Abstract
Serum vitamin D status has been associated with prediabetes and metabolic syndrome. Evidence for the increased risk of metabolic disorders in individuals with prediabetes and a low vitamin D status is limited and uncertain. Furthermore, it has not been confirmed whether this possible relationship occurs in the Korean population. The aim of this study was to assess serum vitamin D status and to examine the relationship between serum vitamin D levels and metabolic risk factors in Korean adults with prediabetes. This cross-sectional study was conducted among 60 subjects aged 20–65 years. Participants had fasting glucose levels of 100 to 125 mg/dl. A questionnaire was used to assess vitamin D synthesis from sun exposure and a dietary intake examined using 3-days dietary records. Clinical and biochemical data were also collected. The 2009 harmonized definition of metabolic syndrome was used. Serum vitamin D levels were classified according to criteria from the 2011 Institute of Medicine report. The majority of subjects (75%) had a serum 25(OH)D level < 20 ng/ml, and among them, 31.1% were vitamin D deficiency (< 12 ng/ml). The proportion (42.9%) of subjects having low HDL-cholesterol was the highest among vitamin D deficiency (< 12 ng/ml) group (12 to < 20 ng/ml: 16.1%, ≥ 20 ng/ml: 6.7%). We observed an inverse relationship between 25(OH)D levels and TG, AI (β = -6.355, SE = 2.463; β = -0.020, SE = 0.008) after adjusted confounders. Korean adults with prediabetes were more likely to have low serum 25(OH)D levels. A sufficient 25(OH)D level may have possible beneficial effects on lipid profiles.
Introduction
According to data on the Korea National Health and Nutritional Examination Survey, the prevalence of prediabetes, which is one of metabolic syndrome components, has increased steadily to 25.0% among adults aged 30 years and older in 2013 [1,2]. Although several studies have reported beneficial effects of lifestyle modification including nutrition management for preventing of diabetes and metabolic syndrome (MetS) [3,4], these modifications are inadequate to be applied to Korean with prediabetes, owing to a lack of evidences.
Recently, the role of vitamin D in prediabetes has increased interest [5]. Inadequate vitamin D status is highly prevalent in the general population and is now recognized as a common health issue worldwide [6]. In South Korea, it has been shown that there is a high prevalence of poor vitamin D status [7,8]. Prediabetes is defined as an insulin resistance [9]. Accumulating evidences have suggested that vitamin D may have a protective role in the underlying disorders of prediabetes [10,11]. One proposed mechanism of the beneficial effect of vitamin D in prediabetes is that vitamin D directly modulates gene expression in target cells. Both vitamin D receptor and 25(OH)D-1α hydroxylase involved in this mechanism are discovered in most cells and tissues. On that basis, new potentiality of vitamin D have been presented [6].
More recently, there have been an increasing number of studies that have established the relationship between vitamin D deficiency and MetS including its components [11,12]. However, some studies have suggested conflicting results [13,14], and there is still controversy regarding this relationship.
Some U.S studies have suggested the importance of initial management in the prevention of MetS as well as cardio vascular disease (CVD) in prediabetic adults [1,15]. However, to date, no studies have established the relationship between serum vitamin D status and MetS in South Korea. Although there have been several studies among adults with prediabetes or high risk for T2DM [11,14], these results cannot be directly applied to a Korean population due to ethnic differences in vitamin D metabolism and its nutritional status [16].
Therefore, we evaluated serum vitamin D status among Korean adults aged 20 to 65 years with prediabetes (fasting serum glucose levels 100 to 125 mg/dl). We also examined the association between serum vitamin D levels and metabolic risk factors.
Materials and Methods
Subjects and study design
This cross-sectional study was approved by the Institutional Review Board at Kyung Hee University Hospital in Seoul, South Korea (KMC IRB 1406–02). Individuals were recruited through a notice on the hospital website and bulletin board. A total of 185 individuals were invited to undergo a screening test for 5 months, from May to October 2014. Individuals aged 20 to 65 years, who had a fasting glucose level of 100 to 125 mg/dl, were eligible for this study. Exclusion criteria included: 1) HbA1c levels 6.5% and over, 2) use of oral hypoglycemic agents, 3) pregnancy, 4) alcoholism, and 5) renal dysfunction or dyshepatia. Based on the exclusion criteria, a total of 118 participants were determined to be ineligible. A total of 7 participants withdrew their consent by oneself.
A 60 subjects (31 male and 29 female) was finally enrolled. All subjects signed a written informed consent. This study was conducted using questionnaires, a dietary intake survey, anthropometric investigation, BP measurement, and blood test.
Data collection
A general informational survey was used to record self-reported and verified a fact. Medical history, including any diagnosed conditions and diagnosis date, was collected. A drug history survey was used to assess current medication use including drug name, daily dose with unit, period of dose, and purpose of dose. Smoking and drinking alcohol were classified as never, former, or current.
Participants visited in late spring, summer or fall and self-reported one of three degrees of skin color (light, medium, dark), the duration (after washing face, before going out, while one is out) and body part (face, neck, arm, leg, shoulder, whole body) of sunscreen use, as well as sunscreen reapplication habits, and also documented the frequency and mean duration of daytime outdoor activity.
