Abstract
Background
Increasing evidence indicates that psychosis is associated with abnormal reward processing. Imaging studies in patients with first-episode psychosis (FEP) have revealed reduced activity in diverse brain regions, including the ventral striatum, insula and anterior cingulate cortex (ACC), during reward prediction. However, whether these reductions in local brain activity are due to altered connectivity has rarely been explored.
Methods
We applied dynamic causal modelling and Bayesian model selection to fMRI data during the Salience Attribution Task to investigate whether patients with FEP showed abnormal modulation of connectivity between the ventral striatum, insula and ACC induced by rewarding cues and whether these changes were related to positive psychotic symptoms and atypical antipsychotic medication.
Results
The model including reward-induced modulation of insula–ACC connectivity was the best fitting model in each group. Compared with healthy controls (n = 19), patients with FEP (n = 29) revealed reduced right insula–ACC connectivity. After subdividing patients according to current antipsychotic medication, we found that the reduced insula–ACC connectivity relative to healthy controls was observed only in untreated patients (n = 17), not in patients treated with antipsychotics (n = 12), and that it correlated negatively with unusual thought content in untreated patients with FEP.
Limitations
The modest sample size of untreated patients with FEP was a limitation of our study.
Conclusion
This study indicates that insula–ACC connectivity during reward prediction is reduced in untreated patients with FEP and related to the formation of positive psychotic symptoms. Our study further suggests that atypical antipsychotics may reverse connectivity between the insula and the ACC during reward prediction.
Introduction
Our brain is constantly exposed to a wide variety of stimuli, which compete for limited cognitive resources. External stimuli are processed depending on their salience so as to ignore predictable, state and task-irrelevant events while enhancing resource allocation in order to process unexpected or state- and task-relevant events. Efficient prediction of salient stimuli, such as those of rewards, is thus essential for adapting ongoing behaviour. This process requires the ability to learn that a neutral stimulus becomes emotionally endowed owing to its association with primary reinforcement.1 Behavioural and fMRI studies have demonstrated impairments in patients with psychosis when anticipating reward.2 Relative to controls, behavioural evidence indicated that patients with first-episode psychosis (FEP) exhibited less reactivity to reward-predicting cues.3 Functional MRI studies during reward prediction have reported reduced activity in diverse brain regions, including the ventral striatum (VS),4,5 anterior cingulate cortex (ACC), midbrain, thalamus and cerebellum, of unmedicated patients with FEP compared with controls.5 It has further been shown that VS activation during reward prediction was negatively related to positive psychotic symptoms in patients with FEP.4,5
Reward processing is critically mediated by dopamine,6,7 and the VS response to reward-predicting cues is likely triggered by dopamine activity.8,9 A previous fMRI study in patients with chronic schizophrenia showed that the VS response during reward prediction was reduced only in patients treated with typical antipsychotics, whereas no difference was observed between healthy controls and patients treated with atypical medication.10 In line with this finding in patients with chronic schizophrenia, the reduced baseline VS activation during reward prediction seen in patients with FEP relative to healthy controls has been reported to be normalized after 6 weeks of monotherapy with atypical antipsychotics.11 The largest improvement in positive symptoms was seen in patients with the highest VS signal increase.11 Although not specifically during reward processing, a recent resting-state fMRI study in patients with FEP also showed that atypical antipsychotics increased functional connectivity between striatal regions, the ACC and right anterior insula,12 which correlated positively with symptom improvement. Using the Salience Attribution Task (SAT),13 Smieskova and colleagues14 recently reported that compared with controls patients with FEP showed reduced right insula activity in response to high-versus low-probability reward cues. Furthermore, the right insula and ACC activity was negatively correlated with the severity of hallucinations in unmedicated patients.14 These 3 fMRI studies together show local activity changes mainly in the VS, insula and ACC in patients with FEP during reward prediction4,5,14 as well as alterations in these regions induced by antipsychotic medication.11,14 One previous fMRI study in unmedicated patients with schizophrenia showed reduced connectivity between the prefrontal cortex and the VS during reward processing.15 However, it remains unclear whether the local brain activity changes in patients with FEP during reward prediction may result from alterations in the underlying connectivity.
In the present study, we applied dynamic causal modelling (DCM)16 and Bayesian model selection (BMS)17 to the fMRI data reported by Smieskova and colleagues14 to address the following questions. First, among connectional models including the visual cortex, VS, insula and ACC, we investigated the regions where the high-probability reward cues operate and modulate connectivity strengths. We included the visual cortex as a sensory input region in our models based on evidence showing that reward also modulates responses in the visual cortex.18 Second, we investigated differences between healthy controls and patients with FEP in the connectivity strengths obtained from the best fitting model and investigated possible effects of atypical antipsychotics. Finally, we explored the association between the modulation of connectivity induced by high-probability reward cues and the expression of positive symptoms in patients with FEP.
Methods
Participants
Participants were recruited in a specialized clinic for the early detection of psychosis at the University Hospital of Psychiatry, Basel, Switzerland. All participants provided written informed consent and received compensation for participating. The study was approved by the local ethics committee (Ethikkommission Nordwest- und Zentralschweiz [EKNZ]). All patients were competent to give informed consent. They were able to understand relevant study information, including the reasons why they were being asked to participate and the procedures of the study, and they understood the consequences of accepting or declining the invitation to participate and how to discontinue their participation.
