Abstract
The culture supernatant of Paenibacillus sp. TKU036, a bacterium isolated from Taiwanese soils, showed high antioxidant activity (85%) when cultured in a squid pen powder (SPP)-containing medium at 37 °C for three days. Homogentisic acid (2,5-dihydroxyphenylacetic acid, HGA) was isolated and found to be the major antioxidant in the culture supernatant of the SPP-containing medium fermented by Paenibacillus sp. TKU036. Tryptophan was also present in the culture supernatant. The results of high-performance liquid chromatography (HPLC) fingerprinting showed that HGA and tryptophan were produced via fermentation but did not pre-exist in the unfermented SPP-containing medium. Neither HGA nor tryptophan was found in the culture supernatants obtained from the fermentation of nutrient broth or other chitinous material, i.e., medium containing shrimp head powder, by Paenibacillus sp. TKU036. The production of HGA via microorganisms has rarely been reported. In this study, we found that squid pen was a potential carbon and nitrogen source for Paenibacillus sp. Tryptophan (105 mg/L) and HGA (60 mg/L) were recovered from the culture supernatant. The isolated HGA was found to have higher antioxidant activity (IC50 = 6.9 μg/mL) than α-tocopherol (IC50 = 17.6 μg/mL). The anti-inflammatory activity of the isolated HGA (IC50 = 10.14 μg/mL) was lower than that of quercetin (IC50 = 1.14 μg/mL). As a result, squid pen, a fishery processing byproduct, is a valuable material for the production of tryptophan and the antioxidant and anti-inflammatory HGA via microbial conversion.
Keywords: squid pen, chitin, homogentisic acid, tryptophan, Paenibacillus, antioxidant, anti-inflammatory
1. Introduction
Chitin is one of the most abundant biopolymers in the world, and these natural polymers have versatile properties, such as biocompatibility and non-toxicity. Among the natural chitinous resources, fishery processings (shrimp shells, crab shells, and squid pens) have the highest chitin content. Conventionally, chitin is obtained from shrimp shells, crab shells, and squid pens using a strong alkali or an inorganic acid for deproteinization or demineralization, respectively [1]. However, these chemical processes have several drawbacks, such as the creation of pollutant alkali or acid liquid. Furthermore, the unutilized bioresource of the deproteinized liquid is reduced due to the presence of an alkali [1].
Among the chitin-containing fishery processings, squid pens contain the highest ratio of protein (approximately 70%) [2]. For recycling squid pens in order to produce additional highly value-added products other than chitin or chitosan, we investigated the reutilization of this fishery processings via microbial conversion in order to produce enzymes [2,3,4,5], exopolysaccharides [6,7], chitooligomers [3], antioxidants [8,9], insecticidal materials [10], and biosorbents [11,12].
Many strains of Paenibacillus have been reported to use squid pen powder (SPP) as the sole carbon and nitrogen (C/N) source. Recently, we isolated strains of Paenibacillus species that converted squid pen to exopolysaccharides [7], chitosanase [3], and chitooligomers [3].
Microbial fermentation can result in the production of some antioxidants, such as ellagic acid produced by Aspergillus niger [13], gallic acid produced by Bacillus sphaericus [14], ferulic and acid produced by Saccharomyces cerevisiae [15]. In this study, we screened antioxidant-producing bacteria from Taiwanese soils by using squid pen as the sole C/N source. A potential bacterial strain, TKU036, was isolated and identified as Paenibacillus sp. The optimized culture conditions for antioxidant production via Paenibacillus sp. TKU036 was studied.
Here, the antioxidant compound produced in the culture supernatant of Paenibacillus sp. TKU036 was isolated and identified as HGA. HGA has shown to have antioxidant and anti-inflammatory activities [16,17]. In this study, the antioxidant and anti-inflammatory activities of the isolated HGA were investigated and compared with those activities of other well-known antioxidant (α–tocopherol) and anti-inflammatory compound (quercetin).
2. Results and Discussion
2.1. Screening and Identification of Strain TKU036
Over 350 bacterial strains isolated from the soils of Northern Taiwan were cultivated at 37 °C in a medium containing 1% squid pen powder (SPP). Among these strains, strain TKU036 exhibited the strongest antioxidant activity and was chosen for more intensive examination. Based on morphological and biochemical studies, as well as 16S rDNA sequences [7], this strain was confirmed to be Paenibacillus sp. Analytical profile index (API) identification was further used to identify the species name [7]; however, no match was found. Therefore, the TKU036 strain was identified as Paenibacillus sp. and was used for further investigation.
