Abstract
At home injectable chemotherapy for patients receiving treatment for hematological diseases is still in debate. Given the expense of new innovative medicines, at home treatment has been proposed as a suitable option for improving patient quality of life and decreasing treatment costs. We decided to assess the cost of bortezomib administration in France among multiple myeloma patients from an economic standpoint. Patients in this study were treated within a regional hematological network combining outpatient hospital care and Hospital care at Home administration. To make the cost comparison, our team simulated outpatient hospital care expenses. Fifty-four consecutive multiple myeloma patients who received at least one injection of bortezomib in Hospital care at Home from January 2009 to December 2011 were included in the study. The median number of injections was 12 (range 1–44) at home and 6 (range 0–30) in the outpatient care unit. When compared with the cost simulation of outpatient hospital care alone, bortezomib administration with combined care was significantly less expensive for the National Health Insurance (NHI) budget. The mean total cost per patient and per injection was 954.20 € for combined outpatient and Hospital care at Home vs 1143.42 € for outpatient hospital care alone. This resulted in an estimated 16.5 % cost saving (Wilcoxon signed-rank test, p < 0.0001). The greatest savings were observed in administration costs (37.5 % less) and transportation costs (68.1 % less). This study reflects results for a regionally implemented program for multiple myeloma patients treated with bortezomib in routine practice in a large rural area.
Electronic supplementary material
The online version of this article (doi:10.1007/s00520-016-3363-3) contains supplementary material, which is available to authorized users.
Keywords: Chemotherapy at home, Hospital care at home, Cost analysis, Bortezomib, Multiple myeloma, Care network, Outpatient department, Outpatient unit, Patient satisfaction
Introduction
Multiple myeloma is a clonal malignancy of plasma cells which mainly affects an elderly population. In France, the median age at death is 76 years old for males and 78 years old for females. In 2013, 10,659 patients were treated for multiple myeloma with chemotherapy (+23.2 % compared with 2011) [1, 2]. Hematology departments in France are the third leading dispensers of chemotherapy, accounting for 17.8 % of all sessions administered [3]. With the development of oral chemotherapy, treatment dispensation at home has been developed in the last decade and subsequently for parenteral administration.
Two studies of home injectable chemotherapy have been led in France for solid tumors [4] as well as for malignant hemopathies. There was a recent study published by the team in Nantes on cost benefits of bortezomib administration at home for the multiple myeloma patients [5].
In France and in many European countries, the concept of Hospital care at Home (HaH) has developed steadily over several decades allowing at home treatment management for patients. Based on the American model of Home Care, the first French HaH structure was created in 1957 at the Public Service - Paris Hospitals (Assistance Publique - Hôpitaux de Paris (AP-HP)). HaH has been legally recognized in France since 1970. In 1991, it became an alternative to hospitalization and since 2009 has served as a form of outpatient hospitalization itself. The mission of HaH is to provide adaptive care with strong communication between providers for a limited amount of time to patients benefiting from treatment at home. Due to its evolution, HaH in France also refers to the structure itself that is administering home care. It can be thought of as an establishment independent of the hospital that provides both Outpatient Department services and home care including injectable chemotherapy sessions as one of its services. In 2011, there were over 11,000 beds available in France with this type of supportive care [6].
