Abstract
Purpose
To assess myocardial strain using cine displacement encoding with stimulated echoes (DENSE) using 1.5T and 3.0T MRI in healthy adults.
Materials and Methods
Healthy adults without any history of cardiovascular disease underwent magnetic resonance imaging (MRI) at 1.5T and 3.0T within 2 days. The MRI protocol included balanced steady‐state free‐precession (b‐SSFP), 2D cine‐echo planar imaging (EPI)‐DENSE, and late gadolinium enhancement in subjects >45 years. Acquisitions were divided into six segments; global and segmental peak longitudinal and circumferential strain were derived and analyzed by field strength, age, and gender.
Results
In all, 89 volunteers (mean age 44.8 ± 18.0 years, range: 18–87 years) underwent MRI at 1.5T, and 88 of these subjects underwent MRI at 3.0T (1.4 ± 1.4 days between the scans). Compared with 3.0T, the magnitudes of global circumferential (–19.5 ± 2.6% vs. –18.47 ± 2.6%; P = 0.001) and longitudinal (–12.47 ± 3.2% vs. –10.53 ± 3.1%; P = 0.004) strain were greater at 1.5T. At 1.5T, longitudinal strain was greater in females than in males: –10.17 ± 3.4% vs. –13.67 ± 2.4%; P = 0.001. Similar observations occurred for circumferential strain at 1.5T (–18.72 ± 2.2% vs. –20.10 ± 2.7%; P = 0.014) and at 3.0T (–17.92 ± 1.8% vs. –19.1 ± 3.1%; P = 0.047). At 1.5T, longitudinal and circumferential strain were not associated with age after accounting for sex (longitudinal strain P = 0.178, circumferential strain P = 0.733). At 3.0T, longitudinal and circumferential strain were associated with age (P < 0.05). Longitudinal strain values were greater in the apico‐septal, basal‐lateral, and mid‐lateral segments and circumferential strain in the inferior, infero‐lateral, and antero‐lateral LV segments.
Conclusion
Myocardial strain parameters as revealed by cine‐DENSE at different MRI field strengths were associated with myocardial region, age, and sex. J. Magn. Reson. Imaging 2016;44:1197–1205.
Keywords: healthy volunteers, myocardial strain, displacement encoding with stimulated echoes
Displacement encoding with stimulated echoes (DENSE) is a magnetic resonance imaging (MRI) method that directly quantifies left ventricular (LV) mechanics within myocardial regions with high spatial and temporal resolution.1 LV ejection fraction (LVEF) and wall motion score reflect displacement of LV borders, provide global measures of heart pump function, and are the standard imaging methods for describing LV function.2 Global longitudinal strain (GLS) has independent prognostic significance compared with LVEF in myocardial infarction survivors,3, 4 and in patients with cardiomyopathy.5, 6 Echocardiography is the standard‐of‐care for imaging LVEF and GLS. However, compared with echocardiography, MRI of LV systolic function is less dependent on operator technique and patient habitus (eg, acoustic windows) and has increased precision.7 MRI also enables characterization of myocardial pathology and therefore enables direct regional measurement of contractility that can be spatially matched with pathology. However, the variation in strain values within a healthy population with age and field strength has still to be established.
There are a number of ways of assessing myocardial strain with MR, including myocardial tagging,8 strain‐encoding imaging (SENC),9 phase contrast (PC) imaging,10 and DENSE.1 As a relatively new MR technique in this family, cine DENSE is capable of revealing pixel‐by‐pixel displacement and subsequently strain information of myocardial tissue from the phase data at each cardiac phase.1, 11, 12, 13 Our aim was to assess the relationship between myocardial strain as revealed by DENSE with age and sex, in a reasonably large group of healthy volunteers at two field strengths. We hypothesized that magnitudes of strain are associated with age and sex, independent of field strength.
Materials and Methods
Study Population
The study was approved by the regional ethics committee. Healthy volunteers aged at least 18 years with no prior medical history (including cardiovascular health problems, medication, or systemic illness) were invited to participate by placing advertisements in public buildings (eg, hospital, university). The other exclusion criteria included standard contraindications to MR (eg, metallic implants and metallic foreign body) and known or suspected pregnancy. Written informed consent was subsequently obtained from prospective participants. A 12‐lead electrocardiogram (ECG) was obtained in all subjects and a normal ECG was an inclusion criterion. Patient characteristics were recorded and body surface area was calculated with the Dubois formula.
MR Acquisition
Participants underwent MRI at 1.5T (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Germany) located in a hospital Radiology Department and at 3.0T (MAGNETOM Verio, Siemens Healthcare) in a university research center. At both field strengths, images were acquired using an anterior phased‐array body coil (12‐element and 16‐element at 1.5T and 3.0T, respectively) and a posterior phased‐array spine coil (24‐element).
MR Protocol
LV dimensions were assessed using balanced steady‐state free‐precession (b‐SSFP) cinematographic breath‐hold sequences. Typical imaging parameters are shown in Table 1. The heart was imaged in multiple parallel short‐axis planes 8‐mm thick separated by 2‐mm gaps, as well as in the 2‐chamber, 3‐chamber, and 4‐chamber long‐axis views.
