Abstract
Background
Trachoma is a blinding disease, initiated in early childhood by repeated conjunctival infection with the obligate intracellular bacterium Chlamydia trachomatis. The population prevalence of the clinical signs of active trachoma; ‘‘follicular conjunctivitis” (TF) and/or ‘‘intense papillary inflammation” (TI), guide programmatic decisions regarding the initiation and cessation of mass drug administration (MDA). However, the persistence of TF following resolution of infection at both the individual and population level raises concerns over the suitability of this clinical sign as a marker for C. trachomatis infection.
Methodology/Principle Findings
We systematically reviewed the literature for population-based studies and those including randomly selected individuals, which reported the prevalence of the clinical signs of active trachoma and ocular C. trachomatis infection by nucleic acid amplification test. We performed a meta-analysis to assess the relationship between active trachoma and C. trachomatis infection before and after MDA. TF and C. trachomatis infection were strongly correlated prior to MDA (r = 0.92, 95%CI 0.83 to 0.96, p<0.0001); the relationship was similar when the analysis was limited to children. A moderate correlation was found between TI and prevalence of infection. Following MDA, the relationship between TF and infection prevalence was weaker (r = 0.60, 95%CI 0.25 to 0.81, p = 0.003) and there was no correlation between TI and C. trachomatis infection.
Conclusions/Significance
Prior to MDA, TF is a good indicator of the community prevalence of C. trachomatis infection. Following MDA, the prevalence of TF tends to overestimate the underlying infection prevalence. In order to prevent unnecessary additional rounds of MDA and to accurately ascertain when elimination goals have been reached, a cost-effective test for C. trachomatis that can be administered in low-resource settings remains desirable.
Author Summary
Trachoma is the leading infectious cause of blindness worldwide, caused by the bacterium Chlamydia trachomatis. Repeated infection of the conjunctiva during childhood can initiate chronic conjunctival inflammation. This can lead to conjunctival scarring, in turning of the eyelashes, abrasion of the eyelashes on the cornea and eventually blindness later in adulthood. The World Health Organization recommends mass drug administration (MDA) for infection control when the prevalence of the clinical sign of Active Trachoma (TF) is ≥10% in 1–9 year olds. This systematic review of the literature examined the relationship between TF and C. trachomatis infection before and after MDA in order to evaluate the usefulness of TF for guiding trachoma control programmes. The population prevalence of TF and C. trachomatis infection were strongly correlated prior to MDA, however the relationship was weaker after MDA with a greater tendency for TF to overestimate the underlying infection prevalence. A cost effective test for C. trachomatis suitable for use in low resource settings could prevent unnecessary additional rounds of MDA in the population and could identify when trachoma elimination goals have been reached at an earlier time point.
Introduction
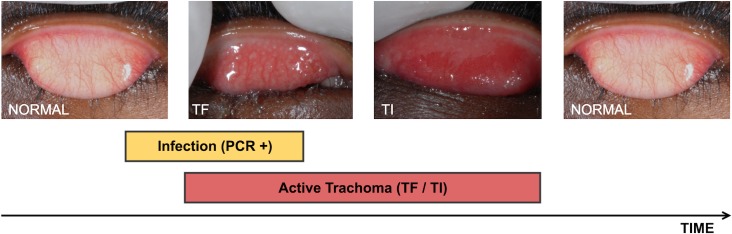
Sight loss from trachoma is the end result of a scarring disease process that is initiated in early childhood by the obligate intracellular bacterium Chlamydia trachomatis [1]. Repeated infection of the conjunctiva by C. trachomatis causes a recurrent chronic follicular conjunctivitis (TF) of the upper eyelid mucosal surface (Fig 1) [2]. This can sometimes be particularly severe with intense papillary inflammation (TI). Together, TF and / or TI are collectively referred to as “Active Trachoma”. Conjunctival scarring gradually develops, usually becoming visible from early adulthood. Eventually the scarring causes the eyelashes to turn in and scratch the surface of the eye. If left uncorrected, trichiasis traumatises the cornea surface, resulting in opacification and sight loss.
Fig 1. The natural history of an episode of ocular C. trachomatis infection and the associated conjunctival inflammatory response.
Trachomatous Inflammation–Follicular (TF): the presence of five or more follicles in the upper tarsal conjunctiva. Trachomatous Inflammation–Intense (TI): pronounced inflammatory thickening of the upper tarsal conjunctiva that obscures more than half of the normal deep tarsal vessels [2].
There is a variable relationship between the clinical signs of Active Trachoma and the presence of C. trachomatis infection. The natural history of an infection episode in children is probably characterised by a brief “pre-clinical” phase, in which there is detectable infection but the clinical signs of the inflammatory response are yet to develop (Fig 1). Human volunteer experiments in which the conjunctiva was inoculated with C. trachomatis indicated that the signs of disease typically take about 10 days to develop in previously uninfected individuals [3]. This is followed by a variable period of time in which both infection and disease can be detected at the same time; this may last for several days to many weeks. The immune response brings the infection under control, completely clearing it or reducing it to undetectable levels. However, inflammatory clinical signs persist, typically lasting several weeks after the resolution of the infection. In children aged 4–15 years, data from longitudinal cohort studies estimate the median duration of infection range between 23 days and 8 weeks, and the median duration of disease between 54 days and 18 weeks [4–6]. The duration of disease and infection declines with increasing age. Therefore, at an individual level there is frequently a large mismatch between when infection can be detected and the clinical signs of disease are found [7].
The most recent estimates from the World Health Organization (WHO) Alliance for the Global Elimination of Trachoma by 2020 (GET2020) estimates indicate that about 200 million people live in trachoma endemic areas in 42 countries, 2.2 million have visual impairment or blindness, and about 3.2 million have trichiasis [8]. To meet this large public health challenge, the GET2020 Alliance recommends the implementation of the SAFE Strategy which tackles the disease at different stages: Surgery to correct trichiasis, Antibiotics to treat chlamydial infection and Facial cleanliness and Environmental improvements to suppress transmission of the infection [1].
The antibiotic azithromycin is being used in community-wide mass drug administration (MDA); it is given as a single oral dose on an annual basis in endemic districts. Decisions around when to initiate and stop MDA are guided by the prevalence of TF in children in endemic communities. According to the current guidelines, azithromycin MDA is indicated for entire districts where the prevalence of TF in 1–9 year olds is ≥10%. Moreover, the determination of whether a program has controlled the active stage of trachoma as a public health problem also rests on the district level prevalence of TF [9].
Therefore, in view of the significance attached to TF for making programmatic decisions, it is important to understand the relationship between active disease and chlamydial infection in endemic communities. It is probable that this relationship changes after the introduction of MDA and will vary with different levels of endemicity. Several studies have specifically investigated what clinical signs can tell us about infection [7, 10–19]. However, in addition there are many other population-based studies, which report on both disease and infection, that can contribute information. Here we systematically review the published literature for reports that can inform our understanding of the relationship between clinical signs of active disease and C. trachomatis infection, both before and after the introduction of MDA.
Methods
In this review we included population-based studies and studies involving a random selection of participants that report the relationship between signs of Active Trachoma (TF, TI, TF/TI) and the detection of ocular C. trachomatis by nucleic acid amplification tests (NAAT), including polymerase chain reaction (PCR), polymerase chain reaction/enzyme immunoassay (PCR-EIA), ligase chain reaction (LCR) and transcription-mediated amplification (TMA). We excluded studies which did not test individuals without Active Trachoma for infection. References were identified through searching PubMed for articles using the terms (i) “trachoma” AND “Chlamydia trachomatis”, (ii) “Trachoma” AND “PCR”, (iii) “Trachoma” AND “LCR”, (iv) “Trachoma” AND “azithromycin”. The search was limited to 1991 onwards, the year of the first report of the use of PCR to test for ocular C. trachomatis infection in a trachoma endemic population [20]. The search was last updated on 5th May 2016.
The titles and abstracts of all articles resulting from these searches were screened by two authors (AMR, MJB) for potentially eligible publications. These two authors then independently assessed the potentially eligible articles for inclusion and extracted the data. The bibliographies of publications meeting the inclusion criteria were also reviewed for any additional publications not already identified. Studies were excluded where the participant sample was not population-based. Several studies were identified with multiple related published reports arising from the same data. These are considered as a single study, but for completeness we include all the relevant references.
Core information was extracted using a standardised form. The core information included country, year of publication, study design, study population size, age group, TF prevalence, TI prevalence, TF/TI prevalence (if TF alone was not reported), C. trachomatis infection prevalence, diagnostic test used, grading system, use of antibiotic for infection control. The clinical grading system was recorded: the 1981 Detailed WHO FPC System or the 1987 Simplified WHO Trachoma Grading System [2, 21]. For the purpose of this review the prevalence levels of TF in the populations studied were categorised as follows: Hypoendemic <10%, Mesoendemic 10–20% and Hyperendemic >20% [12].
Due to the heterogeneity in the study designs and the reporting of data, only a limited meta-analysis was performed. Studies report the results for different age groups. Where available we separately present both the “All Age” results and those for children (≤15 years). In the meta-analysis we use the results from children only where these are available; where these are not available, the all age results are used. Summary graphs are presented of the relationship between: (i) the community prevalence of disease signs and C. trachomatis infection, (ii) the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) of TF for infection, by the community prevalence of disease signs. The degree of correlation between these was tested using Pearson’s correlation coefficient, weighted by study size. The relationship between signs and infection was analysed separately for pre-treatment and post-treatment data, then a test for interaction was performed on the two datasets combined to test whether the association between signs and infection was similar in the pre and post-treatment data. Forest plots were generated to illustrate the strength and heterogeneity of the relationship between disease signs and detection of infection; study heterogeneity was estimated using the I2 statistic. Hierarchical summary receiver operating characteristics (HSROC) curves were plotted to illustrate the relationship between the sensitivity and specificity of signs of TF for the presence of C. trachomatis infection and a pooled estimate of sensitivity and specificity was made in both the pre and the post MDA studies. All analyses were performed using Stata 13. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist and flow diagram are included in the supporting information (S1 Checklist and S1 Fig).
Results
The search returned a list of 718 publications. The findings from pre and post-MDA studies are presented separately. Thirty-six separate population-based studies reported findings prior to the introduction of MDA. Several had multiple related publications; yielding a total of 48 publications (Table 1). Twenty-one studies reported findings following the introduction of MDA met inclusion criteria, in a total of 35 publications (Table 2).