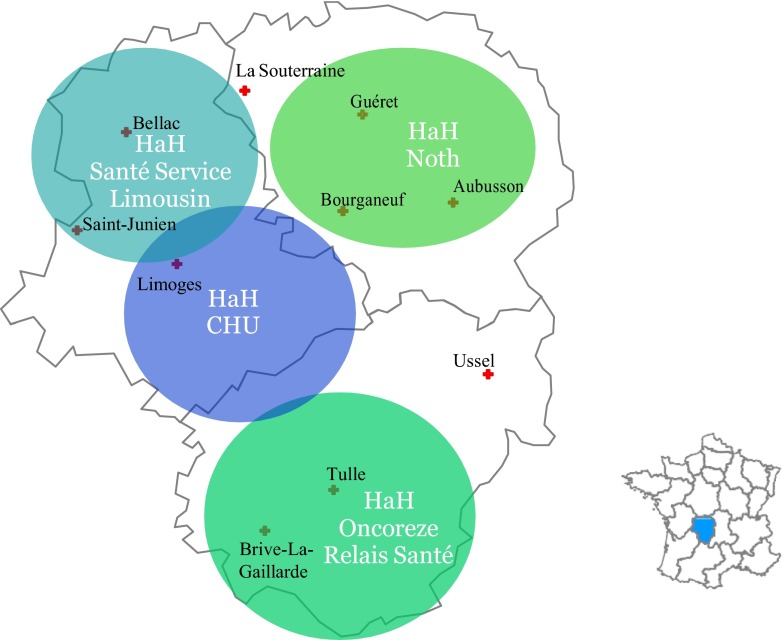
Limousin is a French region covering an area of 17,000 km2 and is characterized by low population density and the oldest population in France. There is one university hospital and two local state-run hospitals each with a hematology department. In 1994, these three hospitals set up a network which was later organized in 1999 with a medical coordinator. The HEMATOLIM network was officially established in 2007 to adhere to the national “Cancer Plan” that outlines cancer care organization in France.
In 2009, a regional organization within the HEMATOLIM network, externalization and securitization of injectable chemotherapy at home for malignant hematological diseases (ESCADHEM), was set up. This organization includes the three previously mentioned hospitals, four HaH structures, and three central pharmacies with an integrated preparation unit for cancer treatments (Fig. 1). Both written procedures and risk management processes for at home chemotherapy administration were established and validated (Fig. 2).
Fig. 1.
Map of the distribution of HaH involved in injectable chemotherapy at home in Limousin region and map of France showing situation of Limousin region
Fig. 2.
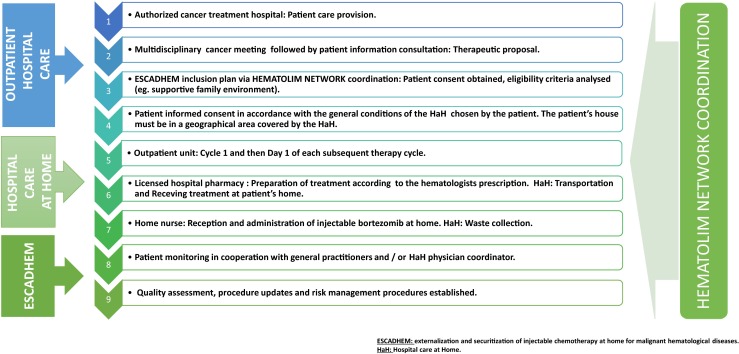
Organizing injectable chemotherapy via Hospital care at Home with ESCADHEM
In France, the decision to offer chemotherapy as a therapeutic option is made in a multidisciplinary meeting of specialists. The patient is informed about this option at a special appointment (informational consultation). When the decision to treat with bortezomib has been made and the patient profile corresponds with the eligibility criteria, the ESCADHEM program is proposed as part of the treatment option. A document explaining at home chemotherapy administered by the HaH is given to the patient during the informational consultation. To be cared for by ESCADHEM, three standard criteria must be met: (1) a supportive family environment and the patient’s consent; (2) a signed agreement between the general practitioner, the HaH structure, and the visiting nurse; and (3) the first chemotherapy cycle and D1 injection of subsequent cycles are administered at the hospital.
Chemotherapy at home begins with the medical prescription written by the hematologist at one of the region’s three hospitals. The patient must then pass a hematology exam to insure that the patient is fit to receive the prescribed treatment. Then, the chemotherapy preparation starts in the hospital pharmacy in accordance with regulations using specific software. Next, the treatment is prepared and delivered to the HaH. The HaH must insure (1) chemotherapy drugs prepared for transportation, (2) reception and administration of the treatment in the patient’s home, and (3) patient supervision at home by the family doctor and/or the HaH’s coordinating doctor or the HaH nurse or the floating nurse. The follow-up document and treatment tracking and the patient’s tolerance are given to the hospital pharmacy and to the hematologist by the HaH coordinating doctor; (4) waste collection is conducted according to regulation (Fig. 2).