2.2. Comparing the Non-Exopolysaccharide Antioxidants Produced by Paenibacillus Species
Many strains of Paenibacillus, such as P. mucilaginosus TKU032 [7], Paenibacillus sp. TKU023 [18], and P. macerans TKU029 [19], have been reported as the sole C/N source for the production of exopolysaccharides (EPOs) using SPP, and some of these EPOs showed antioxidant activity. In this study, EPOs were also found in the culture broth of SPP-containing medium fermented by Paenibacillus sp. TKU036 (data not shown). To investigate whether the antioxidant activity was from the EPOs, the EPO-containing culture supernatant of strain TKU036 underwent ethanol precipitation (final concentration of 70%, v/v) to remove the EPOs. The obtained EPO-deficient culture supernatants were than lyophilized to remove the ethanol and were used for analyzing antioxidant activity. The EPO-deficient culture supernatant of Paenibacillus sp. TKU036 showed high antioxidant activity (85%). The culture conditions for the production of antioxidants and the isolation of the non-EPO antioxidants were studied subsequently.
2.3. Culture Conditions for Antioxidant Production
Different concentrations (0.5%, 1.0%, and 1.5% w/v) of squid pen powder (SPP), shrimp head powder (SHP), and cicada shell powder (CSP) were used as the sole C/N source for the production of antioxidant by Paenibacillus sp. TKU036. The effects of the medium volume, the medium pH, and the culture temperature on antioxidant activity were also examined. Commercial nutrient broth (NB) medium, which does not contained chitin, was used for comparison. The result showed that the highest antioxidant activity (85%) was obtained by fermentation within the 0.5% SPP-containing medium (100 mL medium in 250 mL Erlenmeyer flask) at 37 °C in a reciprocal shaker at 150 rpm for three days.
The results of this study are remarkably different from those of other reports, such as studies of Bacillus subtilis using red bean [20], Aspergillus awamori and Aspergillus oryzae using soybean [21], Aspergillus usami using sesamin [22], A. awamori using black bean [23], and Monascus pilosus using potato dextrose broth [24] as a C/N source for antioxidant production.
A novel antioxidant (serraticin) with antitumor activity was isolated from the culture supernatant of SPP-containing medium fermented by Serratia ureilytica TKU013 [8]. S. ureilytica TKU013 used SPP (1.5%) for the production of the antioxidant, but the maximal antioxidant activity was 82% after four days of fermentation [25]. In this study, Paenibacillus sp. TKU036 used a cheaper C/N source of 0.5% SPP and produced a higher antioxidant activity (85%) in a shorter time (three days). The studied culture condition was then used for antioxidant production.
2.4. Isolation of Antioxidant Compounds
As described in the Materials and Methods section below, the 95% ethanol extract was separated into 14 fractions by column chromatography. All the fractions were evaluated for antioxidant activity using a scavenging 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical test. As shown in Figure 1, Fraction 4 had the highest antioxidant activity.
Figure 1.
DPPH radical scavenging activity of the 14 fractions eluted by different concentrations of methanol. —●—, Fraction 1 (1.1414 g, eluted with 0% methanol); —○—, Fraction 2 (0.4464 g, eluted with 5% methanol); — —, Fraction 3 (0.1765 g, eluted with 10% methanol); —★—, Fraction 4 (0.1698 g, eluted with 15% methanol); —■—, Fraction 5 (0.1253 g, eluted with 20% methanol); —□—, Fraction 6 (0.0864 g, eluted with 25% methanol); —◆—, Fraction 7 (0.0599 g, eluted with 30% methanol) ;—◇—, Fraction 8 (0.0482 g, eluted with 35% methanol); —▲—, Fraction 9 (0.0363 g, eluted with 40% methanol); —△—, Fraction 10 (0.0275 g, eluted with 45% methanol); —▼—, Fraction 11 (0.0263 g, eluted with 50% methanol); —▽—, Fraction 12 (0.0321 g, eluted with 55% methanol); —✖—, Fraction 13 (0.0324 g, eluted with 60% methanol); and —✕—, Fraction 14 (0.0424 g, eluted with 100% methanol).
