Abstract
Oxytocin (OT) is a neuromodulator that facilitates pair-bonding, maternal care, and social approach. OT is thought to promote these social behaviors by enhancing the salience and reinforcing effects of relevant social stimuli. There is the additional possibility that OT per se may be rewarding. To test this, we investigated whether female rats would voluntarily self-administer OT. Female Long-Evans rats were ovariectomized, and received an estrogen implant and an intracerebroventricular cannula. Rats were tested in an operant chamber with active and inactive levers. They were initially tested 4h/day on a fixed-ratio 5 schedule for self-administration of artificial cerebral spinal fluid (aCSF) for 5 days, followed by aCSF, or OT at 1 or 10 ng/ul for another 5 days. Rats self-administering aCSF made 36.2±6.2 active lever responses/4h vs. 14.9±3.4 inactive responses. Responses for 1 ng/ul OT were similar. However, rats self-administering 10 ng/ul OT made significantly more active lever responses (67.8±12.0 per 4h), and received 121.4±21.0 ng OT/4h. To determine if reduced anxiety contributes to the reinforcing effects of OT, rats received an infusion of aCSF or OT at 0.3 or 3.0 ug immediately before testing on the elevated plus maze. There was no effect of OT on anxiety as reflected by percent time on the open arms, and no effect of OT on locomotion as measured either by the number of closed arm entries or the number of total arm entries. These results suggest that OT may be rewarding, and that this is not due to anxiolytic effects of OT.
Keywords: Oxytocin, Self-Administration, Intracerebroventricular, Operant behavior, Reinforcement, Rats
INTRODUCTION
In mammals, oxytocin (OT) is essential for delivery of offspring and milk ejection (1). In addition to its systemic hormonal effects, OT also acts as a neuromodulator in the brain to stimulate social behaviors important for reproduction and offspring survival, such as pair-bonding and maternal behavior (reviewed in 1, 2). OT can induce maternal behavior towards foster pups in virgin female rats (3), and expedites pair-bonding and partner preference in female prairie voles (4). Conversely, a selective OT receptor antagonist in the nucleus accumbens (Acb) core and prefrontal cortex of female voles inhibits partner preference and pair bond formation (5). As reviewed by Insel (6), these social behaviors are rewarding because they have motivational properties and activate the mesolimbic dopamine system. For example, prairie voles prefer the scent of their partner over that of a stranger (7). Rat dams also show a conditioned place preference (CPP) for a location previously paired with pups (8), and will work for access to pups (9). OT increases expression of pair-bonding and maternal behavior presumably by enhancing the salience and reinforcing effects of social stimuli essential for these behaviors (10). There is the additional possibility that OT per se may be rewarding, independent of social behavior. If so, reinforcing effects of endogenous OT released during mating or lactation may amplify the reinforcing effects of social bonding. The present study tested if rats would voluntarily self-administer OT as a measure of OT reward.
Furthermore, successful reproduction requires skills and behaviors that are not restricted to the social domain. Therefore, OT’s effects likely extend beyond social behaviors. Already, evidence for OT’s influence on non-social behaviors is accumulating. In male rats and male and female mice, OT can be anxiolytic as measured by open-field, zero-maze, and elevated plus maze activity (11–13). Conversely, female mice lacking OT exhibit increased anxiety in the elevated plus maze (14). OT also decreases stress-induced corticosterone release in female rats (15). In male mice, OT exerts an antidepressant effect, as measured by decreased time immobile in the Porsolt swim test, and decreased latency to escape in the learned helplessness test (16). Moreover, OT improves reference memory in female mice tested in the radial arm maze (17).
Considering that OT has anxiolytic and anti-depressive effects in a non-social setting, it is reasonable to expect that OT may be reinforcing even in the absence of social cues. Our lab has previously shown that female mice form both a CPP and a conditioned social preference for OT (18), suggesting that OT is rewarding in both non-social and social contexts. The present study extends our previous findings on OT reward by testing OVX+E female rats for self-administration. Voluntary self-administration suggests that a drug is reinforcing (19). If OT is reinforcing, rats should be willing to work to obtain it. Many drugs of abuse, such as alcohol (20), and benzodiazepines (21, 22), have anxiolytic properties in addition to their rewarding effects. Since OT has been implicated in anxiety (11–15), testing the effects of OT in the elevated plus maze will determine if reinforcing effects of OT are related to a reduction in anxiety.