When ESCADHEM was in its developmental stages, three drugs were chosen for externalization according to their in-use stability and ease as well as frequency of administration: bortezomib, azacitidine, and, less frequently, alemtuzumab. This decision was made due to the facility and frequent administration of these drugs. Their externalization avoids multiple visits to the outpatient care unit. These visits often present long waiting times and a significant amount of time in transit for patients living far from the hospital.
Bortezomib is indicated as monotherapy for the treatment of progressive multiple myeloma in adult patients who have received at least one prior therapy and who have already undergone or do not meet the requirements for bone marrow transplantation. It is also indicated in combination with melphalan and prednisone for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for high-dose chemotherapy with bone marrow transplantation. Bortezomib has a 3-week treatment cycle. The recommended starting dose [7] is 1.3 mg/m2 body surface area to be administered via intravenous (IV) or subcutaneous (SC) [8] injection twice weekly on days 1, 4, 8, and 11, followed by a 10-day rest period on days 12–21. There is a 72-h wait time between consecutive doses of bortezomib. This proteasome inhibitor may be combined with other alkylant molecules such as melphalan or cyclophosphamide and corticosteroids. The frequency of injections may be weekly (instead of twice weekly) under certain protocols with cycles of 4, 5, or 6 weeks (instead of 3 weeks) [9–11]. Doses can be withheld or adjustments at 1 and 0.7 mg/m2 are planned depending on toxicities. The chemical and physical in-use stability of the reconstituted solution has been demonstrated to be valid for at least 8 h at 25 °C stored in the original vial and/or syringe. The total storage time for the reconstituted medicinal product should not exceed 8 h prior to administration.
The aim of this study was to assess the cost of a combined treatment of Outpatient Hospital (OH) care and at home chemotherapy with injectable bortezomib over three consecutive years in the rural Limousin region of France. In comparison, cost assessment was calculated as if patients had been treated in OH care alone.
Patients and methods
Inclusion criteria
In 2012, all ESCADHEM patients consecutively treated with bortezomib injection for multiple myeloma starting in January 2009 and ending in December 2011 were contacted to participate in this retrospective study. Of the 88 patients who received at least one bortezomib injection, 54 patients gave their consent and were able to declare their mode of transport. All patient data were collected from electronic and paper hospital files.
The first cycle of bortezomib and the first injection of each cycle were administered via OH care. The decision to administer each subsequent injection at home or at the outpatient care unit was determined by the healthcare professionals.
Cost assessment
In France, there are several organizations for the administration of anticancer chemotherapy which include OH care and HaH. The cost for the NHI is determined by the health authorities [12, 13] in which 100 % of cancer medical costs are funded by the government (national fees). There is no extra charge paid by the patient. The average cost of bortezomib administration corresponds to the cost of patient receiving an anticancer chemotherapy and is dependent on the administration route, OH, or an OH/HaH combination. For our study, only direct costs were recorded.
When determining OH cost, the patient is classified in the diagnosis-related group (DRG) so-called GHM in French, which means homogeneous group of patients, labelled “Chemotherapy for tumor, in sessions” referred to specific coding of care 28Z07Z which indicates treatment cost to the NHI. In 2012, our patient population corresponded to an OH “hospital stay group” (GHS) with a cost of 397.58 € in a public health institution. This GHS covers the following items: cost of medical and non-medical staff, logistic and general management, medical logistics, and the direct costs excluding expensive drugs. Expensive drugs, such as bortezomib, are registered outside of the GHS rate, and the NHI pays the hospital directly for the medicine. Similarly, direct non-medical costs such as transportation are not included in the GHS and are covered by the NHI according to the mode of transport (ambulance, taxi, car, public transport, etc.) and distance travelled. Distance was calculated using Google Maps. Costs associated with exclusive OH care management were estimated thanks to a model based on the same methodology as the combined OH plus HaH management.