We recruited patients with FEP who fulfilled the criteria for acute psychotic disorder according to the ICD-10 or DSM-IV, but who did not yet fultull the criteria for schizophrenia.19 At study intake, we assessed patients using the Brief Psychiatric Rating Scale (BPRS), the Scale for the Assessment of Negative Symptoms (SANS) and the Global Assessment of Functioning (GAF). Inclusion required scores of 4 or above on the hallucination item or 5 or above on the unusual thought content, suspiciousness or conceptual disorganization items of the BPRS,19 with symptoms occurring at least several times a week and persisting for more than 1 week. We obtained data on current nicotine, cannabis and other illegal drug consumption using a semi-structured interview adapted from the Early Psychosis Prevention and Intervention Centre Drug and Alcohol Assessment Schedule (www.eppic.org.au) and applied the following exclusion criteria: history of previous psychotic disorder, psychotic symptomatology secondary to an organic disorder, recent substance abuse according to ICD-10 research criteria, psychotic symptomatology associated with an affective psychosis or a borderline personality disorder, age younger than 18 years, inadequate knowledge of the German language, and IQ lower than 70.
We recruited healthy controls from the same geographical area as patients. To be included in the study, controls had to have no current psychiatric disorder; no history of psychiatric illness, head trauma, neurologic illness, serious medical or surgical illness or substance abuse; and no family history of any psychiatric disorder as assessed by an experienced psychiatrist in a detailed clinical assessment.
Salience Attribution Task
The SAT has been previously described in more detail.13,20,21 In brief, the SAT is a speeded-response game with a monetary reward, and it measures responses to task-relevant and task-irrelevant cue features.21 Participants had to respond to a briefly presented square. Before the onset of the square, participants saw different categories of cues indicating the likelihood of reward on a given trial. Participants received a monetary reward on 50% of trials, with more money for faster responses. The cues varied in 2 different visual dimensions — colour (red or blue) and shape (animals or household objects) — with 1 of these cue dimensions being task-relevant and the other task-irrelevant. In the task-relevant dimension, 1 cue dimension was highly associated with receiving a reward, with 87.5% of these trial types rewarded (e.g., blue animals and households), while only 12.5% of the alternative cue dimension was rewarded (e.g., red animals and households). In the task-irrelevant dimension, 50% of both cue types were rewarded (e.g., 50% of all animals and 50% of all households). Participants were not informed about the contingencies, which remained the same over blocks, and had to learn them during the task. They were also asked to estimate reward probabilities for each of the 4 stimulus categories after each session using visual analogue scales (VAS) ranging from 0% to 100%. The SAT provides behavioural (in terms of VAS ratings and reaction times) and neuronal measures of adaptive (task-relevant features) and aberrant (task-irrelevant features) reward prediction. An example trial during the SAT is shown in Appendix 1, available at jpn.ca. Based on our previous findings showing neuronal differences between healthy controls and patients with FEP during adaptive reward prediction,14 the present connectivity analysis focused on behavioural and neural effects during adaptive reward prediction (high-probability v. low-probability rewarding cues).
Image acquisition and analysis
Scanning was performed with a whole-body 3 T MRI system (Magnetom Verio, Siemens Healthcare). During the SAT, we acquired T2*-weighted echo-planar images (EPI) with the following parameters: 38 axial slices of 3 mm thickness, 0.5 mm interslice gap, field of view 228 × 228 cm2, in-plane resolution of 3 × 3 mm2, repetition time (TR) 2.5 s, and echo time (TE) 28 ms. The EPIs were analyzed using SPM8 software (www.fil.ion.ucl.ac.uk/spm). During preprocessing, images were realigned and unwarped, spatially normalized to the Mon-treal Neurological Institute (MNI) space template (including reslicing to 2 × 2 × 2 mm voxels) and smoothed with an 8 mm full-width at half-maximum Gaussian kernel. We first checked the realignment parameters of each individual to identify scans on which sharp movements (bigger than half of the voxel size [1.5 mm] and/or more than 1.5°) had occurred and inspected those scans manually. Corrupted images were excluded and replaced with the average of the neighbouring images. No participant had more than 10% corrupted images owing to movement. Maximum likelihood parameter estimates were then calculated at the first level at each voxel using a general linear model (GLM). Our design matrix included an autoregressive (AR(1)) model of serial correlations and a high-pass filter with a cutoff of 128 s. The onsets of each event (duration 2 s for the cue and 1.5 s for the outcome regressor) were convolved with the hemodynamic response function and its temporal and dispersion derivatives. The first-level design matrix included 4 cue regressors (blue/red animals, blue/red objects), an outcome regressor and its parametric modulation by magnitude of reward.
Volumes of interest
We selected the bilateral visual cortex (left: x, y, z = −24, −98, −8; right: x, y, z = 22, −98, −6), VS (left: x, y, z = −14, 6, −4; right: x, y, z = 14, 6, −8), insula (left: x, y, z = −34, 14, 0; right: x, y, z = 34, 24, 6), as well as the dorsal ACC (x, y, z = −4, 16, 28) as volumes of interest (VOIs) based on following information: 1) the previously published second-level SPM analysis of these data showing reduced right insula and ACC activity in patients with FEP,14 2) previous fMRI studies in patients with FEP showing reduced activity in the VS4,5 and ACC5 during reward prediction, and 3) evidence demonstrating that reward prediction responses in the VS were normalized after atypical antipsychotic medication in patients with FEP.11 The visual cortex coordinates were based on the activation induced by all stimuli (high- and low-probability rewarding cues) collapsed across groups, while the coordinates for the VS, insula and ACC were specified from the contrast of high-probability minus low-probability rewarding cues (cluster-forming threshold of p = 0.001, uncorrected, family-wise error (FWE)–corrected at the cluster level at p < 0.05). For each participant, regional time series from these VOIs were extracted within spheres of 4 mm radii centred on the peak of the contrasts of interest within the same anatomic area, as defined by the PickAtlas toolbox22 (p < 0.01, uncorrected, adjusted for effects of interest F contrasts).