MATERIALS AND METHODS
Subjects
Female Long-Evans rats (ca. 150g) were obtained from Charles River Laboratories (Wilmington, MA). They were pair-housed prior to surgery, and then individually housed afterward. Rats were maintained in a temperature- and humidity-controlled room on a reversed 14:10 light/dark cycle (lights off at 9 a.m.). All testing occurred during the first 4h of the dark phase when activity peaks. Food and water were available ad libitum, except during daily 4h tests of self-administration and during elevated plus maze testing. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and with the approval of the University of Southern California Institutional Animal Care and Use Committee (IACUC).
Drugs
Since OT does not easily cross the blood-brain barrier (23), it was delivered by intracerebroventricular (icv) infusion for precise dose control. A wide range of OT doses has been tested in previous studies with rodents, from 600 µg systemically in rats to inhibit food and fluid intake (24) to 1 ng icv in OT knockout mice to increase social recognition (25). In the present study, each 1 ul infusion delivered OT (Sigma-Aldrich St. Louis, MO) at 1 or 10 ng in an artificial cerebral spinal fluid (aCSF) vehicle. These doses have been shown previously to facilitate lordosis in female rats (26, 27). For testing anxiety in the elevated plus maze, rats were pretreated with a 10 ul infusion of OT at 0.3 or 3.0 ug. 0.3 ug OT is anxiolytic in male mice (12), and is similar to the amount of OT consumed during 4h of self-administration at 10 ng/ul. We also tested potential anxiolytic effects of OT at a 10x dose (3.0 ug).
Surgery
All surgical procedures were carried out under aseptic conditions according to Principles of Laboratory Animal Care (National Institutes of Health, 1985). Five days before testing, each rat was surgically implanted with a 22-gauge stainless steel guide cannula (Plastics One, Roanoke, VA) into the lateral ventricle (AP: −0.5 mm, ML: +1.5 mm, DV: approx. −4.0 mm from bregma) under stereotaxic guidance, as described previously (28). Rats were anesthetized with a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg; Sigma-Aldrich) ip. The cannula assembly was anchored to the skull with stainless steel skull screws (Lomat Inc, Montreal, QC) and dental acrylic (Plastics One).
Estrogen interacts with OT (29). Accordingly, variations in estrogen across the estrous cycle could modify the reinforcing effects of OT, and introduce additional variability into OT self-administration. Therefore, each rat was bilaterally ovariectomized at the time of icv cannula placement, and received a Silastic implant sc (I.D.: 1.57 mm, O.D.: 3.18 mm; Dow Corning, Midland, MI) containing 5 mm of crystalline 17β-estradiol (Sigma-Aldrich; OVX+E). This treatment produces constant physiological levels of estrogen (30).
Self-Administration
Rats were tested for icv self-administration 4h/day, 5 days/week in an operant chamber (Med Associates, St. Albans, VT). Initially, all rats self-administered aCSF on an ascending fixed-ratio (FR) schedule: 2 days each at FR1-FR4, followed by 5 days at FR5. Rats were then allowed to continue aCSF self-administration (n=10), or to self-administer OT at 1 ng/ul (n=7) or 10 ng/ul (n=10) for 5 days at FR5.
