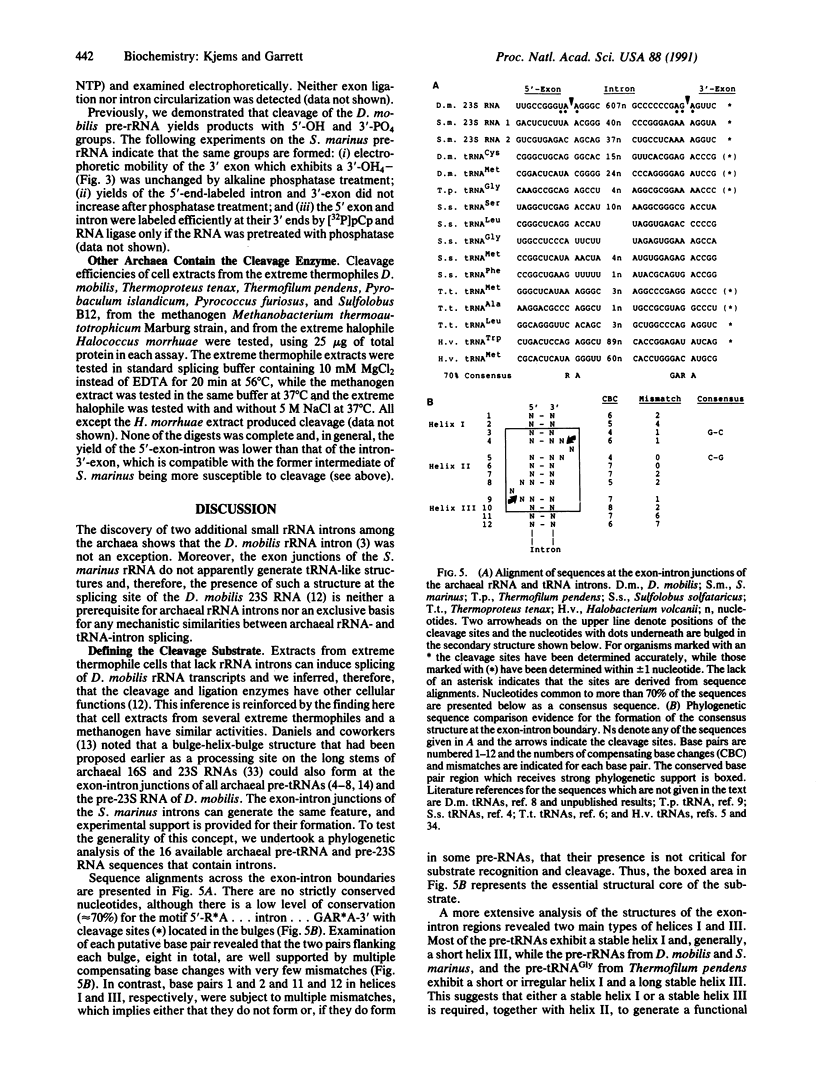
Abstract
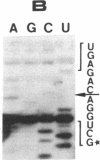
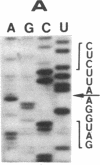
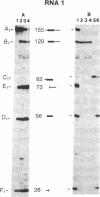
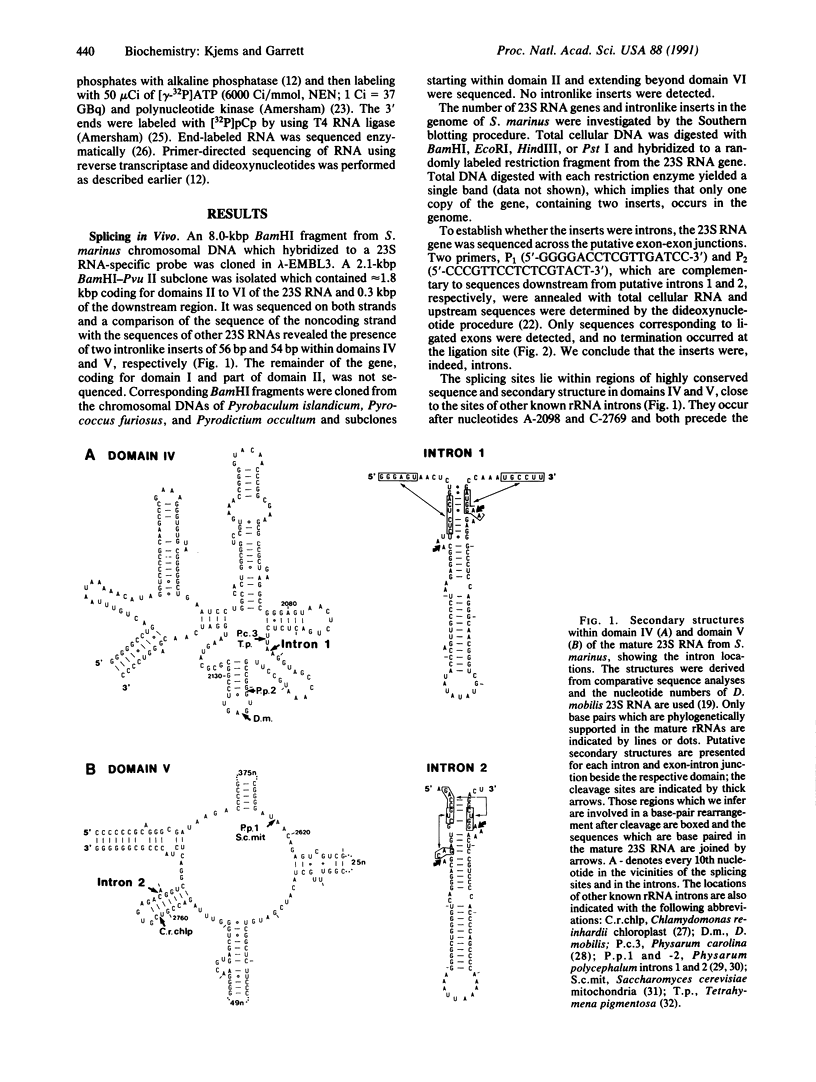
The single 23S rRNA gene of the archaeon Staphylothermus marinus exhibits two introns which, at the RNA level, are located in highly conserved regions of domains IV and V. The RNA introns, which are 56 and 54 nucleotides long, respectively, can form single hairpin structures. In vivo, RNA splicing occurs efficiently, whereas in vitro pre-rRNA transcripts containing each intron were cleaved efficiently when incubated with archaeal cell extracts but were poorly ligated. The introns are cleaved by a mechanism which differs from the mechanisms of eukaryotic rRNA introns but resembles those of the rRNA intron of Desulfurococcus mobilis and the archaeal tRNA introns. The cleavage enzyme recognizes and cuts a putative bulge-helix-bulge structure that can form at the archaeal exon-intron junctions. Using a phylogenetic sequence comparison approach, we define the parts of this structural feature that are essential for cleavage. We also provide evidence for conformational changes occurring in the S. marinus 23S RNA, after cleavage, at both exon-exon junctions, which may account for the low yields of ligation observed in vitro.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Rochaix J. D. Structure analysis at the ends of the intervening DNA sequences in the chloroplast 23S ribosomal genes of C. reinhardii. Cell. 1979 Sep;18(1):55–60. doi: 10.1016/0092-8674(79)90353-2. [DOI] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 1978 Oct;5(10):3665–3677. doi: 10.1093/nar/5.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Bass B. L. Biological catalysis by RNA. Annu Rev Biochem. 1986;55:599–629. doi: 10.1146/annurev.bi.55.070186.003123. [DOI] [PubMed] [Google Scholar]
- Chu F. K., Maley G. F., Maley F., Belfort M. Intervening sequence in the thymidylate synthase gene of bacteriophage T4. Proc Natl Acad Sci U S A. 1984 May;81(10):3049–3053. doi: 10.1073/pnas.81.10.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C. J., Gupta R., Doolittle W. F. Transcription and excision of a large intron in the tRNATrp gene of an archaebacterium, Halobacterium volcanii. J Biol Chem. 1985 Mar 10;260(5):3132–3134. [PubMed] [Google Scholar]