Dietary intake was investigated through 3 days of dietary records. To account for weekly variations in dietary intake, the 3 days of dietary records included one weekend day or holiday. The 3-day dietary records were compared with actual intake using model food during personal interviews. Data from dietary records were analyzed using a computer-aided nutritional analysis program, CAN Pro version 4.0 (The Korean Nutrition Society 2010, Seoul, Korea) to determine subjects’ dietary intake for multiple nutrient categories including vitamin D.
Standing height, weight and body composition were obtained on an empty stomach by bioelectrical impedance analysis (Inbody720, Biospace, Seoul, South Korea). Measurements were recorded to the nearest 0.1cm or 0.1kg. WC was measured horizontally at the navel using a tape measure (Hoechstmass, Sulzbach, Germany). At rest while seated, BP of the upper arms was measured 2 times at 30-seconds intervals using an automatic electronic sphygmomanometer (BPBI0320S, Inbody, Seoul, South Korea) and the average of measurements was used for analysis. To reduce measuring error, the same equipment and instrument was used according to standard methods.
Blood samples were collected in the morning after fasting for 12 hours. After 30 minutes venous blood collection, serum was separated in serum separation tubes by centrifugal filtration (3000rpm for 15minutes), separated serum put into eppendorf tubes using pipeat. Eppendorf tubes and ethylene diamine tetra acetic acid tubes were properly stored, and shipped to the blood analysis facility for analysis. The fasting serum concentration of glucose was analyzed using UV assay (HK). Levels of insulin were analyzed using electro-chemiluminescence immunoassay (ECLIA). Whole blood HbA1c was analyzed using turbidimetric immunoassay (TIA).
Insulin resistance (IR) was approximately estimated by both the Homeostatic Model of Insulin Resistance (HOMA-IR) and Quantitative Insulin Sensitivity Check Index (QUICKI). HOMA-IR was calculated by the following formula:
[17].
The reciprocal of HOMA-IR was used for analysis due to greater reproducibility [18]. QUICKI is a simple, accurate method for evaluating IR that is useful for clinical investigations [19]. It was calculated as the following:
[19].
Serum concentrations of total cholesterol and triglycerides were analyzed using enzymatic colorimetric assay. Levels of high density lipoprotein cholesterol (HDL-cholesterol) and low density lipoprotein cholesterol (LDL- cholesterol) were analyzed using homogeneous enzymatic colorimetric assay. Serum 25(OH)D concentrations were measured using chemiluminescent immunoassay (CLIA). Atherogenic index (AI) was calculated as the following:
[20].
Definition of metabolic syndrome
In this study, MetS was defined according to the harmonizing definition from the International Diabetes Federation, the American Heart Association, and the National Heart, Lung, and Blood Institute (AHA/IDF/NHLBI harmonizing definition, 2009) [21].
The presence of 3 or more of the following criteria was used to define MetS:
Central obesity, waist circumference (≥ 90 cm for men and ≥ 85 cm for women) [22]
Triglycerides (≥ 150 mg/dl) or current use of drug treatment for elevated triglycerides
HDL-cholesterol (< 40 mg/dl for men and < 50 mg/dl for women) or current use of drug treatment for reduced HDL-cholesterol
Blood pressure (systolic ≥ 130 mmHg or diastolic≥ 85mmHg) or current use of antihypertensive drug treatment
Fasting glucose (≥ 100mg/dl) or current use of insulin, oral hypoglycemia drug.
Diagnostic criteria of vitamin D status
Serum 1, 25(OH)2D has a shorter half-life of 4 hours than serum 25(OH)D and should not be used as a measure of vitamin D deficiency. In addition, levels of 1, 25(OH)2D may be normal or even elevated as a result of secondary hyperparathyroidism [23]. Therefore, evidence suggests that serum 25(OH)D is the best parameter to assess vitamin D status due to its relatively long half-life of approximately 2–3 weeks [7]. There is a lack of consensus regarding the optimal serum 25(OH)D levels. According to a 2011 report by the Institute of Medicine (IOM), review of the available data suggests that there is a risk for vitamin D deficiency at serum 25(OH)D levels less than 30 nmol/L (12 ng/mL). There is a potential risk for vitamin D inadequacy at serum 25(OH)D levels between 30 and 50 nmol/L (12 and 20 ng/mL). Practically, vitamin D normal occurs at serum 25(OH)D levels of at least 50 nmol/L (20 ng/mL) [24].
Statistical analysis
Data was presented as mean ± standard error (SE) for numerical data and as numbers and percentage for categorical data. Comparison of general characteristics, mean of metabolic outcomes and proportion of metabolic risk factors by vitamin D status groups including mean of serum 25(OH)D levels of MetS combinations were performed using Fisher’s exact test and non-parametric statistics such as Kruskal-Wallis tests (post hoc by Bonferroni’s method). Partial correlation analysis and multiple linear regression analysis were used to demonstrate a linear relationship between serum 25(OH)D levels and metabolic outcomes. The covariates for the adjusted r and β include age, sex, smoking, drinking alcohol, daytime outdoor activity and energy intake.
All statistical analyses were performed using SPSS (version 21.0, SPSS Inc., Chicago, IL, USA). Statistical significance was defined as a p-value of < 0.05.