Operant chambers were equipped with a house light, clicker, two levers with stimulus lights, and a computer-controlled syringe pump with balance arm and fluid swivel (Instech, Plymouth Meeting, PA). Each chamber was enclosed in a sound-attenuating cabinet with a fan for ventilation. One of the levers was designated as the active lever. Placement of the active lever to the front or the back of the chamber was balanced to control for side preferences. A response on this lever counted toward the response requirement for infusion. Once the response requirement was met, the house light was extinguished, the clicker was activated, and the stimulus light above the active lever was illuminated during the infusion period. Infusions (OT or aCSF) from a 100-µl glass syringe (Hamilton Co., Reno, NV) were delivered through Tygon tubing connected to the fluid swivel and then to a 28-gauge infusion cannula, which was inserted into the guide cannula at the start of each test session. Each infusion delivered 1 µl at 0.2 µl/s for 5 s. Additional responses on the active lever during the infusion period were recorded, but did not count toward further reinforcement. Responses on the inactive lever were recorded, but did not result in drug infusion, activation of the clicker, or illumination of the stimulus light. Data from both levers were recorded by Med PC IV software (Med Associates) on a Windows PC.
Elevated Plus Maze
To determine if OT reduces anxiety or locomotor behavior, rats were tested in the in the elevated plus maze (31). The apparatus consisted of a plus-shaped maze with two arms (50×10 cm) closed by opaque plastic sidewalls, and two arms without walls. The maze was located 50 cm above the floor and visually isolated by a curtain enclosure. The day after the final self-administration session, rats received an additional icv infusion of aCSF or OT immediately before testing in the elevated plus maze. Rats that had previously self-administered aCSF (n=8) received a 10 ul infusion of aCSF. Rats that had previously self-administered OT received a 10 ul infusion of aCSF (n=5), or OT at 0.3 (n=6) or 3.0 ug (n=5). Rats were placed in the center of the maze facing an open arm. Animals were allowed to explore freely for 5 min. Exploratory activity was recorded on video camera, and scored by an observer who was blind to the treatment groups. Results were analyzed as in File et al (31), where the percentage of time spent in the open arms was scored as a measure of anxiety, and the number of closed arm entries was used as a measure of overall locomotor activity. An entry was recorded when all four paws entered the arm.
Data Analysis