Network analysis: DCM
We used DCM10 (revision No. 4290) in SPM8 to explore causal interactions among our VOIs. Dynamic causal modelling16 is a hypothesis-driven method that does not explore all possible models, but tests a specified model space based on prior knowledge about the system of interest. The bilinear DCM for fMRI infers dynamics at the neuronal level by translating modelled neuronal responses into predicted blood-oxygen level–dependent (BOLD) measurements. Specifically, DCM allows modelling how neural states (reflecting specific brain regions) change as a function of endogenous interregional connections, modulatory effects on these connections, and driving inputs.16 In this study, we particularly applied DCM to probe how the endogenous connections induced by all stimuli are modulated by high-probability rewarding cues (modulatory effect).
Model space construction
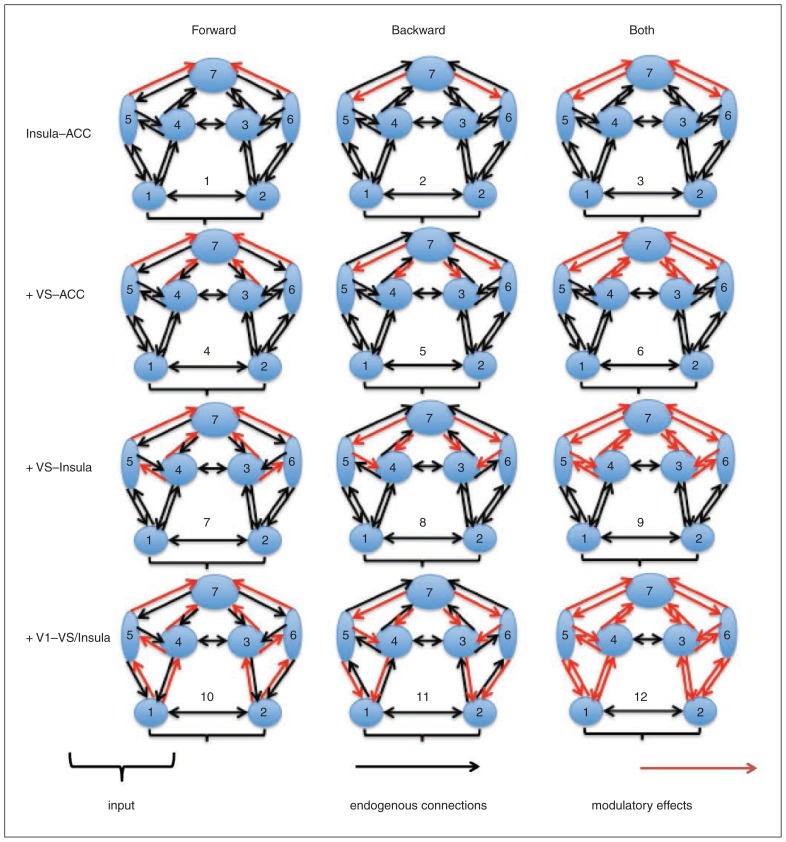
Across all models tested, we assumed the same network layout with reciprocal connections between the VS, insula and ACC. The bilateral visual cortex was further incorporated as sensory input regions, which were reciprocally connected with the insula and VS. Bilateral visual cortices and the VS exhibited interhemispheric connections as well. This base model was then elaborated systematically to produce alternative variants, which varied in where the effect of high-probability reward cues modulated connections among our VOIs (Fig. 1). These variations were guided by studies highlighting functional ACC–insula,23,24 ACC–VS25 and insula–VS18,26 interactions during reward processing and by studies providing evidence for an involvement of the visual cortex (and their connections to the insula and VS) in reward processing.18,27 In particular, we allowed high-probability reward cues to modulate 1) only ACC–insula connectivity, 2) ACC–insula and ACC–VS connectivity, 3) ACC–insula, ACC–VS and insula–striatum connectivity, and 4) ACC–insula, ACC–VS, insula–striatum, visual cortex–insula and visual cortex–VS connectivity. These 4 options were crossed with the possibility that high-probability reward cues affected either forward, backward or both forward and backward connections within the hierarchical network. This additional fractioning was driven by the principle of predictive coding,28,29 which proposes neuronal message passing among different levels of cortical hierarchies.
Fig. 1.
Model space construction. Numbers 1 through 7 indicate the left and right visual cortex, right and left striatum, left and right insula, and anterior cingulate cortex (ACC), respectively. Twelve different variations of dynamic causal modelling were created depending on where the modulation of high-probability reward cues exerted its effect (red arrows) on the endogenous connections (black arrows). VS = ventral striatum.
Bayesian model selection
We used BMS17 to determine the most plausible model of the ones we considered. The BMS method rests on comparing the (log) evidence of a predefined set of models (see the previous section on model space construction). The model evidence is the probability of observing the empirical data, given a model, and represents a principled measure of model quality derived from probability theory.17 We used a random-effects BMS approach for group studies, which is capable of quantifying the degree of heterogeneity in a population while being extremely robust to potential outliers.30 A common way to summarize the results of random-effects BMS is to report the exceedance probability (EP) of each model (i.e., the probability that this model is more likely than any other of the models tested, given the group data).
