Abstract
The H2 subclass of histamine receptors mediates gastric acid secretion, and antagonists for this receptor have proven to be effective therapy for acid peptic disorders of the gastrointestinal tract. The physiological action of histamine has been shown to be mediated via a guanine nucleotide-binding protein linked to adenylate cyclase activation and cellular cAMP generation. We capitalized on the technique of polymerase chain reaction, using degenerate oligonucleotide primers based on the known homology between cellular receptors linked to guanine nucleotide-binding proteins to obtain a partial-length clone from canine gastric parietal cell cDNA. This clone was used to obtain a full-length receptor gene from a canine genomic library. Histamine increased in a dose-dependent manner cellular cAMP content in L cells permanently transfected with this gene, and preincubation of the cells with the H2-selective antagonist cimetidine shifted the dose-response curve to the right. Cimetidine inhibited the binding of the radiolabeled H2 receptor-selective ligand [methyl-3H]tiotidine to the transfected cells in a dose-dependent fashion, but the H1-selective antagonist diphenhydramine did not. These data indicate that we have cloned a gene that encodes the H2 subclass of histamine receptors.
Full text
PDF


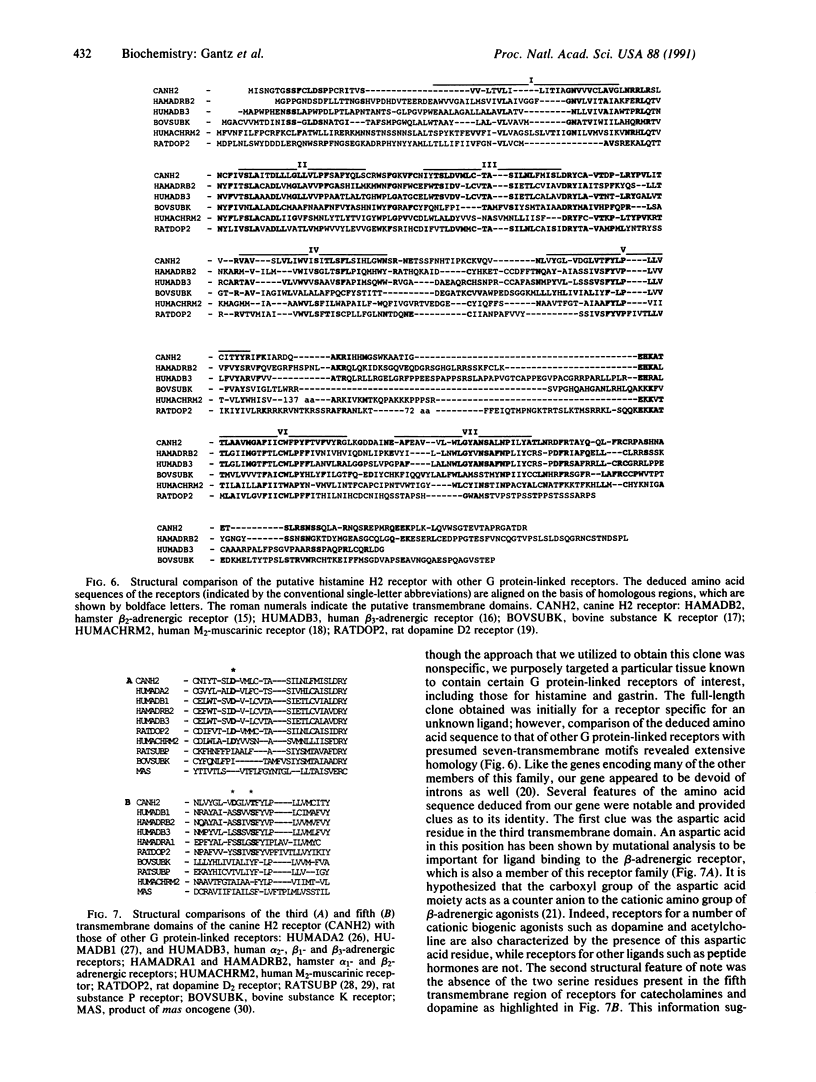

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. M., Abrass I. B., Palmiter R. D. Beta 2-adrenergic receptor regulation after transfection into a cell line deficient in the cAMP-dependent protein kinase. Mol Pharmacol. 1989 Aug;36(2):248–255. [PubMed] [Google Scholar]
- Arrang J. M., Garbarg M., Lancelot J. C., Lecomte J. M., Pollard H., Robba M., Schunack W., Schwartz J. C. Highly potent and selective ligands for histamine H3-receptors. Nature. 1987 May 14;327(6118):117–123. doi: 10.1038/327117a0. [DOI] [PubMed] [Google Scholar]
- Brown N. A., Stofko R. E., Uhler M. D. Induction of alkaline phosphatase in mouse L cells by overexpression of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1990 Aug 5;265(22):13181–13189. [PubMed] [Google Scholar]
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Campbell V. W., Del Valle J., Hawn M., Park J., Yamada T. Carbonic anhydrase II gene expression in isolated canine gastric parietal cells. Am J Physiol. 1989 Mar;256(3 Pt 1):G631–G636. doi: 10.1152/ajpgi.1989.256.3.G631. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crouse G. F., Frischauf A., Lehrach H. An integrated and simplified approach to cloning into plasmids and single-stranded phages. Methods Enzymol. 1983;101:78–89. doi: 10.1016/0076-6879(83)01006-x. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Emorine L. J., Marullo S., Briend-Sutren M. M., Patey G., Tate K., Delavier-Klutchko C., Strosberg A. D. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989 Sep 8;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frielle T., Collins S., Daniel K. W., Caron M. G., Lefkowitz R. J., Kobilka B. K. Cloning of the cDNA for the human beta 1-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7920–7924. doi: 10.1073/pnas.84.22.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey A. D., Krause J. E. Molecular characterization of a functional cDNA encoding the rat substance P receptor. Science. 1990 Feb 23;247(4945):958–962. doi: 10.1126/science.2154852. [DOI] [PubMed] [Google Scholar]
- Jackson T. R., Blair L. A., Marshall J., Goedert M., Hanley M. R. The mas oncogene encodes an angiotensin receptor. Nature. 1988 Sep 29;335(6189):437–440. doi: 10.1038/335437a0. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., MacGregor C., Daniel K., Kobilka T. S., Caron M. G., Lefkowitz R. J. Functional activity and regulation of human beta 2-adrenergic receptors expressed in Xenopus oocytes. J Biol Chem. 1987 Nov 15;262(32):15796–15802. [PubMed] [Google Scholar]
- Kobilka B. K., Matsui H., Kobilka T. S., Yang-Feng T. L., Francke U., Caron M. G., Lefkowitz R. J., Regan J. W. Cloning, sequencing, and expression of the gene coding for the human platelet alpha 2-adrenergic receptor. Science. 1987 Oct 30;238(4827):650–656. doi: 10.1126/science.2823383. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Caron M. G. Adrenergic receptors. Models for the study of receptors coupled to guanine nucleotide regulatory proteins. J Biol Chem. 1988 Apr 15;263(11):4993–4996. [PubMed] [Google Scholar]
- Libert F., Parmentier M., Lefort A., Dinsart C., Van Sande J., Maenhaut C., Simons M. J., Dumont J. E., Vassart G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989 May 5;244(4904):569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- Masu Y., Nakayama K., Tamaki H., Harada Y., Kuno M., Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. 1987 Oct 29-Nov 4Nature. 329(6142):836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Smith D. H., Ramachandran J., Capon D. J. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987 Dec 20;6(13):3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H. The actions of secretagogues on oxygen uptake by isolated mammalian parietal cells. J Clin Invest. 1978 Feb;61(2):370–380. doi: 10.1172/JCI108947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Candelore M. R., Rands E., Hill W. S., Dixon R. A. Conserved aspartic acid residues 79 and 113 of the beta-adrenergic receptor have different roles in receptor function. J Biol Chem. 1988 Jul 25;263(21):10267–10271. [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Dixon R. A. Structural basis of beta-adrenergic receptor function. FASEB J. 1989 May;3(7):1825–1832. doi: 10.1096/fasebj.3.7.2541037. [DOI] [PubMed] [Google Scholar]
- Yokota Y., Sasai Y., Tanaka K., Fujiwara T., Tsuchida K., Shigemoto R., Kakizuka A., Ohkubo H., Nakanishi S. Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem. 1989 Oct 25;264(30):17649–17652. [PubMed] [Google Scholar]