- Datta P. K., Hawkins L. K., Gupta R. Presence of an intron in elongator methionine-tRNA of Halobacterium volcanii. Can J Microbiol. 1989 Jan;35(1):189–194. doi: 10.1139/m89-029. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon B. Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell. 1980 May;20(1):185–197. doi: 10.1016/0092-8674(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Guo L. H., Yang R. C., Wu R. An improved strategy for rapid direct sequencing of both strands of long DNA molecules cloned in a plasmid. Nucleic Acids Res. 1983 Aug 25;11(16):5521–5540. doi: 10.1093/nar/11.16.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaine B. P., Gupta R., Woese C. R. Putative introns in tRNA genes of prokaryotes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3309–3312. doi: 10.1073/pnas.80.11.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjems J., Garrett R. A. Novel splicing mechanism for the ribosomal RNA intron in the archaebacterium Desulfurococcus mobilis. Cell. 1988 Aug 26;54(5):693–703. doi: 10.1016/s0092-8674(88)80014-x. [DOI] [PubMed] [Google Scholar]
- Kjems J., Jensen J., Olesen T., Garrett R. A. Comparison of transfer RNA and ribosomal RNA intron splicing in the extreme thermophile and archaebacterium Desulfurococcus mobilis. Can J Microbiol. 1989 Jan;35(1):210–214. doi: 10.1139/m89-033. [DOI] [PubMed] [Google Scholar]
- Kjems J., Leffers H., Garrett R. A., Wich G., Leinfelder W., Böck A. Gene organization, transcription signals and processing of the single ribosomal RNA operon of the archaebacterium Thermoproteus tenax. Nucleic Acids Res. 1987 Jun 25;15(12):4821–4835. doi: 10.1093/nar/15.12.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjems J., Leffers H., Olesen T., Garrett R. A. A unique tRNA intron in the variable loop of the extreme thermophile Thermofilum pendens and its possible evolutionary implications. J Biol Chem. 1989 Oct 25;264(30):17834–17837. [PubMed] [Google Scholar]
- Leffers H., Kjems J., Ostergaard L., Larsen N., Garrett R. A. Evolutionary relationships amongst archaebacteria. A comparative study of 23 S ribosomal RNAs of a sulphur-dependent extreme thermophile, an extreme halophile and a thermophilic methanogen. J Mol Biol. 1987 May 5;195(1):43–61. doi: 10.1016/0022-2836(87)90326-3. [DOI] [PubMed] [Google Scholar]