Operant responses for self-administration of aCSF or OT at 1 or 10 ng/ul were evaluated using repeated measures analysis of variance (RM-ANOVA), with lever (active vs inactive) as the repeated measure. Active and inactive lever responses were compared across treatment groups using ANOVA with Dunnett’s post-hoc test. For the elevated plus maze, entries into closed arms, total arm entries, and percentage of time spent in the open arms were analyzed by ANOVA. Statistical analyses were completed using JMP Pro 11 (SAS Institute Inc., Cary, NC). Data are presented as mean±SEM. Differences were considered significant at p<0.05.
RESULTS
Self-Administration
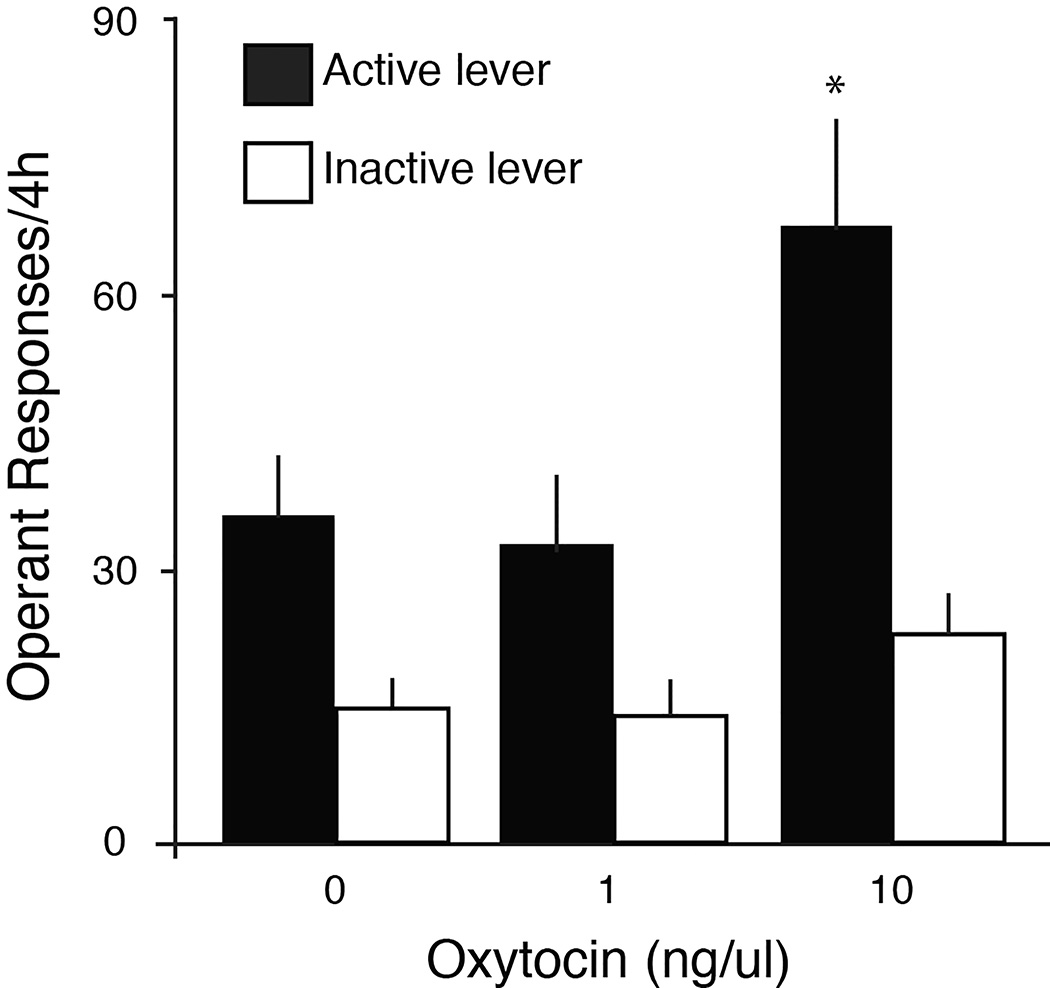
Operant responses for OT and aCSF on active and inactive levers at FR5 are shown in Fig. 1. By RM-ANOVA with lever (active vs. inactive) as the repeated measure, there was a significant effect of lever (F1,24=27.34, p<0.05) and of drug dose (F2,24=5.08, p<0.05). The interaction between lever and dose missed significance (F2,24=2.44, p=0.11). Rats self-administering aCSF made 36.2±6.2 active responses/4h (mean±SEM) vs. 14.9±3.4 inactive responses. Responses for OT at 1 ng/ul were similar (33.0±7.5 active/4h; 14.1±3.7 inactive/4h). Comparing active responses among groups by ANOVA with Dunnett’s post-hoc test, rats self-administering 10 ng/ul OT made significantly more active lever responses (67.8±12.0/4h) than rats self-administering aCSF (p<0.05). However, responses on the inactive lever were similar in all groups (23.3±4.3 inactive responses/4h for 10 ng/ul OT, N.S.). At 10 ng/ul OT, rats self-administered 121.4±21.0 ng OT during the 4h session. At 1 ng/ul OT, rats received 5.7±1.4 ng OT/4h.
Fig. 1.
Operant responses/4h for icv infusion of OT in female rats. Responses at FR5 (mean±SEM) for active (closed bars) and inactive (open bars) levers averaged over 5 days. By RM-ANOVA, there was a significant effect of OT and a significant effect of lever on operant responses. Asterisk indicates significant increase in active lever responses for OT at 10 ng/ul vs vehicle (0 ng/ul OT).
Elevated Plus Maze
OT had no acute or chronic effects on anxiety-related behavior in the elevated plus maze (F3,23=0.22, N.S.), similar to previous reports in gonad-intact female rats with acute OT (32) and for OVX+E rats with chronic OT-treatment (15). OT-naïve rats infused acutely with aCSF spent 20.5±6.0% of the 5-min test in the open arms of the maze. There was no lasting effect of prior OT exposure on open arm entries. Rats that had previously self-administered OT and received aCSF immediately before testing spent 24.0±10.6% of time on the open arms. Likewise, acute OT infusion did not alter behavior in the elevated plus maze. With 0.3 ug OT, rats spent 26.0±6.8% of the test in the open arms, and rats that received 3 ug OT spent 18.0±6.5%.
