Abstract
During exercise, cardiac oxygen consumption increases and the resulting low oxygen level in the myocardium triggers coronary vasodilation. This response to hypoxia is controlled notably by the vasodilator adenosine and its A2A receptor (A2AR). According to the “spare receptor” pharmacological model, a strong A2AR-mediated response can occur in the context of a large number of receptors remaining unoccupied, the activation of only a weak fraction of A2AR (evaluated using KD), which results in maximal cAMP production (evaluated using EC50), and hence in maximal coronary vasodilation. In coronary artery disease (CAD), myocardial ischemia limits adaptation to exercise, which is commonly detected using the exercise stress test (EST). We hypothesized that spare A2AR is present in CAD patients to correct ischemia. Seventeen patients with angiographically documented CAD and 17 control subjects were studied. We addressed adenosine-plasma concentration and A2AR-expression at the mononuclear cell-surface, which reflects cardiovascular expression. The presence of spare A2AR was tested using an innovative pharmacological approach based on a homemade monoclonal antibody with agonist properties. EST was positive in 82% of patients and in none of the controls. Adenosine plasma concentration increased by 60% at peak exercise in patients and in none of the controls (p < 0.01). Most patients (65%), and none of the controls, had spare A2AR (identified when EC50/KD ≤ 0.1) and a low A2AR-expression (mean: –37% versus controls; p < 0.01). All patients with spare A2AR had a positive EST whereas the subjects without spare A2AR had a negative EST (p < 0.05). Spare A2AR is therefore associated with positive EST in CAD patients and its detection may be used as a diagnostic marker.
INTRODUCTION
During muscle exercise, heart work and the resulting myocardial energetic consumption increase. The ensuing low oxygen level in the myocardium triggers coronary vasodilation (1). This adaptive response is partly controlled by the vasodilator adenosine that regulates coronary blood flow, in particular via activation of the adenosine A2A receptor (A2AR), and coupling to the cAMP pathway (2–10). Cyclic AMP production and coronary vasodilation are correlated (11).
Sometimes a strong A2AR-mediated response occurs in the context of a large reserve of unoccupied receptors called “spare receptors” according to the Stephenson’s receptor theory (12). The presence of spare A2AR is evidenced when activation of only a weak fraction of A2AR (evaluated using the KD variable) results in maximal cAMP production (evaluated using the EC50 variable), and hence in maximal coronary vasodilation (13–15). Thus, the presence of spare A2AR allows for rapid, transient responses that are sensitive to low agonist concentrations. In other words, the presence of spare A2AR is expected to provide a high-efficiency vasodilation mechanism.
In coronary artery disease (CAD), the vasodilatory response to myocardial hypoxia appears to be generally unable to correct myocardial ischemia that is detected using the exercise stress test (EST) (1). The presence of spare A2AR in CAD patients and its role in CAD pathophysiology in which the regulation of myocardial blood flow is altered have never been addressed, and we hypothesized that spare A2AR is present in CAD patients to try to correct myocardial ischemia. We therefore undertook in this study to test the pharmacological characteristics of A2AR present on peripheral blood mononuclear cells (PBMC) because i) this cell population is readily accessible compared with coronary tissues and ii) properties of A2AR on PBMC appear to be similar to those of A2AR in heart tissue as changes in PBMC-surface expression of A2AR occur in cardiovascular diseases are associated with adenosine metabolism abnormalities (16–18), which suggests that regulation of A2AR expression may be a systemic mechanism.
MATERIALS AND METHODS
Compliance with Ethical Standards
The protocol was approved by the Ethics Committee of our institution (CPP Sud Méditerranée, Marseille, France). The study conformed to the standards set out in the 1983 Declaration of Helsinki. Written informed consent to participate in the study was obtained for all subjects.
Study Population
Seventeen patients (11 men and
6 women; mean age/range, 64 years [40–80]) with angiographically documented CAD were consecutively enrolled in the study as part of their medical follow-up, which included exercise stress testing (EST) (Table 1): i) 8 subjects were previously revascularized and EST was performed to determine the incidence of restenosis due to symptoms such as dyspnea and angina pectoris; ii) 5 type-2 diabetic subjects were screened for silent myocardial ischemia; and iii) 4 subjects with a suspicion of CAD were subjected to EST. Seventeen control patients (10 men and 7 women; mean age, 60 years [37–69]) with no history of CAD and who underwent cardiac examination prior to plastic surgery were included as controls. The patients in the control group underwent voluntary coronary computed tomography angiography (CCTA) evaluation and EST. The coronary arteries were assessed using the 17-segment AHA model. Disease of the epicardial coronary arteries was considered to be significant if the stenosis was ≥ 70% in a major coronary artery. The treatment of the patients was conservative.