Statistical analysis
We used 1-way analysis of variance (ANOVA) and χ2 tests to examine between-group differences in clinical, demographic and behavioural characteristics, and we applied Bonferroni post hoc testing to correct for multiple comparisons. The connectivity analysis was based on the summary statistics approach in DCM (i.e., model selection followed by interrogation of posterior estimates).31 In particular, we used the posterior means reflecting the modulatory effect from the best fitting model obtained from BMS for the ANOVA analysis. In a first step, all patients with FEP were treated as 1 group. A second ANOVA with 3 groups was then applied to address the effect of antipsychotics. Finally, we used Pearson correlation analysis to assess the association between significant group differences in connectivity strengths and positive psychotic symptoms (indexed by BPRS items 9, 10, 11 and 15) in treated and untreated patients with FEP. The statistical threshold was adjusted for the number of correlations performed for both patient groups separately (n = 4; p < 0.5 ÷ 4). We tested the influence of potential outliers for each correlation using the Cook distance test (critical value: 4/(n – k – 1) = 0.33 and 0.57, respectively). No outliers were detected.
Results
Participants
We recruited 30 patients with FEP for participation in the study.19 The upper limit of the duration of psychosis was 5 years, and the mean duration of illness was 7.76 ± 15.77 months. One patient was not able to continue the MRI examination, leaving 29 patients with FEP for our analyses. We recruited 23 controls for participation in the study; 4 had to be excluded owing to brain vascular abnormalities (n = 3) and arachnoid cyst (n = 1), leaving 19 controls for our analyses. The demographic and clinical characteristics of the final study sample are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the study population
| Group; mean ± SD or no. (%) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Characteristic | Control n = 19 |
FEP, treated n = 12 |
FEP, untreated n = 17 |
Statistic | p value | Bonferroni post hoc |
| Age, yr | 26.42 ± 4.11 | 27.42 ± 7.93 | 24.82 ± 1.38 | F2,47 = 0.749 | p = 0.48 | — |
| Female sex | 9 (47) | 6 (50) | 4 (24) | χ22 = 2.858 | p = 0.24 | — |
| Right-handedness | 18 (95) | 11 (92) | 16 (94) | χ22 = 0.124 | p = 0.940 | — |
| MWT | 113 ± 9.88 | 105 ± 19.63 | 103 ± 12.27 | F2,47 = 2.570 | p = 0.09 | — |
| BPRS total | 24.53 ± 1.7 | 42.75 ± 14.75 | 51.71 ± 15.53 | F2,47 = 24.687 | p < 0.001 | HC < FEP, treated HC < FEP, untreated |
| Suspiciousness (BPRS 9) | 1.00 ± 0.00 | 3.00 ± 1.71 | 3.47 ± 1.38 | F2,47 = 22.059 | p < 0.001 | HC < FEP, treated HC < FEP, untreated |
| Hallucinations (BPRS 10) | 1.00 ± 0.00 | 2.42 ± 2.15 | 3.53 ± 2.0 | F2,47 = 11.781 | p < 0.001 | HC < FEP, treated HC < FEP, untreated |
| Unusual thought content (BPRS 11) | 1.00 ± 0.00 | 3.25 ± 1.87 | 3.71 ± 1.9 | F2,47 = 17.431 | p < 0.001 | HC < FEP, treated HC < FEP, untreated |
| Conceptual disorganization (BPRS 15) | 1.00 ± 0.00 | 2.08 ± 1.31 | 2.06 ± 1.30 | F2,47 = 6.561 | p = 0.003 | HC < FEP, treated HC < FEP, untreated |
| SANS total | 0.00 ± 0.0 | 17.08 ± 16.21 | 21.82 ± 14.88 | F2,47 = 16.396 | p < 0.001 | HC < FEP, treated HC < FEP, untreated |
| GAF total | 88.63 ± 4.52 | 63.50 ± 9.65 | 53.06 ± 17.95 | F2,47 = 41.171 | p < 0.001 | HC < FEP, treated HC < FEP, untreated |
| Antidepressants | 0 (0) | 3 (25) | 4 (24) | χ22 = 5.381 | p = 0.07 | — |
| Cannabis | 4 (21) | 1 (8) | 7 (41) | χ22 = 4.308 | p = 0.12 | — |
| Cigarettes, no./d | 2.47 ± 5.834 | 9.42 ± 8.207 | 10.88 ± 11.522 | F2,47 = 4.618 | p = 0.015 | HC < FEP, untreated |
BPRS = brief psychiatric rating scale; FEP = first-episode psychosis; GAF = Global Assessment of Functioning; HC = healthy controls; MWT = Mehrfachwahl-Wortschatz-Intelligenz-Test, (a multiple choice vocabulary intelligence test); SANS = Scale for the Assessment of Negative Symptoms; SD = standard deviation.
Among patients with FEP, 12 were taking the following atypical antipsychotics: quetiapine (n = 6), olanzapine/aripiprazole (n = 2) and paliperidone/risperidone (n = 1). The remaining 17 patients were not taking antipsychotic medication at the time of the study; 11 of them were antipsychotic-naive and 6 were antipsychotic-free. Seven patients were taking antidepressants.