Likewise, OT had no acute or chronic effects on locomotor activity on the elevated plus maze as measured either by the number of closed arm entries (F3,23=1.4, N.S.), or the number of total arm entries (open+closed) (F3,23=1.9, N.S.). aCSF controls made 9.9±0.7 closed-arm entries and 14.4±1.0 total entries per 5 min. In rats infused with aCSF following previous OT exposure via self-administration, closed arm entries averaged 12.4±1.0, and total entries averaged 17.0±1.3. Acute OT infusion at 0.3 ug (13.0±1.8 closed entries, 18.7±2.2 total entries) or 3 ug (11.4±1.4 closed entries, 14.4±1.5 total entries) had no effect.
DISCUSSION
In the present study, OVX+E female rats voluntarily self-administered OT icv at a dose of 10 ng/ul. This finding complements our recent study demonstrating OT-induced CPP in female mice (18). Together, CPP and self-administration provide evidence that OT is both rewarding and reinforcing, at least in females. CPP and self-administration provide complementary insights into OT as a motivating stimulus. CPP is thought to reflect the rewarding (hedonic value) effects of a substance while self-administration measures drug-seeking and drug-taking (reinforcing effects) (33). Our studies of CPP and self-administration each delivered OT icv in OVX+E females. Mice demonstrated CPP for OT at 200 ng (18), a dose comparable to the amount of OT self-administered by rats in 4h (ca. 120 ng). In the present study, rats showed a substantial preference for the active lever paired with OT infusion at 10 ug/ul. It is notable that the control group also showed a modest but significant preference for the active lever over the inactive lever, as reported previously (34). This may reflect the additional stimuli (illumination of the stimulus light and activation of the clicker) associated with the active lever. Nonetheless, icv infusion of 10 ug/ul OT increased responses on the active lever above that of aCSF controls.
Reward and reinforcement are linked through dopamine release from neurons in the ventral tegmental area (VTA) that project to Acb (35). Already, a variety of evidence links OT with the mesolimbic dopamine system. Receptors for OT are present in Acb, with higher concentrations during proestrus/estrus compared to non-estrus in female rats (36). Likewise, OT infusion into VTA stimulates dopamine in Acb (37). Since drugs of abuse also increase Acb dopamine, it is interesting that OT has potential interactions with some illicit drugs. Prosocial effects of ecstasy may be mediated in part through endogenous OT (38). Conversely, exogenous OT attenuates the dopamine response to methamphetamine in mice (39) and to ethanol in rats (40), thereby blocking the reinforcing properties of these drugs.
Rewarding effects of OT have particular relevance to social behavior. Exogenous OT enhances expression of pair-bonding and maternal behavior (reviewed in 1, 2 and 6). Other studies have measured release of endogenous OT during pair-bonding and maternal behavior (41, 42, 43). Compared with polygamous montane voles, monogamous prairie voles have a high density of OT receptors in Acb (41), and OT is released in Acb as a result of mating (42). In rats bred for high anxiety, maternal aggression is correlated with OT release in the paraventricular nucleus and the central nucleus of the amygdala (43). Since OT release facilitates rewarding social behaviors such as mating (42) and aggression (43), it seems likely that endogenous OT might have reinforcing effects, similar to the effects of exogenous OT infusion reported in the present study. If so, offering the opportunity for self-administration in a socially-rewarding setting could enhance reward through the combination of exogenous OT self-administration and endogenous OT release. The amount of exogenous OT self-administered icv in the present study (121.4±21.0 ng/4h) is higher than endogenous OT release (pg range; 42, 44), and we did not measure OT levels in Acb. However, icv infusion of OT at 10 ng/ul has been shown to restore lordosis in female rats treated with an OT antagonist (27).