HaH cost was evaluated according to the corresponding dedicated home DRG, homogeneous tariff group so-called in France GHT, which is a daily cost [14]. The GHT is based on a score calculated with four criteria: the primary mode of care (i.e., anticancer chemotherapy), secondary associated care (none of the patients), Karnofsky index, and duration of care. Finally, the cost for HaH is achieved by multiplying the length of chemotherapy administration (1 day in the present study) by the daily cost of GHT (Table 1). GHT costs, paid by the NHI, cover the cost of medical and non-medical staff, logistic and general management, and medical logistics (i.e., transportation of bortezomib). For whatever the type of care, HaH or OH, the NHI cost is obtained by adding the costs of transportation and bortezomib to the HaH or OH care cost.
Table 1.
Daily cost of home DRG
| GHT | Daily cost |
|---|---|
| GHT 6 | 133.57 € |
| GHT 9 | 181.57 € |
| GHT 10 | 198.46 € |
| GHT 12 | 230.49 € |
| GHT 13 | 246.51 € |
Home diagnosis-related group (DRG) corresponding in France to GHT which means homogeneous tariff group is a daily cost based on a score calculated with four criteria: the primary mode of care (i.e., anticancer chemotherapy), secondary associated care (none of the patients), Karnofsky index, and duration of care
Statistics
Descriptive analysis was reported with means and standard deviations, medians, and ranges. Wilcoxon rank sum and chi-squared tests were used to determine if there was any significant difference in costs between the combination care of OH/HaH and OH care alone. The statistical analysis was performed with SAS 9.2 software (Cary, North Carolina, USA).
Approval of the local ethics committee was obtained before the beginning of the study.
Results
Between the January 1, 2009 and December 31, 2011, 88 patients with multiple myeloma received at least one injection of bortezomib in HaH. The cohort of the 54 patients included 28 males and 26 females with a median age of 65 years (range, 41–87) and more than a third were >70 years old (Suppl. table: Patient characteristics). The median performance status assessed by Karnofsky index was 70 (range, 50–100). A majority (57 %) of patients had Karnofsky indices <70 %. Twenty-nine patients (54 %) were on first-line treatment, 14 (26 %) in second line, and 11 (20 %) received more than 2 lines of treatment. Bortezomib was combined with other molecules: corticosteroids only (n = 26), melphalan and steroids (n = 16), cyclophosphamide and steroids (n = 14), thalidomide and steroids (n = 7), lenalidomide and steroids (n = 3), and liposomal doxorubicin plus steroids (n = 1). Twenty patients overall received intensive therapy with autologous hematopoietic stem cell.
A total of 1292 infusions were administered: 900 injections were performed via HaH and 392 injections were given via OH care. The range of injections received at home by patients was 1–44 with a median of 12 injections. One third of patients received 20 or more injections (Suppl. Fig. 3). During the study period, there were no major complications related to bortezomib administration at home. The total duration of HaH management lasted from less than 1 month to more than 2 years with a median of 3.2 months and a mean of 5.7 ± 5.9 months. A median of six injections was received by the patients (range, 0–30) at the outpatient unit and 46 % of patients received five or fewer injections. Some patients did not receive the three injections of a given cycle at home.
With regards to transport, the majority of patients used a light health vehicle (50 %) or their own car (31.5 %) and only four patients (7.4 %) were transported by ambulance—the most expensive transport (Table 2). The median home–hospital distance was 34.2 km (range, 2–140), and 44 % of the patients lived less than 20 km from the prescription site.
Table 2.
Transportation characteristics from home to outpatient hospital care unit
| Transportation modalities | Average distance per patient (km) | Average cost per patient (km) | Number of patients | % |
|---|---|---|---|---|
| Light service vehicle | 66.71 | 77.26 | 27 | 50.0 |
| Own car with reimbursement of cost request | 90.2 | 27.66 | 9 | 16.7 |
| Own car without reimbursement of cost request | 67.0 | 0 | 8 | 14.8 |
| Taxi | 71.44 | 123.06 | 5 | 9.3 |
| Ambulance | 45.11 | 198.32 | 4 | 7.4 |
| Other | 6 | 2.6 | 1 | 1.9 |
| Total | 54 | 100 |
The mean total cost per patient and per injection was 954.20 € for combined management OH/HaH and 1143.42 € for OH alone (Wilcoxon signed-rank test, p < 0.0001) (Table 3). The OH/HaH combination produced a cost reduction of 16.5 % compared with the model used to simulate daily hospital care. Differences were mainly due to the mean cost of administration (255.54 € vs 397.57 €) and the cost of patient transportation. The transportation cost accounted for 2.3 % of the total cost per injection in combined OH/HaH management, whereas it reached more than 6 % of the total cost for the administration of treatment via OH care alone. The empirical distribution of the total costs and the total cost distribution are shown in Suppl. Figs. 4, 5, and 6.