Behavioural scores on adaptive reward prediction
Compared with healthy controls, patients with FEP showed reduced VAS ratings at a trend level (F1,47 = 2.906, p = 0.10). We found no group difference for reaction times (F1,47 = 2.561, p = 0.12). Subsequent ANOVA analysis with 3 groups revealed no differences among healthy controls, treated and untreated patients with FEP for both VAS ratings (F1,47 = 2.165, p = 0.13) and reaction times (F1,47 = 1.379, p = 0.26).
Network analysis (DCM results)
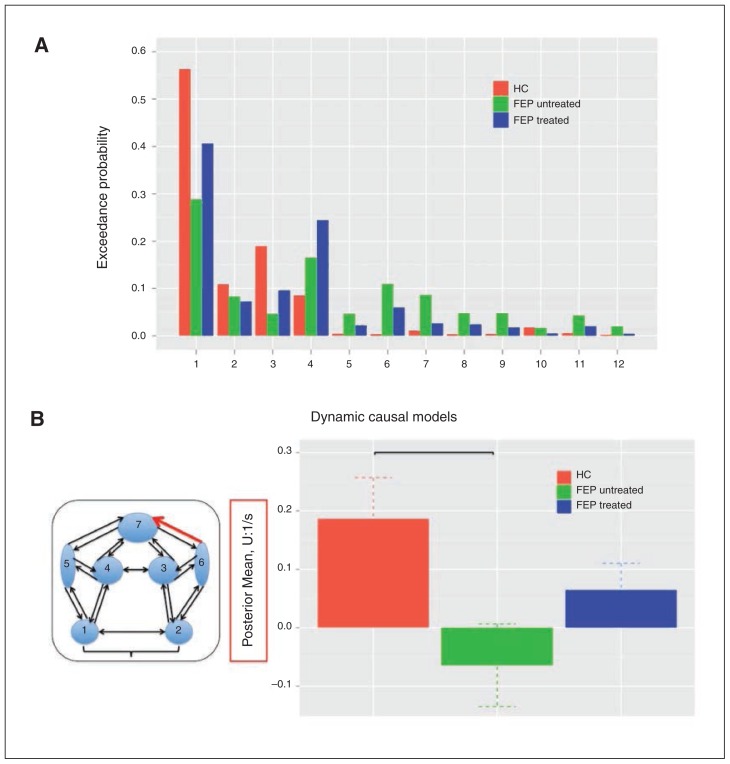
Random-effects BMS revealed model 1 as the best fitting model in healthy controls (EP: 56%) and all patients with FEP (EP: 65%). Model 1 was also superior to all other models tested if patients were separated into treated (EP: 29%) and untreated (EP: 41%) categories (Fig. 2A).
Fig. 2.
(A) Bayesian model selection (BMS) results among all 12 dynamic causal models (DCMs) for each group separately. Results are expressed in terms of exceedance probability, the relative probability that this model is more likely than any other of the models tested, given the group data. (B) Significant group differences in the modulation of right insula–anterior cingulate cortex (ACC) connectivity induced by high-probability reward cues. In particular, the modulation of right insula–ACC connectivity was significantly reduced in untreated patients with first-episode psychosis (FEP) compared with healthy controls (HC), whose connectivity strengths did not differ from those of treated patients with FEP.
Group differences in effective connectivity
In our final group-level analysis, we were able to test for differences in 2 parameters describing the modulation of connections induced by high-probability reward cues (model 1). We found a significant reduction in the modulation of right insula–ACC connectivity (F1,47 = 5.976, p = 0.018) but not in the modulation of left insula–ACC connectivity (F1,47 = 0.320, p = 0.57) in all patients with FEP relative to healthy controls.
Effects of antipsychotics on effective connectivity
The subsequent 3-group ANOVA analysis revealed a significant group effect on the modulation of right insula–ACC connectivity (F2,47 = 3.823, p = 0.029) but not left insula–ACC connectivity (F2,47 = 0.281, p = 0.76). Compared with healthy controls, post hoc testing showed that the modulation of right insula–ACC connectivity induced by high-probability reward cues was significantly reduced in untreated (p = 0.025) but not antipsychotic-treated patients (p = 0.70; Fig. 2B, Table 2).
Table 2.
Dynamic causal modelling parameters from the best fitting model
| Group; mean ± SD* | |||
|---|---|---|---|
|
|
|||
| Connectivity | Control n = 19 |
FEP, treated n = 12 |
FEP, untreated n = 17 |
| Right insula– ACC† | 0.1867 ± 0.3064‡ | 0.0651 ± 0.1567 | −0.0642 ± 0.2877 |
| Left insula– ACC | 0.0976 ± 0.3138 | 0.1182 ± 0.2234 | 0.1879 ± 0.4933 |
ACC = anterior cingulate cortex; FEP = first-episode psychosis; SD = standard deviation.
Modulatory effect induced by high-probability reward cues.
F2,47 = 3.823, p = 0.029 for analysis of variance, and p = 0.025 (healthy controls > untreated FEP) for Bonferroni-corrected post hoc t test.
Significant t tests within each group compared with 0 (p < 0.05).