One of the caveats of OT self-administration in the present study and of CPP (18) is that animals are tested in isolation. Social context influences drug reward in rodents [reviewed in 45]. In this regard, social facilitation increases self-administration of amphetamine at 0.1 mg/kg in rats (46). Although the present study did not address social facilitation of OT self-administration, our previous study in female mice compared CPP vs conditioned social preference (18). Where CPP pairs an unconditioned stimulus (in this case, OT) with an unfamiliar environment (the conditioned stimulus, CS+), conditioned social preference uses an unfamiliar conspecific as the CS+. Importantly, OT was rewarding by conditioned social preference at both 100 and 200 ng, while CPP was evident only at the higher dose (18).
Our results with the elevated plus maze suggest that reinforcing effects of OT cannot be explained simply by a reduction in anxiety. While there is evidence for anxiolytic effects of OT in mice and male rats, our findings are consistent with published reports in virgin female rats (15, 32, 47, 48). In the present study, OT had no effect on anxiety behavior in the elevated plus maze for OVX+E rats exposed chronically to OT during self-administration, similar to OVX+E females chronically infused icv with OT (15). While our study did not include rats that self-administered aCSF only prior to acute OT infusion, acute OT infusion (1 ug icv) had no effect on anxiety in ovary-intact virgin female rats (32). Moreover, an OT antagonist was anxiogenic only in pregnant and lactating female rats, but not in virgin females (47, 48). Although anxiolytic effects of chronic OT have been observed in female rats bred for high anxiety, there was no effect in rats bred for low-anxiety (32). Conversely, systemic treatment with OT at high doses (3 mg/kg) is reported to have an anxiolytic effect in male rats measured in an open field test (11). Likewise, OT reduces anxiety behavior in male and female mice when delivered either centrally or systemically (12–13). However, mice may be more sensitive than rats to anxiolytic effects of OT. Based on the results we obtained with OXV+E rats in the elevated plus maze following OT self-administration, OT’s rewarding properties are likely to be independent of its anxiolytic effects.
In the present study, self-administration of OT in the absence of social stimuli suggests that OT may be rewarding per se. If so, OT may have potential to cause dependence, either alone or in combination with other drugs of abuse. The rewarding effects of OT are unlikely to compare with that of cocaine or other highly-addictive drugs, at least based on rates of operant responding reported here compared with cocaine self-administration (for example, see [49]). Instead, OT reward may be comparable to other neuroactive hormones, such as estrogen (50) and testosterone (28, 34). In this regard, evidence from human and animal studies suggests that testosterone can cause dependence (51). OVX rats show CPP for estrogen (50), and estrogen promotes rewarding effects of amphetamine (52). Like OT, estrogen increases social interaction (53). Furthermore, OT and estrogen synergize to promote social and sexual behaviors: OT facilitates lordosis behavior in estrogen-treated rats (54), and estrogen increases central OT receptors (55). OT reward and reinforcement as demonstrated by CPP and self-administration on an FR schedule are initial steps to explore potential OT dependence. Further studies testing drug discrimination or self-administration on a progressive ratio schedule and in a reinstatement paradigm (reviewed in 19) would be necessary.
Acknowledgments
Supported by NIH R21-AA020575 to RIW.
