Abstract
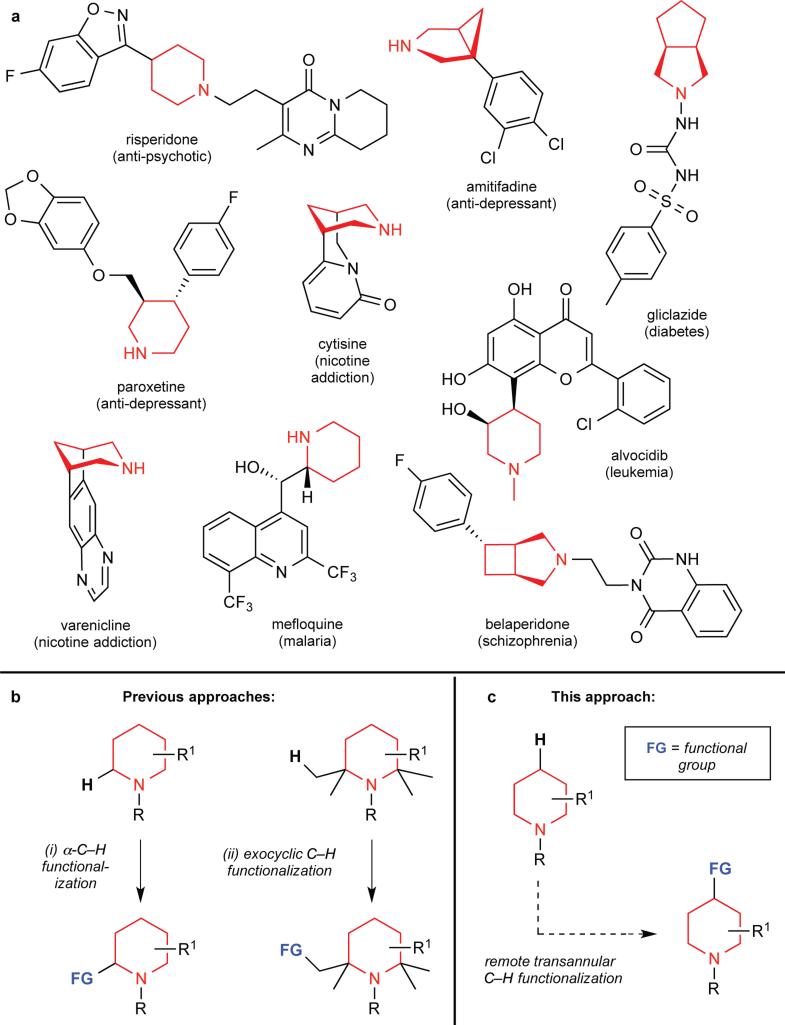
The discovery of pharmaceutical candidates is a resource-intensive enterprise that frequently requires the parallel synthesis of hundreds or even thousands of molecules. Carbon-hydrogen bonds are present in almost all pharmaceutical agents. As such, the development of selective, rapid, and efficient methods for converting carbon-hydrogen bonds into new chemical entities has the potential to dramatically streamline pharmaceutical development1,2,3,4. Saturated nitrogen-containing heterocycles (alicyclic amines) feature prominently in pharmaceuticals, including treatments for depression (paroxetine, amitifadine), diabetes (gliclazide), leukemia (alvocidib), schizophrenia (risperidone, belaperidone), and nicotine addiction (cytisine and varenicline)5. However, existing methods for the C–H functionalization of saturated nitrogen heterocycles, particularly at sites remote to nitrogen, remain extremely limited 6,7. Here we report a new approach to selectively manipulate the carbon–hydrogen bonds of alicyclic amines at sites remote to nitrogen. Our reaction leverages the boat conformation of the substrates to achieve the palladium-catalyzed amine-directed conversion of C–H bonds to C–C bonds on various alicyclic amine scaffolds. This approach is applied to the synthesis of novel derivatives of several bioactive molecules, including the top-selling smoking cessation drug varenicline (Chantix®). We anticipate that this method should prove broadly useful in medicinal chemistry.