Association between abnormal connectivity and positive symptoms
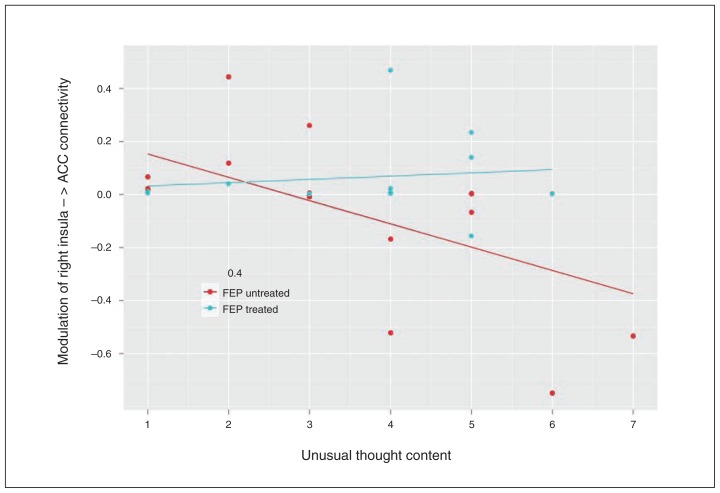
Pearson correlation analysis indicated a significant negative correlation between the modulatory effect on right insula–ACC connectivity induced by high-probability reward cues and the formation of unusual thought content (BPRS item 11) in untreated (r = −0.593, p = 0.012, corrected for multiple testing) but not in treated patients with FEP (r = 0.127, p = 0.69; Fig. 3). No correlations between right insula–ACC connectivity and BPRS items 9, 10 and 15 were found.
Fig. 3.
Negative correlation between the modulation of right insula–anterior cingulate cortex (ACC) connectivity and unusual thought content across untreated (r = −0.593, p = 0.012), but not treated (r = 0.127, p = 0.70) patients with first-episode psychosis (FEP). The X axis represents patients’ unusual thought content as indexed by the Brief Psychiatric Rating Scale item 11. The Y axis represents the posterior mean (1/s) of the modulation of right insula–ACC connectivity induced by high-probability reward cues.
Discussion
This study demonstrates that right insula–ACC connectivity during reward prediction is significantly reduced in patients with FEP compared with healthy controls. Importantly, this reduced insula–ACC connectivity is evident only in untreated, not treated patients, and is negatively associated wtih the formation of unusual thought content in untreated patients.
Irrespective of the diagnostic group, the BMS results revealed that reward cues essentially modulated insula–ACC connectivity within our network, supporting the key role of this functional coupling during salience processing.23,32 This finding dovetails with the concept of proximal salience.24 This concept proposes that the processing of incoming stimuli induces a proximal salience signal in the insula depending on its predictability, which indicates whether further downstream processing is required to adjust one’s predictive model. The downstream processing includes motor action, updating the prefrontal fund of knowledge or stopping an activity that is ongoing. All of these downstream activities require resource allocation to appropriate networks and are initiated by insula– ACC interactions. With respect to the SAT, high-probability reward cues are the ones that require further downstream processing and action. The observation that these stimuli modulate insula–ACC connectivity adds support to the notion that the role of the insula–ACC network lies in the formation of stimulus-response association (proximal salience), which precedes the learning of stimulus-reinforcement associations (motivational salience) in which hippocampal–midbrain– striatal connections may play a more crucial role.
We further found a reduced right insula–ACC connectivity in untreated patients with FEP compared with healthy controls. Moreover, the degree of insula–ACC connectivity was negatively correlated with the formation of unusual thought content in these patients. These findings extend our previous results of reduced ACC activity in unmedicated patients with FEP and the association between positive symptoms in untreated patients with FEP and regional activity in the right in-sula and ACC in response to high-probability reward cues.14 Given that the psychopathological assessment occurred at study intake and that imaging occurred later, dysfunctional insular connectivity could thus reflect vulnerability to positive symptom formation. Although functional connectivity studies extract a bilateral salience network pattern involving both the right and left insula and ACC,23 the right-hemispheric asymmetry is reminiscent of studies that use temporal information (e.g., Granger causality or DCM).33–35 A meta-analysis revealed that both the insula and ACC were accompanied by significant grey matter reductions in patients with FEP,36 which might provide a scaffold for the reduction of insula–ACC connectivity observed here. In accordance with this, deficits in grey matter volumes in the insula and ACC have also been negatively associated with delusion and hallucinations in psychotic patients.37 However, grey matter losses in the ACC and insula have been detected across different psychiatric diagnoses and may not be specific to psychosis.38 Within the framework of proximal salience, deficient insular detection of external salient events, such as those of rewarding cues, might lead to a faulty allocation of salience to internally generated thoughts and impede the attention to relevant external information.24 The internal mental state might be further enhanced by inappropriate salience, promoting the formation of various psychotic symptoms, such as hallucinations and delusions.24 Unlike hallucinations and delusions, illogical thinking may be more pronounced when study participants are interacting with stimuli, such as in carrying out a task inside a scanner. The association between ACC–insula dysconnectivity when processing rewarding cues and the severity of thought content that we observed suggests that aberrant assignment of salience to task-relevant stimuli may enhance the emergence of illogical and bizarre ideas in patients with FEP.