Table 1.
Typical Imaging Parameters, at 1.5T and 3.0T MR Field Strengths
| b‐SSFP | 1.5T | 3.0T |
|---|---|---|
| TR (msec) | 3.3 | 3.4 |
| TE (msec) | 1.2 | 1.5 |
| FoV (mm) | 340 | 340 |
| Flip angle (degree) | 80 | 50 |
| Slice thickness (mm) | 7 | 7 |
| Resolution (mm) | 180×256 | 256×256 |
| Bandwidth (Hz/pixel) | 930 | 977 |
| DENSE | 1.5T | 3.0T |
| TR (msec) | 32.5 | 27.34 |
| TE (msec) | 7.97 | 6.63 |
| FoV (mm) | 360 | 360 |
| Voxel size (mm) | 3.2×3.2×8 | 3.2×3.2×8 |
| Flip angle (degree) | 20 | 20 |
| Bandwidth (Hz/pixel) | 1207 | 1207 |
| Displacement encoding Frequency (π/mm) | 0.2 | 0.2 |
| Triggers per breath‐hold | 8 | 8 |
| EPI factor | 8 | 8 |
| Segments per cardiac frame | 16 | 16 |
| Shimming method | Automatic | Manual |
TR: repetition time (msec); TE: echo time (msec); FoV: field of view (mm).
A 2D echo planar imaging (EPI) DENSE sequence (work‐in‐progress sequence 611, Siemens Healthcare) was used to acquire mid‐ventricular short‐axis and 4‐chamber long‐axis views. Typical imaging parameters are shown in Table 1. Through‐plane dephasing and 2‐point complementary spatial modulation of magnetization (CSPAMM) were used for artifact suppression during DENSE acquisition.14 Fat suppression was carried out using a fast water excitation option provided by the vendor. The readout and phase‐encoding direction of displacement were acquired in a single breath‐hold.
Participants over 45 years of age had their renal function checked and if the estimated glomerular filtration rate (eGFR) was >30 mls/min/1.73 m2 gadolinium contrast was administered (0.15 mmol/kg per bolus of gadolinium diethyltriaminepentaacetic acid [Gd‐DTPA], Magnevist, Bayer Healthcare, Berlin, Germany). Late gadolinium enhancement images covering the entire LV were acquired 10–15 minutes after intravenous contrast agent administration using segmented phase‐sensitive inversion recovery (PSIR) turbo fast low‐angle shot sequence.
Image Analysis
Datasets were anonymized to ensure operators were blinded to all other data. Project data were coordinated by S.R. The absence of late gadolinium enhancement (myocardial fibrosis or scar) was determined qualitatively by visual assessment by D.C. (>3 years MRI experience) and C.B. (>10 years MRI experience). The absence of myocardial late gadolinium enhancement was another requirement for inclusion of the data in this analysis.
LV mass and function were analyzed in randomly ordered, deidentified scans by three MRI‐trained cardiologists (MRI experience: G.C. 5 years, K.M. 3 years, D.S.C. 3 years) using computer‐assisted planimetry (Syngo MR, Siemens Healthcare).
The LV was segmented using the anterior right ventricular‐LV insertion point as the reference point. The mid‐left ventricular short axis (six segments: anterior, antero‐septal, infero‐septal, inferior, infero‐lateral, and antero‐lateral) and horizontal long axis (6 segments: basal infero‐septal, mid‐infero‐septal, apical septal, apical lateral, mid‐antero‐lateral, and basal‐antero‐lateral) DENSE data were analyzed using the CIM_DENSE2D software (University of Auckland, New Zealand),15 which provided longitudinal and circumferential strain. The underlying principles of the analysis included segmentation, phase unwrapping, displacement extraction, and strain calculation. Global and segmental myocardial circumferential (Ecc) and longitudinal (Ell) strain were analyzed by three trained observers (K.M., G.C., C.M.) in a random order. Image quality and artifact scoring were carried out by two operators (K.M., G.C.) and when there was discordance, X.Z. acted as a blinded independent adjudicator.
Statistical Analysis
Statistical analysis was performed using SPSS software (Chicago, IL, v. 22). Normality was tested using the Shapiro‐Wilk test. Continuous variables are expressed as mean ± standard deviation (SD). For comparison of two or more normally distributed variables, Student's t‐test and analysis of variance (ANOVA) with Tukey post‐hoc analysis were used. The population was divided per tertile of age to compare strain between groups. Interobserver reproducibility was assessed using Bland–Altman statistics. P < 0.05 was considered statistically significant.
RESULTS
Characteristics of the Study Participants
A total of 90 subjects underwent cardiac MRI. One male had an incidental finding of high T 1 in the antero‐septal LV wall in the territory of the left anterior descending coronary artery. A clinical assessment disclosed a history of exertional chest pain suggestive of angina. The elevated T 1 may have reflected myocardial edema secondary to ischemia. This patient was excluded from the analysis and referred for clinical investigation.