Table 1.
Characteristics of patients and controls.
| Patients n=17 | Controls n=17 | |
|---|---|---|
| Demographics | ||
| Age, year (median, range) | 64 (40–80) | 60 (37–69) |
| Men/women | 11/6 | 10/7 |
| Cardiac Risk Factors | ||
| Diabetes | 6 | 4 |
| Current/former smoker | 10 | 2 |
| Hyperlipidemia | 10 | 4 |
| Hypertension | 8 | 4 |
| Syntax score (mean ± SD) | 14.7 ± 9.1 | |
| EST results | ||
| Chest pain | 3 | |
| ST-depression, mv > 0.2 | 14 | |
| Duration, min (median, range) | 6.8 [6–9] | 8.9 [9–18] |
| Workload, METa (median, range) | 6.9 [6–11] | 8.9 [7–14] |
| Workload, Watt (median, range) | 125 [90–150] | 135 [90–180] |
| Peak HRb (median, range) | 138 [120–150] | 145 [130–165] |
| Peak SBPc (median, range) | 188 [180–220] | 184 [180–250] |
| Treatment | ||
| Ticagrelor | 4 (26%) | |
| Clopidogrel | 11 (74%) | |
| Aspirin | 8 (47%) | |
| Statins | 7 (41%) | 4 (26%) |
| Ezetimibe | 10 (58%) | |
| Beta-blocker | 1 (6%) | 4 (26%) |
| Renin-angiotensin system inhibitor | 4 (26%) | |
| Esomeprazole | 11 (74%) | 4 (26%) |
| Metformin | 6 (40%) | |
| Extent of coronary disease | ||
| – 1 vessel | 9 | |
| – 2 vessel | 5 | |
| – 3 vessel | 3 | |
| – 0 vessel | 17 | |
| Coronary vessel narrowed | ||
| – Left-main | 1 | |
| – Left-anterior descending | 9 | |
| – Left-circumflex | 9 | |
| – Right | 8 | |
MET: mean metabolic equivalents of task level.
HR: heart rate.
SBP: systolic blood pressure.
EST Procedure
The treadmill test was symptom-
limited and was terminated if the patient had exercise-limiting chest pain, shortness of breath or other symptoms as assessed by the supervising clinician independently of the heart rate level. In accordance with the American Heart Association and the American College of Cardiology guidelines, testing could be terminated early at the discretion of the supervising clinician for significant arrhythmias, abnormal hemodynamic responses, ST-segment changes or if the participant was unwilling or unable to continue. Patients with baseline ECG abnormalities that could interfere with the interpretation of the criteria described above did not enter the study. Maximum heart rate level was assessed according to the formula: 220 – age. The bicycle ergometer was used in a sitting position (19). Increments were of 30 watts/3-min stage starting from a base of 30 watts. Results were expressed in terms of maximum watts and metabolic equivalents of task level (MET) based on the workload derived from the maximal speed and grade achieved during the total treadmill time. The EST was considered positive when the patient developed ECG signs of ischemia (significant ST segment/T wave changes/downsloping (> 2 mm) or horizontal ST-segment depression) and/or typical chest pain.
Adenosine Measurement
Blood samples (3 mL) were collected during a routine medical examination and processed as described previously using laboratory-prepared tubes containing 2 mL of a cold stop solution under vacuum. This method allows whole blood to mix quickly with the stop solution, thus preventing red blood cell uptake and adenosine degradation (20–22). After collection, samples were centrifuged and deproteinized prior to analysis using high-performance liquid chromatography. Adenosine was identified by its elution time and spectrum and quantified by comparison of peak areas with those of known quantities of adenosine.
A2AR Expression on PBMC
The procedure has been described previously (14, 15). In brief, blood samples from the brachial vein were collected in tubes containing sodium citrate, a polyester gel and a density gradient liquid (Vacutainer CPT, Beckton Dickinson). Blood samples were then centrifuged (20 min; 1,700g at room temperature), and the PBMC layer was collected and washed twice using phosphate-buffered saline prior to treatment with lysis buffer and sonication. Samples (equivalent to 0.25 × 106 cells) were then submitted to standard 12% polyacrylamide gel electrophoresis under reducing conditions followed by transfer onto a PVDF membrane. The filter was then incubated with Adonis (1 μg/mL), a homemade IgM, κ mouse monoclonal antibody directed against a linear epitope on A2AR (23), and staining was performed using horseradish peroxidase-labeled anti-mouse antibodies and enhanced chemiluminescence substrate. The 45-kDa bands corresponding to A2AR were submitted to densitometry analysis using the ImageJ 1.42q software (National Institutes of Health) and results were expressed as arbitrary units (AU), the ratio of pixels generated by the A2AR band to pixels generated by the background signal.