Table 1. Active Trachoma and Chlamydia trachomatis (Ct) infection prior to mass antibiotic treatment.
| Country, Year, Ref. | Study design | Participants | Active Trachoma % | Ct % | Ct+/TF+ | Ct+/TF- | Comments |
|---|---|---|---|---|---|---|---|
| Tanzania, 1991, [20] | Baseline cross-sectional population-based data for a treatment trial. One child aged 1–7 years was randomly selected from 234 households in a village. | 1–7 years: 234 | 1–7 years: ● TF/TI 58.5% (137/234 ● TI 15.8% (37/234) |
1–7 years: 47.9% (112/234) | 1–7 years: 65.0% (89/137) | 1–7 years: 23.7% (23/97) | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: in-house PCR-EIA for OMP1. |
| Gambia, 1994, [29] | Cross-section survey of the entire population of two villages | ● All ages: 1332 ● 0–15 years: 714 |
All ages: ● TF/TI 15.0% (200/1332)0–15 years: ● TF/TI 29.8% (183/714) |
● All ages: 17.2% (229/1332) ● 0–15 years: 29.8% (183/714) |
● All ages: 72.0% (144/200) ● 0–15 years: 73.2%% (134/183) |
● All ages: 7.5% (85/1132) ● 0–15 years: 9.2% (49/531) |
● Mesoendemic setting ● Clinical grading: detailed WHO-FPC system ● Chlamydia test: in-house PCR for plasmid, detected by agarose gel electrophoresis. |
| Gambia, 1994, [22] | Cross-section survey of the entire population of one village. Only 133 individuals were tested for Ct infection by PCR. All active cases were tested. A sample of 37 normal individuals from two households were tested. | All ages: 844 | All ages: ● TF/TI 11.4% (96/844) |
Data not available | All ages: 51.0% (49/96) | All ages: 5.4% (2/37) | ● Mesoendemic setting ● Clinical grading: detailed WHO-FPC system ● Chlamydia test: in-house PCR for plasmid, detected by agarose gel electrophoresis. ● The clinically normal group are not necessarily representative of the whole community as they are drawn from two households only. |
| Nepal, 1998, [30] | All children under 11 years in primary school and all children from four randomly selected households. | 0–10 years: 70 | 0–10 years: ● TF/TI 38.6% (27/70) ● TI 7.1% (5/70) |
0–10 years: 57.1% (40/70) | 0–10 years: 66.7% (18/27) | 0–10 years: 51.2% (22/43) | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: in-house PCR for OMP1. ● The sample was not necessarily representative of the child population of this community. |
| Egypt, 1999, [10, 31] | Baseline cross-sectional, population-based survey; pre-treatment data from a RCT of azithromycin vs tetracycline. | ● All ages: 2069 ● 1–10 years: 731 |
All ages: ● TF/TI 19.7% (408/2069) ● TI 5.8% (120/2069) ● 1–10 years:TF/TI 48.3% (353/731) ● TI 13.0% (95/731) |
● All ages: 35.7% (739/2069) ● 1–10 years: 48.6% (355/731) |
● All ages: 66.9% (273/408) ● 1–10 years: 69.4% (245/353) |
● All ages: 31.6% (466/1661) ● 1–10 years: 29.1% (110/378) |
● Hyperendemic setting ● Clinical grading: detailed WHO-FPC system ● Chlamydia test: LCR, Abbott Laboratories. |
| Gambia, 1999, [31] | Baseline cross-sectional, population-based survey; pre-treatment data from a RCT of azithromycin vs tetracycline. | ● All ages: 1747 ● 0–10 years: 730 |
All ages: ● TF/TI 15.8% (277/1747) ● TI 7.0% (122/1747)0–10 years: ● TF/TI 33.8% (247/730) ● TI 13.7% (100*/730) |
● All ages: 35.9% (628/1747) ● 0–10 years: 39.3% (287/730) |
All ages: 59.9% (166/277) | All ages: 31.4% (462/1470) | ● Hyperendemic setting ● Clinical grading: detailed WHO-FPC system ● Chlamydia test: LCR, Abbott Laboratories. ● Main results presented for all ages, possible to derive some of the indicators for the 0–10 year group. |
| Tanzania, 1999, [31] | Baseline cross-sectional, population-based pre-treatment survey; data from a RCT of azithromycin vs tetracycline. | ● All ages: 2653 ● 0–10 years: 940 |
All ages: ● TF/TI 31.8% (844/2653) ● TI 16.4% (436/2653) 0–10 years: ● TF/TI 60.1% (565/940) ● TI 24.7% (233*/940) |
● All ages: 18.5% (491/2653) ● 0–10 years: 34.6% (325/940) |
All ages: 48.3% (408/844) | All ages: 4.6% (83/1809) | ● Hyperendemic setting ● Clinical grading: detailed WHO-FPC system ● Chlamydia test: LCR, Abbott Laboratories. ● Main results presented for all ages, possible to derive some of the indicators for the 0–10 year group. ● In this study there was some concern about sample degradation, leading to an underestimate of infection |
| Nepal, 1999, [11] | Cross-sectional survey of all children in six villages. Swabs and LCR test done on all active cases and 1/8 without active disease. | 0–10 years: 726 | years: ● TF/TI 6.3% (46/726) |
0–10 years: 0%(0/90) | 0–10 years: 0%(0/46) | 0–10 years: 0% (0/44) | ● Hypoendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: LCR, Abbott Laboratories. |
| Nepal, 2001, [23] | Baseline cross-sectional population-based data from a RCT of mass vs targeted antibiotic treatment. Children recruited from 17 wards. Only the children with active disease and a similar sized random sample of the others were tested by LCR. | 1–7 years: 619 | 1–7 years: ● TF/TI 19.0% (118/619) |
Data not available | 1–7 years: 24.8% (29/117) | 1–7 years: 4.2% (5/118) | ● Mesoendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: LCR, Abbott Laboratories. |
| Gambia, 2003, [7, 27, 32, 33] | Baseline population-based survey of a longitudinal study of MDA. All residents of 14 villages. | ● All ages: 1319 ● 1–9 years: 492 |
All ages: ● TF/TI 7.8% (103/1319) ● TI 1.4% 19/13191–9 years: ● TF 16.3% (80/492) ● TI 2.6% (13/492) |
● All ages: 7.2% (95/1319) ● 1–9 years: 9.3% (46/492) |
● All ages: 22.3% (23/103) ● 1–9 years: 23.8% (19/80) |
● All ages: 5.9% (72/1216) ● 1–9 years: 6.6% (27/412) |
● Mesoendemic setting ● Clinical grading: detailed WHO-FPC system ● Chlamydia test: Amplicor PCR, Roche. |
| Tanzania, 2003, [27, 34, 35] | Baseline population-based survey for a longitudinal study of MDA. All residents of a single sub-village. | All ages: 956 | All ages: ● TF/TI 18.2% (174/956) ● TI 11.6% (111/956) |
All ages: 9.5% (91/956) | All ages: 33.3% (58/174) | All ages: 4.2% (33/782) | ● Hyperendemic Setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Tanzania, 2003, [27, 36–39] | Baseline population-based survey for a longitudinal study of MDA. All residents of a single village. | ● All ages: 871 ● 0–10 Years: 325 |
All ages: ● TF/TI 35.8% (312/871) ● TI 10.9% (95/871) ● 0–10 Years:F/TI 72.6% (236*/325) |
● All ages: 56.9% (496/871) ● 0–10 Years: 64.9% (211*/325) |
All ages: 77.2% (241/312) | All ages: 45.6% (255/559) | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Ethiopia, 2004, [17] | Random selection of 100 children aged 1–6 years from four villages. | 1–6 years: 100 | 1–6 years: ● TF 79% (79/100) ● TI 55% (55/100) |
1–6 years: 63.0% (63/100) | 1–6 years: 72.2% (57/79) | 1–6 years: 28.6% (6/21) | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Gambia, 2006, [40] | Cross-sectional population-based study of school children aged 4–15 years from nine villages. | 4–15 years: 331 | 4–15 years: ● TF 18.2% (60/331) ● TI 1.5% (5/331) |
4–15 years: 21.8% (72/331) | 4–15 years: 41.7% (25/60) | 4–15 years: 15.3% (38/249) | ● Mesoendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: in-house RT-PCR assay for chlamydial 16S rRNA |
| Tanzania, 2006a, [41] | Cross-sectional population-based study of children aged 1–9 years from two hyperendemic villages. | 1–9 years: 464 | 1–9 years: ● TF 44.0% (204/464) |
1–9 years: 24.8% (115/464) | 1–9 years: 36.3% (74/204) | 1–9 years: 13.6% (41/301) | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Tanzania, 2006b, [41] | Cross-sectional population-based study of children aged 1–9 years from one hypoendemic village. | 1–9 years: 200 | 1–9 years: ● TF 12.5% (25/200) |
1–9 years: 6.5% (13/200) | 1–9 years: 32.0% (8/25) | 1–9 years: 2.9% (5/175) | ● Hypoendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Niger, 2007, [42] | Cross-sectional study of randomly selected children from 12 villages. | 1–5 years: 651 | 1–5years: ● TF/TI 43.0% (280*/651) ● TI 16% (104*/651) |
1–5years: 21.0% (137*/651) | 1–5years: 37.3% (103*/267) | 1–5years: 9.0% (33*/365) | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Ethiopia, 2007, [43] | Cross-sectional population-based study of children aged 0–10 years from two hyperendemic villages. | 0–10 years: 56 | 0–10 years: ● TF/TI 78.6% (44/56) ● TI 46.4% (26/56) |
0–10 years: 39.3% (22/56) | 0–10 years: 43.1% (19/44) | 0–10 years: 25.0% (3/12) | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Nepal, 2008, [44] | Cross-sectional study of 9 randomly selected households from one village | All ages: 127 | All ages: ● TF 26.8% (34/127) ● TI 38.6% (49/127) |
All ages: 38.6% (49/127) | All ages: 44.1% (15/34) | All ages: 36.6% (34/93) | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Gambia, 2009, [45, 46] | Cross-sectional survey using a two-stage cluster random sampling strategy with probability of selection proportional to size, in 19 enumeration areas in Lower River Region, The Gambia. | 1–9 years: 876 | 1–9 years: ● TF 12.3% (108/876) ● TI 0.1% (1/876) |
1–9 years: 0.3% (3/876) | 1–9 years: 0.9% (1/108) | 1–9 years: 0.3% (2/768) | ● Mesoendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Ethiopia, 2009, [47] | Cross-sectional population-based sample of 8 randomly selected children (1–5 years) per village from eight villages. | 1–5 years: 120 | 1–5 years: ● TF/TI 60.8% (73/120) ● TI 44.2% (53/120) |
1–5 years: 40.8% (49/120) | Data not available | Data not available | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Ethiopia, 2010, [18, 48] | Cross-sectional population-based sample of children (1–5 years) living in 24 villages. | 1–5 years: 1200 | 1–5 years: ● TF/TI 86.0% (mean village prevalence) |
1–5 years: 52.9% (mean village prevalence) | Data not available | Data not available | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche ● NB: The data are reported a mean of the prevalence across all 24 villages. |
| Gambia, 2010, [25, 49] | Cross-sectional population-based survey of ~100 randomly selected children aged 0–5 years from 48 enumeration areas. | 0–5 years: 5033 | 0–5 years: ● TF 6.3% (316/5033) ● TI 0.6% (28/5033) |
0–5 years: 0.8% (39/5033) | 0–5 years: 0.9% (3/316) | 0–5 years: 0.7% (36/4717) | ● Hypoendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche. |
| Tanzania, 2010, [49–51] | Cross-sectional population-based survey of ~100 randomly selected children aged 0–5 years from 32 enumeration areas. The villages were purposely selected based on having a preliminary survey prevalence of >20%. | 0–5 years: 3122 | 0–5 years: ● TF 30.8% (963/3122) ● cTI 7.8% (244/3122) |
0–5 years: 21.9% (684/3122) | 0–5 years: 48.9% (471/963) | 0–5 years: 9.8% (213/2159) | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche. |
| Niger, 2010, [52] | Cross-sectional study of randomly selected children from 12 villages | 1–5 years: 557 | 1–5 years: ● TF/TI 41.6% (232/557) |
1–5 years: 20.1% (112/557) | Data not available | Data not available | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Australia, 2011, [14] | Population-based cross-sectional study in five Aboriginal communities. | All ages: 1282 | All ages: ● TF 8.4% (108/1282) |
All ages: 3.6% (46/1282) | All ages: 17.6% (19/108) | All ages: 2.3% (27/1174) | ● Hypoendemic setting ● Clinical grading: variation of the detailed WHO-FPC system [14] ● Chlamydia test: Amplicor PCR, Roche |
| Tanzania, 2011, [26, 53] | Cross-sectional population-based survey in three villages | 0–9 years: 473 | 0–9 years: ● TF 13.7% (65/473) ● TI 1.3% (6/473) |
0–9 years: 5.3% (25/473) | 0–9 years: 6.1% (4/65) | 0–9 years: 5.1% (21/408) | ● Mesoendemic setting ● Clinical grading: detailed WHO-FPC system ● Chlamydia test: Amplicor PCR, Roche. |
| Tanzania, 2011, [54, 55] | Cross-sectional baseline population-based survey in 4 villages. | 0–8 years: 1991 | 0–8 years: ● TF 27.8% (553/1991) |
0–8 years: 23.7% (463/1956) | Data not available | Data not available | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Tanzania, 2011, [56] | Cross-sectional baseline, population-based survey of four communities | 0–9 years: 2118 | 0–9 years: ● TF/TI 27.7%: (586/2118) |
0–9 years: 23.6% (499/2118) | 0–9 years: 33.1% (194*/586) | 0–9 years: 13.6% (209/1532) | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Ethiopia, 2011, [57] | Cross-sectional population-based survey in 23 communities. Both arms of a cluster RCT at baseline. | 0–9 years: 1168 | 0–9 years: ● TF/TI 66.5% (770/1158) |