The characteristics of the remaining participating subjects (n = 89, including nine individuals ≥70 years) and their LV mass and function are presented in Tables 2 and 3, respectively. Global and segmental strain values are depicted in Figure 1.
Table 2.
Demographics of the Healthy Volunteers (n = 89)
| Demographic | |
|---|---|
| Age (years)* | 44.8 ± 18.0 |
| Age ≥70 years, n (%) | 9 (10) |
| Sex (male) n (%) | 45 (50) |
| Height (cm)* | 170 ± 10 |
| Weight (kg)* | 75.9 ± 15.0 |
| Body mass index, kgm−2 | 26 ± 4 |
| Body surface area (m2)* | 1.87 ± 0.20 |
*Mean ± SD.
Table 3.
Comparison of Heart Rate and LV Parameters at 1.5T and 3.0T
| 1.5T | 3.0T | P‐value* | |
|---|---|---|---|
| Heart rate (bpm) | 65.6 ± 11 | 64.2 ± 11 | 0.131 |
| LVEF (%) | 63.6 ± 5 | 63.4 ± 5 | 0.508 |
| LVEDV index (mL/m2) | 69.8 ± 11 | 70.7 ± 11 | 0.205 |
| LVESV index (mL/m2) | 25.6 ± 6 | 26.2 ± 6 | 0.106 |
| LV mass index (g/m2) | 40.3 ± 10 | 41.1 ± 10 | 0.051 |
LVEF: Left ventricle ejection fraction; LVESV: Left ventricle end‐diastolic volume; LVESV: Left ventricle end‐systolic volume.
*Measured using Student's t‐test.
Figure 1.
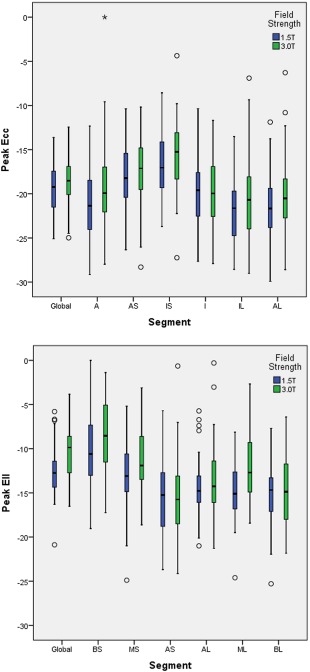
Global and segmental circumferential (a) and longitudinal (b) strain values at different field strengths. The circles in the figures represent participants outwith the confidence intervals.
Artifact Analysis and Interobserver Analysis
Overall image quality was high for circumferential (94%) and longitudinal strain (92%) at 1.5T (Table 4). Overall quality was adequate at 3.0T for both circumferential (54%) and longitudinal strain (57%). 7% of circumferential and 9% of longitudinal strain data could not be analyzed due to poor quality at 3.0T, while all images were of diagnostic quality at 1.5T. A higher proportion of images had artifact at 3.0T when compared with 1.5T, as can be seen in Table 4.
Table 4.
Image Quality and Artifact Scoring
| DENSE sequences | 1.5T | 3.0T | ||
|---|---|---|---|---|
| Image quality | Ecc (n = 81) | Ell (n = 34) | Ecc (n = 88) | Ell (n = 86) |
| High qualitya | 76 (94%) | 31 (92%) | 34 (39%) | 28 (33%) |
| Adequate qualityb | 5 (6%) | 3 (8%) | 48 (54%) | 49 (57%) |
| Nondiagnosticc | 0 (0%) | 0 (0%) | 6 (7%) | 9 (10%) |
| Number of images with motion artifact | 2 (2%) | 2 (6%) | 40 (45%) | 43 (50%) |
| Number of images with field effect artifact | 1 (1%) | 0 (0%) | 11 (13%) | 13 (15%) |
High quality: Well defined endo‐ and epicardial borders at end‐systole. No ghosting due to patient breathing. No flow artifact.
Adequate quality: One or more of the following were present: Slight blurring of endo‐ and epicardial borders at end‐systole. Slight artifact due to flow within blood pool but not affecting myocardium. Slight ghosting due to patient breathing.
Nondiagnostic: One or more of the following are present: Loss of endo‐ and epicardial borders at end‐systole; ghosting due to respiration.
Interoperator repeatability analysis was performed on the strain analysis, and the intraclass correlation coefficient was above 0.85 for all parameters (Table 5).
Table 5.