Identification of Spare A2AR
Adonis is an agonist-like monoclonal antibody that binds with an high affinity linear epitope localized in the second extracellular loop of the human A2AR (23). Consequently: i) Adonis behaves as an irreversible ligand of A2AR, at least during the length of the experiment, irreversible binding being necessary for the pharmacological identification of spare receptors (13); ii) Adonis similarly recognizes various forms of A2AR expressed on cells; iii) Adonis binds to the PBMC surface triggering cAMP production (23). Therefore, Adonis allows determination of both binding (KD) and functional (EC50) parameters of A2AR (24,25). For both analyses, PBMC were prepared using the vacutainer CPT system as described above and incubated with increasing concentrations of Adonis (0–1.8 μmol/L in 0.5-mL culture medium; 90 min; room temperature with shaking). PBMC were then either washed for KD determination or centrifuged without washing for cAMP dosage (see below).
KD determination
The KD was defined as the concentration of ligand (that is, Adonis) at which 50% of the binding sites (that is, A2AR) were occupied (24–26). We used Western blotting to establish the binding curve of Adonis to A2AR on PBMC and to determine the KD value. As described above, PBMC (0.25 × 106) were incubated with increasing concentrations of Adonis and then washed to remove free Adonis prior to treatment of the cell pellet with lysis buffer and sonication. Samples were then submitted to standard 12% electrophoresis under reducing conditions prior to transfer onto a PVDF membrane. These conditions led to the dissociation of Adonis associated at the PBMC surface via A2AR into its heavy and light chains (14,15), with only the kappa light chain (25 kDa) being stained here using specific peroxidase-labeled antibodies and chemiluminescent substrate. The staining intensity was measured using densitometry analysis and expressed as AU (that is, the ratio of pixels generated by the light chain band to pixels generated by the background signal). KD values for Adonis binding were estimated using nonlinear regression analysis (Prism software; GraphPad Software).
cAMP dosage (EC50)
We undertook to address the cAMP production level induced by incubation of increasing concentrations of the agonist-like Adonis with PBMC (0.75 × 106 cells) using the Amersham Biotrak Kit (GE Healthcare Bio-Sciences). Dodecyltrimethylammonium bromide acetate buffer was used to stop the incubation step. Two independent experiments with triplicates were performed and results expressed as the percentage of the maximal cAMP production. EC50 was defined as the concentration of Adonis that leads to half maximal stimulation of cAMP production (24,25).
Identification of the presence of spare A2AR
A spare A2AR mechanism was identified when the EC50/KD ratio ≤ 0.1 (25).
Statistical Analysis
Quantitative variables are expressed as means and standard deviations (SD) or range, or as medians and interquartile range (IQR). Variance analysis (two-way ANOVA) was used for intergroup comparisons. The Wilcoxon test was used to address adenosine plasma concentration kinetic. All statistical tests were two-sided. Analyses were performed with the Prism software.
RESULTS
Population of the Study
The subjects were consecutively enrolled. There was no significant difference in age between patients and controls (P > 0.05). The control subjects had a normal (without plaque) CCTA and a calcium score < 100. Angiographically documented CAD patients and controls were not matched for cardiovascular risk factors because all controls had normal CCTA. The clinical characteristics of the population and the treatments are given in Table 1. We found that 14/17 (82%) patients had a positive EST (ECG signs of ischemia and/or painful ischemia) and that the 17 (100%) controls had a negative EST. The occurrence of signs of myocardial ischemia was significant in these patients because 10/17 patients had left main or left anterior descending artery disease, and hence a significant amount of myocardium particularly prone to ischemia during EST.