Despite the ubiquity of alicyclic amines, there are very few methods available for the late-stage functionalization of these structures. Late stage functionalization approaches are particularly valuable in the context of drug development, since they enable the rapid synthesis of analogues to optimize pharmacokinetic properties. Transition metal-catalyzed C–H bond functionalization offers a powerful approach for the late stage functionalization of bioactive molecules,1-4 and recent progress in this field has led to thousands of new synthetic methods for selective C–H functionalization in a variety of molecular contexts3-6,8,9. However, methods for the C–H functionalization of saturated nitrogen heterocycles remain extremely limited 5,6, and are dominated by functionalization of the highly activated C–H bonds α-to nitrogen10,11,12,13,14 (Fig. 1B, i) or of C–H bonds on exocyclic alkyl groups15,16 (Fig. 1B, ii). In contrast, this report describes our approach for achieving the C–H functionalization of alicyclic amine cores at sites remote from nitrogen (Fig. 1C) via nitrogen-directed transannular C–H activation.
Figure 1. Relevance of alicyclic amines and strategies for their late stage functionalization.
a, Representative pharmaceutical agents containing alicyclic amines. b, Previous synthetic approaches for the late-stage functionalization of alicyclic amines. R, R1, generic substituent; FG, new functional group. c, Proposed approach for late stage transannular C–H functionalization of alicyclic amines.
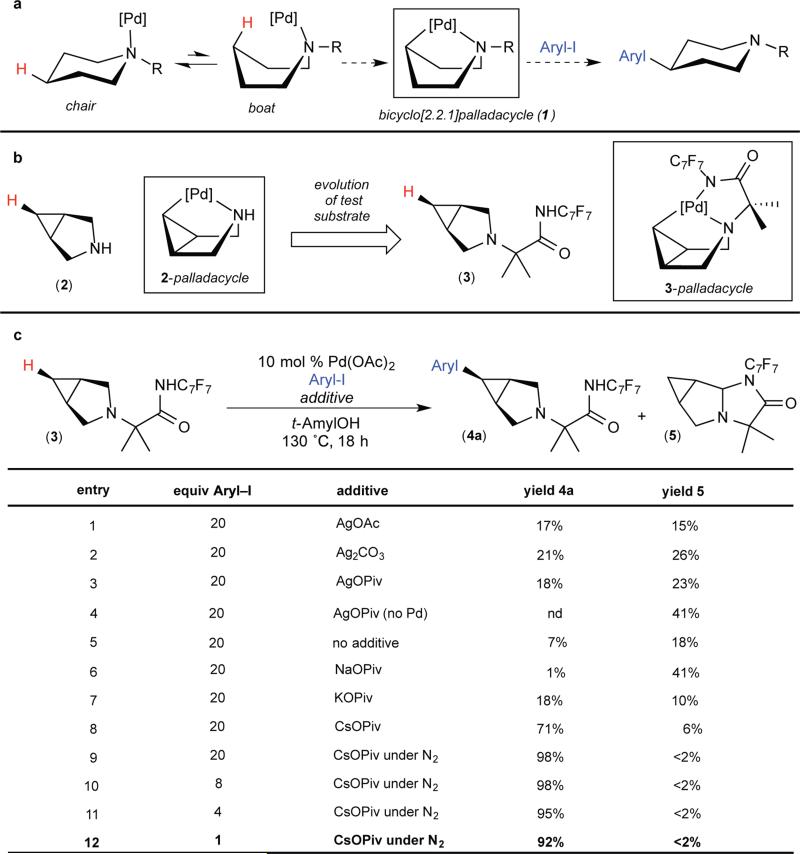
We envisioned that coordination of the nitrogen of an alicyclic amine such as piperidine to palladium could enable selective transannular C–H activation17, 18 to generate a bicyclo[2.2.1]palladacycle (exemplified by 1 in Fig. 2A). However, there are several challenges associated with this approach, including: (1) the low equilibrium population of the required boat conformer, (2) the requirement for cleavage of an unactivated 2° Csp3–H bond, and (3) the potential susceptibility of the basic amine towards α-oxidation or N-oxidation. With these considerations in mind, we initially selected 3-azabicyclo[3.1.0]hexane 2 as a test substrate (Fig. 2B). We anticipated that the bicyclic core of 2 would prearrange it in a boat-like conformation and that the high s-character of the cyclopropyl C–H bonds should lower the barrier for C–H activation relative to a typical 2° Csp3–H site19.