Reproducibility of DENSE Analysis
| Variable | Mean bias ± SD (%) | 95% Limits of agreement | P‐value | CoV | ICC |
|---|---|---|---|---|---|
| Ecc 1.5T | 0.2 ± 1.2 | −2.1 to 2.5 | 0.45 | 4.3 | 0.93 |
| Ell 1.5T | 0.5 ± 2.1 | −3.7 to 4.7 | 0.33 | 12.5 | 0.86 |
| Ecc 3.0T | 0.4 ± 1.4 | −2.4 to 3.1 | 0.27 | 5.6 | 0.93 |
| Ell 3.0T | 0.6 ± 2.3 | −3.9 to 5.0 | 0.29 | 13.7 | 0.89 |
Ecc: circumferential strain; Ell: longitudinal strain; ICC: intra‐class correlation co‐efficient. The inter observer variability analysis. A sample size of 20 images was taken per variable. The intra‐class correlation coefficient is above 0.85 for all of them.
Sex Differences in LV Strain at 1.5 and 3.0T
Global circumferential and longitudinal strains were greater in magnitude in women than in men at both field strengths (Table 6). Regional differences in strain were also observed. Longitudinal strain values were greater in the apico‐septal, basal‐lateral, and mid‐lateral segments and circumferential strain in the inferior, infero‐lateral, and antero‐lateral LV segments.
Table 6.
Circumferential and Longitudinal Strain at 1.5 and 3.0T in Males and Females
| 1.5T | 3.0T | |||||
|---|---|---|---|---|---|---|
| Parameter | Male | Female | P‐value | Male | Female | p‐value |
| Ecc* | (n = 39) | (n = 42) | (n = 41) | (n = 41) | ||
| Global (%) | ‐18.7 ± 2.2 | ‐20.1 ± 2.7 | 0.014 | ‐17.9 ± 1.8 | ‐19.1 ± 3.1 | 0.047 |
| Anterior (%) | −20.3 ± 3.5 | −21.6 ± 4.2 | 0.153 | −18.7 ± 3.7 | −21.0 ± 3.9 | 0.008 |
| Antero‐septal (%) | −18.2 ± 3.7 | −17.9 ± 3.3 | 0.667 | −17.0 ± 2.9 | −17.9 ± 3.7 | 0.245 |
| Infero‐septal (%) | −16.0 ± 3.6 | −17.4 ± 3.0 | 0.055 | −15.4 ± 3.0 | −15.9 ± 4.1 | 0.511 |
| Inferior (%) | −18.5 ± 3.3 | −20.8 ± 3.6 | 0.003 | −19.3 ± 3.4 | −20.3 ± 4.3 | 0.246 |
| Infero‐lateral (%) | −21.1 ± 2.9 | −22.7 ± 3.7 | 0.030 | −21.0 ± 3.1 | −20.8 ± 4.7 | 0.851 |
| Antero‐lateral (%) | −20.9 ± 3.2 | −22.3 ± 3.2 | 0.047 | −19.2 ± 3.6 | −21.4 ± 4.2 | 0.012 |
| Ell* | (n = 14) | (n = 20) | (n = 39) | (n = 38) | ||
| Global (%) | ‐10.2 ± 3.4 | ‐13.7 ± 2.4 | 0.001 | ‐10.7 ± 2.4 | ‐11.9 ± 2.7 | 0.052 |
| Basal‐septal (%) | −8.2 ± 5.1 | −10.7 ± 4.8 | 0.151 | −9.0 ± 4.2 | −10.3 ± 4.3 | 0.163 |
| Mid‐septal (%) | −12.4 ± 2.8 | −13.2 ± 4.4 | 0.550 | −12.2 ± 3.6 | −11.9 ± 4.2 | 0.803 |
| Apico‐septal (%) | −12.7 ± 4.5 | −16.4 ± 4.8 | 0.03 | −15.1 ± 4.9 | −16.7 ± 4.0 | 0.115 |
| Basal‐lateral (%) | −12.9 ± 3.1 | −15.8 ± 4.0 | 0.027 | −12.8 ± 4.4 | −15.5 ± 4.7 | 0.01 |
| Mid‐lateral (%) | −12.7 ± 4.5 | −15.9 ± 3.3 | 0.02 | −12.5 ± 4.3 | −12.8 ± 4.4 | 0.747 |
| Apico‐lateral (%) | −13.3 ± 3.9 | −15.0 ± 3.4 | 0.176 | −13.6 ± 4.3 | −13.5 ± 4.6 | 0.933 |
Mean (± SD); Ecc: Circumferential strain; Ell: Longitudinal strain.
Age Differences in LV Strain at 1.5 And 3.0T
Due to the sex difference in strain we carried out a regression model with multiple variables (age and sex) (Tables 6, 7). At 1.5T, longitudinal and circumferential strain were not associated with age after accounting for sex. At 3.0T, there was a statistically significant relationship between both longitudinal and circumferential strain and age (P < 0.05). We have also presented global and segmental strain according to three age tertiles (Table 8).
Table 7.
Significance of Relationship of Strain With Age and Sex at 1.5T and 3.0T
| 1.5T | 3.0T | |||
|---|---|---|---|---|
| Ecc | Ell | Ecc | Ell | |
| P value for regression | 0.046 | 0.002 | 0.008 | 0.004 |
| P value for age | 0.733 | 0.178 | 0.017 | 0.007 |
| P value for sex | 0.015 | 0.001 | 0.029 | 0.033 |
Ecc: circumferential strain; Ell: longitudinal strain.