—, Fraction 3 (0.1765 g, eluted with 10% methanol); —★—, Fraction 4 (0.1698 g, eluted with 15% methanol); —■—, Fraction 5 (0.1253 g, eluted with 20% methanol); —□—, Fraction 6 (0.0864 g, eluted with 25% methanol); —◆—, Fraction 7 (0.0599 g, eluted with 30% methanol) ;—◇—, Fraction 8 (0.0482 g, eluted with 35% methanol); —▲—, Fraction 9 (0.0363 g, eluted with 40% methanol); —△—, Fraction 10 (0.0275 g, eluted with 45% methanol); —▼—, Fraction 11 (0.0263 g, eluted with 50% methanol); —▽—, Fraction 12 (0.0321 g, eluted with 55% methanol); —✖—, Fraction 13 (0.0324 g, eluted with 60% methanol); and —✕—, Fraction 14 (0.0424 g, eluted with 100% methanol).
At a concentration of 200 μg/mL, Fraction 4 showed approximately 99% antioxidant activity. Fraction 4 was further purified with a preparative HPLC column. In total, five sub-fractions (4-1, 4-2, 4-3, 4-4, and 4-5) (Figure 2) were obtained, and the antioxidant activity of these fractions were analyzed (Figure 3). As shown in Figure 3, Fraction 4-4 showed the highest antioxidant activity (IC50 of 6.9 μg/mL) compared with those of Fraction 4-5 (62.8 μg/mL, which showed a little antioxidant activity due to containing minor 4-4) and the other three fractions, as well as the antioxidant activity of the positive control, α-tocopherol (17.6 μg/mL). The results showed Fraction 4-4 contained a potential antioxidant that was valuable for further identification.
Figure 2.
The HPLC profile of Fraction 4 (13% acetonitrile, 254 nm).
Figure 3.
DPPH radical scavenging activities of 4-1 to 4-5. —●—, 4-1; —○—, 4-2; —▼—, 4-3; —△—, 4-4; —■—, 4-5; and —□—, α-tocopherol.
2.5. Identification of HGA and Tryptophan by NMR
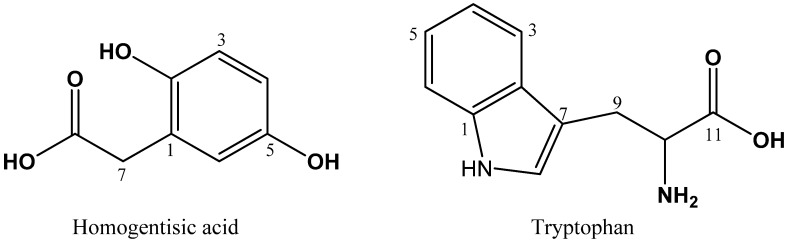
The chemical structures of the isolated compounds were elucidated using detailed spectroscopic analyses, including 1D (1H NMR, 13C NMR) and 2D NMR experiments (1H–1H COSY, HSQC, and HMBC), together with the spectroscopic comparisons of previously reported compounds. Fractions 4-4 and 4-5 were shown to contain HGA [26] and tryptophan [27], respectively (Figure 4).
Figure 4.
The chemical structures of HGA (left) and l-tryptophan (right).
HGA (4-4) was obtained as a white amorphous powder. 1H NMR data (400 MHz, MeOH-d4, δH ppm): 6.62 (d, J = 8.4 Hz, H-3), 6.59 (d, J = 2.8 Hz, H-6), 6.53 (dd, J = 8.4, 2.8 Hz, H-4), and 3.50 (s, 2H, H-7). 13C NMR data (100 MHz, MeOH-d4, δC ppm): 177.0 (C-8), 151.6 (C-5), 150.4 (C-2), 124.3 (C-1), 119.0 (C-6), 117.4 (C-3), 116.0 (C-4), and 37.7 (C-7).
Tryptophan was obtained as a white amorphous powder. 1H NMR data (600 MHz, MeOH-d4, δH ppm): 7.69 (d, J = 7.8 Hz, H-3), 7.35 (d, J = 7.8 Hz, H-6), 7.19 (s, H-8), 7.11 (td, J = 7.8, 0.6 Hz, H-5), 7.04 (td, J = 7.8, 0.6 Hz, H-4), 3.86 (dd, J = 9.6, 4.2 Hz, H-10), 3.51 (dd, J = 15.6, 9.6 Hz, H-9), 3.15 (dd, J = 15.6, 4.2 Hz, H-9). 13C NMR data (150 MHz, MeOH-d4, δC ppm): 174.5 (C-11), 138.4 (C-1), 128.5 (C-2), 125.2 (C-8), 122.7 (C-5), 120.1 (C-4), 119.3 (C-3), 112.4 (C-6), 109.5 (C-7), 56.7 (C-10), and 28.5 (C-9).
