs. mature host tissues (Christensen et al., 2008).
Figure 8.
Regulation of hyphal cell wall synthesis by cAMP during leaf colonization. The cell wall of 12 hyphae each from developing leaves (A) and mature second leaf sheaths (B) of L. perenne was measured at eight positions around each hypha. The gray symbols represent the average cell wall thickness of each hypha. The overall mean cell wall thickness and its 95% confidence interval is presented for each strain. Analysis of variance was used to compare the strains. Within each plant tissue (A,B), different letters indicate means that are different at the 5% significance level. Transmission electron micrographs show representative hyphae of E. festucae wild-type, mutants ΔacyA34, and ΔacyA42, and the complementation strain ΔacyA34/acyA strain in the two host tissue types. All images are at the same magnification. The scale bar is 500 nm.
Discussion
In this study we tested the hypothesis that cAMP signaling is required for regulated growth of the mutualistic fungal endophyte E. festucae in host grasses, and for compatibility between the host and endophyte. Our data show that disruption of the acyA gene severely reduced E. festucae radial colony growth in axenic culture, however this growth defect did not affect initial plant infection processes. The acyA mutant strains were hyper-branched in culture and unable to accumulate superoxide radicals. When infecting plants, the mycelia became progressively more dense compared to wild-type as plant tissues aged. Disruption of cAMP synthesis therefore disrupted the ability of the endophyte to grow in synchrony with developing leaves, an attribute which prevents overgrowth of the host during symbiosis.
The reduction in colony radial growth rate of E. festucae acyA mutants is consistent with deletion of the AC gene in plant pathogenic fungi such as F. verticillioides (Choi and Xu, 2010), F. fujikuroi (García-Martínez et al., 2012), F. proliferatum (Kohut et al., 2010) and Botrytis cinerea (Klimpel et al., 2002). Complementation of the mutant phenotype with cAMP confirmed that the relatively slow radial growth rate in the mutants was due to cAMP depletion. Significantly, strains ΔacyA34 and ΔacyA42 had similar radial growth rates in the absence of exogenous cAMP, while ΔacyA47 grew at a faster rate despite having an identical acyA disruption locus. Similar spontaneous revertants have been obtained from adenylate cyclase MAC1 mutants of Magnaporthe oryzae (Adachi and Hamer, 1998) and cr-1 mutants of Neurospora crassa (Garnjobst and Tatum, 1970). This phenomenon has also been reported in B. cinerea where the slow radial growth rate of the AC mutants reverted to wild-type levels over time (Klimpel et al., 2002). The reason for this phenomenon was not investigated in our study but is speculated to be due to the accumulation of suppressor mutations that complement the mutant growth phenotype. By-pass suppressors of the MAC1 (AC) phenotype (sum) that fully restore growth and morphogenesis in M. oryzae have been identified (Adachi and Hamer, 1998). One such mutation in the PKA catalytic subunit (sum1–99) alters a conserved amino acid in the cAMP binding domain (Adachi and Hamer, 1998). As shown here and reported elsewhere, the fungal cAMP signaling pathway is highly responsive to AC deletion/disruption and perturbations in cAMP synthesis. Suppressor mutations mask AC deletion phenotypes and confound the interpretation of cAMP signaling experiments, potentially contributing to the diversity in morphology and growth rates reported in different fungi.
Microscopic examination of E. festucae ΔacyA strains in culture revealed that the cAMP signaling pathway plays a significant role in colony architecture. Mutant hyphae were aggregated, convoluted and hyper-branched. The cAMP cascade therefore enforces apical dominance in this species by preventing development of new hyphal branch points near the apex. The morphology of the acyA mutant colonies quite closely resembled the E. festucae small GTPase racA mutant (Tanaka et al., 2008) which also had a slower growth rate compared to the wild-type, had convoluted hyphae, produced lateral branches with higher frequency, and formed branches at atypical sites. Notably, similarly to the E. festucae ΔacyA phenotype, hyphal morphology and colony size in the ΔracA reverted to wild-type over time in culture (Kayano et al., 2013). RacA is a small GTPase of the Rho subfamily involved in hyphal growth and morphogenesis (Zhang et al., 2013). Production of superoxide from molecular oxygen by the plasma membrane-localized NADPH oxidase complex is important in regulating polar growth in E. festucae as exemplified by deletion of several genes encoding proteins of the NADPH oxidase complex (Takemoto et al., 2007; Scott and Eaton, 2008; Eaton et al., 2011; Tanaka et al., 2012), noxA (Tanaka et al., 2006), noxR (Takemoto et al., 2006), racA (Tanaka et al., 2008), and bemA (Takemoto et al., 2011). E. festucae RacA GTPase binds to NoxR, a regulator of NADPH oxidase, and activates the NADPH oxidase complex that synthesizes superoxide (Tanaka et al., 2008). A further similarity in morphology between the E. festucae racA and acyA mutants in culture was the absence of ROS in hyphal tips. In the strains complemented with the wild-type acyA gene, superoxide levels were the same as, or more concentrated than wild-type, confirming that cAMP signaling positively regulates superoxide accumulation in E. festucae. This result contrasts with the E. festucae Fl1 sakA mutant where deletion of the stress-activated mitogen-activated protein (MAP) kinase resulted in elevated H2O2, both in culture and in plants (Eaton et al., 