Adenosine Plasma Concentration and A2AR Expression
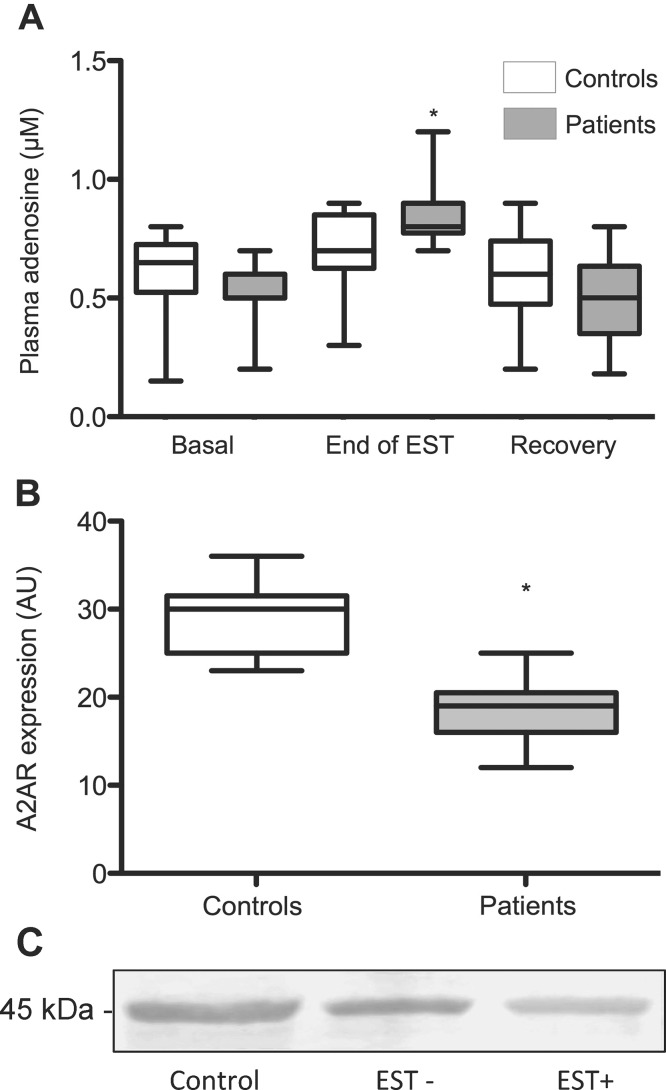
At rest, no significant difference of adenosine concentration was found in patients versus controls (median [IQR]: 0.50 [0.50–0.60] versus 0.65 [0.52–0.72] μmol/L; P > 0.05; Figure 1A). The concentration increased significantly during EST in patients (0.80 [0.77–0.90] μmol/L; P < 0.01) but not in controls (0.70 [0.62–0.85] μmol/L; P = 0.13). After a 10 min recovery period, adenosine concentration decreased in CAD patients to reach the range of values found at rest (0.50 [0.35–0.65] μmol/L; P < 0.01). Patients with positive EST were distinguished from those with negative EST and the results are presented in Table 2. A2AR expression in PBMC isolated from patients and control subjects was investigated using Western blot analysis of cell lysates and probing with the anti-A2AR monoclonal antibody Adonis prior to densitometry analysis of the 45-kDa band. At the basal state, A2AR expression was 37% lower in patients versus controls (19 [16–20.5] versus 30 [25–31.5] AU; P < 0.01) (Figure 1B). The analysis of EST-positive and EST-negative patients is shown in Table 2. Western blot analysis of A2AR expression in a representative sample close to the mean value of each group of interest is given and shows that controls and patients with negative EST had higher A2AR expression versus patients with positive EST (29.1, 23.4 and 15.2 AU, respectively; Figure 1C).
Figure 1.
Purinergic profile of CAD patients and controls. (A) Adenosine plasma concentration (APC) in basal conditions, at the end of EST and after a 10-min recovery period was measured in 17 CAD patients and 17 controls (*: P < 0.01 compared with basal state). (B) A2A receptor expression was evaluated using Western blotting in 17 controls and 17 CAD patients (*: P < 0.01). Data (AU) are expressed as mean, median and IQR. (C) Western blot analysis of A2AR expression in a representative sample close to the mean value of each group of interest (Controls, EST–/EST+ patients) are shown.
Table 2.
Biological variables of patients with positive and negative exercise stress test.
| APCa basal (μmol/L) | APC end of test (μmol/L) | APC recovery (μmol/L) | A2ARb expression (AU) | KD (μmol/L) | EC50 (μmol/L) | |
|---|---|---|---|---|---|---|
| EST+c | 0.52 ± 0.12d | 0.86 ± 0.13 | 0.53 ± 0.16 | 17 ± 3 | 0.40 ± 0.20 | 0.21 ± 0.27 |
| EST–e | 0.60 ± 0.10 | 0.80 ± 0.07 | 0.32 ± 0.06 | 23 ± 2 | 0.30 ± 0.13 | 0.40 ± 0.20 |
APC: adenosine plasma concentration.