HGA is an important intermediate in the metabolism of phenylalanine and tyrosine [28]. The production HGA via microorganisms has only been shown in a few reports, such as Aspergillus niger (using phenyl acetic acid as a C/N source) [29], Vibrio cholerae (using marine broth with 4 mM tyrosine as a C/N source) [30], and Yarrowia lipolytica (using tyrosine as a C/N source) [28]. Tryptophan is widely used in human food and medicine, as well as in animal feed. The production of tryptophan has been reported in two typical bacteria strains: Escherichia coli FB-04 (using glucose, yeast, tryptone, and citric acid as C/N sources) [31] and Corynebacterium glutamicum KY9218 (using sucrose, corn steep liquor, tyrosine, phenylalanine etc. as C/N sources) [32]. In this study, Paenibacillus sp. TKU036 cultured with SPP, a seafood processing, was used for the production of HGA and tryptophan and may have potential for further investigation.
2.6. The Effect of HGA on Cytotoxicity and Anti-Inflammation
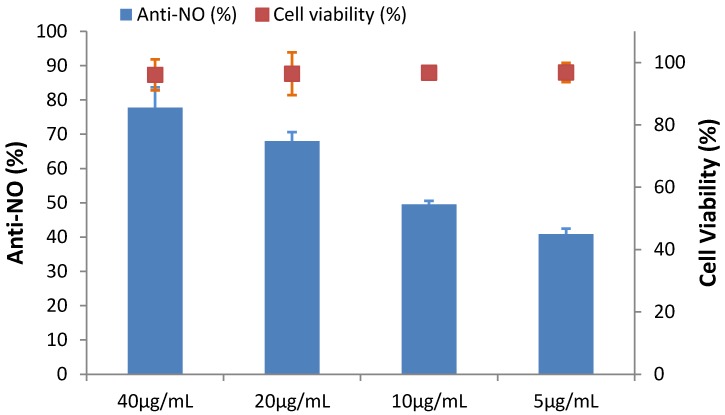
Nitric oxide (NO) is recognized as a key pro-inflammatory mediator that is involved in certain inflammatory disorders, including chronic hepatitis, pulmonary fibrosis, and rheumatoid arthritis [33]. In our previous study [3], we discovered chitosan oligomers with a low degree of polymerization that showed both antioxidant and anti-inflammatory activity. In this study, the anti-inflammatory activity of Fraction 4-4 (HGA) was estimated using an in vitro model: LPS-stimulated RAW 264.7 cells. The inhibition of LPS-stimulated NO secretion was due to anti-inflammatory activity. First, to examine the potential cell cytotoxicity induced by Fraction 4-4, a MTT assay was conducted. When RAW 264.7 macrophages were treated with Fraction 4-4 at concentrations of 0, 5, 10, 20, and 40 μg/mL, along with 1 μg/mL LPS, the resulting viabilities of RAW 264.7 cells were recorded and are summarized in Figure 5. The results of a statistical analysis indicated that treatment with Fraction 4-4 (40 μg/mL) had no noticeable toxic effect on cell growth when compared with the cell growth of the 0.05% DMSO treated group (100%). Fraction 4-4 (40 μg/mL) was capable of inhibiting NO production by 77.79% in LPS-stimulated cells. The IC50 value of Fraction 4-4, representing the anti-inflammatory effect, was 10.14 μg/mL (Figure 5). A similar result was found when purchased HGA was used. Quercetin is a potent dietary antioxidant that also displays anti-inflammatory activity [34]. Thus, the anti-inflammatory activity (IC50) of quercetin was investigated. These results indicated that HGA exhibited an acceptable anti-inflammatory activity (IC50 = 10.14 μg/mL) compared with the anti-inflammatory activity of quercetin (IC50 = 1.14 μg/mL).
Figure 5.
NO inhibitory activities of HGA isolated from culture supernatant of Paenibacillus sp. TKU036 in the SPP-containing medium. Cell lines: Murine RAW 264.7 monocyte/macrophage cells. The cells were treated with LPS (1 μg/mL) or in combination with the tested agents (40, 20, 10, and 5 μg/mL) for 24 h.