The putative imbalance between active inference processes about external phenomena and self-generated internal reflections may result from a failure of the insula–ACC network and in particular of the insula to switch between these 2 alternating systems. This interpretation is motivated by a recent model proposing that activation in the insula–ACC network is negatively correlated with the engagement of the default mode network,23 a system that is active during the construction of self-relevant mental simulations.39 Reduced negative correlation between the default mode network and the task-positive network has already been observed in individuals at clinically high risk for psychosis. Notably, a negative association was found between the correlation of default mode network and the task-positive network and the expression of cognitive impairments.40
Importantly, the reduced insula–ACC connectivity was evident only in untreated, not antipsychotic-treated, patients with FEP, suggesting a normalization of this functional coupling via dopamine D2 receptor antagonism together with serotonin 2A receptor antagonism.41 This result corresponds to conclusions from a recent review that the BOLD signal in specific neural regions normalizes over the course of antipsychotic treatment42 and to a recent resting-state fMRI study showing that antipsychotic-induced improvement of psychotic symptoms was accompanied by increased functional connectivity among striatal regions, the ACC and the anterior insula.12 The antipsychotic effect in treated patients can perhaps be explained by the underlying structure as well, given that insular and ACC volumes increase with increasing antipsychotic exposure in psychotic patients.43,44 However, meta-analytical evidence indicates that ACC and insula volume is particularly decreased in treated patients with FEP.45 More studies are needed to understand the structure–function relationship of the insula–ACC network in patients with psychosis and the alterations induced by antipsychotics.
Limitations
There are some limitations to be considered in the present study. We restricted our analysis to striatal–insular–ACC connectivity although there are also other regions activated in response to high-probability reward cues during the SAT, such as the midbrain, medial dorsal thalamus and prefrontal cortex,20 and a previous study during the processing of aversive outcomes showed reduced functional connectivity between the medial prefrontal cortex and the VS in unmedicated patients with schizophrenia compared with healthy controls.15 More research is required to study (abnormal) functional connectivity during reward processing, including feedback phases and the processing of aversive stimuli. We cannot completely rule out that smoking has confounded our findings given the impact of smoking on the connectivity between the ACC and insula in patients with schizophrenia.46 However, there were no correlations between the left (r = −0.67, p = 0.65) and right (r = −0.65, p = 0.66) insula–ACC connectivity and smoking behaviour across all participants. Furthermore, abnormal insula–ACC connectivity seems to be task-specific. While insula–ACC dysconnectivity is not prominent in resting-state conditions,33 our results showed that when high-probability rewarding cues were presented, this network was not generating the neural readiness required for further action on the reward predicting stimuli as, for example, the formation of stimulus-reinforcement association. Another point of contention is that we found connectivity differences across groups in association with antipsychotic medication, although no significant effects were found for the behavioural indices. However, a significant effect on brain activations but not behavioural performance is a common finding in fMRI studies and can be explained by the fact that functional neuroimaging techniques detect changes at the physiologic level and are more sensitive than behavioural measures.47 Finally, this study analyzed a relatively modest number of treated and untreated patients with FEP. Larger samples sizes are needed to replicate our findings.
Conclusion
Our study demonstrates that patients with FEP exhibit reduced right insula–ACC connectivity during reward prediction and that abnormal insula–ACC connectivity may make patients more vulnerable to the development of psychotic symptoms. Our findings also suggest that atypical antipsychotics reverse insula–ACC connectivity during reward prediction in patients with FEP. Longitudinal studies with larger samples are needed in order to draw robust inferences on medication effects on insula–ACC connectivity and to validate whether the assessment of effective insula connectivity during reward prediction may reflect an important brain marker of treatment effectiveness in psychosis.
Acknowledgements
This work was supported by the Swiss National Science Foundation (grant number P2ZHP3_155184 awarded to A. Schmidt; grant number 3232BO_119382 awarded to R. Smieskova and S.J. Borgwardt). The authors thank Jon Roiser for providing the SAT task.
Footnotes
Competing interests: L. Palaniyappan declares royalties from Oxford University Press for books published for MRCPsych revision, fees for preparing MRCPsych teaching materials, travel support from Magstim Limited, and grants unrelated to the present work from the Medical Research Council UK and EU CASCADE Programme. He also declares that his spouse’s pension fund holds shares in GlaxoSmith-Kline. No other competing interests declared.
Contributors: R. Smieskova, A. Riecher-Rössler, P. McGuire and S. Borgwardt designed the study. R. Smieskova, A. Simon and A. Riecher-Rössler acquired the data, which A. Schmidt, L. Palaniyappan, U. Lang, P. Fusar-Poli and P. McGuire analyzed. A. Schmidt, L. Palaniyappan and R. Smieskova wrote the article, which all authors reviewed and approved for publication.