Table 8.
Magnitudes of Strain Per Age Tertile
| 1.5T | 3.0T | |||||
|---|---|---|---|---|---|---|
| Parameter | 1st tertile (<35 y) | 2nd tertile (35–55 y) | 3rd tertile (>55y) | 1st tertile (<35 y) | 2nd tertile (35–55 y) | 3rd tertile (>55y) |
| Ecc* | n = 31 | n = 27 | n = 26 | n = 31 | n = 30 | n = 26 |
| Global (%) | −19.4 | −19.4 | −19.3 | −18.7 | −18.6 | −18.4 |
| Anterior (%) | −18.1 | −17.9 | −17.9 | −17.6 | −17.5 | −17.5 |
| Antero‐septal (%) | −21.0 | −20.9 | −20.8 | −19.7 | −19.7 | −19.8 |
| Infero‐septal (%) | −21.5 | −21.6 | −20.6 | −20.2 | −20.4 | −20.1 |
| Inferior (%) | −22.0 | −21.9 | −21.8 | −21.1 | −21.0 | −20.7 |
| Infero‐lateral (%) | −19.7 | −19.4 | −19.5 | −20.1 | −20.0 | −19.5 |
| Antero‐lateral (%) | −16.7 | −16.6 | −16.7 | −15.7 | −15.7 | −15.4 |
| Ell* | n = 12 | n = 11 | n = 11 | n = 30 | n = 30 | n = 22 |
| Global (%) | −11.4 | −12.3 | −12.2 | −12.3 | −11.3 | −11.0 |
| Basal‐septal (%) | −9.8 | −9.7 | −9.5 | −9.8 | −9.6 | −9.3 |
| Mid‐septal (%) | −12.2 | −12.9 | −12.8 | −12.8 | −12.0 | −11.6 |
| Apico‐septal (%) | −15.7 | −14.9 | −14.7 | −15.1 | −15.7 | −15.6 |
| Basal‐lateral (%) | −14.0 | −14.6 | −14.7 | −14.6 | −14.1 | −14.0 |
| Mid‐lateral (%) | −12.8 | −14.6 | −14.5 | −14.3 | −13.0 | −12.4 |
| Apico‐lateral (%) | −13.7 | −14.3 | −14.5 | −14.2 | −13.4 | −13.6 |
Ecc: circumferential strain; Ell: longitudinal strain.
Myocardial Strain: Variations With Field Strength
Global and regional strain values differed slightly between 1.5T and 3.0T (Fig. 1). These differences mainly related to circumferential strain values in the anterior, infero‐lateral, and antero‐lateral LV segments, where strain is greater at 1.5T. Global longitudinal strain and regional longitudinal strain in the mid‐septal and medio‐lateral segments were higher at 1.5T compared with 3.0T.
Discussion
We described myocardial strains at different MRI field strengths in 89 healthy adults across a broad age range, including 10% of elderly individuals ≥70 years. Circumferential and longitudinal strain values revealed by 2D‐DENSE had low interobserver variability, implying good reliability. We observed that longitudinal and circumferential strains varied in a regional distribution, with higher strain values in the anterior and lateral LV territories. Strains were higher in females than in males. After adjusting for the association with sex, strain was only associated with age when assessed at 3.0T. We also observed small differences in the magnitude of longitudinal and circumferential strains at different field strengths, with the magnitude of strain being slightly higher at 1.5T than 3.0T.
We observed that females had higher magnitudes of peak strain when compared with males. These differences were statistically significant for circumferential strain at both 1.5T and 3.0T and for longitudinal strain at 1.5T. Longitudinal strain at 3.0T was numerically higher for females, with a P value approaching statistical significance. These results are similar to what has been published in previous studies by Lawton et al16 using tagging, and Taylor et al17 using feature‐tracking. On the other hand, other authors, including Augustine et al,18 using feature‐tracking, Neizel et al19 using SENC, and Kuznetsova et al20 using echocardiography found that women had similar circumferential and longitudinal strain to men. DENSE,1 tagging,8 phase contrast MR,10 and feature tracking21 use different techniques to analyze strain, which could account for some of the differences in strain values observed using these different methods.22
We used a multivariate regression model to account for sex differences to assess whether there was an age‐related change in strain. Interestingly, there was a statistically significant relationship between age and strain at 3.0T but not at 1.5T. On reviewing the literature, it seems that there is no clear consensus on the relationship between age and strain. Oxenham et al23 and Neizel et al19 (Supplementary Table 1) did not observe any association between age and strain when assessed using tagged MR. Taylor et al17 described a positive association between circumferential strain but not longitudinal strain with age in individuals over the age of 50 years. Kuznetsova et al20 using echocardiography in 236 healthy subjects found that strain was inversely associated with age, consistent with the results at 3.0T in our study.
We observed that myocardial strain measured using DENSE was higher at 1.5T compared with 3.0T. Looking to the literature, Schuster et al.24 and Wehner et al.25 did not observe any differences in strain between field strengths; however, the sample size in both studies was small.