2.7. Confirmation of HGA and Tryptophan Produced from SPP by Fermentation
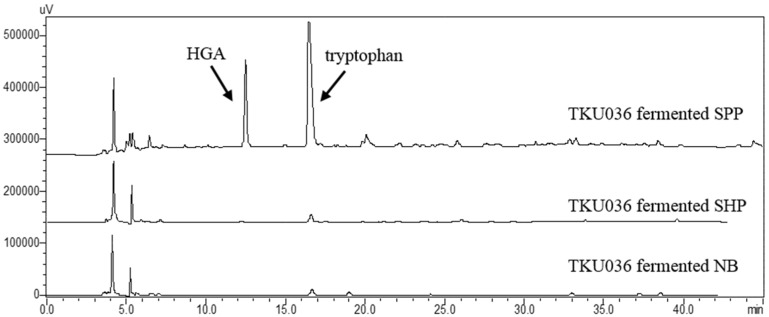
The 95% ethanol extracts were extracted from the culture fermented supernatant of Paenibacillus sp. TKU036 and were then compared with the extract of unfermented medium via HPLC analysis. The results found that HGA and tryptophan appeared in the fermentation supernatant at Rt 12.75 min and at Rt 16.25 min, respectively, revealing that the two components did not pre-exist in the SPP-containing medium (data not shown).
To confirm whether HGA and tryptophan were also produced using other chitin-containing materials as the sole C/N source, the 0.5% SHP-containing medium was also studied. Furthermore, nutrient broth (NB), a commercial medium for bacteria cultivation, was also tested. As shown in Figure 6, after fermentation by Paenibacillus sp. TKU036 for three days, no HGA and tryptophan were detected from the ethanol extract of the culture supernatant. To the best of our knowledge, there have been no reports of materials harmful to humans produced by Paenibacillus species [35]. The transformation of squid pen to functional foods of HGA and tryptophan via Paenibacillus sp. strain TKU036 may have the potential to be intensively investigated.
Figure 6.
The HPLC fingerprints of the ethanol extracts from the Paenibacillus sp. TKU036 fermented SPP-containing medium, the Paenibacillus sp. TKU036 fermented SHP-containing medium, and Paenibacillus sp. TKU036 fermented nutrient broth (NB).
3. Materials and Methods
3.1. Materials
Squid pens were obtained from Shin-Ma Frozen Food Co. (I-Lan, Taiwan). Shrimp head power (SHP) was obtained from Fwu-Sow Industry. (Taichun, Taiwan). Cicada shells were collected at the Tamsui Campus of Tamkang University (New Taipei, Taiwan). HGA, tryptophan, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nutrient broth was obtained from Difco. Octadecylsilane (ODS) gel was purchased from Merck (Darmstadt, Germany).
3.2. Antioxidant Activity Assay
The antioxidant samples (1.2 mL) were mixed with 0.3 mL of a methanolic solution containing 0.75 mM DPPH radicals. The mixture was vigorously shaken and incubated for 30 min in the dark, and the absorbance was then measured at 517 nm against a blank [3]. The scavenging ability was calculated as described in our previous paper [3].
3.3. Screening of Antioxidant-Producing Strain
The bacteria were isolated from soil samples that were collected at different locations in Northern Taiwan. They were cultivated in a medium containing squid pen powder (SPP) (pH 7.2) supplemented with 0.05% MgSO4·7H2O and 0.1% K2HPO4 to screen for antioxidant activity. The strains were cultivated in a 250 mL Erlenmeyer flask that contained 50 mL of medium at 37 °C in a reciprocal shaker at 150 rpm for 1–2 days. The supernatants obtained via centrifugation were used for the estimation of antioxidant activity using the protocol described in our previous paper [7]. Strain TKU036, which showed the highest activity, was selected for further study.
3.4. Extraction and Isolation of HGA and Tryptophan
The culture supernatant (2 L) of Paenibacillus sp. TKU036 was lyophilized (5.824 g) and extracted via ultrasonication in 300 mL of 95% ethanol at 60 °C in triplicate. The extract was concentrated under reduced pressure. The obtained ethanol extract (2.4905 g) was dissolved in H2O, and it was loaded onto an open ODS column and eluted with 0%–60% MeOH in H2O (to maintain the acetic acid concentration at 0.4%), resulting in 14 fractions (Fractions 1 to 14). The tryptophan was found in Fractions 5 (0.1253 g) and 6 (0.0864 g). Fraction 4 was further purified via preparative HPLC, equipped with a 250 mm × 20 mm i.d. preparative Cosmosil 5C18-AR-II column (Nacalai Tesque, Kyoto, Japan) and a UV detector at 254 nm, and it was eluted with 13% ACN in H2O (0.4% acetic acid), yielding 5 fractions (Fractions 4-1 to 4-5). HGA (0.012 g) and tryptophan (0.008 g) were obtained from Fractions 4-4 and 4-5, respectively. In total, HGA (0.012 g) and tryptophan (0.21 g) were produced from 2 L of the culture supernatant (5.824 g) by employing the method described above. The recovery of HGA (60 mg/L) in this study was comparable to that of HGA in strawberry tree honey, which has been reported to contain HGA at a concentration of 414 mg/kL [19].