A2AR: adenosine A2A receptor.
EST+: positive exercise stress test (n = 14).
Means and standard deviations (mean ± SD) of biological parameters evaluated in patients with coronary artery disease.
EST–: negative exercise stress test (n = 3).
Pharmacological Properties of A2AR
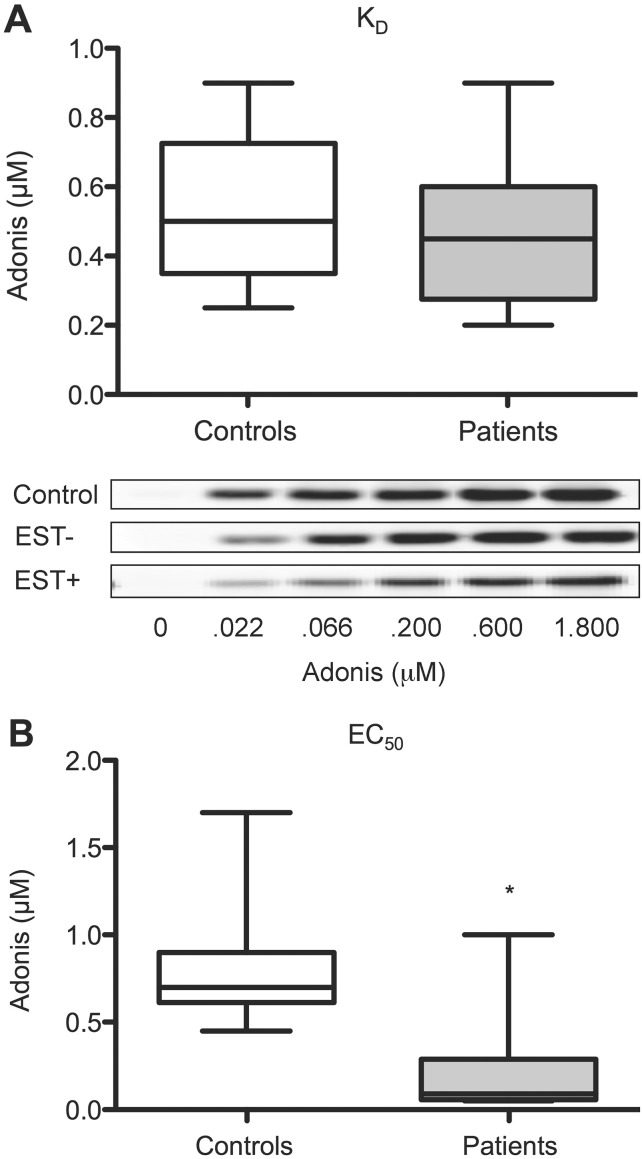
We then performed a pharmacological analysis of A2AR: i) the KD value was defined as the concentration of Adonis at which half-maximal labeling of cell-surface A2AR occurred as assessed by Western blot quantitation of Adonis bound to PBMC; ii) the EC50 value was defined as the concentration of the agonist Adonis at which half-maximal cAMP production occurred. According to these procedures, we observed that: i) KD values were similar in patients versus controls (0.45 [0.27–0.60] versus 0.50 [0.35–0.72] μmol/L; P = 0.3) (Figure 2A), and Western blot analysis of the light chain (25 kDa) of Adonis are shown for the representative samples presented in Figure 1C (KD: Control: 0.46 μmol/L; EST– patient: 0.40 μmol/L; EST+ patient: 0.32 μmol/L; Figure 2A insert). (The analysis of EST-positive and EST-negative patients is shown in Table 2); ii) EC50 values were markedly lower in patients versus controls (0.09 [0.03–0.29] versus 0.70 [0.61–0.90] μmol/L; P < 0.01) (Figure 2B)
Figure 2.
Adenosine A2A receptor characterization. (A) KD and EC50 were interpolated from dose-response curves obtained using increasing concentrations of Adonis, an anti-A2AR monoclonal antibody with agonist properties (see methods). Comparison of KD values (controls versus patients: P = 0.3; Western blot analysis of the light chain [25 kDa] of Adonis in a representative sample of each group of interest are shown in the insert). (B) Comparison of EC50 values (*: P < 0.01).