The 3.0T scanner used in this study had a 70 cm bore, while the 1.5T had a 60 cm bore. During scan acquisition, we found that it was more difficult to obtain a high level of magnetic field homogeneity in a wide bore scanner and hence artifacts were more common. The breath‐hold duration with this DENSE application may have lasted up to 20 seconds, depending on the heart rate, which may have resulted in motion artifacts due to respiration. Markl et al26 (n = 16) compared image quality using tagging at both field strengths and reported an increase in off‐resonance artifact at 3.0T, without an effect on overall image quality. Kramer et al27 (n = 14) and Valeti et al28 (n = 13) reported better image quality with tagging at 3.0T.
Another potentially relevant factor is that the timing parameters, eg, TR, of the DENSE pulse sequence were slightly different between the two field strengths. Since sampling of strain during the cardiac cycle occurred at slightly different timepoints, it is possible that there may have been a small disparity in what was perceived to be the “peak” strain at 1.5T and 3.0T. Importantly, the magnitude of the differences in strain between the field strengths was small and unlikely to be clinically significant; however, this result means that we need to interpret the observed relationship between age and strain at 3.0T with caution.
A summary of studies looking at strain assessment in healthy volunteers is provided in Supplementary Table 1. Compared with these studies, the distinct features of our study are 1) healthy adults with no history of cardiovascular disease or treatment were recruited; 2) further verification and exclusion of subclinical cardiac disease by the requirement of a normal ECG and exclusion of patients with incidental myocardial fibrosis or scar; 3) a reasonably large cohort of adults equally balanced between male:female and representative of a broad range of ages; and 4) performance of MR at 1.5T and 3.0T within a short period of time (average 1.4 days).
Reproducibility with EPI DENSE in our study seems to be higher than with feature Tracking and Tagging. For feature tracking at 1.5T,16 an interobserver coefficient of variation between 5.48% and 17.3% was reported for longitudinal strain, and between 4.9% and 13.3% for circumferential strain. For feature tracking at 3.0T, an interobserver coefficient of variation between 16.3% and 26.4% for longitudinal strain and between 9.9% and 20.3% for circumferential strain17, 18, 29, 30 was reported. Reproducibility with DENSE is higher than with Tagging.30 Compared to spiral cine DENSE,25 the reproducibility in our study was similar for circumferential strain and lower for longitudinal strain.
Strain measurements with MRI provide clinically useful information linked with myocardial viability31, 32 and predictive of longer‐term prognosis.33, 34, 35 Strain derived from DENSE provides higher spatial density of displacement and a higher temporal resolution compared to other MRI sequences.24 On the other hand, MRI has a lower temporal resolution compare to echocardiography, but MR permits tissue characterization. DENSE MRI has potential for wider adoption in clinical practice, and our study provides further information that adds to what is known about myocardial contractility revealed by DENSE MRI, including field‐ and sex‐specific differences.
Strain imaging is emerging as an alternative to LVEF and wall motion for the assessment of myocardial function. In the American Society of Echocardiography and the European Association of Cardiovascular Imaging guidelines, serial longitudinal strain assessment is recommended for assessment of patients treated with cytotoxic chemotherapy.37 Strain‐echocardiography has emerging utility for the discrimination of hypertrophic cardiomyopathy from hypertensive LV hypertrophy.38 DENSE MRI adds diagnostically over echocardiography because of the potential to characterize tissue pathology and register with contractility in the same scan. Accordingly, DENSE MRI has future potential in clinical practice. There have been further methods development using DENSE since the 2D EPI‐DENSE, including increasing signal‐to‐noise ratio by spiral k‐space acquisition,39 increasing phase signal‐to‐noise ratio by balanced encoding13 artifact suppression14 to improve 2D DENSE, as well as work on 3D‐DENSE whole heart acquisition.40
Fewer participants had longitudinal DENSE data acquired at 1.5T because 1.5T scans were performed on an MRI scanner in a busy cardiac regional center, whereas the 3.0T scans were obtained on a research‐dedicated scanner that had greater flexibility for the duration of the scan. Our study was exploratory. The results are hypothesis generating and further research is warranted.
In conclusion, we described myocardial strains at different MR field strengths in reasonably large sample of healthy adults across a broad age range with cine DENSE MR. We observed that longitudinal and circumferential strains varied in a regional distribution, with higher strain values in the anterior and lateral LV territories. Strains were higher in females than in males. Accounting for the between‐sex difference an association between age and strain was only apparent at 3.0T. We also observed small differences in the magnitude of longitudinal and circumferential strains at different field strengths, with the magnitude of strain being slightly higher at 1.5T than 3.0T.
Potential Conflict of Interest
The University of Glasgow holds a research agreement with Siemens Healthcare, who provided the DENSE work‐in‐progress CMR sequence and data analysis software.