2008, 2010) suggesting the stress-activated MAP kinase and cAMP pathways play opposing roles in ROS regulation. Transcriptomics analysis of the sakA mutant demonstrated that genes for 27 ROS decomposition enzymes such as peroxidases and catalases are also more highly expressed in relation to the wild-type, possibly in response to the elevated ROS produced by this mutant (Eaton et al., 2010). Regulation of ROS by cAMP has also been reported in C. albicans where cAMP negatively regulates oxidative stress response genes such as MCR1 (cytochrome b5 reductase), SOD2 (Mn superoxide dismutase), HSP12 (heat shock protein) and CCP1 (cytochrome c peroxidase) (Bahn et al., 2007). Likewise, repression of the Ras-cAMP-PKA cascade by farnesol (a small signaling molecule) results in up-regulation of catalase and superoxide dismutase, with a consequential increase in resistance against oxidative stress (Deveau et al., 2010). Increased resistance to H2O2 has also been observed in F. proliferatum after deletion of FpacyA (Kohut et al., 2010). Despite similarities between racA and acyA mutants in morphology and ROS regulation during saprotrophic growth, deletion of noxA, noxA/noxB, noxR, or bemA in E. festucae results in only a slight reduction in colony growth on PDA and no marked effects on hyphal morphology (Tanaka et al., 2006; Kayano et al., 2013) indicating that the substantial reduction in radial colony growth rate and the hyper-branched phenotype of the acyA mutants on PDA is therefore not mediated through ROS but through other, yet unidentified, mechanisms. This is also true for traits associated with apical dominance in other fungi where apical dominance and polar growth is not overcome by disruption of NADPH oxidase genes when growing on enriched media (Scott and Eaton, 2008; Semighini and Harris, 2008). In E. festucae noxA, noxA/noxB, and noxR (but not bemA) are critical for regulation of apical dominance and hyphal organization during growth on water agar suggesting that ROS regulation of hyphal branching is more important in nutrient limited conditions (Kayano et al., 2013).
The cAMP signaling cascade regulates key processes in fungal pathogenesis, and in most pathogenic fungi AC deletion attenuates or eliminates virulence in animal and plant pathogens (Choi and Dean, 1997; Klimpel et al., 2002; Kohut et al., 2010), although others report the virulence unchanged, as in F. fujikuroi (ΔacyA) on tomato (García-Martínez et al., 2012). In contrast with most fungal pathogens, AC was not required for establishment of a stable symbiosis between E. festucae and L. perenne. Only ΔacyA34 and 42 were competent to form a symbiosis, and ΔacyA47 did not appear to be infectious, despite growing more rapidly in culture as mentioned above. The spontaneous growth revertant, ΔacyA47var, was capable of forming compatible interactions with host plants. We speculate that this change in infectivity was also due to suppressor mutation(s). The hyphae of acyA mutants in plants were hyper-branched in mature host tissues, suggesting that the endophytes produced many more hyphal apices in plants, as they did in culture. This phenotype largely reverted to wild-type in strains complemented with the functional acyA gene. E. festucae is predominantly a foliar symbiont, and infects aerial plant tissues as they are developing on the host SAM at the base of the plant. The youngest host (and endophyte) tissues are those nearest the SAM while the older tissues are those furthest from it (the tips of the leaves for example). Hyphae were visible between meristematic cells at the base of developing leaves, and morphology and distribution of ΔacyA42/EGFP appeared similar to the wild-type in this very young tissue. However hyphal biomass appeared to increase at each successive stage in leaf development examined. This contrasts with the wild-type colonization process where branching is mostly confined to hyphae colonizing meristematic host tissues, and is quite tightly constrained during intercalary growth in the host expansion zone (Christensen et al., 2008).
It is not possible to deduce whether breakdown in regulated control of hyphal biomass during host colonization through cAMP disruption was a consequence of lower ROS levels as this was not tested directly. However, E. festucae strains with deletions in components of the NADPH oxidase complex exhibit similar hyper-colonization phenotypes (Takemoto et al., 2006, 2011; Tanaka et al., 2006, 2008) suggesting that changes in ROS accumulation should be considered as one potential contributor to the E. festucae cAMP disruption phenotype in plants. Another E. festucae Fl1 mutant (ΔsidN, deficient in the biosynthesis of the iron-chelating siderophore epichloënin A), also phenocopies the defective polarized growth of the E. festucae racA and acyA deletion mutants, but under iron depleted conditions (Johnson et al., 2013b). Deletion of ΔsidN in E. festucae reduced racA transcripts between 2.2 and 3.3 fold in infected plants and the mutants also over colonized the host (Johnson et al., 2013b). Iron homeostatic regulation of the NADPH oxidase complex is therefore another layer of complexity potentially involved in ROS regulation of hyphal branching. Whether there are direct interactions between the NADPH oxidase complex, racA, iron homeostasis and the cAMP pathway remains to be determined. Unlike the other acyA phenotypes which revert to wild-type over time in culture, ROS suppression in acyA deletion mutants was highly stable. Cyclic AMP may therefore exert its effect on ROS accumulation through a different downstream mechanism to those used to regulate other traits such as colony growth and hyphal morphology.