Table 3.
Cost per patient
| Criteria | Exclusive outpatient care | Outpatient and hospital at home cares | |
|---|---|---|---|
| Drug | Cost of the drug per mg, mean (SD), Euros | 320.75 (0) | 320.75 (0) |
| Dosage of the drug, mean (SD), mg | 2.11 (0.33) | 2.11 (0.33) | |
| Administration | Cost of administration, mean (SD), Euros | 397.57 (0) | 255.54 (48.03) |
| Number of drug injections in HO, mean (SD) | 16.67 (10.90) | ||
| Number of drug injection at outpatient care unit, mean (SD) | 23.93 (13.13) | 7.26 (5.51) | |
| Transport | Cost of transportation, per km per injection, mean (SD), Euros | 1.01 (1.77) | 0.32 (0.56) |
| Distance home-hospital (round trip), mean (SD) | 68.38 (67.54) | 68.38 (67.54) | |
| Total cost per patient and per injection, Euros | 1143.42 € | 954.20 € |
Costs were calculated with current Euro to 2012
Discussion
This study showed that administration of bortezomib with combined care is less costly for the NHI budget than an administration of the drug using OH care alone. Time and cost benefits are the main advantages to be highlighted from this new way of care for cancer patients.
The strong point of our study is that it demonstrates a significant savings of a combination of HaH and OH care with the aim to improve patient comfort, especially for elderly and frailer patients. Although an increasing number of anticancer molecules are available in oral form, we will continue to treat patients with injectable molecules (IV or SC) requiring the implementation of organization for the externalization of chemotherapy in a home-safe way. There are currently new drug chemotherapy treatments with similar administration profiles that could be externalized.
With the administration of two thirds of injections at home, a cost savings of 16.5 % might be achieved, representing an economy of 189 € per injection of bortezomib. Savings have been made in the administration category (37.5 % less) and in the transport category (68.1 % less). This study showed that the cost of patient care in France is dependent on various methods of treatment (combined OH and HaH vs OH alone) but also revealed the impact of transportation cost in the total treatment cost.
There are a couple limitations to our study. One limitation is the retrospective nature of data collection that may have induced minor difficulties in terms of finding accurate patient records; however, this limitation is compensated for by the use of electronic files that can easily be accessed and updated. Another limitation is that this study was performed in one region. While one might extrapolate findings to other regions, such extrapolations need to be adapted to the specific care provided by medical institutions in those regions.
Other teams have described experiences similar to ours. In one case, bortezomib administration in myeloma patients was performed at home from the first dose without significant problems [15]. Given the current national guidelines established in 2003, we have favored the first day of each cycle of therapy with bortezomib at the outpatient care unit [16].
The Nantes team [5] has recently published the results of a medico-economic study/satisfaction survey of bortezomib home administration managed by a territorial network funded for home care. Their results are similar to ours with 20 % savings between outpatient care and home administration that is less expensive. The difference with our experience is that bortezomib is managed by a territorial network and provided from the pharmacy directly to the nurse in charge of home administration without depending on a HaH structure as is the case in Limousin.
Savings for chemotherapy administered at home has also been demonstrated in solid tumors like colorectal, non-small cell lung cancer with a decrease in expenses of up to 53 % for intensive chemotherapies mainly administered for hematological malignancies [4, 5, 17].
One study had an innovative idea for bringing treatment to patients. A hospital in South Wales delivers chemotherapy as close as possible to the patient’s home using a mobile unit with a bus [18].