0–9 years: 44.7% (516/1168) | Data not available | Data not available | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Ethiopia, 2012, [19, 58, 59] | Cross-sectional baseline, population-based survey of 0–9 year olds in 12 communities | 0–9 years: 583 | 0–9 years: ● TF/TI 68.3% (393/575) |
0–9 years: 42.4% (248/584) | Data not available | Data not available | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Cameroon, 2012, [24] | Cross-sectional study, with a random selection of children from 30 villages, with probability proportional to size. Only children with signs of Active Trachoma were tested for infection by PCR. | 1–9 years: 2397 | 0–9 years: ● TF/TI 26.2% (628/2397) ● TI 5.2% (124/2397) |
Data not available | 0–9 years: 35.0% (220*/628) | Data not available | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: in-house PCR assay for chlamydial rRNA gene. |
| Niger, 2012, [60, 61] | Cross-sectional study for randomly selected children from 48 communities. | 0–5 years: 4484 | 0-5years: ● TF 26.0% (1166*/4484) |
0-5years: 20.7% (928*/4484) | Data not available | Data not available | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Guinea Bissau, 2013, [62, 63] | Cross-sectional population-based survey across multiple communities | ● All ages: 1508 ● 1–9 years: 618 |
All ages: ● TF/TI 11.1% (167/1508) ● TI 2.0% (29/1508)1–9 years: ● TF/TI 22.0% (136/618) ● TI 3.2% (21/618) |
● All ages: 17.9% (269/1507) ● 1–9 years: 25.4% (157/618) |
● All ages: 63.3% (100/158) ● 1–9 years: 86/129 (66.7%) |
● All ages: 12.1% (164/1351) ● 1–9 years: 83/537 (15.5%) |
● Mesoendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Tanzania, 2013, [64] | Cross-sectional population-based survey of children from one village. | 0–9 years: 27 | 0–9 years: ● TF 44.9% (57/127) ● TI 16.5% (21/127) |
0–9 years: 27.6% (35/127) | 0–9 years: 29/57 (50.9%) | 0–9 years: 6/70 (8.6%) | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche. |
| Tanzania, 2014, [65] | Cross-sectional population-based survey of all 1–6 year olds in a single village | 1–6 years: 208 | 1–6 years: ● TF/TI 47.0% (98/208) |
1–6 years: 25.0% (52/208) | Data not available | Data not available | ● .Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche. |
* Estimated value inferred from available data in publication.
Table 2. Active Trachoma and Chlamydia trachomatis (Ct) infection after the introduction of mass antibiotic treatment.
| Country, Year | Study design | Time Point post 1st MDA | Participants | Active Trachoma % | Ct % | Ct+/TF+ | Ct+/TF- | Comments |
|---|---|---|---|---|---|---|---|---|
| Tanzania, 1993, [20, 66] | Cross-section survey of randomly selected children one month after the completion of a one month tetracycline treatment course, given as MDA to the entire community. | 1 month | 1–7 years: 227 | 1–7 years: ● TF/TI 41.9% (95/227) ● TI 2.6% (6/227) |
1–7 years: 23.8% (54/227) | Data not available | Data not available | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: in-house PCR-EIA for OMP1. |
| Egypt, 2003, [10, 31] | Cross-section population-based survey 14 months after azithromycin MDA. | 14 months | 1–10 years: 354 | 1–10 years: ● TF/TI 26.0% (92/354) |
1–10 years: 5.1% (18/354) | 1–10 years: 9.8% (9/92) | 1–10 years: 3.4% (9/262) | ● Hyperendemic setting ● Clinical grading: detailed WHO-FPC system ● Chlamydia test: LCR, Abbott Laboratories. ● Only the azithromycin arm is included. |
| Gambia, 1999, [31] | Cross-section population-based survey 12 months after azithromycin MDA. | 12 months | All ages: ● 675 (exam) ● 540 (tested) |
All ages: ● TF/TI 8.6% (58*/675) ● TI 2.2% (15*/675) |
All ages: 8.3% (45/540) | Data not available | Data not available | ● Hyperendemic setting, pre treatment ● Clinical grading: detailed WHO-FPC system ● Chlamydia test: LCR, Abbott Laboratories. ● Only the azithromycin arm is included. |
| Tanzania, 1999, [31] | Cross-section population-based survey 12 months after azithromycin MDA. | 12 months | All ages: ● 1308 (exam) ● 1162 (tested) |
All ages: ● TF/TI 24.6% (322*/1308) ● TI 6.3% (83*/1308) |
All ages: 7.0% (82/1162) | Data not available | Data not available | ● Hyperendemic setting ● Clinical grading: detailed WHO-FPC system ● Chlamydia test: LCR, Abbott Laboratories. ● Only the azithromycin arm is included. |
| Nepal, 2001, [23] | Cross-section survey of randomly selected normal children and a purposive sample of children with Active Trachoma, 6 months after azithromycin MDA. | 6 months | 1–7 years: ● 5262 (exam) ● 394 (tested) |
1–7 years: ● TF/TI 16% (841*/5262) |
Data not available | 1–7 years: 11.8% (31/263) | 1–7 years: 5.1% (6/118) | ● Mesoendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: LCR, Abbott Laboratories. |
| Tanzania, 2004, [34, 35] | Cross-sectional survey of all residents of a sub-village, 24 months after MDA | 24 months | All ages: 842 | All ages: ● TF/TI 5.8% (49/842) |
All ages: 0.1% (1/842) | All ages: 2.0% (1/49) | All ages: 0.0% (0/793) | ● Hyperendemic setting, pre-treatment ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Gambia, 2005, [7, 27, 32, 33] | Cross-sectional survey of all residents of 14 small villages, 12 months after MDA | 12 months | ● All ages: 1210 ● 1–9 years: 440 |
All ages: ● TF/TI 3.9% (47/1210)1–9 years: ● TF 6.8% 30/440) ● TI 0.9% (4/440) |
All ages: 2.3% (28/1210)1–9 years: 5.4% (24/440) | All ages: 29.8% (14/47)1–9 years: 36.7% (11/30) | All ages: 1.2% (14/1163)1–9 years: 3.2% (13/410) | ● Mesoendemic setting, pre-treatment ● Clinical grading: detailed WHO-FPC system ● Chlamydia test: Amplicor PCR, Roche. |
| Tanzania, 2005, [27, 36–39] | Cross-sectional survey of residents of a village, 12 months after MDA | 12 months | 0–7 years: 287 | 0–7 years: ● TF/TI 46.8% (134*/287) |
0–7 years: 12.8% (37*/287) | Data not available | Data not available | ● Hyperendemic ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Tanzania, 2007, [39] | Cross-sectional of population-based survey 5 years after baseline MDA and 3.5 years after a second MDA. | 5 years | 0–10 years: 464 | 0–10 years: ● TF 30.2% (140/464) ● TI 10.6% (49/464) |
0–10 years: 25.9% (120/464) | Data not available | Data not available | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Ethiopia, 2008, [67] | Cross-sectional population based sample of children from 32 communities. These had received between 1 and 3 rounds of MDA, with the most recent does less than 6 months in about half the communities. | Variable | 3–9 years: 1459 | 3–9 years: ● TF 23.6% (345/1459) |
3–9 years: 3.0% (44/1459) | 3–9 years: 6.1% (21/345) | 3–9 years: 6.1% (23/1114) | ● Hyperendemic setting ● Heterogeneous treatment history ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Ethiopia, 2009, [47] | Cross-sectional population-based sample of 8 randomly selected children (1–5 years) per village from eight villages. The villages had received MDA biannually for 2 years, with the last dose 18 months prior to the survey. | 42 months | 0–5 years: 120 | 0–5 years: ● TF/TI 47.5%: (57/120) ● TI 30.0% (36/120) |
0–5 years: 15% (18/120) | Data not available | Data not available | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Ethiopia, 2010, [18, 48] | Cross-sectional population-based sample of children (1–5 years) living in 24 villages. Communities had received 4 biannual MDA, the most recent 6 months before survey | 24 months | 1–5 years: 1234 | 1–5 years: ● TF/TI 39.2% (mean village prevalence) |
1–5 years: 2.0% (mean village prevalence) | Data not available | Data not available | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche ● NB: The data are reported a mean of the prevalence across all 24 villages. |
| Tanzania, 2011, [15] | Cross-sectional population-based sample of children from 71 communities. All communities had received 3 to 7 rounds of MDA. | Variable | 0–5 years: 7817 | 0–5 years: ● TF 10.0% (784/7817) ● TI 3.2% (252/7817) |
0–5 years: 5.5%% (429/7817) | 0–5 years: 23.5% (184/784) | 0–5 years: 3.5% (245/7033) | ● Mesoendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche Heterogeneous MDA history. Wide range of community level prevalence of infection and disease. Summary data here pools these communities |
| Ethiopia, 2011, [57] | Cross-sectional population-based survey in 24 communities. Both arms of a cluster RCT of latrine provision received a single round of MDA at baseline. | 24 months | 0–9 years: 1211 | 0–9 years: ● TF/TI 47.0% (567/1207) |
0–9 years: 14.6% (177/1211) | Data not available | Data not available | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche ● Data from both arms combined. |
| Ethiopia, 2012, [19, 58, 59, 68, 69] | Cross-sectional survey of 50 children per village under 10 years in 12 villages. Three annual rounds of MDA were given. The final survey was months after the last MDA. | 36 months | 0–9 years: 577 | 0–9 years: ● TF 43.5% (251/577) |
0–9 years: 4.3% (25/577) | Data not available | Data not available | ● Hyperendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Gambia, 2013, [25, 70] | Cross-sectional population-based sample of children from 48 communities enrolled in an cluster RCT from four districts. 24 communities had 3 annual MDA and 24 communities had a single round of MDA at baseline. Survey was 3 years after the first round of MDA. | 36 months | 0–5 years: ● District 1: 1128 ● District 2: 1199 ● District 3: 1243 ● District 4: 1246 |
0–5 years: ● TF 0.2% (2/1128) ● TF 0.3% (3/1199) ● TF 3.8% (47/1243) ● TF 6.4% (80/1246) |
0–5 years: ● 0.5% (6/1128) ● 0.1% (1/1198) ● 1.1% (13/1241) ● 0.3% (4/1235) |
0–5 years: ● 0.0% (0/2) ● 0.0% (0/3) ● 6.4% (3/47) ● 0.0% (0/80) |
0–5 years: ● 0.5% (6/1126) ● 0.1% (1/1196) ● 0.8% (10/1196) ● 0.3% (4/1166) |
● Hypoendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche. ● Data from both trial arms were combined, and presented separately for each district. |
| Tanzania, 2014, [51, 71] | Cross-sectional survey of all children under 10 years in four villages. Four annual rounds of MDA were given. The final survey was 6 months after the last MDA. | 42 months | 0–9 years: 2234 | 0–9 years: ● TF/TI 7.9% (176/2234) |
0–9 years: 5.1% (114/2234) | Data not available | Data not available | ● Hyperendemic setting, pre-treatment ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Tanzania, 2014, [71, 72] | Cross-sectional population-based sample of children aged 0–5 years in 32 villages, 100 children per village. Survey was done 12 months after the third MDA. | 36 months | 0–5 years: 3136 | 0–5 years: ● TF 7.6% (237/3136) |
0–5 years: 4.5% (142*/3136) | 0–5 years: 29.5% (70/237) | 0–5 years: 2.5% (72*/2899) | ● Hyperendemic setting, pre-treatment ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Tanzania,: 2015,[73] | Cross-sectional population-based of residents of a village, which had received 2 rounds of MDA 12 and 10 years previously. | 12 years | ● All ages: 571 ● 1–9 years: 200 |
All ages: ● TF 2.5% (14/571)1–9 years: ● TF 6.5% (13/200) ● TI 1.5% (3/200) |
All ages: 0% (0/571)1–9 years: 0% (0/200) | Data not available | Data not available | ● Hyperendemic setting, pre-treatment ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Nepal, 2016,[74] | Cross-section survey of 1–9 year olds in 24 randomly selected communities. Four rounds of MDA. The survey was conducted 5 years after the first round. | 5 years | 1–9 years: 1124 | 1–9 years: ● TF/TI 0.3% (3/1124) |
1–9 years: 0% (0/1124) | Data not available | Data not available | ● Hypoendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Amplicor PCR, Roche |
| Tanzania, 2016, [75] | Random sample of children aged 1–9 years, from 30 hamlets. 50 children were sampled per hamlet. Communities had received variable rounds of MDA, between 4 and seven years previously. | 7 years | 1–9 years: 1474 | 1–9 years: ● TF 0.4% (6*/1474) |
1–9 years: 1.1% (16/1474) | Data not available | Data not available | ● Hypoendemic setting ● Clinical grading: simplified WHO system ● Chlamydia test: Aptima Combo2, Hologic |
* Estimated value inferred from available data in publication.