- Mankin A. S., Teterina N. L., Rubtsov P. M., Baratova L. A., Kagramanova V. K. Putative promoter region of rRNA operon from archaebacterium Halobacterium halobium. Nucleic Acids Res. 1984 Aug 24;12(16):6537–6546. doi: 10.1093/nar/12.16.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscarella D. E., Vogt V. M. A mobile group I intron in the nuclear rDNA of Physarum polycephalum. Cell. 1989 Feb 10;56(3):443–454. doi: 10.1016/0092-8674(89)90247-x. [DOI] [PubMed] [Google Scholar]
- Nomiyama H., Kuhara S., Kukita T., Otsuka T., Sakaki Y. Nucleotide sequence of the ribosomal RNA gene of Physarum polycephalum: intron 2 and its flanking regions of the 26S rRNA gene. Nucleic Acids Res. 1981 Nov 11;9(21):5507–5520. doi: 10.1093/nar/9.21.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomiyama H., Sakaki Y., Takagi Y. Nucleotide sequence of a ribosomal RNA gene intron from slime mold Physarum polycephalum. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1376–1380. doi: 10.1073/pnas.78.3.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R., Maniatis T. A role for exon sequences and splice-site proximity in splice-site selection. Cell. 1986 Aug 29;46(5):681–690. doi: 10.1016/0092-8674(86)90343-0. [DOI] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg B. M., Hahne S., Mathews C. Z., Mathews C. K., Rand K. N., Gait M. J. The bacteriophage T4 gene for the small subunit of ribonucleotide reductase contains an intron. EMBO J. 1986 Aug;5(8):2031–2036. doi: 10.1002/j.1460-2075.1986.tb04460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Meissner F., Moll J., Vorderwülbecke T. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1987;15 (Suppl):r53–188. doi: 10.1093/nar/15.suppl.r53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Thompson L. D., Brandon L. D., Nieuwlandt D. T., Daniels C. J. Transfer RNA intron processing in the halophilic archaebacteria. Can J Microbiol. 1989 Jan;35(1):36–42. doi: 10.1139/m89-006. [DOI] [PubMed] [Google Scholar]
- Thompson L. D., Daniels C. J. A tRNA(Trp) intron endonuclease from Halobacterium volcanii. Unique substrate recognition properties. J Biol Chem. 1988 Dec 5;263(34):17951–17959. [PubMed] [Google Scholar]
- Wich G., Leinfelder W., Böck A. Genes for stable RNA in the extreme thermophile Thermoproteus tenax: introns and transcription signals. EMBO J. 1987 Feb;6(2):523–528. doi: 10.1002/j.1460-2075.1987.tb04784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild M. A., Sommer R. Sequence of a ribosomal RNA gene intron from Tetrahymena. Nature. 1980 Feb 14;283(5748):693–694. doi: 10.1038/283693a0. [DOI] [PubMed] [Google Scholar]