The hyphal walls of the E. festucae ΔacyA mutants were significantly thinner compared to wild-type or acyA-complemented mutant strains, however this was only the case for hyphae in developing host tissues (where the endophytes grow by intercalary extension). This suggests that, while cell wall growth is slower in the acyA mutants vs. the wild-type, the differential is sufficiently small to enable them to maintain cell walls of similar thickness once hyphal and plant growth has ceased (in mature leaves). These data indicate that, similar to the wild-type, cell wall synthesis in acyA mutant strains also continues after extension growth has ceased.
The mutant strains had a small but variable impact on the morphology of host plants (Supplementary Figure 2). In contract to the results presented here, excessive hyphal branching by E. festucae mutants in plants almost always induces abnormalities in host morphology, such as stunted tillers (Takemoto et al., 2006, 2011; Tanaka et al., 2008; Eaton et al., 2010; Johnson et al., 2013b; Green et al., 2016). We assume that the acyA mutants did not stunt the host because hyphal distribution in the shoot apex was similar to the wild-type. The architecture of grasses is largely regulated by this tissue (where the leaves, inflorescences and tillers are differentiated) and competition for resources, hyphal overgrowth or disruption of host cell organization at this critical stage in development is likely to have a negative impact on host morphology. Since the distribution of hyphae in the shoot apex has not been investigated for many E. festucae mutants the discrepancy between hyphal branching and host morphology disturbance is unresolved.
Most fungi contain a single AC gene, however ancestral interspecific hybridizations between different Epichloë species have generated a number of strains with additional genomes (Schardl et al., 1994; Kuldau et al., 1999). Analyses of allopolyploids AR3046, and AR1006 (Craven et al., 2001; Moon et al., 2004) indicates that these strains each have two homeologs of acyA. A report describing the consequences of genomic and transcriptomics shock in a third relatively recent natural Epichloë hybrid (Lp1) suggests that both homeologs of most genes in the parental strains are also retained in this hybrid, and that there is little evidence for higher expression levels in one homeolog vs. the other (Cox et al., 2014). Similarly, in AR1006 one of the acyA genes was truncated and is likely a pseudogene, while in AR3046 both genes appear functional. Allopolyploids are often more competitive than their parental progenitors (Cox et al., 2014) and duplicate copies of key signaling pathway genes may, if both are retained, provide allopolyploid E. festucae strains (all asexual) resilience against mutations or allow for the evolution of new functions.
In summary, the cAMP cascade regulates saprotrophic growth of E. festucae in culture, with a role in enforcing the dominance of the hyphal apex and restricting development of lateral branches. Although ROS accumulation in E. festucae on PDA is positively regulated through cAMP, depletion of ROS alone cannot account for the hyper-branched phenotype in E. festucae ΔacyA as ROS synthesis mutants of E. festucae (ΔracA excepted) have a wild type phenotype when growing on PDA. In plants, wild-type E. festucae hyphal growth is tightly regulated and hyphal overgrowth is often observed in antagonistic Epichloë-L. perenne symbioses, such as those with disruptions in ROS synthesis (Takemoto et al., 2006; Tanaka et al., 2006). E. festucae ΔacyA mutants produce progressively more hyphae in host leaves as they age, presumably through the continued production of new hyphal tips that are normally restricted in wild type strains. Cyclic AMP is therefore critical in restricting potential overgrowth of the symbiont during colonization of developing host tissues, and in regulating the synchronicity in growth of the two organisms.
Author contributions
CV, RJ, GB, LJ, and JK conceived the ideas for the study. All the authors participated in data analysis and interpretation, and contributed to the writing and editing of the manuscript. CV, MC, LJ, NF, SC, MG and WS contributed to the experimentation.