Results
General characteristics by serum vitamin D status
The general characteristics of Korean prediabetic adults by serum 25(OH)D level are shown in Table 1. Among 60 subjects, 31 (51.7%) were male and 29 (48.3%) were female. The mean age of all subjects was 45.1 ± 1.4 years. Overall, most subjects (75%) had a serum 25(OH)D level < 20 ng/ml, and among these subjects, 31.1% were vitamin D deficient (< 12 ng/ml) according to IOM criteria. The mean serum 25(OH)D level among all subjects was 17.3 ± 0.8 ng/ml.
Table 1. General characteristics of Korean prediabetic adults by serum 25(OH)D level.
| Serum 25(OH)D groups by IOM criteria a | |||||
|---|---|---|---|---|---|
| Total | < 12 ng/ml | 12 to < 20 ng/ml | ≥ 20 ng/ml | P value | |
| (n = 60) | (n = 14) | (n = 31) | (n = 15) | ||
| Sex (%) | |||||
| Male | 31 (51.7) b | 5 (35.7) | 18 (58.1) | 8 (53.3) | 0.387 |
| Age (y) | 45.1 ± 1.4 c | 43.5 ± 3.2 | 43.7 ± 1.8 | 49.4 ± 3.1 | |
| Smoking (%) | |||||
| Never | 40 (66.7) | 12 (85.7) | 17 (54.8) | 17 (73.3) | 0.090 |
| Former | 2 (3.3) | 1 (7.1) | 1 (3.2) | 0 (0.0) | |
| Current | 18 (30.0) | 1 (7.2) | 13 (42.0) | 4 (26.7) | |
| Drinking alcohol (%) | |||||
| Never | 16 (26.7) | 5 (35.7) | 6 (19.4) | 5 (33.3) | 0.693 |
| Former | 1 (1.7) | 0 (0.0) | 1 (3.2) | 0 (0.0) | |
| Current | 43 (71.7) | 9 (64.3) | 24 (77.4) | 10 (66.7) | |
| Season of blood draw (%) | |||||
| Late spring and summer | 24 (40.0) | 8 (57.1) | 13 (41.9) | 3 (20.0) | 0.042 |
| Fall | 36 (60.0) | 6 (42.9) | 18 (58.1) | 12 (80.0) | |
| Daytime outdoor activity (%) | |||||
| 6~7/week | 12 (20.0) | 1 (7.1) | 4 (12.9) | 7 (46.7) | 0.019 |
| 3~5/week | 7 (11.7) | 3 (21.4) | 1 (3.2) | 3 (20.0) | |
| 1~2/week | 19 (31.7) | 5 (35.7) | 12 (38.7) | 2 (13.3) | |
| Never | 22 (36.7) | 5 (35.7) | 14 (45.2) | 3 (20.0) | |
| Skin color (%) | |||||
| Light | 9 (15.0) | 4 (28.6) | 5 (16.1) | 0 (0.0) | 0.081 |
| Medium | 39 (65.0) | 9 (64.3) | 17 (54.8) | 13 (86.7) | |
| Dark | 12 (20.0) | 1 (7.1) | 9 (29.0) | 2 (13.3) | |
| Sunblock (%) | |||||
| User | 24 (40.0) | 5 (35.7) | 13 (41.9) | 6 (40.0) | 0.941 |
| Energy intake (kcal/day) | 1565.0 ± 63.8 | 1514.4 ± 76.5 | 1551.9 ± 108.8 | 1639.2 ± 103.0 | 0.363 |
| Total fat intake (g/day) | 41.8 ± 2.1 | 39.3 ± 3.4 | 40.4 ± 3.2 | 47.1 ± 3.8 | 0.196 |
| Vitamin D intake (μg/day) | 3.1 ± 0.4 | 2.9 ± 0.5 | 2.6 ± 0.3 | 3.9 ± 1.2 | 0.741 |
| Vitamin D intake (%, KDRIs) d | 30.0 ± 3.7 | 29.3 ± 4.7 | 25.8 ± 3.5 | 39.4 ± 12.3 | 0.689 |
25(OH)D, 25-hydroxyvitamin D; IOM, Institute of Medicine
a 25(OH)D levels were classified according to diagnostic criteria defined by IOM in 2011.
b Values are n (%); difference among serum 25(OH)D group was assessed by the Fisher’s exact test.
c Values are mean ± SE; difference among serum 25(OH)D group was assessed by the Kruskal-Wallis test.
d Percentage of daily intake of vitamin D compared with the quantity suggested by dietary reference intakes for Koreans (KDRIs).
The proportion of subjects sampled in the late spring or summer decrease as serum 25(OH)D levels increased (p = 0.042). Approximately one third (36.7%) of subjects reported no outdoor activity during the daytime. The proportion of daytime outdoor activity stayed as much as 6–7 times a week in subjects with vitamin D deficiency (< 12 ng/ml) was the lowest among the three serum 25(OH)D groups (p = 0.019). Skin color and use of sunblock did not differ among serum 25(OH)D levels (all p ≥ 0.05).
The mean daily intake of vitamin D was 3.1 ± 0.4 μg as measured by 3 days of dietary records. These intakes are approximately 30% of the quantity suggested by Dietary Reference Intakes for Koreans (KDRIs).
Metabolic outcomes by serum vitamin D status
The metabolic outcomes of Korean prediabetic adults by serum 25(OH)D level are shown in Table 2. The mean BMI of all subjects was 25.4 ± 0.5 kg/m2. There was significant association between serum 25(OH)D level and atherogenic index (AI) in females only (p = 0.038).
