Abstract
Click chemistry provides fast, convenient, versatile and reliable chemical reactions that take place between pairs of functional groups of small molecules that can be purified without chromatographic methods. Due to the fast kinetics and low or no elimination of byproducts, click chemistry is a promising approach that is rapidly gaining acceptance in drug discovery, radiochemistry, bioconjugation, and nanoscience applications. Increasing use of click chemistry in synthetic procedures or as a bioconjugation technique in diagnostic imaging is occurring because click reactions are fast, provide a quantitative yield, and produce minimal amount of nontoxic byproducts. This review summarizes the recent application of click chemistry in magnetic resonance imaging and discusses the directions for applying novel click reactions and strategies for further improving MRI performance.
Keywords: click chemistry, magnetic resonance imaging (MRI), paramagnetic chemical shift saturation transfer (ParaCEST), Copper-catalyzed azide-alkyne cycloaddition (CuAAC)
Introduction
Magnetic resonance imaging (MRI) is a powerful non-invasive and non-destructive diagnostic imaging technique. The primary use of small molecular chemistry in magnetic resonance imaging is to develop and synthesize contrast agents to enhance the diagnostic power of imaging. The important advantages of MRI for diagnostic imaging are high spatial resolution and improved contrast in soft tissues. Using advanced image acquisition, reconstruction techniques and appropriate contrast media, MRI can provide well-resolved images of multiple organs and lesions in clinic. The physical chemistry, chemical structure or nanostructure of contrast agents plays a vital role in developing highly specific MRI agents. Therefore, the design and development of novel contrast agents and MRI probes is an important and rapidly progressing research area.
Click Chemistry
Incorporation of novel synthetic methodologies in designing contrast agents has become a promising tool box for MRI applications in diagnostics and drug development. Among them, click chemistry plays a fundamental role because of the unique features of this synthetic method. The term “click chemistry” was first introduced by Barry Sharpless in 2001 for rapid, stereospecific and simple reactions with quantitative yield, wide scope of applicability, and elimination of byproducts that can be simply removed without advanced purification methods, such as chromatography.1,2 The click reactions are hemoselective, hence, no protecting group is required before using conjugation of functional moieties on the target nanoconstruct. The click chemistry concept has received acceptance in pharmaceutical, material and other industries because of time saving in the development of molecular libraries and screening.3–5 Molecular and cellular imaging, which is an important branch in diagnosis, drug discovery and development has also benefited significantly from this chemical technology. Specifically, fast, quantitative yielding, and pure conjugation chemistries are required for design and synthesis of molecular or nano-scale imaging probes. Fulfilling these needs, click chemistry has become a popular synthetic and conjugation tool in the design and development of novel contrast agents.
Click chemistry in synthesis of MRI contrast agents
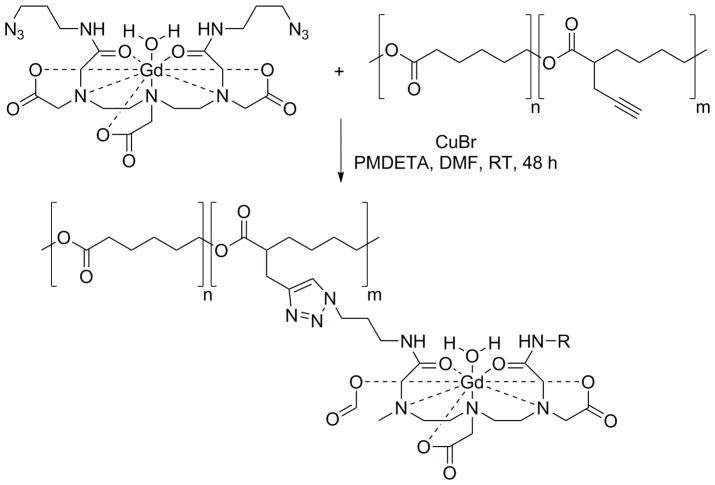
A review of recent literature demonstrates an increased use of click chemistry for development of contrast agents because of the favorable characteristics of click reactions, or characteristics of the linker generated by the click reaction, or both. Novel biocompatible and bioorthogonal variants of click chemistry have been recently developed.6 However, traditional, copper-catalyzed azide-alkyne cycloaddition (CuAAC) is still the most commonly used click reaction in the synthesis of contrast agents for MRI due to the limited commercial availability and high cost of other recently developed precursors and components for advanced click chemistry. CuAAC components can be synthesized in bulk for semi-commercial production. Habnouni et al. have synthesized azide-containing Gd-DTPA contrast agent and propargyl-functionalized poly(ε-caprolactone) (PCL) carrier and synthesized MRI-PCLs that self-assembles into micellar structures via Cu(I) [3+2] cycloaddition reaction.7 This preparation can be characterized as macromolecular contrast agents (MMCAs). They have optimized the ligation of contrast agents on the polymer via CuAAC chemistry (Scheme 1) with high quantitative yield. Usually this type of click reaction requires elevated temperatures to be completed in less than 12 h; however, 48 h is required to complete the reaction at room temperature. The T1 relaxations of MRI-PCLs were determined using a film of constructs embedded in agarose.