Figure 2. Design and realization of transannular C–H activation of alicyclic amines.
a, Conceptual approach to transannular C-H arylation of via a bicyclo[2.2.1]metallacycle intermediate. [Pd], Pd complex. b, Evolution of model substrate 2 to 3. C7F7, 4-(CF3)C6F4. c, Reaction optimization using 4-iodobiphenyl (Aryl-I). t-AmylOH, 2-methyl-2-butanol; nd, not detected. All yields determined by gas chromatography (GC). See supplementary information for full details.
The palladium (Pd)-catalyzed reaction of 2 with 4-iodobiphenyl provided only traces of C–H arylated products under a variety of conditions. However, when a second coordinating group (namely an amide derived from Yu's p-CF3C6F4 aniline; Fig. 2C, 3)20,21,22 was appended to nitrogen, the reaction afforded 4a in modest to excellent yield. Notably, no products derived from C–H functionalization of the methyl groups of the fluoroamide directing group were observed in this reaction. This is in marked contrast to other reported applications of this directing group, where C–H functionalization at β-methyl sites is strongly favored21,22. This highlights the complementarity of our approach of leveraging bidentate coordination of an innate sp3-hybridized nitrogen of an alicyclic amine substrate along with the fluoroamide to achieve selectivity (i.e., transannular 2° Csp3–H functionalization).
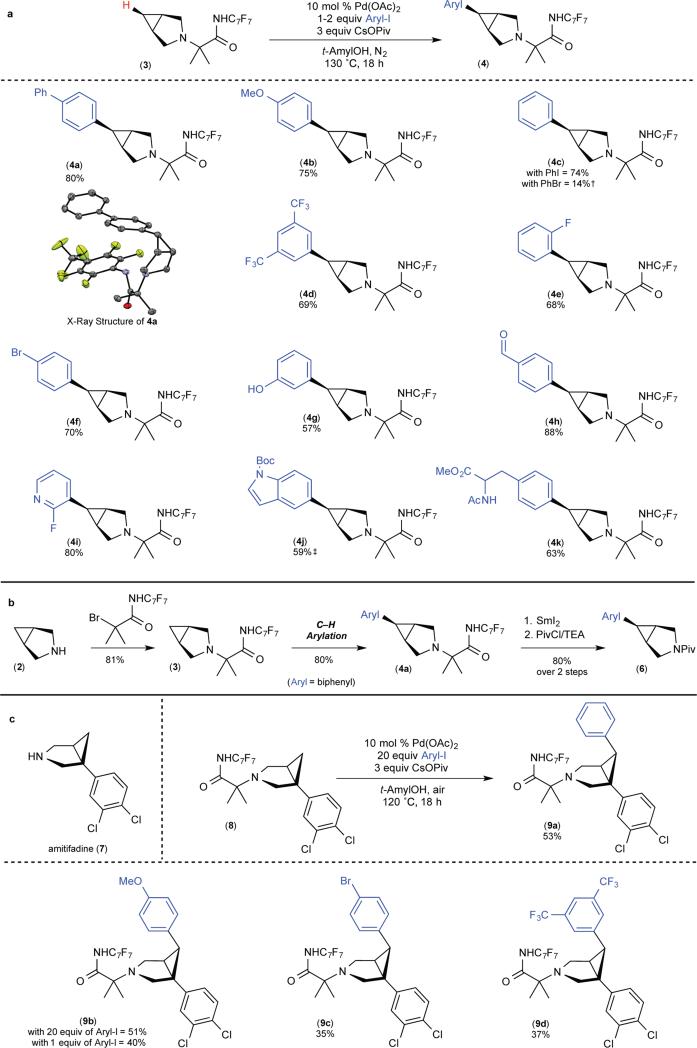
The use of 10 mol % of Pd(OAc)2 (Ac = acetate) and 1 equiv of AgOAc (an additive commonly used to promote C–H arylation)23 provided 17% of 4a (Fig. 2C, entry 1). The modest yield of 4a under these conditions is due to competing formation of aminal 5, which is believed to arise from α-oxidation of 3 to the corresponding iminium ion followed by intramolecular trapping with the amide nitrogen. Notably, the Ag additive mediates this transformation, and aminal 5 was obtained in 41% yield in the absence of Pd (entry 4). The role of the Ag carboxylate salt in these transformations is to regenerate the Pd carboxylate catalyst by abstraction of iodide from the Pd center24,25. As such, we hypothesized that the Ag salt could be replaced by a non-oxidizing metal carboxylate. A survey of alkali metal pivalate salts revealed that CsOPiv (Piv = pivalate) delivers the arylated product 4a while suppressing the formation of aminal 5 (Fig. 2C, entry 8). Under the optimal conditions, 4a was obtained in 92% yield as a single detectable stereoisomer (Fig. 2C, entry 12). X-ray crystallographic characterization of 4a confirmed that the aryl group is installed on the concave face of the azabicycle (Fig. 3A).