Table 2. Metabolic outcomes of Korean prediabetic adults by serum 25(OH)D level.
| Serum 25(OH)D groups by IOM criteria a | |||||
|---|---|---|---|---|---|
| Total | < 12 ng/ml | 12 to < 20 ng/ml | ≥ 20 ng/ml | P value f | |
| (n = 60) | (n = 14) | (n = 31) | (n = 15) | ||
| Clinical parameters | |||||
| BMI (kg/m2) | 25.4 ± 0.5 b | 25.9 ± 1.3 | 24.9 ± 0.6 | 26.0 ± 0.7 | 0.434 |
| Percent body fat (%) | 29.6 ± 0.9 | 32.8 ± 2.3 | 28.7 ± 1.0 | 28.8 ± 2.1 | 0.238 |
| Waist circumference (cm) | |||||
| Male | 89.9 ± 1.5 | 90.4 ± 4.9 | 90.7 ± 1.8 | 87.9 ± 2.9 | 0.871 |
| Female | 79.6 ± 1.8 | 79.5 ± 3.1 | 78.1 ± 2.2 | 82.5 ± 5.3 | 0.888 |
| Systolic blood pressure (mmHg) | 122.8 ± 2.1 | 121.9 ± 3.7 | 123.9 ± 3.1 | 121.5 ± 4.0 | 0.941 |
| Diastolic blood pressure (mmHg) | 82.9 ± 1.5 | 82.5 ± 2.9 | 83.8 ± 1.8 | 81.4 ± 3.9 | 0.752 |
| Biochemical parameters | |||||
| Fasting glucose (mg/dl) | 106.3 ± 0.7 | 103.8 ± 0.8 | 106.6 ± 1.0 | 107.9 ± 1.6 | 0.160 |
| Fasting insulin (mU/l) | 6.2 ± 0.6 | 7.5 ± 1.8 | 5.5 ± 0.5 | 6.5 ± 1.2 | 0.783 |
| 1/HOMA-IR c | 1.46 ± 0.31 | 0.94 ± 0.17 | 1.45 ± 0.36 | 1.96 ± 1.02 | 0.752 |
| QUICKI d | 0.38 ± 0.01 | 0.37 ± 0.01 | 0.39 ± 0.01 | 0.39 ± 0.03 | 0.714 |
| Triglyceride (mg/dl) | 148.0 ± 13.9 | 180.2 ± 31.7 | 146.9 ± 21.4 | 119.9 ± 15.1 | 0.262 |
| Total cholesterol (mg/dl) | 205.2 ± 4.9 | 208.7 ± 10.0 | 211.1 ± 7.0 | 189.7 ± 8.6 | 0.183 |
| LDL-cholesterol (mg/dl) | 126.5 ± 4.5 | 126.9 ± 9.8 | 132.6 ± 6.5 | 113.6 ± 7.8 | 0.230 |
| HDL-cholesterol (mg/dl) | |||||
| Male | 51.6 ± 2.1 | 53.2 ± 6.6 | 50.7 ± 2.5 | 52.6 ± 5.0 | 0.961 |
| Female | 62.5 ± 3.2 | 53.4 ± 4.6 | 63.2 ± 5.4 | 72.9 ± 4.1 | 0.077 |
| Atherogenic index e | |||||
| Male | 0.46 ± 0.06 | 0.44 ± 0.18 | 0.50 ± 0.07 | 0.38 ± 0.11 | 0.708 |
| Female | 0.24 ± 0.06 | 0.48 ± 0.12 | 0.14 ± 0.09 | 0.11 ± 0.06 | 0.038 |
25(OH)D, 25-hydroxyvitamin D; IOM, Institute of Medicine; BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance; QUICKI, Quantitative Insulin Sensitivity Check Index; LDL-cholesterol, low density lipoprotein cholesterol; HDL-cholesterol, high density lipoprotein cholesterol.
a 25(OH)D levels were classified according to diagnostic criteria defined by IOM in 2011.
b Values are mean ± SE.
c HOMA-IR = (fasting insulin (mU/L) x fasting glucose (mg/dl))/405.
d QUICKI = 1/log(fasting insulin(μU/mL)) + log(fasting glucose(mg/dl)).
e Atherogenic index (AI) = (total cholesterol–HDL-cholesterol)/HDL-cholesterol.
f Difference among serum 25(OH)D group was assessed by the Kruskal-Wallis test.
Metabolic risk factors by serum vitamin D status
Table 3 shows the metabolic risk factors of Korean prediabetic adults by serum 25(OH)D level. Approximately 43.3% of all subjects were classified as having MetS. A significant difference in the prevalence of MetS by serum 25(OH)D levels was not found. Low HDL-cholesterol increased as serum 25(OH)D levels decreased (p = 0.047).