In one nursing study [15], from the patient and family point of view, the advantages of chemotherapy injection at home are numerous: being in one’s own environment leading to less disturbances to daily life, less time in transit and less fatigue, and the ability to continue a professional activity. All these aspects of treatment management at home have been shown to improve or maintain quality of life [19–22].
In 2014, we conducted a satisfaction survey (unpublished data) with patients who received treatment in HaH structures via ESCADHEM in 2013. Of the 84 patients addressed by the survey, 61 (73 %) responded including 34 patients with multiple myeloma treated by bortezomib. The overall satisfaction rate was 95 %. All surveyed patients (100 %) were willing to recommend this mode of care to close relations. The four HaHs participating in ESCADHEM organization were rated 8.7 out of 10. Of the benefits reported by the patients, 55 (90 %) confirmed a sense of security, 54 (88 %) reported home environment, 54 (88 %) mentioned less time in transit, 52 (85 %) appreciated no hospital wait, and 46 (75 %) patients mentioned continuing daily life. Of the patients surveyed, 54 (88 %) reported no disadvantage while 3 (5 %) feel they are a burden to their family, and 4 (6 %) mentioned poor communication with the hospital team. These results concur with those of the satisfaction survey conducted by the team from Nantes [5] which shows that home bortezomib administration is preferred by patients.
In the last decade, interest in developing administration of chemotherapy at home has increased with the aim of increasing patient quality of life. While this may be the case, one of the side results has been an alleviation of overcrowding in inpatient oncology, hematology, and outpatient units. The externalization of treatment theoretically induces a loss of resources for hospitals, and this has proven an obstacle in some hospitals. In France, many hematology departments are overloaded. With this organization, patient care is improved and a wider range of patients are admitted. In the end, as in our department, there is an increase in the number of outpatient hospital venues.
In January 2015, the French Health Authorities [Haute Autorité de Santé (HAS)] [23] published an online report supporting the development of chemotherapy via HaH structures. This project in which the network HEMATOLIM [24] has collaborated indicates that in 2016, the administration of injectable chemotherapy at home still remains an activity underdeveloped and unevenly distributed throughout France. The tariff model of participating institutions is often a disincentive to the development of home chemotherapy and thus slows the development of this type of health care organization. However, the report concludes that HaH is a relevant modality of patient care that should be developed in order to allow the practice of some injectable chemotherapy molecules in the home environment.
The cost of chemotherapy in HaH vs using OH depends on multiple parameters (institution status, coding of care, duration of care, Karnofsky index). However, there is no single answer to suggest that HaH is always the less costly option compared to OH. For example, the administration of injectable chemotherapy at home appears more costly than OH for treatment with bortezomib D1, D4, D8, and D11 if the patient stays in HaH between injections and if the D-1 is the balance sheet encoded by the HaH. This analysis done by HAS has shown that HaH sometimes cannot be less costly for health insurance than the OH alternative. The parameter impacting the results is the protocol procedures, especially the interval between injections. According to the report, the cost associated with HaH is generally lower than the conventional OH. However, this finding is related to the pricing methods used by HaH (per day vs per sequence) and is protocol dependent.
Conclusion
One of the aims of hematological malignancy care is to deliver innovative drugs at home, especially for an aging patient population. This treatment management needs to be coordinated by a regional hematological care network with processes validated for ensuring safe patient conditions. Our study of at home bortezomib injections with combination HaH/OH demonstrated a significant cost savings compared with OH care alone. This indicates that exclusive HaH or exclusive OH care is not the most economic approach. A combination of the two could strike an economic balance that ensures patient safety and quality of life.
Electronic Supplementary Material
(RTF 11.9 mb)
(DOC 55 kb)
(RTF 4.02 mb)
(RTF 663 kb)
(DOCX 18.8 kb)
Acknowledgments
We acknowledge the contribution of the following institutions and associations for their help in this study: Centre Hospitalier Universitaire (CHU) de Limoges, Agence Régionale de Santé (ARS) Aquitaine Limousin Poitou-Charentes, Unité de Recherche Clinique en Hématologie (URC-H du CHU de Limoges), and Limousin Association pour le Développement et la Recherche en HEmatologie Clinique (LADRHEC). We would like to thank Nicole Fort for her help in writing the earlier version of the manuscript and Rachel Tipton for English proofreading and light editing. We also thank the patients and their families for their contributions to this work.