a Additional data sub-divided by district were provided by the authors of this study.
Pre-Treatment
Of the 36 pre-treatment study populations, 24 were hyperendemic, 8 mesoendemic and 4 hypoendemic (Table 1). The studies were conducted in populations from Tanzania (12), The Gambia (7), Ethiopia (6), Nepal (4), Niger (3), Cameroon (1), Guinea Bissau (1), Egypt (1) and Australia (1). The Simplified WHO Trachoma Grading System was used in 28 studies and 8 used the Detailed WHO FPC System. The majority of studies (25) used the commercially produced Amplicor CT/NG (Roche) PCR assay, five used ligase chain reaction (LCR, Abbott Laboratories), and six used in-house PCR assays. Four of the 36 studies listed in Table 1 did not report the community prevalence of C. trachomatis infection, however they report infection on a selected subset of individuals [11, 22–24]. These four studies were therefore retained in Table 1 as they contribute useful information, however they were excluded from the meta-analysis. Of the remaining 32 studies included in the meta-analysis, 29 studies reported data for childhood age groups (variable age ranges), seven of which also reported all age data, and three studies reported only all age data.
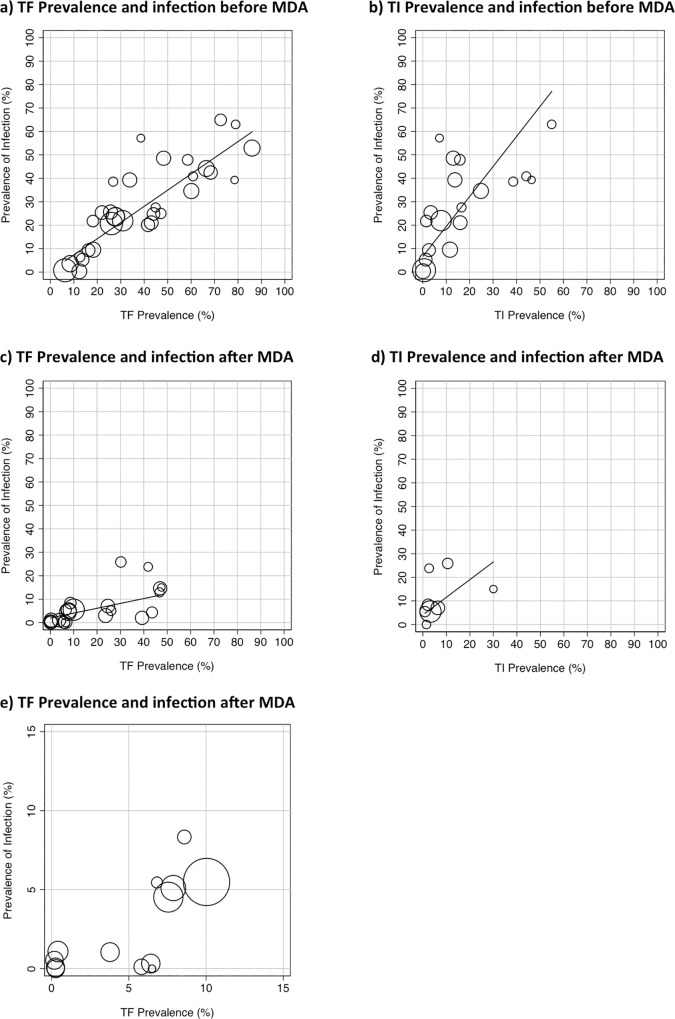
Prior to the introduction of MDA there was evidence of a strong positive correlation between the community-level prevalence of Active Trachoma and the community-level prevalence of detected C. trachomatis, using data from all 32 studies (r = 0.92, 95%CI 0.83 to 0.96, p<0.0001), Fig 2A. The correlation was similar when the analysis was limited to children only data (29 studies; r = 0.91, 95%CI 0.82 to 0.96, p<0.0001). The correlation was much weaker for all age data (10 studies; r = 0.51, 95%CI -0.18 to 0.86 p = 0.13). Overall, the community prevalence of TF is typically greater than the underlying community prevalence of detected C. trachomatis, with TF having a higher prevalence than C. trachomatis in 25 out of the 32 studies. The mean difference in prevalence was 10.4% (95% CI 5.9%-15.0%, p = 0.001).
Fig 2. The relationship between the prevalence of disease signs and infection before and after the introduction of MDA.
(a) Community prevalence of TF (or TF/TI) vs. the community prevalence of C. trachomatis infection before the introduction of MDA. (b) Community prevalence of TI vs. the community prevalence of C. trachomatis infection before the introduction of MDA. (c) Community prevalence of TF (or TF/TI) vs. the community prevalence of C. trachomatis infection after the introduction of MDA. (d) Community prevalence of TI vs. the community prevalence of C. trachomatis infection after the introduction of MDA. (e) Community prevalence of TF (or TF/TI) vs. the community prevalence of C. trachomatis infection after the introduction of MDA, showing only the communities with less than 15% TF. Data from population-based studies, summarised in Tables 1 and 2. The size of the circles reflects the sample size. Line fitted by linear regression, weighted by the size of the studies.
There were 19 studies that reported the prevalence of TI separately from TF. There was evidence of a moderate positive correlation between the community-level prevalence of TI and the community-level prevalence of detected C. trachomatis (r = 0.75, 95%CI 0.45 to 0.90 p = 0.0002), Fig 2B. Although there are only a limited number of studies, in all communities where the TI prevalence was >20% the prevalence of C. trachomatis was at least 30%. In contrast, where the TI prevalence was below 20% there was a considerable degree of variation in the underlying community prevalence of infection.