3.5. The Analysis of HGA and Tryptophan by HPLC:
The chromatographic separation was carried out on an Agilent HC-C18 (5 μm, 250 mm × 4.6 mm i.d., Agilent Technologies, Tokyo, Japan). The binary gradient elution system consisted of 0.4% acetic acid aq. (A) and 0.4% acetic acid in acetonitrile (B), and the HPLC profile separation was achieved using the following gradient: 0–20 min, 5%–15% B; 20–35 min, 15%–35% B; and 35–40 min, 35%–100% B. The UV detection wavelength was 292 nm. The column was kept at room temperature. The flow rate was 0.8 mL/min, and the injection volume was 10 μL.
4. Conclusions
To efficiently reutilize seafood processings via microbial transformation, squid pen was used as the sole C/N source for screening antioxidant-producing bacteria from Taiwanese soils. The culture supernatant of strain TKU036 produced potential antioxidant activity and was identified as Paenibacillus species. The antioxidant compound in the culture supernatant was identified as HGA, which showed anti-inflammatory effects as well. Tryptophan was also identified in the culture supernatant. Neither HGA nor tryptophan was found in the unfermented SPP-containing medium or in other chitinous materials (shrimp head powder)-containing medium. The results showed that squid pen is a promising material for the production of antioxidants and anti-inflammatories by Paenibacillus sp. TKU036.
HGA showed higher antioxidant activity (IC50 = 6.9 μg/mL) than α-tocopherol (IC50 = 17.6 μg/mL). The anti-inflammation activity of HGA (IC50 = 10.14 μg/mL) was lower than that of quercetin (IC50 = 1.14 μg/mL). HGA has been reported as the most abundant phenolic compound in strawberry tree honey (414 mg/kg) [36]. The recovery of 60 mg of HGA per liter of culture supernatant, compared with that of strawberry tree honey, seems to have potential for HGA production.
Acknowledgments
This work was supported in part by a grant from the Ministry of Science and Technology, Taiwan (MOST 105-2313-B-032-001, MOST 104-2811-B-032-001, MOST 104-2320-B-077-006-MY3, and MOST 101-2320-B-077-006-MY3).
Author Contributions
S.-L.W. conceived and designed the experiment; H.-T.L. and Z.-H.L. performed the experiments; S.-L.W., Y.-H.K. and L.-J.Z. analyzed the data; S.-L.W. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wang S.L., Liang T.W. Microbial reclamation of squid pen and shrimp shell. Res. Chem. Intermed. 2016 doi: 10.1007/s11164-016-2425-y. [DOI] [Google Scholar]
- 2.Wang S.L. Microbial reclamation of squid pen. Biocatal. Agric. Biotechnol. 2012;1:177–180. doi: 10.1016/j.bcab.2012.01.002. [DOI] [Google Scholar]
- 3.Liang T.W., Chen W.T., Lin Z.H., Kuo Y.H., Nguyen A.D., Pan P.S., Wang S.L. An amphiprotic novel chitosanase from Bacillus mycoides and its application in the production of chitooligomers with their antioxidant and anti-inflammatory evaluation. Int. J. Mol. Sci. 2016;17:1302. doi: 10.3390/ijms17081302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen A.D., Huang C.C., Liang T.W., Nguyen V.B., Pan P.S., Wang S.L. Production and purification of a fungal chitosanase and chitooligomers from Penicillium janthinellum D4 and discovery of the enzyme activators. Carbohydr. Polym. 2014;108:331–337. doi: 10.1016/j.carbpol.2014.02.053. [DOI] [PubMed] [Google Scholar]
- 5.Wang S.L., Liang T.W., Yen Y.H. Bioconversion of chitin-containing wastes for the production of enzymes and bioactive materials. Carbohydr. Polym. 2011;84:732–742. doi: 10.1016/j.carbpol.2010.06.022. [DOI] [Google Scholar]
- 6.Liang T.W., Wang S.L. Recent advances in exopolysaccharides from Paenibacillus spp.: Production, isolation, structure, and bioactivities. Mar. Drugs. 2015;13:1847–1863. doi: 10.3390/md13041847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang T.W., Tseng S.C., Wang S.L. Production and characterization of antioxidant properties of exopolysaccharides from Paenibacillus mucilaginosus TKU032. Mar. Drugs. 2016;14:40–51. doi: 10.3390/md14020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo Y.H., Liang T.W., Liu K.C., Hsu Y.W., Hsu H.C., Wang S.L. Isolation and identification of a novel antioxidant with antitumor activity from Serratia ureilytica using squid pen as fermentation substrate. Mar. Biotechnol. 2011;13:451–461. doi: 10.1007/s10126-010-9316-9. [DOI] [PubMed] [Google Scholar]