Funding
This research was supported by Core Funding from AgResearch Ltd., and the New Zealand Ministry of Business and Primary Industries (to CV).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Kelly Dunstan, Sophie Borchert, Charlotte Gaborit, Jennifer Pratt, Catherine Tootle, Nadia Houssain and Lydia Koolaard for technical services, Pauline Hunt for preparation of the figures, and Rosemary Van Essen and Joy Dick for library services (all AgResearch Ltd., Grasslands Research Centre, New Zealand). Transmission electron microscopy services were provided by Jianyu Chen and Jordan Taylor (Manawatu Microscopy and Imaging Centre, Massey University, New Zealand). We also gratefully acknowledge Professor Chris Schardl (University of Kentucky) for providing generous early access to the genome sequences of E. festucae and other fungi of the Clavicipitaceae.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01546/full#supplementary-material
References
- Adachi K., Hamer J. E. (1998). Divergent cAMP signaling pathways regulate growth and pathogenesis in the rice blast fungus Magnaporthe grisea. Plant Cell Online 10, 1361–1373. 10.1105/tpc.10.8.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh J. A., Pukkila-Worley R., Harashima T., Cavallo L. M., Funnell D., Cox G. M., et al. (2002). Adenylyl cyclase functions downstream of the G alpha protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell 1, 75–84. 10.1128/EC.1.1.75-84.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arachevaleta M., Bacon C. W., Hoveland C. S., Radcliffe D. E. (1989). Effect of the tall fescue endophyte on plant response to environmental stress. Agron. J. 81, 83–90. 10.2134/agronj1989.00021962008100010015x [DOI] [Google Scholar]
- Bacon C. W., Porter J. K., Robbins J. D., Luttrell E. S. (1977). Epichloë typhina from toxic tall fescue grasses. Appl. Environ. Microbiol. 34, 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn Y.-S., Molenda M., Staab J. F., Lyman C. A., Gordon L. J., Sundstrom P. (2007). Genome-wide transcriptional profiling of the cyclic AMP-Dependent signaling pathway during morphogenic transitions of Candida albicans. Eukaryot. Cell 6, 2376–2390. 10.1128/EC.00318-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn Y. S., Mühlschlegel F. A. (2006). CO2 sensing in fungi and beyond. Curr. Opin. Microbiol. 9, 572–578. 10.1016/j.mib.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Bahn Y. S., Sundstrom P. (2001). CAP1, an adenylate cyclase-associated protein gene, regulates bud-hypha transitions, filamentous growth, and cyclic AMP levels and is required for virulence of Candida albicans. J. Bacteriol. 183, 3211–3223. 10.1128/JB.183.10.3211-3223.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. A., Kelly J. M. (2004). Structure, function and evolution of microbial adenylyl and guanylyl cyclases. Mol. Microbiol. 52, 1229–1242. 10.1111/j.1365-2958.2004.04067.x [DOI] [PubMed] [Google Scholar]
- Ball O. J.-P., Prestidge R. A. (1993). Endophyte-associated alkaloids, insect resistance and animal disorders: an interrelated complex. N.Z. Vet. J. 41, 216. [Google Scholar]
- Bormann J., Boenisch M. J., Brückner E., Firat D., Schäfer W. (2014). The adenylyl cyclase plays a regulatory role in the morphogenetic switch from vegetative to pathogenic lifestyle of Fusarium graminearum on wheat. PLoS ONE 9:e91135. 10.1371/journal.pone.0091135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakhage A., Liebmann B. (2005). Aspergillus fumigatus conidial pigment and cAMP signal transduction: significance for virulence. Med. Mycol. 43, S75–S82. 10.1080/13693780400028967 [DOI] [PubMed] [Google Scholar]
- Byrd A. D., Schardl C. L., Songlin P. J., Mogen K. L., Siegel M. R. (1990). The β-tubulin gene of Epichloë typhina from perennial ryegrass (Lolium perenne). Curr. Genet. 18, 347–354. 10.1007/BF00318216 [DOI] [PubMed] [Google Scholar]
- Card S. D., Faville M. J., Simpson W. R., Johnson R. D., Voisey C. R., de Bonth A. C. M., et al. (2014). Mutualistic fungal endophytes in the Triticeae–survey and description. FEMS Microbiol. Ecol. 88, 94–106. 10.1111/1574-6941.12273 [DOI] [PubMed] [Google Scholar]