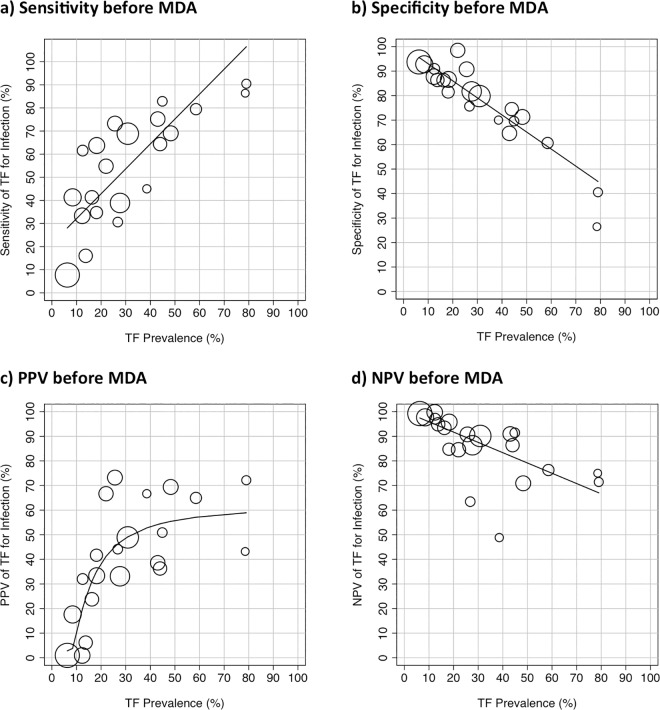
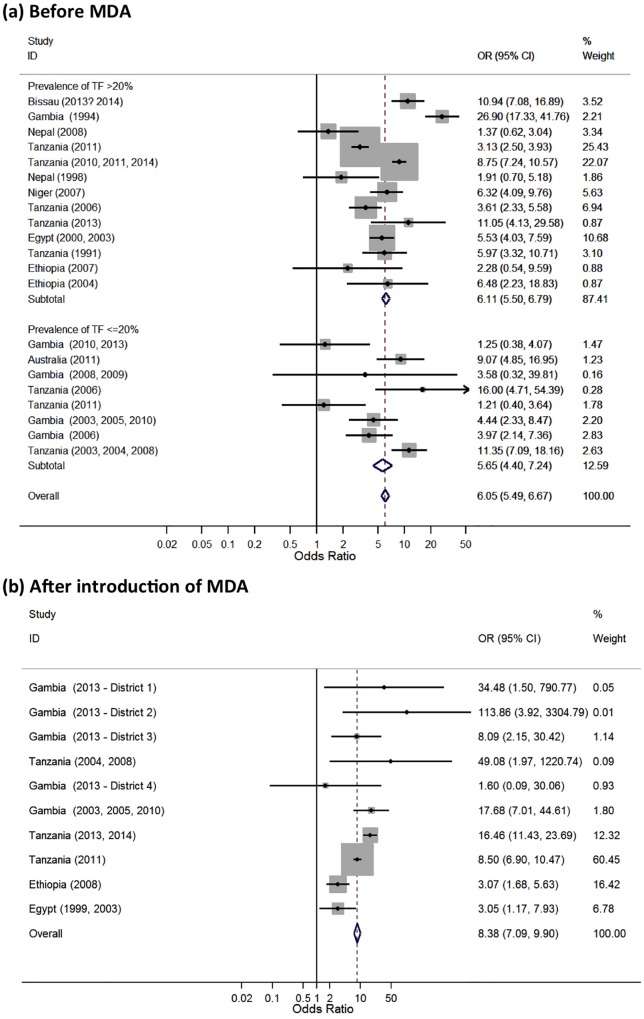
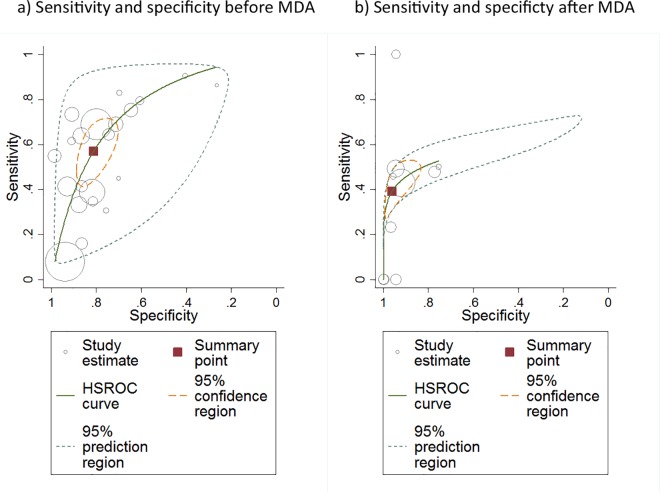
There was sufficient data at the individual level presented to estimate the sensitivity, specificity, PPV and NPV for 21 studies. The sensitivity of TF for identifying the presence of C. trachomatis infection varied substantially with the prevalence of TF in the community, Fig 3A. There was evidence of a strong positive correlation (r = 0.82, 95%CI 0.59 to 0.92, p<0.0001). In contrast, there was a strong negative correlation between the specificity of TF for infection and the community prevalence of TF (r = -0.92, 95%CI -0.97 to -0.80, p<0.0001), Fig 3B. The Forest plot of the pre-treatment relationship between disease and detection of infection at the individual level showed an overall strong association (OR 6.05, 95% CI 5.49 to 6.67, p<0.0001), although there was marked heterogeneity (I2 = 87.1%, p<0.001), Fig 4A. A plot of sensitivity against specificity of the pre-treatment studies using TF as a test for C. trachomatis infection at the individual level showed that the overall estimated sensitivity was moderate (57.1% (45.4–68.1%) and the overall specificity was good (81.1% (73.4–87.0%), Fig 5A.
Fig 3. The relationship between the individual level presence of Active Trachoma (TF or TF/TI) and the detection of C. trachomatis infection by community TF prevalence before the introduction of MDA.
(a) Sensitivity of TF for infection. (b) Specificity of TF for infection. (c) Positive Predictive Value (PPV) of TF for C. trachomatis infection. (d) Negative Predictive Value (NPV) of TF for C. trachomatis infection. Data from population-based studies, summarised in Table 1. The size of the circles reflects the sample size. Line fitted by linear regression, weighted by the size of the studies, except for the PPV which was fitted by polynomial.
Fig 4.
A Forest plot showing the relationship between Active Trachoma (TF or TF/TI) and the detection of C. trachomatis infection at the individual level (a) before and (b) after the introduction of MDA, grouped by community TF prevalence level. Studies are ordered by increasing prevalence of TF, the size of the grey boxes represent the how much weight each study contributes to the overall estimate, the blue diamonds represent the subtotal and overall pooled odds ratio estimates. The odds ratios are for ocular C. trachomatis infection as the outcome and TF as the explanatory variable.
Fig 5.
Sensitivity versus specificity of each study, using TF to diagnose C. trachomatis infection at the individual level (a) before and (b) after the introduction of MDA. Each circle represents the estimate for a single study, with the size of the circle representing the size of the study. The red square is the estimated pooled sensitivity and specificity for all studies (Pre- or Post-MDA). The orange dashed line represents the 95% CI (or confidence region in 2 dimensions) for the sensitivity and specificity. The grey curve (hierarchical summary receiver operating characteristic (HSROC) curve) represents the estimated relationship between sensitivity and specificity in these studies, with the grey dashed line indicating the region in which we would expect 95% of studies to fall.
The relationship between the community-level prevalence of Active Trachoma and the proportion of people with TF who were infected with C. trachomatis (Positive Predictive Value, PPV) showed a positive correlation (r = 0.80, 95%CI 0.56 to 0.91, p<0.0001), Fig 3C. The pattern of this distribution suggests that when the community-level prevalence of TF is below 20% the PPV of TF for the presence of C. trachomatis infection drops substantially. Above a community-level prevalence of TF of 20% the PPV is typically 40–70%, across a wide range of TF prevalence.
The relationship between the community-level prevalence of Active Trachoma and the proportion of people without TF who were not infected with C. trachomatis (Negative Predictive Value, NPV) showed a strong negative correlation (r = -0.81, 95%CI -0.92 to -0.57, p<0.0001), Fig 3D. When the community-level TF prevalence was greater than 20%, the NPV was more variable.
Post-Treatment
Of the 21 studies from populations following the introduction of MDA, prior to the first treatment, 15 were hyperendemic, 3 mesoendemic and 3 hypoendemic (Table 2). The studies were conducted in populations from Tanzania (10), Ethiopia (5), The Gambia (3), Nepal (2), and Egypt (1). The Simplified WHO Trachoma Grading System was used in 17 studies and four used the Detailed WHO FPC System. The majority of studies (15) used the Amplicor CT/NG (Roche) PCR assay, four used LCR (Abbott), one used an in-house PCR and one used the Aptima Combo2 (Hologic) TMA assay. There was one study included in Table 2 that did not report the community prevalence of C. trachomatis infection, and was therefore not included in the meta-analysis [23]. One of the Gambian studies involved surveys in four separate districts; we have included these as four separate sets of data in the analysis [25]. This resulted in 23 discrete studies included in the meta-analysis: for 20 studies data was available on children only (variable age ranges), two of which also reported all age data, and three other studies reported only all age data.
Following the introduction of MDA there was evidence of a moderate positive correlation between the community-level prevalence of Active Trachoma and the community-level prevalence of detected C. trachomatis, using data from all 23 studies (r = 0.60, 95%CI 0.25 to 0.81, p = 0.003), Fig 2C. The relationship was similar when the analysis was limited to the 20 studies of children only (r = 0.60, 95%CI 0.21 to 0.82, p = 0.005). However, for the five studies reporting results for all ages there was no significant correlation (r = 0.72, 95%CI -0.18 to 0.86 p = 0.18). The overall impression is that the relationship between disease and infection is more uncertain post-MDA, such that the community-level prevalence of TF can substantially overestimate the underlying community-level prevalence of C. trachomatis; the community prevalence of TF can remain high (>20%) even when the prevalence of infection has declined (<10%), Fig 2C and 2E.
We found evidence that the relationship between the prevalence of TF and the prevalence of C. trachomatis infection differs between pre and post-MDA. Assuming a linear relationship between C. trachomatis and TF, the prevalence of C. trachomatis was associated with an expected 6.5% increase for every 10% increase in TF in pre-MDA studies, compared with an increase of 2.8% in post-MDA (test for interaction p = 0.004).This demonstrates that as the prevalence of TF increases, the expected increase in C. trachomatis is higher in pre-MDA communities than in post-MDA communities.
There were only eight studies that reported the prevalence of TI separately from TF after the introduction of MDA. There was no evidence of a correlation between the community-level prevalence of TI and the community-level prevalence of detected C. trachomatis (r = 0.50, 95%CI -0.31 to 0.89 p = 0.20), Fig 2D. However, it is noteworthy that the prevalence of TI was low (<10%) in all but two communities.
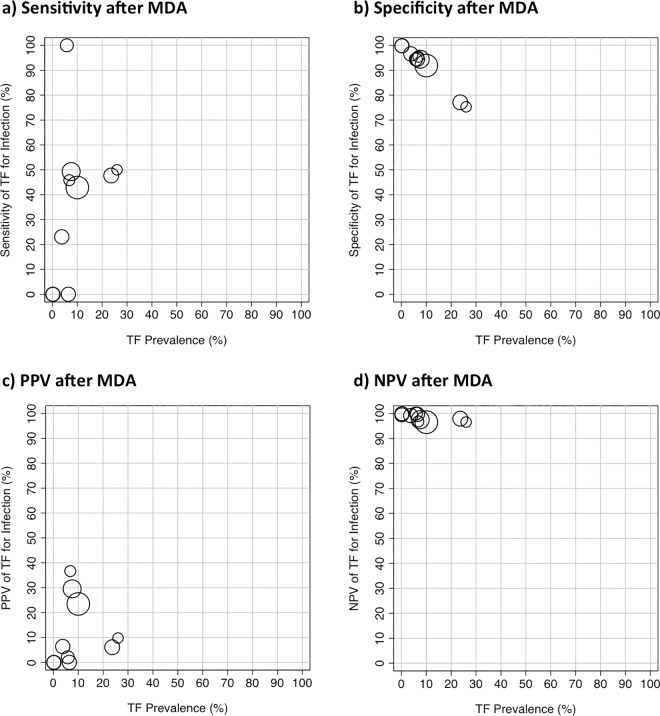
There was sufficient data presented to estimate the sensitivity, specificity, PPV and NPV for only 10 studies at the individual level after introduction of MDA. Therefore, only limited inference can be drawn. The sensitivity of TF for identifying the presence of C. trachomatis infection after the introduction of MDA varied widely, across a range of community-level TF prevalence levels, Fig 6A. In contrast, there was evidence of a strong negative correlation between the specificity of TF for infection and the community prevalence of TF (r = -0.99, 95%CI -0.99 to -0.96, p<0.0001), Fig 6B. The Forest plot of the post-treatment relationship between disease and detection of infection at the individual level showed an overall strong association (OR 8.38, 95% CI 7.09 to 9.90, p<0.0001), although there was marked heterogeneity (I2 = 75%, p<0.001), Fig 4B. Although this overall OR is slightly higher post-MDA compared to pre-MDA, it should be noted that this result is calculated from individual-level data, whereas the correlations between TF and C. trachomatis infection prevalence (Fig 2) are calculated from population-level data. Compared to the pre-MDA situation (Fig 5A), after the introduction of MDA the individual level sensitivity was weaker (39.2% (30.9%, 48.2%) while specificity was stronger (96.1% (89.6%, 98.6%), Fig 5B.