- 9.Kuo Y.H., Hsu H.C., Chen Y.C., Liang T.W., Wang S.L. A novel compound with antioxidant activity produced by Serratia ureilytica TKU013. J. Agric. Food Chem. 2012;60:9043–9047. doi: 10.1021/jf302481n. [DOI] [PubMed] [Google Scholar]
- 10.Liang T.W., Chen C.H., Wang S.L. Production of insecticidal materials from Pseudomonas tamsuii. Res. Chem. Intermed. 2015;41:7965–7971. doi: 10.1007/s11164-014-1869-1. [DOI] [Google Scholar]
- 11.Wang S.L., Chen S.Y., Yen Y.H., Liang T.W. Utilization of chitinous materials in pigment adsorption. Food Chem. 2012;135:1134–1140. doi: 10.1016/j.foodchem.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 12.Liang T.W., Lo B.C., Wang S.L. Chitinolytic bacteria-assisted conversion of squid pen and its effect on dyes and pigments adsorption. Mar. Drugs. 2015;13:4576–4593. doi: 10.3390/md13084576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sepúlveda L., Aguilera-Carbó A., Ascacio-Valdés J.A., Rodríguez-Herrera R., Martínez-Hernández J.L., Aguilar C.N. Optimization of ellagic acid accumulation by Aspergillus niger GH1 in solid state culture using pomegranate shell powder as a support. Process Biochem. 2012;47:2199–2203. doi: 10.1016/j.procbio.2012.08.013. [DOI] [Google Scholar]
- 14.Raghuwanshi S., Dutt K., Gupta P., Misra S., Saxena R.K. Bacillus sphaericus: The highest bacterial tannase producer with potential for gallic acid synthesis. J. Biosci. Bioeng. 2011;111:635–640. doi: 10.1016/j.jbiosc.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Lambert F., Zucca J., Ness F., Aigle M. Production of ferulic acid and coniferyl alcohol by conversion of eugenol using a recombinant strain of Saccharomyces cerevisiae. Flavour Fragr. J. 2014;29:14–21. doi: 10.1002/ffj.3173. [DOI] [Google Scholar]
- 16.Kang K.A., Chae S., Lee K.H., Zhang R., Jung M.S., You H.J., Kim J.S., Hyun J.W. Antioxidant effect of homogentisic acid on hydrogen peroxide induced oxidative stress in human lung fibroblast cells. Biotechnol. Bioprocess. Eng. 2005;10:556–563. doi: 10.1007/BF02932294. [DOI] [Google Scholar]
- 17.Chen P., Wang Y., Chen L., Jiang W., Niu Y., Shao Q., Gao L., Zhao Q., Yan L., Wang S. Comparison of the anti-inflammatory active constituents and hepatotoxic pyrrolizidine alkaloids in two Senecio plants and their preparations by LC-UV and LC-MS. J. Pharm. Biomed. Anal. 2015;115:260–271. doi: 10.1016/j.jpba.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Wang C.L., Huang T.H., Liang T.W., Wang S.L. Production and characterization of exopolysaccharides and antioxidant from Paenibacillus sp. TKU023. New Biotechnol. 2011;28:559–565. doi: 10.1016/j.nbt.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Liang T.W., Wu C.C., Cheng W.T., Chen Y.C., Wang C.L., Wang I.L., Wang S.L. Exopolysaccharides and antimicrobial biosurfactants produced by Paenibacillus macerans TKU029. Appl. Biochem. Biotechnol. 2014;172:933–950. doi: 10.1007/s12010-013-0568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung Y.C., Chang C.T., Chao W.W., Lin C.F., Chou S.T. Antioxidative activity and safety of the 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J. Agric. Food Chem. 2002;50:2454–2458. doi: 10.1021/jf011369q. [DOI] [PubMed] [Google Scholar]
- 21.Dyah H.W., Joe A.V., Severino S.P. Mathematical modeling of the development of antioxidant activity in soybeans fermented with Aspergillus oryzae and Aspergillus awamori in the solid state. J. Agric. Food Chem. 2009;57:540–544. doi: 10.1021/jf802492s. [DOI] [PubMed] [Google Scholar]