Table 3. Metabolic risk factors of Korean prediabetic adults by serum 25(OH)D level.
| Serum 25(OH)D groups by IOM criteria a | |||||
|---|---|---|---|---|---|
| Total | < 12 ng/ml | 12 to < 20 ng/ml | ≥ 20 ng/ml | P value d | |
| (n = 60) | (n = 14) | (n = 30) | (n = 15) | ||
| Metabolic syndrome c | 26 (43.3) b | 7 (50.0) | 13 (41.9) | 6 (40.0) | 0.594 |
| Central obesity | 23 (38.3) | 5 (35.7) | 12 (38.7) | 6 (40.0) | 0.815 |
| High blood pressure | 28 (46.7) | 6 (42.9) | 15 (48.4) | 7 (46.7) | 0.844 |
| Hypertriglyceridemia | 25 (41.7) | 6 (42.9) | 13 (41.9) | 6 (40.0) | 0.876 |
| Low HDL-cholesterol | 12 (20.0) | 6 (42.9) | 5 (16.1) | 1 (6.7) | 0.047 |
25(OH)D, 25-hydroxyvitamin D; IOM, Institute of Medicine; HDL-cholesterol, high density lipoprotein cholesterol.
a 25(OH)D levels were classified according to diagnostic criteria defined by IOM in 2011.
b Values are n (%).
c Metabolic syndrome was Defined using the harmonizing definition of IDF and AHA/NHlBI in 2009.
d Difference among serum 25(OH)D group was assessed by the Fisher’s exact test.
Serum 25(OH)D levels according to the combinations of metabolic risk factors
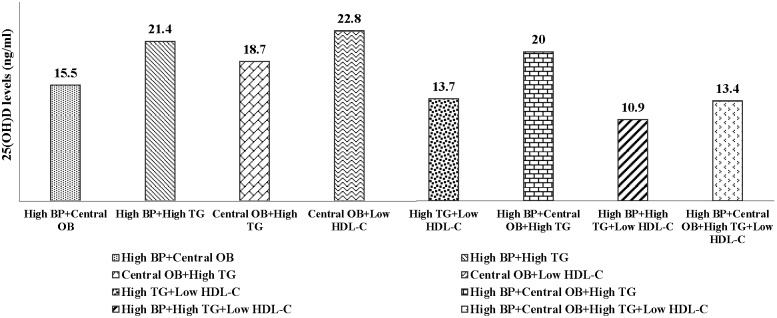
Comparison of mean serum 25(OH)D levels according to MetS combinations among prediabetic adults are shown in Fig 1. There were no subjects with combinations of (1) high blood pressure, low HDL-cholesterol (High BP+Low HDL-C), (2) high blood pressure, low HDL-cholesterol, central obesity (High BP+low HDL-C +Central OB) and (3) central obesity, hypertriglyceridemia, low HDL-cholesterol (central OB+High TG+Low HDL-C). Subjects with combination of high blood pressure, hypertriglyceridemia, low HDL-cholesterol (High BP+High TG+Low HDL-C) had the lowest serum 25(OH)D levels (10.9 ng/ml). Mean 25(OH)D levels did not differ by MetS combinations.
Fig 1. Comparison of mean serum 25(OH)D levels of MetS combinations.
25(OH)D, 25-hydroxyvitam in D; MetS, metabolic syndrome; BP, blood pressure; OB, obesity; TG, triglyceride; HDL-C, high density lipoprotein cholesterol.
Comparison of mean serum 25(OH)D levels of subjects with various MetS combinations.
NS: Non Significantly difference by serum 25(OH)D levels for the kruskal-wallis test.
Linear relationship between serum 25(OH)D levels and metabolic outcomes
The results of the linear regression analysis to identify the relationship between serum 25(OH)D levels and metabolic outcomes are shown in Table 4. Serum 25(OH)D levels were inversely associated with TG (β = -6.355) and AI (β = -0.020) after adjustment for age, sex, smoking, drinking alcohol, daytime outdoor activity and total energy intake (TG, p = 0.013; AI, p = 0.010, respectively). S1 Table shows the linear relationship between serum 25(OH)D levels and 1/HOMA-IR and QUICKI. Serum 25(OH)D levels showed a significantly positive correlation to 1/HOMA-IR (r = 0.389) and QUICKI (r = 0.306) after adjustment for age, sex, smoking, drinking alcohol, daytime outdoor activity, and total energy intake (1/HOMA-IR, p = 0.007; QUICKI, p = 0.022, respectively).
Table 4. Linear regression coefficients between serum 25(OH)D level and metabolic outcomes among Korean prediabetic adults.
| Unadjusted | Adjusted b | |||
|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | |
| Waist circumference (cm) | 0.254 (0.226) | 0.266 | 0.062 (0.230) | 0.787 |
| Systolic blood pressure (mmHg) | 0.492 (0.349) | 0.164 | 0.175 (0.363) | 0.632 |
| Diastolic blood pressure (mmHg) | 0.348 (0.252) | 0.173 | 0.305 (0.264) | 0.254 |
| Triglyceride (mg/dl) | -3.510 (2.339) | 0.139 | -6.355 (2.463) | 0.013 |
| HDL-cholesterol (mg/dl) | 0.431 (0.341) | 0.212 | 0.738 (0.389) | 0.064 |
| Atherogenic index a | -0.010 (0.007) | 0.199 | -0.020 (0.008) | 0.010 |
25(OH)D, 25-hydroxyvitamin D; HDL-cholesterol, high density lipoprotein cholesterol.
a Atherogenic index (AI) = (total cholesterol–HDL-cholesterol)/HDL-cholesterol.
b adjusted for age, sex, smoking (never, former, current), drinking alcohol (never, former, current), daytime outdoor activity (never, 1–2, 3–5, 6–7) and total energy intake.