Fig 6. The relationship between the individual level presence of Active Trachoma (TF or TF/TI) and the detection of C. trachomatis infection by community TF prevalence after the introduction of MDA.
(a) Sensitivity of TF for infection. (b) Specificity of TF for infection. (c) Positive Predictive Value (PPV) of TF for C. trachomatis infection. (d) Negative Predictive Value (NPV) of TF for C. trachomatis infection. Data from 6 population-based studies, summarised in Table 2. The size of the circles reflects the sample size.
The community-level prevalence of Active Trachoma and the proportion of people with TF who were infected with C. trachomatis (PPV) did not appear to be correlated (r = 0.16, 95%CI -0.52 to 0.72, p = 0.65), Fig 6C. Finally, the proportion of people without TF who were not infected with C. trachomatis (NPV) was high (>90%), across the limited range of community-level prevalence of Active Trachoma in these studies, Fig 6D.
Discussion
It has long been observed that the relationship between the signs of Active Trachoma and the detection of C. trachomatis infection at the individual level is not highly concordant [12]. Surveys, including those in this review, consistently find that within endemic populations there are many individuals with signs of disease who do not have detectable infection and conversely there are people who do not meet the diagnostic criteria for Active Trachoma (TF or TI) who do have detectable infection. Therefore, at the individual level, signs of infection cannot be depended upon to determine which members of an endemic community have ocular C. trachomatis infection.
In a trachoma endemic population, the main reason for this mismatch between active disease and infection is probably the different time courses of the typical infection and disease episodes, outlined in the introduction and illustrated in Fig 1. In addition, it is also possible that other factors contribute to this mismatch. Some individuals who have detectable infection but do not meet the diagnostic criteria for TF may have a mild trachomatous follicular conjunctivitis. Others may have previously acquired immunity and are able clear the infection without developing any detectable inflammatory signs. Alternatively, a positive NAAT test for C. trachomatis in a clinically normal individual could arise through cross-contamination during sample collection or processing. Clinical signs similar to those of Active Trachoma can also arise for other reasons, such as viral or bacterial infections, vernal conjunctivitis and hypersensitivity reactions [26].
It is clear that clinical signs are not a reliable indicator for C. trachomatis at the individual level. There is no point-of-care diagnostic test available for programmes to use to determine which individual members of a community are infected, and who would therefore benefit from targeted antibiotic treatment. Moreover, a strategy of testing everybody is not considered a practical option. Therefore, the WHO guidance and the standard practice is to conduct community-wide antibiotic distribution of the entire population of endemic districts.
Decisions around the initiation and cessation of trachoma control measures are based on the prevalence of TF in children aged 1–9 years, determined through district level surveys, such as those conducted by the Global Trachoma Mapping Project. District-wide antibiotic treatment programmes and F&E measures to suppress transmission are initiated where the initial prevalence of TF is ≥10%. Below 10% TF the advice is to conduct sub-district level surveys. If a sub-district has ≥10% TF MDA and F&E are implemented. For sub-districts between 5% and 9.9% TF the guidance is to consider targeted MDA and F&E measures. For sub-districts <5% no MDA is needed and implementation of F&E can be considered.
The dependence on the clinical signs of disease to guide programmatic decisions raises the important question of how accurate these clinical signs are as a proxy measure for C. trachomatis infection at the population level. This question is particularly relevant after the introduction of MDA, when the association between clinical signs and infection prevalence at the population level is weaker, and as we try to determine when elimination targets have been reached.
In this systematic review, we found that prior to the introduction of antibiotic treatment the relationship between the community-level prevalence of TF in children correlated well with that of infection. The prevalence of TF was usually slightly greater than that of infection. Therefore, over a wide prevalence range, the community-level prevalence of TF in children is a reliable indicator that broadly reflects the underlying population burden of infection. The association between disease and infection for all ages was much weaker than that for children only; this supports the rationale for measuring TF in 1–9 year olds as the key indicator group for determining the need of antibiotic. The prevalence of TI was less well correlated than TF with infection before MDA, with the disease prevalence generally underestimating the level of infection.
At the individual level the utility of TF as a marker for infection is highly sensitive to the underlying prevalence of TF. Both the sensitivity and PPV rise substantially with increasing TF prevalence, and the specificity and NPV both drop. This sensitivity increase is therefore offset by a corresponding decrease in the specificity of this test in high TF prevalence communities. The usefulness of TF as an indicator of an individual’s infection status is dependent on both the sensitivity and the specificity of the test; too low a sensitivity leads to C. trachomatis infected individuals not receiving treatment, whereas too low a specificity leads to wasted resources treating uninfected individuals. The PPV possibly gives the clearest indication of where TF is useful as an indicator of an individual’s infection status; when the prevalence of TF is low (~5–10%), TF will only indicate an estimated 10% probability that the individual has C. trachomatis infection. This probability increases steadily with prevalence and once the community prevalence reaches 30%, the estimated PPV is in the order of 50–70%. Thus, where the population TF prevalence is above 30%, a positive TF diagnosis gives a 50–70% probability that an individual will be C. trachomatis infected.
After the introduction of MDA the relationship between the community prevalence of TF and chlamydial infection is less certain. Although the relationship between Active Trachoma and C. trachomatis infection appears to remain strong at the individual level, the population-level data suggests that post-MDA, Active Trachoma has a greater tendency to overestimate the underlying population prevalence of C. trachomatis infection. Below the 10% TF level the prevalence of infection was consistently low, and therefore below this level, TF prevalence appears to be a good marker for infection having being brought under control. However, in the studies where the prevalence of TF was above 10% after the introduction of MDA, the underlying prevalence of C. trachomatis infection was much less predictable. In some settings TF prevalence persists at high levels despite relatively little infection being detected. There might be several reasons for this observation. For example, if the loads of infection are substantially lower following the introduction of MDA these may not be so readily detected by diagnostic tests. Other bacterial species might also provoke a follicular conjunctivitis more readily in individuals previously infected with C. trachomatis [26]. However, whatever the explanation, it is likely that in some settings, the prevalence of TF will underestimate the impact MDA has had on the prevalence of C. trachomatis. This might lead to the on-going use of MDA after the infection has been adequately controlled.
There is much less published data on the relationship between TI and infection following the introduction of MDA. In general, the prevalence of TI appears to reduce more readily than TF. A number of studies have investigated the relationship between the load of infection and disease signs. These suggest that TI is particularly associated with high loads of infection [27]. Therefore, the decline in TI may reflect a shift to less intense infections. However, there were a few studies in which the prevalence of TI was low, but the prevalence of infection remained substantial.
We identified a reasonable number of studies reporting the community-level relationship between disease and infection prior to treatment, over a wide TF prevalence range. There were, however, fewer studies documenting the situation following the introduction of MDA, potentially limiting the conclusions that can be drawn after MDA. However, there was generally less detail in these reports about the individual-level relationship between clinical signs and infection. The studies came mostly from several East and West African countries, providing reasonable geographical coverage of the regions with the greatest trachoma burden. Standard WHO definitions of disease were generally used and the large majority of studies used the same commercially produced PCR assay, providing consistency across studies. However, there was some methodological heterogeneity. The age groups reported varied; where possible we use the data for children only in the meta-analysis to try to provide greater consistency between studies. The sample sizes varied considerably (from 56 to 7817); we weighted our analyses to adjust for size. The sampling methodology also varied considerably. The studies included were generally population-based samples or surveys of the entire resident population of a defined area.
Overall, the use of TF prevalence to guide the decision to initiate MDA in previously untreated districts appears to be reliable. In contrast, the situation following treatment is more uncertain, calling into question the reliability of clinical signs for monitoring progress towards the achievement of the elimination targets [28]. There are reports from hyperendemic regions that have received many rounds of high-coverage MDA that suggest that the prevalence of TF can be recalcitrant, even when C. trachomatis infection appears to have been brought under control. Therefore, contextually appropriate, cost-effective tests for C. trachomatis infection that can be administered in low-resource settings, and used to estimate the infection prevalence in a population-based sample, would be very helpful in guiding decisions around the cessation of MDA. Such tests are anticipated to reduce the number of annual rounds of MDA required and lead to the confirmation of trachoma control at an earlier stage.
Supporting Information
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and within the relevant references.
Funding Statement
This work is supported by the Wellcome Trust (https://wellcome.ac.uk), grant number 098481/Z/12/Z, awarded to MJB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Taylor HR, Burton MJ, Haddad D, West S, Wright H. Trachoma. Lancet (London, England). 2014;384(9960):2142–52. Epub 2014/07/22. [DOI] [PubMed] [Google Scholar]
- 2.Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bulletin of the World Health Organization. 1987;65(4):477–83. Epub 1987/01/01. PubMed Central PMCID: PMCPMC2491032. [PMC free article] [PubMed] [Google Scholar]
- 3.Jawetz E, Rose L, Hanna L, Thygeson P. Experimental inclusion conjunctivitis in man: measurements of infectivity and resistance. Jama. 1965;194(6):620–32. Epub 1965/11/08. [PubMed] [Google Scholar]
- 4.Bailey RL, Holland MJ, Whittle HC, Mabey DC. Subjects recovering from human ocular chlamydial infection have enhanced lymphoproliferative responses to chlamydial antigens compared with those of persistently diseased controls. Infection and immunity. 1995;63(2):389–92. Epub 1995/02/01. PubMed Central PMCID: PMCPMC173007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grassly NC, Ward ME, Ferris S, Mabey DC, Bailey RL. The natural history of trachoma infection and disease in a Gambian cohort with frequent follow-up. PLoS neglected tropical diseases. 2008;2(12):e341 Epub 2008/12/03. PubMed Central PMCID: PMCPMC2584235. 10.1371/journal.pntd.0000341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holland MJ, Faal N, Jeffries D, Joof H, Sarr I, Laye M, et al. Human Ocular Chlamydia Trachomatis Disease, Infection and Immune Responses Examined Using Quantitative Conjunctival Gene Expression with Intensive Longitudinal Follow-Up and Gene Co-Expression Analysis. International Chlamydial Symposium; Salzburg, Austria. San Francisco, USA2010. p. 145–8.
- 7.Burton MJ, Holland MJ, Faal N, Aryee EA, Alexander ND, Bah M, et al. Which members of a community need antibiotics to control trachoma? Conjunctival Chlamydia trachomatis infection load in Gambian villages. Investigative ophthalmology & visual science. 2003;44(10):4215–22. Epub 2003/09/26. [DOI] [PubMed] [Google Scholar]
- 8.Eliminating Trachoma: Accelerating Towards 2020. Geneva: WHO Alliance for the Global Elimination of Trachoma by 2020, 2016.
- 9.Report of the 3rd Global Scientific Meeting on Trachoma. World Health Organization, 2010 2010. Report No.