REFERENCES
- 1.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 2.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7(10):1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci USA. 1979;76(12):6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6(3):247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 5.Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40(2):133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 6.Insel TR. Is social attachment an addictive disorder? Physiol Behav. 2003;79:351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 7.Newman KS, Halpin ZT. Individual odours and mate recognition in the prairie vole, Microtus ochrogaster. Animal Behaviour. 1988;36(6):1779–1787. [Google Scholar]
- 8.Fleming A, Korsmit M, Deller M. Rat pups are potent reinforcers to the maternal animal: effects of experience, parity, hormones, and dopamine function. 1994;22(1):44–53. [Google Scholar]
- 9.Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 2000;108(2):215–231. doi: 10.1016/s0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- 10.Young LJ. Oxytocin, social cognition and psychiatry. Neuropsychopharmacology. 2015;40(1):243. doi: 10.1038/npp.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uvnas-Moberg K, Ahlenius S, Hillegaart V, Alster P. High doses of oxytocin cause sedation and low doses cause an anxiolytic-like effect in male rats. Pharmacol Biochem Behav. 1994;49(1):101–106. doi: 10.1016/0091-3057(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 12.Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006;185(2):218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav. 1996;60(5):1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- 14.Mantella RC, Vollmer RR, Li X, Amico JA. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology. 2003;144(6):2291–2296. doi: 10.1210/en.2002-0197. [DOI] [PubMed] [Google Scholar]
- 15.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138(7):2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 16.Arletti R, Bertolini A. Oxytocin acts as an antidepressant in two animal models of depression. Life Sci. 1987;41(14):1725–1730. doi: 10.1016/0024-3205(87)90600-x. [DOI] [PubMed] [Google Scholar]
- 17.Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T, Matsui H. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci. 2003;6(4):384–390. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- 18.Kent K, Arientyl V, Khachatryan MM, Wood RI. Oxytocin induces a conditioned social preference in female mice. J Neuroendocrinol. 2013;25(9):803–810. doi: 10.1111/jne.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch WJ, Nicholson KL, Dance ME, Morgan RW, Foley PL. Animal models of substance abuse and addiction: implications for science, animal welfare, and society. Comp Med. 2010;60(3):177–188. [PMC free article] [PubMed] [Google Scholar]
- 20.Spanagel R, Montkowski A, Allingham K, Stohr T, Shoaib M, Holsboer F, Landgraf R. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology (Berl) 1995;122(4):369–373. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- 21.Szostak C, Finlay JM, Fibiger HC. Intravenous self-administration of the short-acting benzodiazepine midazolam in the rat. Neuropharmacology. 1987;26(12):1673–1676. doi: 10.1016/0028-3908(87)90116-x. [DOI] [PubMed] [Google Scholar]
- 22.Pilotto R, Singer G, Overstreet D. Self-injection of diazepam in naive rats: effects of dose, schedule and blockade of different receptors. Psychopharmacology (Berl) 1984;84(2):174–177. doi: 10.1007/BF00427442. [DOI] [PubMed] [Google Scholar]
- 23.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38(10):1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Arletti R, Benelli A, Bertolini A. Oxytocin inhibits food and fluid intake in rats. Physiol Behav. 1990;48(6):825–830. doi: 10.1016/0031-9384(90)90234-u. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21(20):8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arletti R, Bertolini A. Oxytocin stimulates lordosis behavior in female rats. Neuropeptides. 1985;6(3):247–253. doi: 10.1016/0143-4179(85)90095-2. [DOI] [PubMed] [Google Scholar]
- 27.Benelli A, Poggioli R, Luppi P, Ruini L, Bertolini A, Arletti R. Oxytocin enhances, and oxytocin antagonism decreases, sexual receptivity in intact female rats. Neuropeptides. 1994;27(4):245–250. doi: 10.1016/0143-4179(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 28.Wood RI, Johnson LR, Chu L, Schad C, Self DW. Testosterone reinforcement: intravenous and intracerebroventricular self-administration in male rats and hamsters. Psychopharmacology (Berl) 2004;171(3):298–305. doi: 10.1007/s00213-003-1587-7. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy MM. Estrogen modulation of oxytocin and its relation to behavior. Adv Exp Med Biol. 1995:395235–395245. [PubMed] [Google Scholar]