- Chen D., Janganan T. K., Chen G., Marques E. R., Kress M. R., Goldman G. H., et al. (2007). The cAMP pathway is important for controlling the morphological switch to the pathogenic yeast form of Paracoccidioides brasiliensis. Mol. Microbiol. 65, 761–779. 10.1111/j.1365-2958.2007.05824.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W., Dean R. A. (1997). The adenylate cyclase gene MAC1 of Magnaporthe grisea controls appressorium formation and other aspects of growth and development. Plant Cell 9, 1973–1983. 10.1105/tpc.9.11.1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.-E., Xu J. -R. (2010). The cAMP Signaling pathway in Fusarium verticillioides is important for conidiation, plant infection, and stress responses but not fumonisin production. Mol. Plant Microbe Interact. 23, 522–533. 10.1094/MPMI-23-4-0522 [DOI] [PubMed] [Google Scholar]
- Christensen M. J., Bennett R. J., Ansari H. A., Koga H., Johnson R. D., Bryan G. T., et al. (2008). Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves. Fungal Genet. Biol. 45, 84–93. 10.1016/j.fgb.2007.07.013 [DOI] [PubMed] [Google Scholar]
- Christensen M. J., Voisey C. R. (2009). Tall Fescue-endophyte symbiosis, in Tall Fescue for the Twenty-first Century. Agronomy Monologue, Vol 53, eds Fribourg H. A., Hannaway D. B, West C. P. (Madison, WI: Book and Multimedia Publishing; ), 251–272. [Google Scholar]
- Christensen M., Leuchtmann A., Rowan D., Tapper B. (1993). Taxonomy of Acremonium endophytes of tall fescue (Festuca arundinacea), meadow fescue (F. pratensis), and perennial rye-grass (Lolium perenne). Mycol. Res. 97, 1083–1092. 10.1016/S0953-7562(09)80509-1 [DOI] [Google Scholar]
- Cox M. P., Dong T., Shen G., Dalvi Y., Scott D. B., Ganley A. R. D. (2014). An interspecific fungal hybrid reveals cross-kingdom rules for allopolyploid gene expression patterns. PLoS Genet. 10:e1004180. 10.1371/journal.pgen.1004180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven K. D., Blankenship J. D., Leuchtmann A., Hignight K., Schardl C. L. (2001). Hybrid fungal endophytes symbiotic with the grass Lolium pratense. Sydowia 53, 44–73. [Google Scholar]
- Deveau A., Piispanen A. E., Jackson A. A., Hogan D. A. (2010). Farnesol induces hydrogen peroxide resistance in Candida albicans yeast by inhibiting the Ras-Cyclic AMP signaling pathway. Eukaryot. Cell 9, 569–577. 10.1128/EC.00321-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza C. A., Heitman J. (2001). Conserved cAMP signaling cascades regulate fungal development and virulence. Fems Microbiol. Rev. 25, 349–364. 10.1111/j.1574-6976.2001.tb00582.x [DOI] [PubMed] [Google Scholar]
- Eaton C. J., Cox M. P., Ambrose B., Becker M., Hesse U., Schardl C. L., et al. (2010). Disruption of signaling in a fungal-grass symbiosis leads to pathogenesis. Plant Physiol. 153, 1780–1794. 10.1104/pp.110.158451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton C. J., Cox M. P., Scott B. (2011). What triggers grass endophytes to switch from mutualism to pathogenism? Plant Sci. 180, 190–195. 10.1016/j.plantsci.2010.10.002 [DOI] [PubMed] [Google Scholar]
- Eaton C. J., Jourdain I., Foster S. J., Hyams J. S., Scott B. (2008). Functional analysis of a fungal endophyte stress-activated MAP kinase. Curr. Genet. 53, 163–174. 10.1007/s00294-007-0174-6 [DOI] [PubMed] [Google Scholar]
- Fleetwood D. J., Scott D. B., Lane G. A., Tanaka A., Johnson R. D. (2007). A Complex ergovaline gene cluster in Epichloë endophytes of grasses. Appl. Environ. Microbiol. 73, 2571–2579. 10.1128/AEM.00257-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher L., Owens R. A., Dolan S. K., O'Keeffe G., Schrettl M., Kavanagh K., et al. (2012). The Aspergillus fumigatus Protein GliK protects against oxidative stress and is essential for gliotoxin biosynthesis. Eukaryot. Cell 11, 1226–1238. 10.1128/EC.00113-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Martínez J., Ádám A. L., Avalos J. (2012). Adenylyl cyclase plays a regulatory role in development, stress resistance and secondary metabolism in Fusarium fujikuroi. PLoS ONE 7:e28849. 10.1371/journal.pone.0028849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnjobst L., Tatum E. L. (1970). New crisp genes and crisp-modifiers in Neurospora crassa. Genetics 66, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. A., Becker Y., Fitzsimons H. L., Scott B. (2016). An Epichloë festucae homologue of MOB3, a component of the STRIPAK complex, is required for the establishment of a mutualistic symbiotic interaction with Lolium perenne. Mol. Plant Pathol. 10.1111/mpp.12443. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Johnson R., Scott B. (1994). Integrative transformation of the mycotoxin-producing fungus, Penicillium paxilli. Curr. Genet. 25, 508–513. 10.1007/BF00351670 [DOI] [PubMed] [Google Scholar]