Discussion
This study found that three fourths (75%) of Korean prediabetic adults had a low serum 25(OH)D levels. Although significant association between a low serum vitamin D status and MetS including central obesity and high blood pressure did not find, vitamin D deficient subjects among prediabetic Korean adults were exposed to an increased TG levels and had an elevated risk of arteriosclerosis.
In this study, serum 25(OH)D levels showed a significantly positive correlation to 1/HOMA-IR and QUICKI after adjusted confounders. We did not find a relationship between serum 25(OH)D levels and 1/HOMA-IR, QUICKI by the multiple linear regression analysis in contrast with the results from the majority of studies [10, 11, 24]. Currently, there was a lot of studies to examine whether a causal relationship exists between vitamin D supplementation and the progression to T2DM in individual with prediabetes [25,26]. There are multiple potential pathways underlying the relationship between vitamin D and glucose metabolism [27–30].
Shin et al. [31] suggested that impaired lipid profiles were commonly associated with CVD and can also occur in individual with prediabetes. Lauer et al. [20] presented that individual with low HDL-cholesterol had a higher risk of CVD events irrespective of LDL-cholesterol and AI made an accurate estimate ischemic heart disease events.
We observed that TG and AI decreased per 1ng/ml increase in serum 25(OH)D levels after adjusted confounders. Among vitamin D deficiency (< 12 ng/ml) group, the proportion (42.9%) of subjects with low HDL- cholesterol was higher than insufficiency level (12 to < 20 ng/ml, 16.1%) and normal vitamin D level (≥ 20 ng/ml, 6.7%). The major finding of our study was an association between vitamin D and lipid profiles.
Mostly, our results was consistent with those of the studies among individuals having conditions related IR, adiposity and inflammation such as prediabetes, high risk of T2DM, obese, old age and menopause. For example, a cross-sectional study [11] among 390 Canadian adults (aged median 34 years) with a high risk for T2DM and mean BMI 29.8 kg/m2, showed that TG decreased as much as 0.14 mmol/l per 1nmol/l increase in serum 25(OH)D levels. In another study [32] of 73 European descent adults being morbidly obese (BMI ≥ 40kg/m2), serum 25(OH)D levels were negatively correlated with TG (r = -0.364). Chon et al. [33] suggested that Korean postmenopausal women in the highest serum 25(OH)D level showed a significant decrease in the prevalence of hypertriglyceridemia (ORs = 0.83, 95% CI = 0.71–0.97) and low HDL-cholesterol (ORs = 0.80, 95% CI = 0.69–0.93). Also, Lu et al. [12] reported that among 3262 Chinese individuals aged 50–70 years, TG decreased as much as 0.10 mmol/l and HDL-cholesterol increased as much as 0.06 mmo/l per 1nmol/l increase in serum 25(OH)D levels.
In contrast to results of studies already presented, a cross-sectional study [34] among 355 non-diabetic young adults (aged mean 23.5 years) with a mean BMI 23.8 kg/m2, showed that there was no significant correlation between serum 25(OH)D levels and lipid profiles such as TG and HDL-cholesterol.
Although the mechanistic impact of vitamin D on lipid profiles is not clear, several mechanisms [35,36] have been proposed: vitamin D may increase lipoprotein lipase gene expression, increasing removal of lipoprotein particles; or, hyperparathyroidism due to low 25(OH)D levels may decrease peripheral removal of TG and contribute to activation of microsomal triglyceride transfer protein by hepatocellular Ca+, which is able to induce hypertriglyceridemia. A study [37] has suggested that vitamin D may regulate macrophage function on reverse cholesterol transport and large HDL particles increased by taking over cholesterol from macrophage. In addition, Zhou et al. [38] proposed that vitamin D may improve free fatty acids-induced IR. Kang et al. [39] suggested that vitamin D may stimulate a gene expression of cytokine in macrophage and perform many systemic anti-inflammatory actions. Most recently, Slominski et al. [40] reported that novel vitamin D3-hydroxyderivatives are noncalcemic unlike 1, 25(OH)2D3 and may promote anti-inflammatory activity. Thereby Guasch et al. [41] explained vitamin D indirectly influence lipid metabolism mediated by IR and inflammation.
This evidences discussed earlier may provide support for relationship between vitamin D and TG, HDL-cholesterol, or AI among individuals having conditions related IR, adiposity, and inflammation.
Although Korea is located at latitudes 33–38°N, which does allow for adequate ultraviolet B for vitamin D synthesis, Korea has one of the highest prevalence of vitamin D deficiency [7]. Previous studies [33,42] suggest that 56.0–62.1% of Korean adults are vitamin D insufficient (25[OH]D <20ng/ml). Our study found that the prevalence of 25(OH)D <20ng/ml among adults with prediabetes was higher than in previous studies at 75% of all adults. Gupta et al. [9] and Abbasi et al. [10] suggested that mean 25(OH)D levels are lower in individuals with prediabetes, than in those who are normoglycemia. This study found that approximately one in three prediabetic adults in Korea did not engage in outdoor activity during the daytime and the mean of vitamin D intake was 3.1 ± 0.4 μg/day. Based on this results, the high prevalence of vitamin D deficiency among Korean prediabetic adults could be explained by an indoor lifestyle, low consumption of both vitamin D rich foods and vitamin D fortified foods. [43].