Figure 3. Transannular C–H arylation of 3-azabicyclo[3.1.0]hexane core.
a, Scope of C–H arylation with respect to the aryl iodide. b, Relevant steps in overall transformation: installation of directing group, C–H arylation and SmI2-mediated removal of directing group (Aryl = biphenyl). PivCl, pivaloyl chloride; TEA, triethylamine. c, C–H arylation applied to amitifiadine. All yields are reported for pure isolated material. †, Reaction was conducted using 20 equiv of PhBr; yield determined by GC. ‡, Reaction was conducted under modified conditions. See supplementary information for full details.
This transannular C–H arylation reaction proceeds in high yield with aryl iodides bearing electron-donating, electron-neutral, and electron-withdrawing substituents (products 4a-e, Fig. 3A). Many traditionally sensitive functional groups are compatible with this system, including aryl bromides, unprotected phenols, and aromatic aldehydes (products 4f-h). Both electron-deficient and electron-rich nitrogen heterocycles can be installed (products 4i and 4j). Furthermore, a derivative of the amino acid phenylalanine can be coupled to the bicyclo[3.1.0] scaffold (product 4k). Notably, aryl bromides could also be used as the arylating reagent, albeit with reduced efficiency. For example, the use of phenyl bromide resulted in 14% yield of 4c (see Table S8 in the supplementary information for full details).
The directing group can be removed in high yield via reductive cleavage with SmI2. Overall, a 52% overall yield is obtained for the three relevant steps converting 2 to 6 (81% for installation of the directing group, 80% for C–H arylation with 4-iodobiphenyl, and 80% for removal of the directing group; Fig. 3B).
A particularly attractive application of this method is in the late-stage derivatization of bioactive molecules. Selective C–H functionalization reactions on complex molecular scaffolds provide valuable opportunities for streamlining analog generation and thereby accelerating structure activity relationship studies5,6. The bicyclo[3.1.0] scaffold appears in numerous pharmaceutical candidates, including the serotonin-norepinephrine-dopamine reuptake inhibitor amitifadine (7)26,27. As shown in Fig. 3C, appending our directing group to 7 enables transannular C–H arylation to deliver the novel amitifadine derivatives 9a-d (Fig. 3C).