- 22.Miyake Y., Fukumoto S., Okada M., Sakaida K., Nakamura Y., Osawa T. Antioxidative catechol lignans converted from sesamin and sesaminol triglucoside by culturing with Aspergillus. J. Agric. Food Chem. 2005;53:22–27. doi: 10.1021/jf048743h. [DOI] [PubMed] [Google Scholar]
- 23.Lee I.H., Hung Y.H., Chou C.C. Total phenolic and anthocyanin contents, as well as antioxidant activity, of black been koji fermented by Aspergillus awamori under different culture conditions. Food Chem. 2007;104:936–942. doi: 10.1016/j.foodchem.2006.12.049. [DOI] [Google Scholar]
- 24.Kuo C.F., Hou M.H., Wang T.S., Chyau C.C., Chen Y.Y. Enhanced antioxidant activity of Monascus pilosus fermented products by addition of ginger to the medium. Food Chem. 2009;116:915–922. doi: 10.1016/j.foodchem.2009.03.047. [DOI] [Google Scholar]
- 25.Wang S.L., Lin C.L., Liang T.W., Liu K.C., Kuo Y.H. Conversion of squid pen by Serratia ureilytica for the production of enzymes and antioxidants. Bioresour. Technol. 2009;100:316–323. doi: 10.1016/j.biortech.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Cabras P., Angioni A., Tuberoso C., Floris I., Reniero F., Guillou C., Ghelli S. Homogentisic acid: A phenolic acid as a marker of strawberry-tree (Arbutus unedo) honey. J. Agric. Food Chem. 1999;47:4064–4067. doi: 10.1021/jf990141o. [DOI] [PubMed] [Google Scholar]
- 27.Yan X., Suzuki M., Ohnishi-Kameyama M., Sada Y., Nakanishi T., Nagata T. Extraction and identification of antioxidants in the roots of yacon (Smallanthus sonchifolius) J. Agric. Food Chem. 1999;47:4711–4713. doi: 10.1021/jf981305o. [DOI] [PubMed] [Google Scholar]
- 28.Carreira A., Ferreira L.M., Loureiro V. Brown pigments produced by Yarrowia lipolytica result from extracellular accumulation of homogentisic acid. Appl. Environ. Microbiol. 2001;67:3463–3468. doi: 10.1128/AEM.67.8.3463-3468.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kluyver A.J., van Zijp J.C.M. The production of homogentisic acid out of phenylacetic acid by Aspergillus niger. Antonie Van Leeuwenhoek. 1951;17:315–324. doi: 10.1007/BF02062278. [DOI] [PubMed] [Google Scholar]
- 30.Kotob S.I., Coon S.L., Quintero E.J., Weiner R.M. Homogentisic acid is the primary precursor of melanin synthesis in Vibrio cholerae, a hyphomonas strain, and Shewanella colwelliana. Appl. Environ. Microbiol. 1995;61:1620–1622. doi: 10.1128/aem.61.4.1620-1622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L., Duan X., Wu J. Modulating the direction of carbon flow in Escherichia coli to improve l-tryptophan production by inactivating the global regulator FruR. J. Biotechnol. 2016;231:141–148. doi: 10.1016/j.jbiotec.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda M., Katsumata R. Hyperproduction of tryptophan by Corynebacterium glutamicum with the modified pentose phosphate pathway. Appl. Environ. Microbiol. 1999;65:2497–2502. doi: 10.1128/aem.65.6.2497-2502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung M.J., Park J.K., Park Y.I. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int. Immunopharmcol. 2012;12:453–459. doi: 10.1016/j.intimp.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 34.Boots A.W., Drent M., de Boer V.C., Bast A., Haenen G.R. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin. Nutr. 2011;30:506–512. doi: 10.1016/j.clnu.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Guo Y., Huang E., Yuan C., Zhang L., Yousef A.E. Isolation of a Paenibacillus sp. strain and structural elucidation of its broad-spectrum lipopeptide antibiotic. Appl. Environ. Microbiol. 2012;78:3156–3165. doi: 10.1128/AEM.07782-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosa A., Tuberoso C.I.G., Atzeri A., Melis M.P., Bifulco E., Dessì M.A. Antioxidant profile of strawberry tree honey and its marker homogentisic acid in several models of oxidative stress. Food Chem. 2011;129:1045–1053. doi: 10.1016/j.foodchem.2011.05.072. [DOI] [PubMed] [Google Scholar]