Author contribution
MT, SM, FV, SL, CB, AJ, and DB drafted the manuscript. AL and AD helped write the pharmaceutical section. LL provided cost calculations. AV oversaw the cost calculation methodology. LJ was responsible for statistical analysis.
Compliance with ethical standards
Authorship and Disclosures
This work was supported by an academic grant from JANSSEN-CILAG Company without involvement focus and design of this study. The grant funded the cost calculations provided by the HEVA Company and writing assistance from Nicole Fort who worked on an earlier version of the manuscript.
Footnotes
Article summary
This study is a comparison of the cost of bortezomib chemotherapy in patients with multiple myeloma treated by a combination care in outpatient unit and hospital care at home versus outpatient unit alone. The combined management is significantly less expensive.
References
- 1.National Institute of Cancer INCa 2013. Situation of cancer in France in 2012. http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/La-situation-du-cancer-en-France-en-2012. Accessed 13 Dec 2015
- 2.National Institute of Cancer INCa 2014. Les cancers en France—édition 2014: http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Les-cancers- en-France-Edition-2014. Accessed 31 Dec 2015
- 3.National Institute of Cancer INCa 2013. Situation of cancer chemotherapy in France in 2013. http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Situation-de-la-chimiotherapie-des-cancers-Rapport-2013. Accessed 31 Dec 2015
- 4.Vergnenègre A, Decroisette C, Vincent F, et al. Analyse économique de l’administration d’une chimiothérapie en hospitalisation à domicile (HAD) comparée à l’hospitalisation de jour dans les cancers bronchopulmonaires non à petites cellules de Stade IV. Rev Mal Respir. 2006;23(3 Pt 1):255–263. doi: 10.1016/S0761-8425(06)71575-3. [DOI] [PubMed] [Google Scholar]
- 5.A. Lassalle, P. Thomaré, C. Fronteau et al. Home administration of bortezomib in multiple myeloma is cost-effective and is preferred by patients compared to hospital administration: results of a prospective single-center study. Annals of Oncology Advance Access published November 16, 2015 [DOI] [PubMed]
- 6.FNEHAD, National Federation of French Hospitalization at Home Establishments. Available on http://www.fnehad.fr/. Accessed 1 Apr 2016
- 7.Bortezomib Monograph. Available on http://www.fda.gov/. Accessed 31 Dec 2015
- 8.Moreau P, Pylypenko H, Grosicki, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 9.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 10.Mateos MV, Bringhen S, Richardson PG, et al. Bortezomib cumulative dose, efficacy, and tolerability with three different bortezomib-melphalan-prednisone regimens in previously untreated myeloma patients ineligible for high-dose therapy. Haematologica. 2014;99(6):1114–1122. doi: 10.3324/haematol.2013.099341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mateos MV, Hernandez JM, Hernandez MT, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: updated time-to-events results and prognostic factors for time to progression. Haematologica. 2008;93(4):560–565. doi: 10.3324/haematol.12106. [DOI] [PubMed] [Google Scholar]
- 12.Order of 28 February 2012 fixing for 2012 the tariff items mentioned in I and IV of Article L. 162-22-10 of the Social Security Code and IV and V of the amended Article 33 of Law social security funding for 2004. http://www.legifrance.gouv.fr/affichTexte.do;jsessionid=3E7798D70773D40F58B9F53F961661F6.tpdjo03v_2?cidTexte=JORFTEXT000025413804&dateTexte=&oldAction=rechJO&categorieLien=id&idJO=JORFCONT000025413457. Accessed 31 Dec 2015