- 10.Bird M, Dawson CR, Schachter JS, Miao Y, Shama A, Osman A, et al. Does the diagnosis of trachoma adequately identify ocular chlamydial infection in trachoma-endemic areas? The Journal of infectious diseases. 2003;187(10):1669–73. Epub 2003/05/02. 10.1086/374743 [DOI] [PubMed] [Google Scholar]
- 11.Baral K, Osaki S, Shreshta B, Panta CR, Boulter A, Pang F, et al. Reliability of clinical diagnosis in identifying infectious trachoma in a low-prevalence area of Nepal. Bulletin of the World Health Organization. 1999;77(6):461–6. Epub 1999/07/31. PubMed Central PMCID: PMCPMC2557684. [PMC free article] [PubMed] [Google Scholar]
- 12.Wright HR, Taylor HR. Clinical examination and laboratory tests for estimation of trachoma prevalence in a remote setting: what are they really telling us? The Lancet Infectious diseases. 2005;5(5):313–20. Epub 2005/04/28. 10.1016/S1473-3099(05)70116-X [DOI] [PubMed] [Google Scholar]
- 13.Derrick T, Holland MJ, Cassama E, Markham-David R, Nabicassa M, Marks M, et al. Can corneal pannus with trachomatous inflammation—follicular be used in combination as an improved specific clinical sign for current ocular Chlamydia trachomatis infection? Parasites & vectors. 2016;9:30. Epub 2016/01/28. PubMed Central PMCID: PMCPMC4729176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michel CE, Roper KG, Divena MA, Lee HH, Taylor HR. Correlation of clinical trachoma and infection in Aboriginal communities. PLoS neglected tropical diseases. 2011;5(3):e986 Epub 2011/03/23. PubMed Central PMCID: PMCPMC3057949. 10.1371/journal.pntd.0000986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munoz B, Stare D, Mkocha H, Gaydos C, Quinn T, West SK. Can clinical signs of trachoma be used after multiple rounds of mass antibiotic treatment to indicate infection? Investigative ophthalmology & visual science. 2011;52(12):8806–10. Epub 2011/10/26. PubMed Central PMCID: PMCPMC3230903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thein J, Zhao P, Liu H, Xu J, Jha H, Miao Y, et al. Does clinical diagnosis indicate ocular chlamydial infection in areas with a low prevalence of trachoma? Ophthalmic epidemiology. 2002;9(4):263–9. Epub 2002/08/21. [DOI] [PubMed] [Google Scholar]
- 17.Miller K, Schmidt G, Melese M, Alemayehu W, Yi E, Cevallos V, et al. How reliable is the clinical exam in detecting ocular chlamydial infection? Ophthalmic epidemiology. 2004;11(3):255–62. Epub 2004/09/17. 10.1080/09286580490514577 [DOI] [PubMed] [Google Scholar]
- 18.Keenan JD, Lakew T, Alemayehu W, Melese M, Porco TC, Yi E, et al. Clinical activity and polymerase chain reaction evidence of chlamydial infection after repeated mass antibiotic treatments for trachoma. The American journal of tropical medicine and hygiene. 2010;82(3):482–7. Epub 2010/03/09. PubMed Central PMCID: PMCPMC2829914. 10.4269/ajtmh.2010.09-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keenan JD, See CW, Moncada J, Ayele B, Gebre T, Stoller NE, et al. Diagnostic characteristics of tests for ocular Chlamydia after mass azithromycin distributions. Investigative ophthalmology & visual science. 2012;53(1):235–40. Epub 2011/12/14. PubMed Central PMCID: PMCPMC3292361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bobo L, Munoz B, Viscidi R, Quinn T, Mkocha H, West S. Diagnosis of Chlamydia trachomatis eye infection in Tanzania by polymerase chain reaction/enzyme immunoassay. Lancet (London, England). 1991;338(8771):847–50. Epub 1991/10/05. [DOI] [PubMed] [Google Scholar]
- 21.Dawson CR, Jones BR, Tarizzo ML. Guide to Trachoma Control. Geneva: World Health Organization; 1981. [Google Scholar]
- 22.Bailey RL, Hayes L, Pickett M, Whittle HC, Ward ME, Mabey DC. Molecular epidemiology of trachoma in a Gambian village. The British journal of ophthalmology. 1994;78(11):813–7. Epub 1994/11/01. PubMed Central PMCID: PMCPMC504962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holm SO, Jha HC, Bhatta RC, Chaudhary JS, Thapa BB, Davis D, et al. Comparison of two azithromycin distribution strategies for controlling trachoma in Nepal. Bulletin of the World Health Organization. 2001;79(3):194–200. Epub 2001/04/05. PubMed Central PMCID: PMCPMC2566365. [PMC free article] [PubMed] [Google Scholar]
- 24.Goldschmidt P, Benallaoua D, Amza A, Einterz E, Huguet P, Poisson F, et al. Clinical and microbiological assessment of trachoma in the kolofata health district, far north region, cameroon. Tropical medicine and health. 2012;40(1):7–14. Epub 2012/09/06. PubMed Central PMCID: PMCPMC3426829. 10.2149/tmh.2011-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harding-Esch EM, Sillah A, Edwards T, Burr SE, Hart JD, Joof H, et al. Mass treatment with azithromycin for trachoma: when is one round enough? Results from the PRET Trial in the Gambia. PLoS neglected tropical diseases. 2013;7(6):e2115 Epub 2013/06/21. PubMed Central PMCID: PMCPMC3681669. 10.1371/journal.pntd.0002115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burton MJ, Hu VH, Massae P, Burr SE, Chevallier C, Afwamba IA, et al. What is causing active trachoma? The role of nonchlamydial bacterial pathogens in a low prevalence setting. Investigative ophthalmology & visual science. 2011;52(8):6012–7. Epub 2011/06/23. PubMed Central PMCID: PMCPMC3176035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon AW, Holland MJ, Burton MJ, West SK, Alexander ND, Aguirre A, et al. Strategies for control of trachoma: observational study with quantitative PCR. Lancet (London, England). 2003;362(9379):198–204. Epub 2003/07/30. [DOI] [PubMed] [Google Scholar]
- 28.Technical Consultation on Trachoma Surveillance. World Health Organization, 2014. [Google Scholar]
- 29.Bailey RL, Hampton TJ, Hayes LJ, Ward ME, Whittle HC, Mabey DC. Polymerase chain reaction for the detection of ocular chlamydial infection in trachoma-endemic communities. The Journal of infectious diseases. 1994;170(3):709–12. Epub 1994/09/01. [DOI] [PubMed] [Google Scholar]
- 30.Lietman TM, Dhital SP, Dean D. Conjunctival impression cytology for vitamin A deficiency in the presence of infectious trachoma. The British journal of ophthalmology. 1998;82(10):1139–42. Epub 1999/01/30. PubMed Central PMCID: PMCPMC1722377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schachter J, West SK, Mabey D, Dawson CR, Bobo L, Bailey R, et al. Azithromycin in control of trachoma. Lancet (London, England). 1999;354(9179):630–5. Epub 1999/08/31. [DOI] [PubMed] [Google Scholar]
- 32.Burton MJ, Holland MJ, Makalo P, Aryee EA, Alexander ND, Sillah A, et al. Re-emergence of Chlamydia trachomatis infection after mass antibiotic treatment of a trachoma-endemic Gambian community: a longitudinal study. Lancet (London, England). 2005;365(9467):1321–8. Epub 2005/04/13. [DOI] [PubMed] [Google Scholar]
- 33.Natividad A, Freeman TC, Jeffries D, Burton MJ, Mabey DC, Bailey RL, et al. Human conjunctival transcriptome analysis reveals the prominence of innate defense in Chlamydia trachomatis infection. Infection and immunity. 2010;78(11):4895–911. Epub 2010/09/09. PubMed Central PMCID: PMCPMC2976339. 10.1128/IAI.00844-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solomon AW, Holland MJ, Alexander ND, Massae PA, Aguirre A, Natividad-Sancho A, et al. Mass treatment with single-dose azithromycin for trachoma. The New England journal of medicine. 2004;351(19):1962–71. Epub 2004/11/05. 10.1056/NEJMoa040979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon AW, Harding-Esch E, Alexander ND, Aguirre A, Holland MJ, Bailey RL, et al. Two doses of azithromycin to eliminate trachoma in a Tanzanian community. The New England journal of medicine. 2008;358(17):1870–1. Epub 2008/04/25. 10.1056/NEJMc0706263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West SK, Munoz B, Mkocha H, Holland MJ, Aguirre A, Solomon AW, et al. Infection with Chlamydia trachomatis after mass treatment of a trachoma hyperendemic community in Tanzania: a longitudinal study. Lancet (London, England). 2005;366(9493):1296–300. Epub 2005/10/11. [DOI] [PubMed] [Google Scholar]
- 37.Broman AT, Shum K, Munoz B, Duncan DD, West SK. Spatial clustering of ocular chlamydial infection over time following treatment, among households in a village in Tanzania. Investigative ophthalmology & visual science. 2006;47(1):99–104. Epub 2005/12/31. [DOI] [PubMed] [Google Scholar]
- 38.Merbs SL, West SK, West ES. Pattern of recurrence of trachomatous trichiasis after surgery surgical technique as an explanation. Ophthalmology. 2005;112(4):705–9. Epub 2005/04/06. 10.1016/j.ophtha.2004.10.037 [DOI] [PubMed] [Google Scholar]
- 39.West SK, Munoz B, Mkocha H, Gaydos C, Quinn T. Trachoma and ocular Chlamydia trachomatis were not eliminated three years after two rounds of mass treatment in a trachoma hyperendemic village. Investigative ophthalmology & visual science. 2007;48(4):1492–7. Epub 2007/03/29. [DOI] [PubMed] [Google Scholar]
- 40.Faal N, Bailey RL, Jeffries D, Joof H, Sarr I, Laye M, et al. Conjunctival FOXP3 expression in trachoma: do regulatory T cells have a role in human ocular Chlamydia trachomatis infection? PLoS medicine. 2006;3(8):e266 Epub 2006/08/03. PubMed Central PMCID: PMCPMC1526769. 10.1371/journal.pmed.0030266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michel CE, Solomon AW, Magbanua JP, Massae PA, Huang L, Mosha J, et al. Field evaluation of a rapid point-of-care assay for targeting antibiotic treatment for trachoma control: a comparative study. Lancet (London, England). 2006;367(9522):1585–90. Epub 2006/05/16. [DOI] [PubMed] [Google Scholar]
- 42.Abdou A, Nassirou B, Kadri B, Moussa F, Munoz BE, Opong E, et al. Prevalence and risk factors for trachoma and ocular Chlamydia trachomatis infection in Niger. The British journal of ophthalmology. 2007;91(1):13–7. Epub 2006/08/11. PubMed Central PMCID: PMCPMC1857562. 10.1136/bjo.2006.099507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang JL, Schachter J, Moncada J, Habte D, Zerihun M, House JI, et al. Comparison of an rRNA-based and DNA-based nucleic acid amplification test for the detection of Chlamydia trachomatis in trachoma. The British journal of ophthalmology. 2007;91(3):293–5. Epub 2006/10/20. PubMed Central PMCID: PMCPMC1857674. 10.1136/bjo.2006.099150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dean D, Kandel RP, Adhikari HK, Hessel T. Multiple Chlamydiaceae species in trachoma: implications for disease pathogenesis and control. PLoS medicine. 2008;5(1):e14 Epub 2008/01/08. PubMed Central PMCID: PMCPMC2174965. 10.1371/journal.pmed.0050014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harding-Esch EM, Edwards T, Sillah A, Sarr I, Roberts CH, Snell P, et al. Active trachoma and ocular Chlamydia trachomatis infection in two Gambian regions: on course for elimination by 2020? PLoS neglected tropical diseases. 2009;3(12):e573 Epub 2009/12/23. PubMed Central PMCID: PMCPMC2791206. 10.1371/journal.pntd.0000573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harding-Esch EM, Edwards T, Sillah A, Sarr-Sissoho I, Aryee EA, Snell P, et al. Risk factors for active trachoma in The Gambia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102(12):1255–62. Epub 2008/05/27. PubMed Central PMCID: PMCPMC3836170. 10.1016/j.trstmh.2008.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang JL, Hong KC, Schachter J, Moncada J, Lekew T, House JI, et al. Detection of Chlamydia trachomatis ocular infection in trachoma-endemic communities by rRNA amplification. Investigative ophthalmology & visual science. 2009;50(1):90–4. Epub 2008/08/12. [DOI] [PubMed] [Google Scholar]