Author contributions
CB, CM, DC made substantial contributions to conception and design; DC, CM, KM, RW, VO made substantial contributions to acquisition of data; KM, GC, CM, DSC, SR, JM made substantial contributions to the analysis and interpretation of data; KM, GC, CM, XZ, CB drafted the article; KM, GC, CB, XZ, CM, RW, VO, JM were involved in revising it critically for important intellectual content. All authors gave final approval of the version to be submitted and any revised version.
Supporting information
Supporting Information
Acknowledgments
This research was supported by a Project Grants from the Chief Scientist Office (SC01), Medical Research Scotland (343 FRG) and the British Heart Foundation (BHF‐PG/14/64/31043). K.M. is supported by a Fellowship from the British Heart Foundation (FS/15/54/31639).
We thank the radiographers (Vanessa Orchard, Andrew Saul, Joanne Nicholson, Tracy Steedman, Rosemary Woodward, Evonne McLennan, Caroline Glover) who acquired the images and the volunteers who participated in this study. We also thank Mr. Peter Weale and Mr. Patrick Revell, Siemens Healthcare, Frimley, U.K.
References
- 1. Aletras AH, Ding S, Balaban RS, Wen H. DENSE: displacement encoding with stimulated echoes in cardiac functional MRI. J Magn Reson 1999;137:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Authors/Task Force Members , Steg PG, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force on the management of ST‐segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 3. Park YH, Kang S‐J, Song J‐K, et al. Prognostic value of longitudinal strain after primary reperfusion therapy in patients with anterior‐wall acute myocardial infarction. J Am Soc Echocardiogr 2008;21:262–267. [DOI] [PubMed] [Google Scholar]
- 4. Woo JS, Kim W‐S, Yu T‐K, et al. Prognostic value of serial global longitudinal strain measured by two‐dimensional speckle tracking echocardiography in patients with ST‐segment elevation myocardial infarction. Am J Cardiol 2011;108:340–347. [DOI] [PubMed] [Google Scholar]
- 5. Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta‐analysis of global longitudinal strain and ejection fraction. Heart Br Card Soc 2014;100:1673–1680. [DOI] [PubMed] [Google Scholar]
- 6. Stanton T, Leano R, Marwick TH. Prediction of all‐cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2009;2:356–364. [DOI] [PubMed] [Google Scholar]
- 7. Bellenger N. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are they interchangeable? Eur Heart J 2000;21:1387–1396. [DOI] [PubMed] [Google Scholar]
- 8. Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging—a method for noninvasive assessment of myocardial motion. Radiology 1988;169:59–63. [DOI] [PubMed] [Google Scholar]
- 9. Osman NF, Sampath S, Atalar E, Prince JL. Imaging longitudinal cardiac strain on short‐axis images using strain‐encoded MRI. Magn Reson Med 2001;46:324–334. [DOI] [PubMed] [Google Scholar]
- 10. van Dijk P. Direct cardiac NMR imaging of heart wall and blood flow velocity. J Comput Assist Tomogr 1984;8:429–436. [DOI] [PubMed] [Google Scholar]
- 11. Kim D, Gilson WD, Kramer CM, Epstein FH. Myocardial tissue tracking with two‐dimensional cine displacement‐encoded MR imaging: development and initial evaluation. Radiology 2004;230:862–871. [DOI] [PubMed] [Google Scholar]
- 12. Spottiswoode BS, Zhong X, Hess AT, et al. Tracking myocardial motion from cine DENSE images using spatiotemporal phase unwrapping and temporal fitting. IEEE Trans Med Imaging 2007;26:15–30. [DOI] [PubMed] [Google Scholar]
- 13. Zhong X, Helm PA, Epstein FH. Balanced multipoint displacement encoding for DENSE MRI. Magn Reson Med 2009;61:981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhong X, Spottiswoode BS, Cowart EA, Gilson WD, Epstein FH. Selective suppression of artifact‐generating echoes in cine DENSE using through‐plane dephasing. Magn Reson Med 2006;56:1126–1131. [DOI] [PubMed] [Google Scholar]
- 15. Young AA, Li B, Kirton RS, Cowan BR. Generalized spatiotemporal myocardial strain analysis for DENSE and SPAMM imaging. Magn Reson Med 2012;67:1590–1599. [DOI] [PubMed] [Google Scholar]
- 16. Lawton JS, Cupps BP, Knutsen AK, et al. Magnetic resonance imaging detects significant sex differences in human myocardial strain. Biomed Eng OnLine 2011;10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor RJ, Moody WE, Umar F, et al. Myocardial strain measurement with feature‐tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging 2015;16:871–881. [DOI] [PubMed] [Google Scholar]
- 18. Augustine D, Lewandowski AJ, Lazdam M, et al. Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: comparison with tagging and relevance of gender. J Cardiovasc Magn Reson 2013;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neizel M, Lossnitzer D, Korosoglou G, et al. Strain‐encoded (SENC) magnetic resonance imaging to evaluate regional heterogeneity of myocardial strain in healthy volunteers: Comparison with conventional tagging. J Magn Reson Imaging JMRI 2009;29:99–105. [DOI] [PubMed] [Google Scholar]