Limitations to our study were the small sample size and cross-sectional design, which does not prove a causal relationship. We did not take into consideration parathyroid hormone (PTH) levels, inflammatory markers, and menopausal status which are possible intermediate confounders. Although we performed a stratified analysis by age at menopause (50 years old), there was no significant relationship. Despite these limitations, to our knowledge, this is the first study to investigate the relationship between serum vitamin D status and metabolic risk factors among Korean adults with prediabetes. Sunlight exposure and vitamin D intake were measured and we could verify difference of vitamin D source by serum vitamin D status. In addition, our study considered whether confounders interact with metabolic risk factors.
In conclusion, Korean prediabetic adults generally had a low vitamin D status. Korean adults having both prediabetes and vitamin D deficiency may be interestingly more accompanied with low HDL-cholesterol. Vitamin D may be beneficial role on dyslipidemia, high TG level induced from prediabetes and reduce the risk of atherosclerosis. However, there was no relationship between vitamin D deficiency and MetS including central obesity and high blood pressure. Further longitudinal or intervention studies are needed in order to investigate whether vitamin D may play a role in the prevention of dyslipidemia, MetS, eventually along with CVD among prediabetic adults.
Supporting Information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol 2012;59(7):635–643. 10.1016/j.jacc.2011.08.080 [DOI] [PubMed] [Google Scholar]
- 2.Korean diabetes fact sheet 2015. Korean Diabetes Association.
- 3.Parker AR, Byham-Gray L, Denmark R, Winkle PJ. The effect of medical nutrition therapy by a registered dietitian nutritionist in patients with prediabetes participating in a randomized controlled clinical research trial. Journal of the Academy of Nutrition and Dietetics 2014;114(11):1739–1748. 10.1016/j.jand.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 4.Lin JS, O'Connor E, Evans CV, Senger CA, Rowland MG, Groom HC. Behavioral counseling to promote a healthy lifestyle in persons with cardiovascular risk factors: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2014;161(8):568–578. 10.7326/M14-0130 [DOI] [PubMed] [Google Scholar]
- 5.Barengolts E. Vitamin D role and use in prediabetes. Endocrine Practice 2010;16(3):476–485. 10.4158/EP09195.RA [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357(3):266–281. 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 7.Nah EH, Kim S, Cho H. Vitamin D Levels and Prevalence of Vitamin D Deficiency Associated with Sex, Age, Region, and Season in Koreans. Laboratory Medicine Online 2015;5(2):84–91. [Google Scholar]
- 8.Lips P, Hosking D, Lippuner K, Norquist J, Wehren L, Maalouf G, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med 2006;260(3):245–254. 10.1111/j.1365-2796.2006.01685.x [DOI] [PubMed] [Google Scholar]
- 9.Ferrannini E, Gastaldelli A, Iozzo P. Pathophysiology of prediabetes. Med Clin North Am 2011;95:327–39. 10.1016/j.mcna.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 10.Abbasi F, Blasey C, Feldman D, Caulfield MP, Hantash FM, Reaven GM. Low circulating 25-hydroxyvitamin D concentrations are associated with defects in insulin action and insulin secretion in persons with prediabetes. J Nutr 2015. April;145(4):714–719. 10.3945/jn.114.209171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansuri S, Badawi A, Kayaniyil S, Cole DE, Harris SB, Mamakeesick M, et al. Associations of circulating 25 (OH) D with cardiometabolic disorders underlying type 2 diabetes mellitus in an Aboriginal Canadian community. Diabetes Res Clin Pract 2015;109(2):440–449. 10.1016/j.diabres.2015.04.017 [DOI] [PubMed] [Google Scholar]
- 12.Lu L, Yu Z, Pan A, Hu FB, Franco OH, Li H, et al. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care 2009. July;32(7):1278–1283. 10.2337/dc09-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majumdar V, Nagaraja D, Christopher R. Vitamin D status and metabolic syndrome in Asian Indians. Int J Obes 2011;35(8):1131–1134. [DOI] [PubMed] [Google Scholar]
- 14.Mitri J, Nelson J, Ruthazer R, Garganta C, Nathan DM, Hu FB, et al. Plasma 25-hydroxyvitamin D and risk of metabolic syndrome: an ancillary analysis in the Diabetes Prevention Program. Eur J Clin Nutr 2014;68(3):376–383. 10.1038/ejcn.2013.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 2005;142(8):611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scragg R, Sowers M, Bell C, Third National Health and Nutrition Examination Survey. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004. December;27(12):2813–2818. [DOI] [PubMed] [Google Scholar]
- 17.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004. June;27(6):1487–1495. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama H, Emoto M, Fujiwara S, Motoyama K, Morioka T, Komatsu M, et al. Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment in normal range weight and moderately obese type 2 diabetic patients. Diabetes Care 2003. August;26(8):2426–2432. [DOI] [PubMed] [Google Scholar]
- 19.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. The Journal of Clinical Endocrinology & Metabolism 2000;85(7):2402–2410. [DOI] [PubMed] [Google Scholar]
- 20.Lauer RM, Lee J, Clarke WR. Factors affecting the relationship between childhood and adult cholesterol levels: the Muscatine Study. Pediatrics 1988. September;82(3):309–318. [PubMed] [Google Scholar]