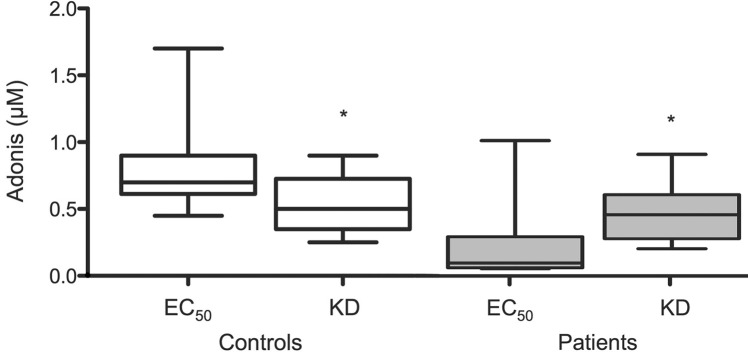
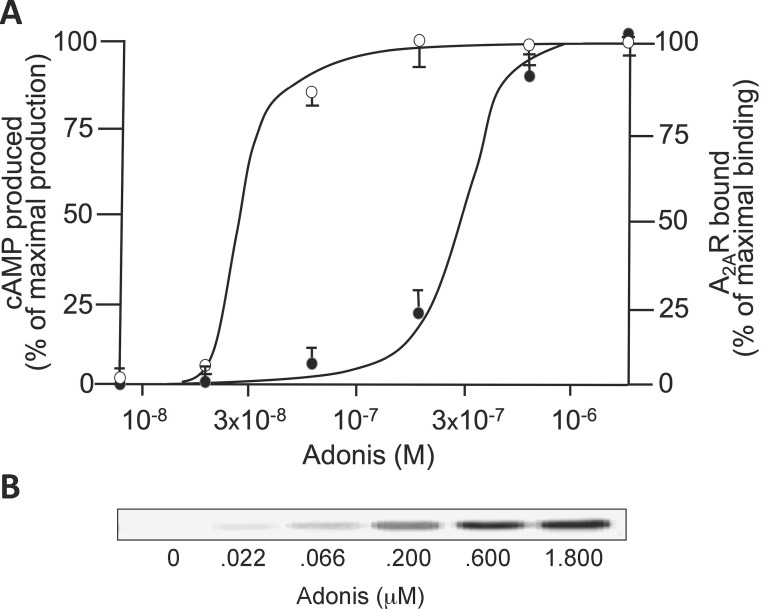
(The analysis of EST-positive and EST-negative patients is shown in Table 2); iii) EC50 values were higher than KD in controls (0.70 [0.61–0.90] versus 0.50 [0.35–0.72] μmol/L; P < 0.05) (Figure 3); whereas iv) EC50 values were markedly lower than KD in patients (0.09 [0.03–0.29] versus 0.45 [0.27–0.60] μmol/L; P < 0.01) (Figure 3). It is noteworthy that i) 11/17 (65%) patients—and none of the controls—had EC50/KD ≤ 0.1, a pharmacological criteria that identifies the presence of spare receptors (Figure 4A) (24), and ii) all patients with spare A2AR had a positive EST whereas the subjects without spare A2AR had a negative EST (P < 0.05). For illustrative purposes, we present the Western blot analysis used for KD determination for a patient with positive EST and spare A2AR (EC50/KD = 0.09) in Figure 4B.
Figure 3.
EC50/KD ratio. Comparison of EC50 and KD values in controls (*: P < 0.05) and in patients (*: P < 0.01).
Figure 4.
Identification of spare A2AR in patients with positive EST. (A) The dose-response curve resulting from Adonis binding to A2AR (KD; black circles; densitometry analysis of the 25 kDa band corresponding to the light chain of Adonis; see methods) and from dosage of the resulting cAMP production (EC50; white circles) for a representative patient with spare A2AR (EC50/KD ≤ 0.1) is shown. Results are mean ± SD of triplicates. (B) The corresponding Western blot analysis used for KD determination is shown.
DISCUSSION
We have recently reported (27) that patients with positive exercise stress test have a low expression of A2AR in the basal state and an increase in adenosine plasma concentration during EST, which is confirmed here. Although it is known that A2AR expression increases in hypoxic/ischemic conditions (2), we considered that it would be pointless to attempt to highlight here a putative ischemia-induced adaptive increase in A2AR expression during EST because the duration of the test is < 10 min in our patients, which is well below the time needed by the de novo synthesis and cell surface expression of A2AR (28).