- Ivey F. D., Hoffman C. S. (2005). Direct activation of fission yeast adenylate cyclase by the Gpa2 Gα of the glucose signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 102, 6108–6113. 10.1073/pnas.0502270102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. J., De Bonth A. C. M., Briggs L. R., Caradus J. R., Finch S. C., Fleetwood D. J., et al. (2013a). The exploitation of epichloae endophytes for agricultural benefit. Fungal Divers 60, 171–188. 10.1007/s13225-013-0239-4 [DOI] [Google Scholar]
- Johnson L. J., Koulman A., Christensen M., Lane G. A., Fraser K., Forester N., et al. (2013b). An extracellular siderophore is required to maintain the mutualistic interaction of Epichloë festucae with Lolium perenne. PLoS Pathog. 9:e1003332. 10.1371/journal.ppat.1003332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Voisey C., Johnson L., Pratt J., Fleetwood D., Khan A., et al. (2007). Distribution of NRPS gene families within the Neotyphodium/Epichloë complex. Fungal Genet. Biol. 44, 1180–1190. 10.1016/j.fgb.2007.04.009 [DOI] [PubMed] [Google Scholar]
- Kamerewerd J., Jansson M., Nowrousian M., Pöggeler S., Kück U. (2008). Three α-subunits of heterotrimeric G proteins and an adenylyl cyclase have distinct roles in fruiting body development in the homothallic fungus Sordaria macrospora. Genetics 180, 191–206. 10.1534/genetics.108.091603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayano Y., Tanaka A., Akano F., Scott B., Takemoto D. (2013). Differential roles of NADPH oxidases and associated regulators in polarized growth, conidiation and hyphal fusion in the symbiotic fungus Epichloë festucae. Fungal Genet. Biol. 56, 87–97. 10.1016/j.fgb.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimpel A., Gronover C. S., Williamson B., Stewart J. A., Tudzynski B. (2002). The adenylate cyclase (BAC) in Botrytis cinerea is required for full pathogenicity. Mol. Plant Pathol. 3, 439–450. 10.1046/j.1364-3703.2002.00137.x [DOI] [PubMed] [Google Scholar]
- Kohut G., Oláh B., Ádám A. L., García-Martínez J., Hornok L. (2010). Adenylyl cyclase regulates heavy metal sensitivity, bikaverin production and plant tissue colonization in Fusarium proliferatum. J. Basic Microbiol. 50, 59–71. 10.1002/jobm.200900113 [DOI] [PubMed] [Google Scholar]
- Kuldau G. A., Tsai H.-F., Schardl C. L. (1999). Genome sizes of Epichloë species and anamorphic hybrids. Mycologia 91, 776–782. 10.2307/3761531 [DOI] [Google Scholar]
- Kulkarni R. D., Dean R. A. (2004). Identification of proteins that interact with two regulators of appressorium development, adenylate cyclase and cAMP-dependent protein kinase A, in the rice blast fungus Magnaporthe grisea. Mol. Genet. Genomics 270, 497–508. 10.1007/s00438-003-0935-y [DOI] [PubMed] [Google Scholar]
- Latch G. C. M., Christensen M. T. (1985). Artificial infection of grasses with endophytes. Ann. Appl. Biol. 107, 17–24. 10.1111/j.1744-7348.1985.tb01543.x [DOI] [Google Scholar]
- Leuchmann A., Bacon C. W., Schardl C. L., White J. F., JR., Tadych M. (2014). Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia 106, 202–215. 10.3852/13-251 [DOI] [PubMed] [Google Scholar]
- Leuchtmann A. (1994). Isozyme relationships of Acremonium endophytes from twelve Festuca species. Mycol. Res. 98, 25–33. 10.1016/S0953-7562(09)80331-6 [DOI] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., et al. (2009). The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Parsons A. J., Xue H., Fraser K., Ryan G. D., Newman J. A., et al. (2011). Competition between foliar Neotyphodium lolii endophytes and mycorrhizal Glomus spp. fungi in Lolium perenne depends on resource supply and host carbohydrate content. Funct. Ecol. 25, 910–920. 10.1111/j.1365-2435.2011.01853.x [DOI] [Google Scholar]
- Liu S., Peng G., Xia Y. (2012). The adenylate cyclase gene MaAC is required for virulence and multi-stress tolerance of Metarhizium acridum. BMC Microbiol 12:163. 10.1186/1471-2180-12-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukito Y., Chujo T., Scott B. (2015). Molecular and cellular analysis of the pH response transcription factor PacC in the fungal symbiont Epichloë festucae. Fungal Genet. Biol. 85, 25–37. 10.1016/j.fgb.2015.10.008 [DOI] [PubMed] [Google Scholar]
- Malinowski D. P., Belesky D. P. (2000). Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci. 40, 923–940. 10.2135/cropsci2000.404923x [DOI] [Google Scholar]
- Martínez-Espinoza A. D., Ruiz-Herrera J., León-Ramírez C. G., Gold S. E. (2004). MAP kinase and cAMP signaling pathways modulate the pH-induced yeast-to-mycelium dimorphic transition in the corn smut fungus Ustilago maydis. Curr. Microbiol. 49, 274–281. 10.1007/s00284-004-4315-6 [DOI] [PubMed] [Google Scholar]