- 21.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009. October 20;120(16):1640–1645. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 22.Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract 2007;75(1):72–80. 10.1016/j.diabres.2006.04.013 [DOI] [PubMed] [Google Scholar]
- 23.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 2004. December;80(6 Suppl):1678S–88S. [DOI] [PubMed] [Google Scholar]
- 24.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. The Journal of Clinical Endocrinology & Metabolism 2011;96(1):53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson MB, Duran P, Lee ML, Friedman TC. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care 2013. February;36(2):260–266. 10.2337/dc12-1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorde R, Sollid ST, Svartberg J, Schirmer H, Joakimsen RM, Njølstad I, et al. Vitamin D 20 000 IU per Week for Five Years Does Not Prevent Progression From Prediabetes to Diabetes. The Journal of Clinical Endocrinology & Metabolism 2016;101(4):1647–1655. [DOI] [PubMed] [Google Scholar]
- 27.Calle C, Maestro B, García-Arencibia M. Genomic actions of 1, 25-dihydroxyvitamin D 3 on insulin receptor gene expression, insulin receptor number and insulin activity in the kidney, liver and adipose tissue of streptozotocin-induced diabetic rats. BMC molecular biology 2008;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sooy K, Schermerhorn T, Noda M, Surana M, Rhoten WB, Meyer M, et al. Calbindin-D(28k) controls [Ca(2+)](i) and insulin release. Evidence obtained from calbindin-d(28k) knockout mice and beta cell lines. J Biol Chem 1999. November 26;274(48):34343–34349. [DOI] [PubMed] [Google Scholar]
- 29.Fujita T, Palmieri GM. Calcium paradox disease: calcium deficiency prompting secondary hyperparathyroidism and cellular calcium overload. J Bone Miner Metab 2000;18(3):109–125. [DOI] [PubMed] [Google Scholar]
- 30.Ding C, Wilding JP, Bing C. 1, 25-dihydroxyvitamin D 3 Protects against Macrophage-Induced Activation of NFκB and MAPK Signalling and Chemokine Release in Human Adipocytes. PLoS One 2013;8(4):e61707 10.1371/journal.pone.0061707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin JY, Lee HR, Lee DC. Increased arterial stiffness in healthy subjects with high-normal glucose levels and in subjects with pre-diabetes. Cardiovasc Diabetol 2011. April 15;10:30 10.1186/1475-2840-10-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, Balsa JA, Vázquez C, Escobar-Morreale HF. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clinical Nutrition 2007;26(5):573–580. 10.1016/j.clnu.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 33.Chon SJ, Yun BH, Jung YS, Cho SH, Choi YS, Kim SY, et al. Association between vitamin D status and risk of metabolic syndrome among Korean postmenopausal women. PloS one 2014;9(2):e89721 10.1371/journal.pone.0089721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C, Chang H, Lu C, Tseng F, Lee L, Huang K. Vitamin D status and risk of metabolic syndrome among non-diabetic young adults. Clinical Nutrition 2015;34(3):484–489. 10.1016/j.clnu.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 35.Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. Journal of molecular medicine 2002;80(12):753–769. 10.1007/s00109-002-0384-9 [DOI] [PubMed] [Google Scholar]
- 36.Cho H, Kang H, Choi S, Ju Y, Lee H, Park H. The possible role of Ca2 on the activation of microsomal triglyceride transfer protein in rat hepatocytes. Biological and Pharmaceutical Bulletin 2005;28(8):1418–1423. [DOI] [PubMed] [Google Scholar]
- 37.Matsuura F, Wang N, Chen W, Jiang XC, Tall AR. HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoE- and ABCG1-dependent pathway. J Clin Invest 2006. May;116(5):1435–1442. 10.1172/JCI27602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou QG, Hou FF, Guo ZJ, Liang M, Wang GB, Zhang X. 1, 25‐Dihydroxyvitamin D improved the free fatty‐acid‐induced insulin resistance in cultured C2C12 cells. Diabetes Metab Res 2008;24(6):459–464. [DOI] [PubMed] [Google Scholar]
- 39.Kang SW, Kim SH, Lee N, Lee WW, Hwang KA, Shin MS, et al. 1,25-Dihyroxyvitamin D3 promotes FOXP3 expression via binding to vitamin D response elements in its conserved noncoding sequence region. J Immunol 2012. June 1;188(11):5276–5282. 10.4049/jimmunol.1101211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EK, et al. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep 2015. October 8;5:14875 10.1038/srep14875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guasch A, Bulló M, Rabassa A, Bonada A, Del Castillo D, Sabench F, Salas-Salvadó J. Plasma vitamin D and parathormone are associated with obesity and atherogenic dyslipidemia: A cross-sectional study. Cardiovasc Diabetol. 2012;11(149). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S, Lim J, Kye S, Joung H. Association between vitamin D status and metabolic syndrome risk among Korean population: based on the Korean National Health and Nutrition Examination Survey IV-2, 2008. Diabetes Res Clin Pract 2012;96(2):230–236. 10.1016/j.diabres.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 43.Chung J, Hong S. Vitamin D status and its association with cardiometabolic risk factors in Korean adults based on a 2008–2010 Korean National Health and Nutrition Examination Survey. Nutrition research and practice 2013;7(6):495–502. 10.4162/nrp.2013.7.6.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.