- 13.Bellanger M. et al. Approaches for cost assessment and price setting in practice. HealthBASKET—Health Benefits and Service Costs in Europe SP21-CT-2004-501588. France September 2005. http://www.ehma.org/files/WP-6-HealthBASKETSP21-CT-2004-501588_D18_France.pdf. Accessed 31 Dec 2015
- 14.Methodological guide for the production of discharge in Hospital at home. Official Bulletin No. 2012/7 bis Special Issue. http://www.sante.gouv.fr/IMG/pdf/sts_20120007_0001_p000.pdf. Accessed 31 Dec 2015
- 15.Meenaghan T, O’Dwyer M, Hayden P, et al. Home administration of bortezomib: making a difference to myeloma patients’ lives. Eur J Oncol Nurs: Off J Eur Oncol Nurs Soc. 2010;14(2):134–136. doi: 10.1016/j.ejon.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 16.ANAES, 2003. Consensus formalisé. Critère d’éligibilité des patients à une chimiothérapie anticancéreuse à domicile. http://www.has-sante.fr/portail/upload/docs/application/pdf/anaes_fiche_de_synth_350se_chimioth_351rapie-2.pdf. Accessed 31 Dec 2015
- 17.Luthi F, Fucina N, Divorne N, et al. Home care—a safe and attractive alternative to inpatient administration of intensive chemotherapies. Support Care Cancer. 2012;20(3):575–581. doi: 10.1007/s00520-011-1125-9. [DOI] [PubMed] [Google Scholar]
- 18.Iredale R, Hilgart J, Hayward J. Patient perceptions of a mobile cancer support unit in South Wales. Eur J Cancer Care (Engl) 2011;20(4):555–560. doi: 10.1111/j.1365-2354.2011.01247.x. [DOI] [PubMed] [Google Scholar]
- 19.Joo EH, Rha SY, Ahn JB, et al. Economic and patient-reported outcomes of outpatient home-based versus inpatient hospital-based chemotherapy for patients with colorectal cancer. Support Care Cancer. 2011;19(7):971–978. doi: 10.1007/s00520-010-0917-7. [DOI] [PubMed] [Google Scholar]
- 20.Lee YM, Hung YK, Mo FK, et al. Comparison between ambulatory infusion mode and inpatient infusion mode from the perspective of quality of life among colorectal cancer patients receiving chemotherapy. Int J Nurs Pract. 2010;16(5):508–516. doi: 10.1111/j.1440-172X.2010.01876.x. [DOI] [PubMed] [Google Scholar]
- 21.Stevens B, McKeever P, Law MP, et al. Children receiving chemotherapy at home: perceptions of children and parents. J Pediatr Oncol Nurs. 2006;23(5):276–285. doi: 10.1177/1043454206291349. [DOI] [PubMed] [Google Scholar]
- 22.Stevens B, Croxford R, McKeever P, Yamada J, Booth M, Daub S, et al. Hospital and home chemotherapy for children with leukemia: a randomized cross-over study. Pediatr Blood Cancer. 2006;47(3):285–292. doi: 10.1002/pbc.20598. [DOI] [PubMed] [Google Scholar]
- 23.Conditions for the development of chemotherapy at home: economic and organizational analysis. http://www.has-sante.fr/portail/upload/docs/application/pdf/2015-03/conditions_du_developpement_de_la_chimiotherapie_en_hospitalisation_a_do micile_-_synthese_et_recommandations.pdf. Accessed 1 Jan 2016. HAS (Haute Autorité Santé). Conditions du développement de la chimiothérapie en Hospitalisation à Domicile : analyse économique et organisationnelle [en ligne]. Paris: HAS, janvier 2015, 28 p.
- 24.At home injectable chemotherapy—Limousin HaH monograph via HEMATOLIM network ESCADHEM. http://www.has-sante.fr/portail/upload/docs/application/pdf/2015- 03/chimiotherapie_injectable_en_had_-_monographie_des_had_du_limousin_dans_le_cadre_du_reseau_dhematologie_du_li mousin_hematolim_via_le_dispositif_escadhem.pdf. (Accessed January 1, 2016). HAS (Haute Autorité Santé).Chimiothérapie injectable en HAD - Monographie des HAD du Limousin dans le cadre du réseau d’hématologie du Limousin HEMATOLIM via le dispositif ESCADHEM
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(RTF 11.9 mb)
(DOC 55 kb)
(RTF 4.02 mb)
(RTF 663 kb)
(DOCX 18.8 kb)