- McDonough K. A., Rodriguez A. (2012). The myriad roles of cyclic AMP in microbial pathogens, from signal to sword. Nat. Rev. Microbiol. 10, 27–38. 10.1038/nrmicro2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen E. G., Janbon G., Chaloupka J., Steegborn C., Man S. F., Moyrand F., et al. (2006). Cryptococcus neoformans senses CO2 through the carbonic anhydrase Can2 and the adenylyl cyclase Cac1. Eukaryot. Cell 5, 103–111. 10.1128/EC.5.1.103-111.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C. D., Craven K. D., Leuchtmann A., Clement S. L., Schardl C. L. (2004). Prevalence of interspecific hybrids amongst asexual fungal endophytes of grasses. Mol. Ecol. 13, 1455–1467. 10.1111/j.1365-294X.2004.02138.x [DOI] [PubMed] [Google Scholar]
- Moon C. D., Tapper B. A., Scott B. (1999). Identification of epichloë endophytes in planta by a microsatellite-based PCR fingerprinting assay with automated analysis. Appl. Environ. Microbiol. 65, 1268–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee M., Mukherjee P. K., Kale S. P. (2007). cAMP signaling is involved in growth, germination, mycoparasitism and secondary metabolism in Trichoderma virens. Microbiology 153, 1734–1742. 10.1099/mic.0.2007/005702-0 [DOI] [PubMed] [Google Scholar]
- Namiki F., Matsunaga M., Okuda M., Inoue I., Nishi K., Fujita Y., et al. (2001). Mutation of an arginine biosynthesis gene causes reduced pathogenicity in Fusarium oxysporum f. sp. melonis. Mol. Plant Microbe Interact. 14, 580–584. 10.1094/MPMI.2001.14.4.580 [DOI] [PubMed] [Google Scholar]
- Punt P. J., Oliver R. P., Dingemanse M. A., Pouwels P. H., van den Hondel C. A. (1987). Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56, 117–124. 10.1016/0378-1119(87)90164-8 [DOI] [PubMed] [Google Scholar]
- Rasmussen S., Parsons A. J., Bassett S., Christensen M. J., Hume D. E., Johnson L. J., et al. (2007). High nitrogen supply and carbohydrate content reduce fungal endophyte and alkaloid concentration in Lolium perenne. New Phytol. 173, 787–797. 10.1111/j.1469-8137.2006.01960.x [DOI] [PubMed] [Google Scholar]
- Schardl C. L., Grossman R. B., Nagabhyru P., Faulkner J. R., Mallik U. P. (2007). Loline alkaloids: currencies of mutualism. Phytochemistry 68, 980–996. 10.1016/j.phytochem.2007.01.010 [DOI] [PubMed] [Google Scholar]
- Schardl C. L., Leuchtmann A., Tsai H. F., Collett M. A., Watt D. M., Scott D. B. (1994). Origin of a fungal symbiont of perennial ryegrass by interspecific hybridization of a mutualist with the ryegrass choke pathogen, Epichloe typhina. Genetics 136, 1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardl C. L., Phillips T. D. (1997). Protective grass endophytes. Where are they from and where are they going? Plant Dis. 81, 430–438. 10.1094/PDIS.1997.81.5.430 [DOI] [PubMed] [Google Scholar]
- Schardl C. L., Young C. A., Hesse U., Amyotte S. G., Andreeva K., Calie P. J., et al. (2013). Plant-symbiotic fungi as chemical engineers: multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet. 9:e1003323. 10.1371/journal.pgen.1003323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardl C. L., Young C. A., Moore N., Krom N., Dupont P.-Y., Pan J., et al. (2014). Chapter ten - genomes of plant-associated clavicipitaceae, in Adv Bot Res, Vol 70, ed Francis M. M. (London: Academic Press; ), 291–327. [Google Scholar]
- Scott B., Eaton C. J. (2008). Role of reactive oxygen species in fungal cellular differentiations. Curr. Opin. Microbiol. 11, 488–493. 10.1016/j.mib.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Semighini C. P., Harris S. D. (2008). Regulation of apical dominance in Aspergillus nidulans hyphae by reactive oxygen species. Genetics 179, 1919–1932. 10.1534/genetics.108.089318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. R., Faville M. J., Moraga R. A., Williams W. M., McManus M. T., Johnson R. D. (2014). Epichloë fungal endophytes and the formation of synthetic symbioses in Hordeeae (=Triticeae) grasses. J. Syst. Evol. 52, 794–806. 10.1111/jse.12107 [DOI] [Google Scholar]
- Simpson W. R., Schmid J., Singh J., Faville M. J., Johnson R. D. (2012). A morphological change in the fungal symbiont Neotyphodium lolii induces dwarfing in its host plant Lolium perenne. Fungal Biol. 116, 234–240. 10.1016/j.funbio.2011.11.006 [DOI] [PubMed] [Google Scholar]