Considering that spare A2AR may be chronically present in CAD patients to try to correct myocardial ischemia in a context of low A2AR expression and high exercise-induced adenosine concentration (27 and Figure 1), we undertook to characterize the pharmacological properties of A2AR in CAD. We observed that these properties differed between CAD patients and healthy subjects. While the KD for A2AR of the agonist used here to mimic adenosine was similar in patients and controls, the EC50 value (related to the biological effect triggered by the agonist and monitored here via cAMP production) was significantly lower in patients. More importantly, while EC50 was greater than KD in healthy subjects, the reverse situation was found for patients: EC50 was 10-fold lower than KD in 65% of the patients, and in 79% of patients with positive EST. In most CAD patients, such an EC50/KD ratio ≤ 0.1 is consistent with the presence of spare receptors (26). Finally, we observed that all patients with spare A2AR had a positive EST. Thus, we concluded that the presence of spare A2AR is associated with signs of myocardial ischemia during exercise.
Spare A2AR was first observed in guinea pig cardiac tissue using an irreversible A2AR antagonist to block response to various agonists (13). Here, we used an agonist-like monoclonal antibody and provided further evidence for the presence of spare A2AR on the surface of human mononuclear cells in relation to cardiac dysfunction, spare A2AR having already been observed in a context of neurocardiogenic syncope, but not in healthy subjects (14,15,18).
It would have been expected that the presence of spare A2AR would increase coronary blood flow in CAD patients as it would in an animal model, where an activation of only 5% of A2AR present in the coronary system was sufficient to produce 50% of the maximal coronary conductance (13). Yet, our results suggest that during EST, CAD patients with spare A2AR failed to adjust coronary vasodilation to workload as shown by chest pain and/or ST depression at peak exercise. We concluded that in healthy subjects, the regulation of coronary vasodilation does not imply spare A2AR, receptor expression and adenosine concentration being sufficient to accommodate the increased workload (27 and Figure 1). In contrast, in CAD patients with positive EST, the presence of spare A2AR is not sufficient to provide efficient vasodilation during exercise in a context of low A2AR expression level and despite an increase in adenosine plasma concentration. Another apparent paradox is that spare A2AR that constitutes a reserve of receptors was detected in patients in which receptor expression was low. Whether the presence of spare A2AR is an adaptive response in CAD patients to chronic myocardial ischemia or results from genetic predisposition needs further investigations.
CONCLUSION
These results show that the presence of spare A2AR is associated with positive EST in CAD patients. Consequently, detecting spare A2AR in the screening of CAD appears to be a promising diagnostic tool.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
This study was supported by grants from Aix-Marseille University.
Cite this article as: Ruf J, et al. (2016) Spare adenosine A2a receptors are associated with positive exercise stress test in coronary artery disease.
REFERENCES
- Banerjee A, Newman DR, Van den Bruel A, Heneghan C. Diagnostic accuracy of exercise stress testing for coronary artery disease: a systematic review and meta-analysis of prospective studies. Int J Clin Pract. 2012;66:477–92. doi: 10.1111/j.1742-1241.2012.02900.x. [DOI] [PubMed] [Google Scholar]
- Grenz A, Homann D, Eltzschig HK. Extracellular adenosine: a safety signal that dampens hypoxia-induced inflammation during ischemia. Antioxid Redox Signal. 2011;15:2221–34. doi: 10.1089/ars.2010.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard ER, et al. KV 7 channels are involved in hypoxia-induced vasodilatation of porcine coronary arteries. Br J Pharmacol. 2014;171:69–82. doi: 10.1111/bph.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick ZC, et al. Contribution of adenosine A(2A) and A(2B) receptors to ischemic coronary dilation: role of K(V) and K(ATP) channels. Microcirculation. 2010;17:600–7. doi: 10.1111/j.1549-8719.2010.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckle T, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–90. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- Eckle T, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18:774–82. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganelli F, et al. Effects of percutaneous transluminal coronary angioplasty on coronary adenosine concentrations in humans. Eur J Clin Invest. 2000;30:105–10. doi: 10.1046/j.1365-2362.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Sanjani MS, et al. Contributions of A2A and A2B adenosine receptors in coronary flow responses in relation to the KATP channel using A2B and A2A/2B double-knockout mice. Am J Physiol Heart Circ Physiol. 2011;301:H2322–33. doi: 10.1152/ajpheart.00052.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shryock JC, Belardinelli L. Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. Am J Cardiol. 1997;79:2–10. doi: 10.1016/s0002-9149(97)00256-7. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, et al. Identification of adenosine A2 receptor-cAMP system in human aortic endothelial cells. Biochem Biophys Res Commun. 1994;199:905–10. doi: 10.1006/bbrc.1994.1314. [DOI] [PubMed] [Google Scholar]
- Cushing DJ, Brown GL, Sabouni MH, Mustafa SJ. Adenosine receptor-mediated coronary artery relaxation and cyclic nucleotide production. Am J Physiol. 1991;261:H343–8. doi: 10.1152/ajpheart.1991.261.2.H343. [DOI] [PubMed] [Google Scholar]
- Stephenson RP. A modification of receptor theory. Br J Pharmacol Chemother. 1956;11:379–93. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shryock JC, et al. A2A-adenosine receptor reserve for coronary vasodilation. Circulation. 1998;98:711–8. doi: 10.1161/01.cir.98.7.711. [DOI] [PubMed] [Google Scholar]
- Franceschi F, et al. A2A adenosine receptor function in patients with vasovagal syncope. Europace. 2013;15:1328–32. doi: 10.1093/europace/eut066. [DOI] [PubMed] [Google Scholar]
- Jacquin L, et al. Search for adenosine A2A spare receptors on peripheral lymphocytes. FEBS Open Bio. 2012;3:1–5. doi: 10.1016/j.fob.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani K, et al. Changes of peripheral A2A adenosine receptors in chronic heart failure and cardiac transplantation. FASEB J. 2003;17:280–2. doi: 10.1096/fj.02-0543fje. [DOI] [PubMed] [Google Scholar]
- Deharo JC, et al. Adenosine plasma level and A2A adenosine receptor expression: correlation with laboratory tests in patients with neurally mediated syncope. Heart. 2012;98:855–9. doi: 10.1136/heartjnl-2011-301411. [DOI] [PubMed] [Google Scholar]
- Guieu R, et al. Adenosine and clinical forms of neurally-mediated syncope. J Am Coll Cardiol. 2015;66:204–5. doi: 10.1016/j.jacc.2015.04.066. [DOI] [PubMed] [Google Scholar]
- Pina IL, et al. Guidelines for clinical exercise testing laboratories. A statement for healthcare professionals from the Committee on Exercise and Cardiac Rehabilitation, American Heart Association. Circulation. 1995;91:912–21. doi: 10.1161/01.cir.91.3.912. [DOI] [PubMed] [Google Scholar]
- Saadjian AY, et al. Role of endogenous adenosine as a modulator of syncope induced during tilt testing. Circulation. 2002;106:569–74. doi: 10.1161/01.cir.0000023924.66889.4c. [DOI] [PubMed] [Google Scholar]
- Bonello L, et al. Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J Am Coll Cardiol. 2014;63:872–7. doi: 10.1016/j.jacc.2013.09.067. [DOI] [PubMed] [Google Scholar]
- Guieu R, et al. Developement of an HPLC/diode array detector method for the determination of human plasma adenosine concentrations. J Liq Chromatogr. 1999;22:1829–41. [Google Scholar]
- By Y, et al. Production of an agonist-like monoclonal antibody to the human A2A receptor of adenosine for clinical use. Mol Immunol. 2009;46:400–5. doi: 10.1016/j.molimm.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Hulme EG. Receptor-Ligand Interactions: A Practical Approach. New York: Oxford University Press; 1992. p. 458. [Google Scholar]
- Lauffenburger DA, Linderman JJ. Receptors For Binding, Trafficking and Signaling. New York: Oxford University Press; 1998. p. 365. [Google Scholar]
- McKinney M, Anderson D, Vella-Rountree L. Different agonist-receptor active conformations for rat brain M1 and M2 muscarinic receptors that are separately coupled to two biochemical effector systems. Mol Pharmacol. 1989;35:39–47. [PubMed] [Google Scholar]
- Guieu R, et al. Low basal expression of A2A adenosine receptors and increase in adenosine plasma concentration are associated with positive exercise stress testing. Int J Cardiol. 2015;180:15–7. doi: 10.1016/j.ijcard.2014.11.089. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Sitkovsky MV. Adenosine A2A receptor is involved in cell surface expression of A2B receptor. J Biol Chem. 2010;285:39271–88. doi: 10.1074/jbc.M109.098293. [DOI] [PMC free article] [PubMed] [Google Scholar]