- 20. Kuznetsova T, Herbots L, Richart T, et al. Left ventricular strain and strain rate in a general population. Eur Heart J 2008;29:2014–2023. [DOI] [PubMed] [Google Scholar]
- 21. TomTec — Imaging Systems [Internet]. [cited 2015. Feb 17]. Available from: http://www.tomtec.de/end_users/cardiac_mr/2d_cardiac_performance_analysis_mrc.html
- 22. Kar J, Knutsen AK, Cupps BP, Pasque MK. A validation of two‐dimensional in vivo regional strain computed from displacement encoding with stimulated echoes (DENSE), in reference to tagged magnetic resonance imaging and studies in repeatability. Ann Biomed Eng 2014;42:541–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oxenham HC, Young AA, Cowan BR, et al. Age‐related changes in myocardial relaxation using three‐dimensional tagged magnetic resonance imaging. J Cardiovasc Magn Reson 2003;5:421–430. [DOI] [PubMed] [Google Scholar]
- 24. Schuster A, Morton G, Hussain ST, et al. The intra‐observer reproducibility of cardiovascular magnetic resonance myocardial feature tracking strain assessment is independent of field strength. Eur J Radiol 2013;82:296–301. [DOI] [PubMed] [Google Scholar]
- 25. Wehner GJ, Suever JD, Haggerty CM, et al. Validation of in vivo 2D displacements from spiral cine DENSE at 3T. J Cardiovasc Magn Reson 2015;17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Markl M, Scherer S, Frydrychowicz A, Burger D, Geibel A, Hennig J. Balanced left ventricular myocardial SSFP‐tagging at 1.5T and 3T. Magn Reson Med 2008;60:631–639. [DOI] [PubMed] [Google Scholar]
- 27. Kramer U, Deshpande V, Fenchel M, et al. [Cardiac MR tagging: optimization of sequence parameters and comparison at 1.5 T and 3.0 T in a volunteer study]. RöFo 2006;178:515–524. [DOI] [PubMed] [Google Scholar]
- 28. Valeti VU, Chun W, Potter DD, et al. Myocardial tagging and strain analysis at 3 Tesla: Comparison with 1.5 Tesla imaging. J Magn Reson Imaging 2006;23:477–480. [DOI] [PubMed] [Google Scholar]
- 29. Schuster A, Kutty S, Padiyath A, et al. Cardiovascular magnetic resonance myocardial feature tracking detects quantitative wall motion during dobutamine stress. J Cardiovasc Magn Reson 2011;13:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh A, Steadman CD, Khan JN, et al. Intertechnique agreement and interstudy reproducibility of strain and diastolic strain rate at 1.5 and 3 Tesla: a comparison of feature‐tracking and tagging in patients with aortic stenosis. J Magn Reson Imaging 2015;41:1129–1137. [DOI] [PubMed] [Google Scholar]
- 31. Bree D. Low‐dose dobutamine tissue‐tagged magnetic resonance imaging with 3‐dimensional strain analysis allows assessment of myocardial viability in patients with ischemic cardiomyopathy. Circulation 2006;114:I33–I36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Potter DD, Araoz PA, McGee KP, Harmsen WS, Mandrekar JN, Sundt TM. Low‐dose dobutamine cardiac magnetic resonance imaging with myocardial strain analysis predicts myocardial recoverability after coronary artery bypass grafting. J Thorac Cardiovasc Surg 2008;135:1342–1347. [DOI] [PubMed] [Google Scholar]
- 33. Buss SJ, Breuninger K, Lehrke S, et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging 2015;16:307–315. [DOI] [PubMed] [Google Scholar]
- 34. Choi E‐Y, Rosen BD, Fernandes VRS, et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi‐Ethnic Study of Atherosclerosis. Eur Heart J 2013;34:2354–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Korosoglou G, Gitsioudis G, Voss A, et al. Strain‐encoded cardiac magnetic resonance during high‐dose dobutamine stress testing for the estimation of cardiac outcomes. J Am Coll Cardiol 2011;58:1140–1149. [DOI] [PubMed] [Google Scholar]
- 36. Gomez AD, Merchant SS, Hsu EW. Accurate high‐resolution measurements of 3‐d tissue dynamics with registration‐enhanced displacement encoded MRI. IEEE Trans Med Imaging 2014;33:1350–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014;27:911–939. [DOI] [PubMed] [Google Scholar]
- 38. Authors/Task Force members , Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 39. Zhong X, Spottiswoode BS, Meyer CH, Kramer CM, Epstein FH. Imaging three‐dimensional myocardial mechanics using navigator‐gated volumetric spiral cine DENSE MRI. Magn Reson Med 2010;64:1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spottiswoode BS, Zhong X, Lorenz CH, Mayosi BM, Meintjes EM, Epstein FH. 3D myocardial tissue tracking with slice followed cine DENSE MRI. J Magn Reson Imaging JMRI 2008;27:1019–1027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