We next sought to expand this reaction from model substrate 3 to piperidine 10 (Fig. 4A). A thermodynamically unfavorable chair-boat isomerization of the piperidine ring in 10 is required prior to C–H activation (Fig. 2A) and is expected to add at least 6 kcal/mol to the activation barrier relative to substrate 328. Under the conditions optimized for 3, the piperidine substrate 10 afforded only 12% yield of the C–H arylation product 11a. However, increasing the temperature and changing the solvent led to a significantly improved 44% yield of 11a (Fig. 4A). Aminals derived from starting material 10 and product 11a were formed as side products of this reaction (see Fig. S2 in the supplementary information for full details), but the reaction mixture could be cleanly converged to a mixture of starting material 10 and product 11a via treatment with NaBH4. Using this work-up procedure, product 11a was isolated in 55% yield (Fig. 4A). Analogous conditions enabled the transannular C–H arylation of a variety of alicyclic amine derivatives, affording products of mono- and/or diarylation (11b-i; Fig. 4a). The structures of 11b-i were established via a combination of NMR spectroscopy and X-ray crystallography.
Figure 4. Transannular C–H arylation of alicyclic amines.
a, Scope of the C–H arylation reaction with respect to the amine. b, Application of this reaction to the derivatization of varenicline. c, Application of this reaction to the derivatization of cytisine. Yields are reported for pure isolated products. †, Reaction was conducted under modified conditions. See supplementary information for full details.
While the yields of 11b-i are moderate in some cases, the de novo synthesis of many of these products would be challenging using traditional synthetic routes. The utility of this transformation is showcased in the late stage C–H arylation of Pfizer's smoking cessation drug varenicline (Chantix®, 12, Fig. 4B). The fluoroamide group was appended to 12 to afford 13 in 81% yield. Under our standard C–H arylation conditions, 13 underwent transannular C–H arylation with a variety of aryl iodides to afford 14a-e. The structure of 14a was assigned by X-ray crystallography (Fig. 4B), which confirms that the aryl group is installed in an axial orientation. This latter point is particularly noteworthy because the synthesis of this stereoisomer would be challenging using other synthetic approaches29. The C–H arylation of 13 with 4-iodo-o-xylene was conducted on scales ranging from 77 mg to 2.5 g of substrate, with nearly identical yields of 14e (43% and 38% isolated yield, respectively). Based on the established synthesis of varenicline, an independent synthesis of these analogues by more traditional methods would require parallel multistep sequences30. In a similar fashion, our method proved effective for the late-stage C–H functionalization of the natural product cytisine (15, a nicotine addiction treatment), converting 16 to 17 in 25% yield. Again, the aryl group is selectively installed at the axial position in this transformation.
In conclusion, this report discloses the transannular C–H arylation of a variety of alicyclic amines. The reaction exhibits high functional group tolerance, and enables the synthesis of novel amino acid derivatives (4k) as well as previously unprecedented analogues of the pharmaceutical candidate amitifadine (9a-d), the top-selling drug varenicline (14a-e), and the natural product cytisine (17). We anticipate that a similar approach will ultimately prove broadly useful for the remote C–H functionalization of diverse cyclic and acyclic secondary amine scaffolds.
Supplementary Material
Acknowledgements
We acknowledge Dr. Jeff W. Kampf for X-ray crystallographic analyses of 4a, 11b, an analog of 11g, 11h and 14a. JTT was supported by an NIH post-doctoral fellowship (F32 GM109479). This work was supported by the NIGMS grant GM073836. Funding from NSF Grant CHE-0840456 for X-ray instrumentation is acknowledged.
Footnotes
Author Contributions J.T., P.C., and N.S. discovered and developed the reaction. J.T., P.C. and M.S. conceived and designed the investigations. M.S. directed and supported the research. J.T., P.C., and M.S. wrote and revised the manuscript. J.T. and P.C. contributed equally to this manuscript and are co-first authors.