- 48.See CW, Alemayehu W, Melese M, Zhou Z, Porco TC, Shiboski S, et al. How reliable are tests for trachoma?—a latent class approach. Investigative ophthalmology & visual science. 2011;52(9):6133–7. Epub 2011/06/21. PubMed Central PMCID: PMCPMC3176003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harding-Esch EM, Edwards T, Mkocha H, Munoz B, Holland MJ, Burr SE, et al. Trachoma prevalence and associated risk factors in the gambia and Tanzania: baseline results of a cluster randomised controlled trial. PLoS neglected tropical diseases. 2010;4(11):e861 Epub 2010/11/13. PubMed Central PMCID: PMCPMC2970530. 10.1371/journal.pntd.0000861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stare D, Harding-Esch E, Munoz B, Bailey R, Mabey D, Holland M, et al. Design and baseline data of a randomized trial to evaluate coverage and frequency of mass treatment with azithromycin: the Partnership for Rapid Elimination of Trachoma (PRET) in Tanzania and The Gambia. Ophthalmic epidemiology. 2011;18(1):20–9. Epub 2011/02/01. 10.3109/09286586.2010.545500 [DOI] [PubMed] [Google Scholar]
- 51.Yohannan J, He B, Wang J, Greene G, Schein Y, Mkocha H, et al. Geospatial distribution and clustering of Chlamydia trachomatis in communities undergoing mass azithromycin treatment. Investigative ophthalmology & visual science. 2014;55(7):4144–50. Epub 2014/06/08. PubMed Central PMCID: PMCPMC4580214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdou A, Munoz BE, Nassirou B, Kadri B, Moussa F, Baare I, et al. How much is not enough? A community randomized trial of a Water and Health Education programme for Trachoma and Ocular C. trachomatis infection in Niger. Tropical medicine & international health: TM & IH. 2010;15(1):98–104. Epub 2010/04/23. PubMed Central PMCID: PMCPMC2867063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burton MJ, Ramadhani A, Weiss HA, Hu V, Massae P, Burr SE, et al. Active trachoma is associated with increased conjunctival expression of IL17A and profibrotic cytokines. Infection and immunity. 2011;79(12):4977–83. Epub 2011/09/14. PubMed Central PMCID: PMCPMC3232643. 10.1128/IAI.05718-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cajas-Monson LC, Mkocha H, Munoz B, Quinn TC, Gaydos CA, West SK. Risk factors for ocular infection with Chlamydia trachomatis in children 6 months following mass treatment in Tanzania. PLoS neglected tropical diseases. 2011;5(3):e978 Epub 2011/03/23. PubMed Central PMCID: PMCPMC3057946. 10.1371/journal.pntd.0000978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West SK, Munoz BE, Mkocha H, Gaydos C, Quinn T. Risk of Infection with Chlamydia trachomatis from Migrants to Communities Undergoing Mass Drug Administration for Trachoma Control. Ophthalmic epidemiology. 2015;22(3):170–5. Epub 2015/07/15. PubMed Central PMCID: PMCPMC4774537. 10.3109/09286586.2015.1010687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West SK, Stare D, Mkocha H, Munoz B, Gaydos C, Quinn TC. Do infants increase the risk of re-emergent infection in households after mass drug administration for trachoma? Investigative ophthalmology & visual science. 2011;52(8):6040–2. Epub 2011/06/18. PubMed Central PMCID: PMCPMC3176068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoller NE, Gebre T, Ayele B, Zerihun M, Assefa Y, Habte D, et al. Efficacy of latrine promotion on emergence of infection with ocular Chlamydia trachomatis after mass antibiotic treatment: a cluster-randomized trial. International health. 2011;3(2):75–84. Epub 2011/07/26. PubMed Central PMCID: PMCPMC3139980. 10.1016/j.inhe.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gebre T, Ayele B, Zerihun M, Genet A, Stoller NE, Zhou Z, et al. Comparison of annual versus twice-yearly mass azithromycin treatment for hyperendemic trachoma in Ethiopia: a cluster-randomised trial. Lancet (London, England). 2012;379(9811):143–51. Epub 2011/12/24. [DOI] [PubMed] [Google Scholar]
- 59.Keenan JD, Ayele B, Gebre T, Moncada J, Stoller NE, Zhou Z, et al. Ribosomal RNA evidence of ocular Chlamydia trachomatis infection following 3 annual mass azithromycin distributions in communities with highly prevalent trachoma. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54(2):253–6. Epub 2011/11/19. PubMed Central PMCID: PMCPMC3245728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amza A, Kadri B, Nassirou B, Stoller NE, Yu SN, Zhou Z, et al. Community risk factors for ocular Chlamydia infection in Niger: pre-treatment results from a cluster-randomized trachoma trial. PLoS neglected tropical diseases. 2012;6(4):e1586 Epub 2012/05/01. PubMed Central PMCID: PMCPMC3335874. 10.1371/journal.pntd.0001586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amza A, Kadri B, Nassirou B, Yu SN, Stoller NE, Bhosai SJ, et al. The easiest children to reach are most likely to be infected with ocular Chlamydia trachomatis in trachoma endemic areas of Niger. PLoS neglected tropical diseases. 2013;7(1):e1983 Epub 2013/01/18. PubMed Central PMCID: PMCPMC3542188. 10.1371/journal.pntd.0001983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roberts CH, Last A, Molina-Gonzalez S, Cassama E, Butcher R, Nabicassa M, et al. Development and evaluation of a next-generation digital PCR diagnostic assay for ocular Chlamydia trachomatis infections. Journal of clinical microbiology. 2013;51(7):2195–203. Epub 2013/05/03. PubMed Central PMCID: PMCPMC3697714. 10.1128/JCM.00622-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Last AR, Burr SE, Weiss HA, Harding-Esch EM, Cassama E, Nabicassa M, et al. Risk factors for active trachoma and ocular Chlamydia trachomatis infection in treatment-naive trachoma-hyperendemic communities of the Bijagos Archipelago, Guinea Bissau. PLoS neglected tropical diseases. 2014;8(6):e2900 Epub 2014/06/27. PubMed Central PMCID: PMCPMC4072588. 10.1371/journal.pntd.0002900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jenson A, Dize L, Mkocha H, Munoz B, Lee J, Gaydos C, et al. Field evaluation of the Cepheid GeneXpert Chlamydia trachomatis assay for detection of infection in a trachoma endemic community in Tanzania. PLoS neglected tropical diseases. 2013;7(7):e2265 Epub 2013/07/19. PubMed Central PMCID: PMCPMC3701699. 10.1371/journal.pntd.0002265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goodhew EB, Morgan SM, Switzer AJ, Munoz B, Dize L, Gaydos C, et al. Longitudinal analysis of antibody responses to trachoma antigens before and after mass drug administration. BMC infectious diseases. 2014;14:216 Epub 2014/04/24. PubMed Central PMCID: PMCPMC4016634. 10.1186/1471-2334-14-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.West S, Munoz B, Bobo L, Quinn TC, Mkocha H, Lynch M, et al. Nonocular Chlamydia infection and risk of ocular reinfection after mass treatment in a trachoma hyperendemic area. Investigative ophthalmology & visual science. 1993;34(11):3194–8. Epub 1993/10/01. [PubMed] [Google Scholar]
- 67.Cumberland P, Edwards T, Hailu G, Harding-Esch E, Andreasen A, Mabey D, et al. The impact of community level treatment and preventative interventions on trachoma prevalence in rural Ethiopia. International journal of epidemiology. 2008;37(3):549–58. Epub 2008/03/22. 10.1093/ije/dyn045 [DOI] [PubMed] [Google Scholar]
- 68.Ayele B, Gebre T, Moncada J, House JI, Stoller NE, Zhou Z, et al. Risk factors for ocular chlamydia after three mass azithromycin distributions. PLoS neglected tropical diseases. 2011;5(12):e1441 Epub 2011/12/20. PubMed Central PMCID: PMCPMC3236733. 10.1371/journal.pntd.0001441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keenan JD, Moncada J, Gebre T, Ayele B, Chen MC, Yu SN, et al. Chlamydial infection during trachoma monitoring: are the most difficult-to-reach children more likely to be infected? Tropical medicine & international health: TM & IH. 2012;17(3):392–6. Epub 2011/11/30. PubMed Central PMCID: PMCPMC3292692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quicke E, Sillah A, Harding-Esch EM, Last A, Joof H, Makalo P, et al. Follicular trachoma and trichiasis prevalence in an urban community in The Gambia, West Africa: is there a need to include urban areas in national trachoma surveillance? Tropical medicine & international health: TM & IH. 2013;18(11):1344–52. Epub 2013/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.West SK, Bailey R, Munoz B, Edwards T, Mkocha H, Gaydos C, et al. A randomized trial of two coverage targets for mass treatment with azithromycin for trachoma. PLoS neglected tropical diseases. 2013;7(8):e2415 Epub 2013/09/07. PubMed Central PMCID: PMCPMC3757067. 10.1371/journal.pntd.0002415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee JS, Munoz BE, Mkocha H, Gaydos CA, Quinn TC, West SK. The effect of multiple rounds of mass drug administration on the association between ocular Chlamydia trachomatis infection and follicular trachoma in preschool-aged children. PLoS neglected tropical diseases. 2014;8(4):e2761 Epub 2014/04/12. PubMed Central PMCID: PMCPMC3983082. 10.1371/journal.pntd.0002761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martin DL, Bid R, Sandi F, Goodhew EB, Massae PA, Lasway A, et al. Serology for trachoma surveillance after cessation of mass drug administration. PLoS neglected tropical diseases. 2015;9(2):e0003555 Epub 2015/02/26. PubMed Central PMCID: PMCPMC4340913. 10.1371/journal.pntd.0003555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pant BP, Bhatta RC, Chaudhary JS, Awasthi S, Mishra S, Sharma S, et al. Control of Trachoma from Achham District, Nepal: A Cross-Sectional Study from the Nepal National Trachoma Program. PLoS neglected tropical diseases. 2016;10(2):e0004462 Epub 2016/02/13. PubMed Central PMCID: PMCPMC4752456. 10.1371/journal.pntd.0004462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.West SK, Munoz B, Weaver J, Mrango Z, Dize L, Gaydos C, et al. Can We Use Antibodies to Chlamydia trachomatis as a Surveillance Tool for National Trachoma Control Programs? Results from a District Survey. PLoS neglected tropical diseases. 2016;10(1):e0004352 Epub 2016/01/16. PubMed Central PMCID: PMCPMC4714879. 10.1371/journal.pntd.0004352 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and within the relevant references.