- 30.Ellis GB, Turek FW. Photoperiod-induced change in responsiveness of the hypothalamic-pituitary axis to exogenous 5 alpha-dihydrotestosterone and 17 beta-estradiol in castrated male hamsters. Neuroendocrinology. 1980;31(3):205–209. doi: 10.1159/000123075. [DOI] [PubMed] [Google Scholar]
- 31.File SE, Lippa AS, Beer B, Lippa MT. Animal tests of anxiety. Curr Protoc Neurosci. 2004 doi: 10.1002/0471142301.ns0803s26. Chapter 8Unit 8.3. [DOI] [PubMed] [Google Scholar]
- 32.Slattery DA, Neumann ID. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology. 2010;58(1):56–61. doi: 10.1016/j.neuropharm.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 33.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 34.Sato SM, Johansen J, Jordan CL, Wood RI. Membrane, not nuclear, androgen receptor mediates androgen reinforcement. Psychoneuroendocrinology. 2010;35(7):1063–1073. doi: 10.1016/j.psyneuen.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, Roitman MF, Lobo MK. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J Neurosci. 2013;33(45):17569–17576. doi: 10.1523/JNEUROSCI.3250-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm Behav. 2013;64(4):693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151(5):2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dumont GJ, Sweep FC, van der Steen R, Hermsen R, Donders AR, Touw DJ, van Gerven JM, Buitelaar JK, Verkes RJ. Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc Neurosci. 2009;4(4):359–366. doi: 10.1080/17470910802649470. [DOI] [PubMed] [Google Scholar]
- 39.Qi J, Yang JY, Song M, Li Y, Wang F, Wu CF. Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn Schmiedebergs Arch Pharmacol. 2008;376(6):441–448. doi: 10.1007/s00210-007-0245-8. [DOI] [PubMed] [Google Scholar]
- 40.Peters ST, Bowen MT, Bohrer K, McGregor IS, Neumann ID. Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addict Biol. 2016 doi: 10.1111/adb.12362. [DOI] [PubMed] [Google Scholar]
- 41.Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40(2):133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 42.Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162(4):892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25(29):6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993;53(1):65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- 45.Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65(1):255–290. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gipson CD, Yates JR, Beckmann JS, Marusich JA, Zentall TR, Bardo MT. Social facilitation of d-amphetamine self-administration in rats. Exp Clin Psychopharmacol. 2011;19(6):409–419. doi: 10.1037/a0024682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 1999;95(2):567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- 48.Figueira RJ, Peabody MF, Lonstein JS. Oxytocin receptor activity in the ventrocaudal periaqueductal gray modulates anxiety-related behavior in postpartum rats. Behav Neuroscience. 2008;122(3):618–628. doi: 10.1037/0735-7044.122.3.618. [DOI] [PubMed] [Google Scholar]
- 49.Perrya AN, Westenbroeka C, Becker JB. Impact of pubertal and adult estradiol treatments on cocaine self-administration. Horm Behav. 2013;64(4):573–578. doi: 10.1016/j.yhbeh.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frye CA, Rhodes ME. Administration of estrogen to ovariectomized rats promotes conditioned place preference and produces moderate levels of estrogen in the nucleus accumbens. Brain Res. 2006;1067(1):209–215. doi: 10.1016/j.brainres.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 51.Pope HG, Jr, Wood RI, Rogol AD, Nyberg F, Bowers LD, Bhasin S. Adverse health consequences of the use of performance-enhancing drugs: an Endocrine Society scientific statement. Endo Rev. 2014;35(3):341–375. doi: 10.1210/er.2013-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silverman JL, Koenig JI. Evidence for involvement of ERb and RGS9-2 in 17-b estradiol enhancement of amphetamine-induced place preference behavior. Horm Behav. 2007;52(2):146–155. doi: 10.1016/j.yhbeh.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walf AA, Frye CA. Conjugated equine estrogen enhances rats' cognitive, anxiety, and social behavior. Neuroreport. 2008;19(7):789–792. doi: 10.1097/WNR.0b013e3282fe209c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caldwell JD, Prange AJ, Jr, Pedersen CA. Oxytocin facilitates the sexual receptivity of estrogen-treated female rats. Neuropeptides. 1986;7(2):175–189. doi: 10.1016/0143-4179(86)90093-4. [DOI] [PubMed] [Google Scholar]
- 55.Bale TL, Dorsa DM. Sex differences in and effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the ventromedial hypothalamus. Endocrinology. 1995;136(1):27–32. doi: 10.1210/endo.136.1.7828541. [DOI] [PubMed] [Google Scholar]