Author Information Metrical parameters for the structures are available free of charge from the Cambridge Crystallographic Data Centre under reference numbers CCDC-1401221, 1401222, 1440132, 1416579, and 1416516. The authors declare no competing financial interests.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Wencel-Delord J, Glorius F. C–H bond activation enables the rapid construction and late stage diversification of functional molecules. Nature Chem. 2013;5:369–375. doi: 10.1038/nchem.1607. [DOI] [PubMed] [Google Scholar]
- 2.McMurray L, O'Hara F, Gaunt MJ. Recent developments in natural product synthesis using metal-catalysed C–H bond functionalization. Chem. Sov. Rev. 2011;40:1885–1898. doi: 10.1039/c1cs15013h. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi J, Yamaguchi AD, Itami K. C-H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 2012;51:8960–9009. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]
- 4.Godula K, Sames D. C-H bond functionalization in complex organic synthesis. Science. 2006;312:67–72. doi: 10.1126/science.1114731. [DOI] [PubMed] [Google Scholar]
- 5.Taylor RD, MacCoss M, Lawson ADG. Rings in drugs. J. Med. Chem. 2014;57:5845–5859. doi: 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]
- 6.Asensio G, Gonzalez-Nunez ME, Bernardini CB, Mello R, Adam W. Regioselective oxyfunctionalization of unactivated tertiary and secondary C–H bonds of alkylamines by methyl(trifluomethyl)dioxirane in acid medium. J. Am. Chem. Soc. 1993;115:7250–7253. [Google Scholar]
- 7.Affron DP, Davis OA, Bull JA. Regio- and stereospecific synthesis of C-3 functionalized proline derivatives by palladium catalyzed directed C(sp3)–H arylation. Org. Lett. 2014;16:4956–4959. doi: 10.1021/ol502511g. [DOI] [PubMed] [Google Scholar]
- 8.Lyons TW, Sanford MS. Palladium-catalyzed ligand-directed C−H functionalization reactions. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Engle KM, Wang D, Yu J-Q. Palladium(II)-catalyzed C-H activation/C-C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 2009;48:5094–5115. doi: 10.1002/anie.200806273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell EA, Peschiulli A, Lefevre N, Meerpoel L, Maes BUW. Direct α-functionalization of saturated cyclic amine. Chem. Eur. J. 2012;18:10092–10142. doi: 10.1002/chem.201201539. [DOI] [PubMed] [Google Scholar]
- 11.Pastine SJ, Gribkov DV, Sames D. sp3 C−H bond arylation directed by amidine protecting group: α-arylation of pyrrolidines and piperidines. J. Am. Chem. Soc. 2006;128:14220–14221. doi: 10.1021/ja064481j. [DOI] [PubMed] [Google Scholar]
- 12.He J, Hamann LG, Davies HML, Beckwith REJ. Late-stage C-H functionalization of complex alkaloids and drug molecules via intermolecular rhodium-carbenoid insertion. Nature Commun. 2015;6 doi: 10.1038/ncomms6943. [DOI] [PubMed] [Google Scholar]
- 13.Shi L, Xia W. Photoredox functionalization of C–H bonds adjacent to a nitrogen atom. Chem. Soc. Rev. 2012;41:7687–7697. doi: 10.1039/c2cs35203f. [DOI] [PubMed] [Google Scholar]
- 14.Spangler JE, Kobayashi Y, Verma P, Wang D-H, Yu J-Q. α-Arylation of saturated azacycles and N-methylamines via palladium(II)-catalyzed C(sp3)−H coupling. J. Am. Chem. Soc. 2015;137:18876–18879. doi: 10.1021/jacs.5b06740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNally A, Haffemayer B, Collins BL, Gaunt MJ. Palladium-catalysed C–H activation of aliphatic amines to give strained nitrogen heterocycles. Nature. 2014;510:129–133. doi: 10.1038/nature13389. [DOI] [PubMed] [Google Scholar]