- Studt L., Humpf H.-U., Tudzynski B. (2013). Signaling governed by G proteins and cAMP is crucial for growth, secondary metabolism and sexual development in Fusarium fujikuroi. PLoS ONE 8:e58185. 10.1371/journal.pone.0058185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugui J. A., Pardo J., Chang Y. C., Zarember K. A., Nardone G., Galvez E. M., et al. (2007). Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot. Cell 6, 1562–1569. 10.1128/EC.00141-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto D., Kamakura S., Saikia S., Becker Y., Wrenn R., Tanaka A., et al. (2011). Polarity proteins Bem1 and Cdc24 are components of the filamentous fungal NADPH oxidase complex. Proc. Natl. Acad. Sci. U.S.A. 108, 2861–2866. 10.1073/pnas.1017309108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto D., Tanaka A., Scott B. (2006). A p67Phox-like regulator is recruited to control hyphal branching in a fungal–grass mutualistic symbiosis. Plant Cell 18, 2807–2821. 10.1105/tpc.106.046169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto D., Tanaka A., Scott B. (2007). NADPH oxidases in fungi: diverse roles of reactive oxygen species in fungal cellular differentiation. Fungal Genet. Biol. 44, 1065–1076. 10.1016/j.fgb.2007.04.011 [DOI] [PubMed] [Google Scholar]
- Tan Y. Y., Spiering M. J., Scott V., Lane G. A., Christensen M. J., Schmid J. (2001). In planta regulation of extension of an endophytic fungus and maintenance of high metabolic rates in its mycelium in the absence of apical extension. Appl. Environ. Microbiol. 67, 5377–5383. 10.1128/AEM.67.12.5377-5383.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Christensen M. J., Takemoto D., Park P., Scott B. (2006). Reactive oxygen species play a role in regulating a fungus-perennial ryegrass mutualistic interaction. Plant Cell 18, 2807–2821. 10.1105/tpc.105.039263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Takemoto D., Chujo T., Scott B. (2012). Fungal endophytes of grasses. Curr. Opin. Plant Biol. 15, 462–468. 10.1016/j.pbi.2012.03.007 [DOI] [PubMed] [Google Scholar]
- Tanaka A., Takemoto D., Hyon G.-S., Park P., Scott B. (2008). NoxA activation by the small GTPase RacA is required to maintain a mutualistic symbiotic association between Epichloë festucae and perennial ryegrass. Mol. Microbiol. 68, 1165–1178. 10.1111/j.1365-2958.2008.06217.x [DOI] [PubMed] [Google Scholar]
- Vanden Wymelenberg A. J., Cullen D., Spear R. N., Schoenike B., Andrews J. H. (1997). Expression of green fluorescent protein in Aureobasidium pullulans and quantification of the fungus on leaf surfaces. BioTechniques 23, 686–690. [DOI] [PubMed] [Google Scholar]
- Vázquez-de-Aldana B. R., García-Ciudad A., García-Criado B., Vicente-Tavera S., Zabalgogeazcoa I. (2013). Fungal endophyte (Epichloë festucae) alters the nutrient content of Festuca rubra regardless of water availability. PLoS ONE 8:e84539 10.1371/journal.pone.0084539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisey C. R. (2010). Intercalary growth in hyphae of filamentous fungi. Fungal Biol. Rev. 24, 123–131. 10.1016/j.fbr.2010.12.001 [DOI] [Google Scholar]
- Vollmer S. J., Yanofsky C. (1986). Efficient cloning of genes of Neurospora crassa. Proc. Natl. Acad. Sci. U.S.A. 83, 4869–4873. 10.1073/pnas.83.13.4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. L., Lee R. T., Fang H. M., Wang Y. M., Li R., Zou H., et al. (2008). Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe 4, 28–39. 10.1016/j.chom.2008.05.014 [DOI] [PubMed] [Google Scholar]
- Yamauchi J., Takayanagi N., Komeda K., Takano Y., Okuno T. (2004). cAMP-pKA signaling regulates multiple steps of fungal infection cooperatively with Cmk1 MAP kinase in Colletotrichum lagenarium. Mol. Plant Microbe Interact. 17, 1355–1365. 10.1094/MPMI.2004.17.12.1355 [DOI] [PubMed] [Google Scholar]
- Young C. A., Bryant M. K., Christensen M. J., Tapper B. A., Bryan G. T., Scott B. (2005). Molecular cloning and genetic analysis of a symbiosis-expressed gene cluster for lolitrem biosynthesis from a mutualistic endophyte of perennial ryegrass. Mol. Genet. Genomics 274, 13–29. 10.1007/s00438-005-1130-0 [DOI] [PubMed] [Google Scholar]
- Young C., Itoh Y., Johnson R., Garthwaite I., Miles C., Munday-Finch S., et al. (1998). Paxilline-negative mutants of Penicillium paxilli generated by heterologous and homologous plasmid integration. Curr. Genet. 33, 368–377. 10.1007/s002940050349 [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang Y., Wang J., Zhai Z., Zhang L., Zheng W., et al. (2013). Functional characterization of Rho family small GTPases in Fusarium graminearum. Fungal Genet. Biol. 61, 90–99. 10.1016/j.fgb.2013.09.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.