References
- 1.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 2.Ziauddeen H, Murray GK. The relevance of reward pathways for schizophrenia. Curr Opin Psychiatry. 2010;23:91–6. doi: 10.1097/YCO.0b013e328336661b. [DOI] [PubMed] [Google Scholar]
- 3.Murray GK, Clark L, Corlett PR, et al. Incentive motivation in first-episode psychosis: a behavioural study. BMC Psychiatry. 2008;8:34. doi: 10.1186/1471-244X-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esslinger C, Englisch S, Inta D, et al. Ventral striatal activation during attribution of stimulus saliency and reward anticipation is correlated in unmedicated first episode schizophrenia patients. Schizophr Res. 2012;140:114–21. doi: 10.1016/j.schres.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen MØ, Rostrup E, Wulff S, et al. Alterations of the brain reward system in antipsychotic naïve schizophrenia patients. Biol Psychiatry. 2012;71:898–905. doi: 10.1016/j.biopsych.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 7.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–13. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 8.Knutson B, Bjork JM, Fong GW, et al. Amphetamine modulates human incentive processing. Neuron. 2004;43:261–9. doi: 10.1016/j.neuron.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 9.da Silva Alves F, Bakker G, Schmitz N, et al. Dopaminergic modulation of the reward system in schizophrenia: a placebo-controlled dopamine depletion fMRI study. Eur Neuropsychopharmacol. 2013;23:1577–86. doi: 10.1016/j.euroneuro.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl) 2006;187:222–8. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen MO, Rostrup E, Wulff S, et al. Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Arch Gen Psychiatry. 2012;69:1195–204. doi: 10.1001/archgenpsychiatry.2012.847. [DOI] [PubMed] [Google Scholar]
- 12.Sarpal DK, Robinson DG, Lencz T, et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 2015;72:5–13. doi: 10.1001/jamapsychiatry.2014.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roiser JP, Stephan KE, den Ouden HE, et al. Do patients with schizophrenia exhibit aberrant salience? Psychol Med. 2009;39:199–209. doi: 10.1017/S0033291708003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smieskova R, Roiser JP, Chaddock CA, et al. Modulation of motivational salience processing during the early stages of psychosis. Schizophr Res. 2015;166:17–23. doi: 10.1016/j.schres.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Schlagenhauf F, Sterzer P, Schmack K, et al. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol Psychiatry. 2009;65:1032–9. doi: 10.1016/j.biopsych.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 17.Penny WD, Stephan KE, Mechelli A, et al. Comparing dynamic causal models. Neuroimage. 2004;22:1157–72. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 18.Rothkirch M, Schmack K, Deserno L, et al. Attentional modulation of reward processing in the human brain. Hum Brain Mapp. 2014;35:3036–51. doi: 10.1002/hbm.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172:14–20. [PubMed] [Google Scholar]
- 20.Roiser JP, Stephan KE, den Ouden HE, et al. Adaptive and aberrant reward prediction signals in the human brain. Neuroimage. 2010;50:657–64. doi: 10.1016/j.neuroimage.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roiser JP, Howes OD, Chaddock CA, et al. Neural and behavioral correlates of aberrant salience in individuals at risk for psychosis. Schizophr Bull. 2013;39:1328–36. doi: 10.1093/schbul/sbs147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maldjian JA, Laurienti PJ, Kraft RA, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 23.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37:17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gluth S, Rieskamp J, Büchel C. Neural evidence for adaptive strategy selection in value-based decision-making. Cereb Cortex. 2014;24:2009–21. doi: 10.1093/cercor/bht049. [DOI] [PubMed] [Google Scholar]
- 26.Chikama M, McFarland NR, Amaral DG, et al. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17:9686–705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pessoa L, Engelmann JB. Embedding reward signals into perception and cognition. Front Neurosci. 2010;4:17. doi: 10.3389/fnins.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friston K. A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci. 2005;360:815–36. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friston K. The free-energy principle: A unified brain theory? Nat Rev Neurosci. 2010;11:127–38. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- 30.Stephan KE, Penny WD, Daunizeau J, et al. Bayesian model selection for group studies. Neuroimage. 2009;46:1004–17. doi: 10.1016/j.neuroimage.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephan KE, Penny WD, Moran RJ, et al. Ten simple rules for dynamic causal modeling. Neuroimage. 2010;49:3099–109. doi: 10.1016/j.neuroimage.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palaniyappan L, Simmonite M, White TP, et al. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79:814–28. doi: 10.1016/j.neuron.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goulden N, Khusnulina A, Davis NJ, et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage. 2014;99:180–90. doi: 10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 36.Ellison-Wright I, Glahn DC, Laird AR, et al. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–23. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palaniyappan L, Mallikarjun P, Joseph V, et al. Reality distortion is related to the structure of the salience network in schizophrenia. Psychol Med. 2011;41:1701–8. doi: 10.1017/S0033291710002205. [DOI] [PubMed] [Google Scholar]
- 38.Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–15. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 40.Wotruba D, Michels L, Buechler R, et al. Aberrant coupling within and across the default mode, task-positive, and salience network in subjects at risk for psychosis. Schizophr Bull. 2014;40:1095–104. doi: 10.1093/schbul/sbt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meltzer HY, Li Z, Kaneda Y, et al. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1159–72. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Abbott CC, Jaramillo A, Wilcox CE, et al. Antipsychotic drug effects in schizophrenia: a review of longitudinal fMRI investigations and neural interpretations. Curr Med Chem. 2013;20:428–37. doi: 10.2174/0929867311320030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pressler M, Nopoulos P, Ho BC, et al. Insular cortex abnormalities in schizophrenia: relationship to symptoms and typical neuroleptic exposure. Biol Psychiatry. 2005;57:394–8. doi: 10.1016/j.biopsych.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Tomelleri L, Jogia J, Perlini C, et al. Brain structural changes associated with chronicity and antipsychotic treatment in schizophrenia. Eur Neuropsychopharmacol. 2009;19:835–40. doi: 10.1016/j.euroneuro.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Radua J, Borgwardt S, Crescini A, et al. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev. 2012;36:2325–33. doi: 10.1016/j.neubiorev.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Moran LV, Sampath H, Kochunov P, et al. Brain circuits that link schizophrenia to high risk of cigarette smoking. Schizophr Bull. 2013;39:1373–81. doi: 10.1093/schbul/sbs149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilkinson D, Halligan P. The relevance of behavioural measures for functional-imaging studies of cognition. Nat Rev Neurosci. 2004;5:67–73. doi: 10.1038/nrn1302. [DOI] [PubMed] [Google Scholar]