- 16.Lee M, Sanford MS. Platinum-catalyzed terminal-selective C(sp3)–H oxidation of aliphatic amines. J. Am. Chem. Soc. 2015;137:12796–12799. doi: 10.1021/jacs.5b09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bercaw JE, et al. Robotic lepidoptery: structural characterization of (mostly) unexpected palladium complexes obtained from high-throughput catalyst screening. Organometallics. 2009;28:5017–5024. [Google Scholar]
- 18.Cui W, Chen S, Wu J-Q, Zhao X, Hu W, Wang H. Palladium-catalyzed remote C(sp3)–H arylation of 3-pinanamine. Org. Lett. 2014;16:4288–4291. doi: 10.1021/ol502011k. [DOI] [PubMed] [Google Scholar]
- 19.Giri R, Chen X, Yu J-Q. Palladium-catalyzed asymmetric iodination of unactivated C-H bonds under mild conditions. Angew. Chem. Int. Ed. 2005;44:2112–2115. doi: 10.1002/anie.200462884. [DOI] [PubMed] [Google Scholar]
- 20.Rouquet G, Chatani N. Catalytic functionalization of C(sp2)-H and C(sp3)-H bonds by using bidentate directing groups. Angew. Chem. Int. Ed. 2013;52:11726–11743. doi: 10.1002/anie.201301451. [DOI] [PubMed] [Google Scholar]
- 21.Wasa M, et al. Ligand-enabled methylene C(sp3)–H bond activation with a Pd(II) catalyst. J. Am. Chem. Soc. 2012;134:18570–18572. doi: 10.1021/ja309325e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He J, et al. Ligand-controlled C(sp3)–H arylation and olefination in synthesis of unnatural chiral α–amino acids. Science. 2014;343:1216–1220. doi: 10.1126/science.1249198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaitsev VG, Shabashov D, Daugulis O. Highly regioselective arylation of sp3 C−H bonds catalyzed by palladium acetate. J. Am. Chem. Soc. 2005;127:13154–13155. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]
- 24.Nadres ET, Santos GIF, Shabashov S, Daugulis O. Scope and limitations of auxiliary-assisted, palladium-catalyzed arylation and alkylation of sp2 and sp3 C–H Bonds. J. Org. Chem. 2013;78:9689–9714. doi: 10.1021/jo4013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lafrance M, Fagnou K. Palladium-catalyzed benzene arylation: incorporation of catalytic pivalic acid as a proton shuttle and a key element in catalyst design. J. Am. Chem. Soc. 2006;128:16496–16497. doi: 10.1021/ja067144j. [DOI] [PubMed] [Google Scholar]
- 26.Beer B, et al. DOV 216,303, a “Triple” reuptake inhibitor: safety, tolerability, and pharmacokinetic profile. J. Clin. Pharmacol. 2004;44:1360–1367. doi: 10.1177/0091270004269560. [DOI] [PubMed] [Google Scholar]
- 27.Epestein JW, et al. 1-Aryl-3-azabicyclo[3.1.0]hexanes, a new series of nonnarcotic analgesic agents. J. Med. Chem. 1981;24:481–490. doi: 10.1021/jm00137a002. [DOI] [PubMed] [Google Scholar]
- 28.Juaristi E. Conformational Behavior of Six-Membered Rings. VCH; New York: 1995. [Google Scholar]
- 29.Hirsch JA. Table of conformational energies – 1967. Topics in Stereochemistry. 1967;1:199–222. [Google Scholar]
- 30.Singer RA, McKinley JD, Barbe G, Farlow RA. Preparation of 1,5-methano-2,3,4,5- tetrahydro-1H-3-benzazepine via Pd-catalyzed cyclization. Org. Lett. 2004;6:2357–2360. doi: 10.1021/ol049316s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.