Scheme 1.
Synthesis of MRI-PCLs by Habnouni et al.7 MRI-PCLs were obtained by a Cu(I)-catalyzed [3+2] cycloaddition between azido-Gd complex and propargylated PCLs in DMF at room temperature in the presence of CuBr and N,N,N′,N″,N‴-pentamethyldiethylenetriamine (PMDETA).
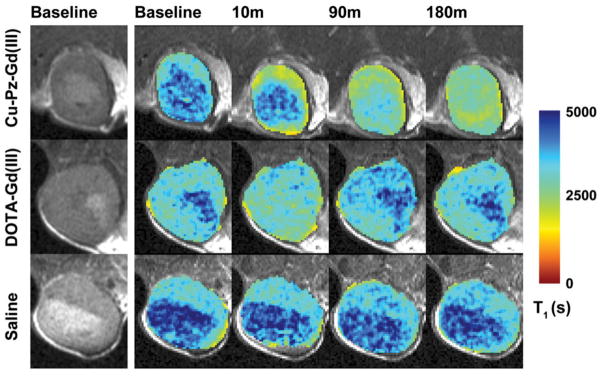
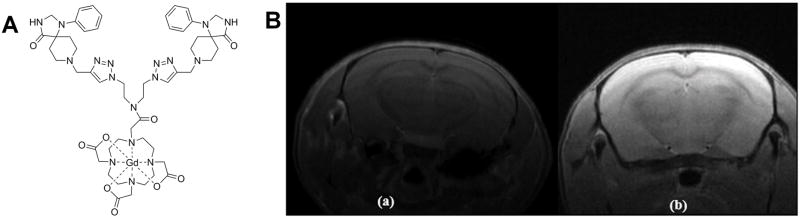
Hoffman’s group has used click chemistry to design and synthesize similar contrast agents based on porphyrazine (Pz) and named them Zn-Pz-Gd(III) and Cu-Pz-Gd(III). This click reaction takes place between azide-functionalized Pz and alkyne-functionalized Gd(III)-DOTA analog.8 The T1 relaxivity of these complexes was significantly higher than for mono-Gd contrast agents used in clinic. Among them Cu-Pz-Gd(III) exhibited promising characteristics as an MRI contrast agent (Figure 1) in MDA-MB-231 orthotopic breast tumor model in athymic nude mice after injection of 150 μL (450 nmol) of imaging agent via the tail vein.
Figure 1.
Selected images from the 3.5 h time course adapted from Trivedi et al.8 The leftmost column shows raw T2-weighted images of the center slice through the tumor. The next four columns show the T1 map of the tumor overlaid on the corresponding T2-weighted image at baseline and at 10, 90 and 180 min after administration of imaging agent. Note that the T1 becomes progressively shorter (indicating Gd(III) accumulation) in the Cu–Pz–Gd(III) treated animal, while it dips transiently but returns quickly to baseline in the DOTA–Gd(III)-treated animal, and remains constant in the saline-treated animal.
Zhang et al. have reported a synthetic route for the preparation of a new ligand system for the clelation with Gd(III). In the synthetic route, they conjugated N2-(hex-5-yne)-diethylenetriamine-tetra-t-butylacetate (DTTA-der) with mPEG-g-PAA-N3 by click cycloaddition.9 The resulted mPEG-g-PAA-DTTA was complexed with Gd(III) after incubation with GdCl3. These macromolecules formed nanomicelles with a molecular size ~30 nm suggesting a potential of passive tumor targeting via enhanced permeability and retention (EPR) effect.10 Compared to Gd-DTPA, these mPEeg-g-PAA-(DTTA-Gd) nanomicellles exhibited higher relaxivity, enhanced T1-weighted signal intensity, and low cytotoxicity.
Use of nanoscale platforms as specific imaging agents is an attractive approach, as their high loading capacity for contrast agents can help to circumvent the intrinsically low sensitivity of MRI. Lu and co-workers have used CuAAC click chemistry for the assembling of peptide-targeted nanoglobular contrast agents.11 Click chemistry was applied to conjugate cyclic decapeptide CGLIIQKNEC (CLT1) to Gd-DOTA loaded nanoglobules made by lysine dendrimers with cubic silsesquioxane core. Due to the high loading of Gd on nanoglobules, a chemically mild, convenient, selective and quantitative conjugation method was needed for decorating of target-specific CLT1. Hence, Lu and co-workers have first functionalized dendrimeric nanoglobules with short-pegylated azide groups before loading Gd complexes. The functionalized dendrimers were reacted with CLT1 functionalized with alkyne groups via CuAAC in TFA/water using CuSO4·H2O and sodium ascorbate from 60 °C to room temperature over 8 h. MRI contrast enhancement and relaxivity of mPEG-g-PAA-(DTTA-Gd) was studied in vitro in human nasopharyngeal cells. T1-weighted MR images showed that the new probe provides better MRI contrast at lower concentrations than Gd-DTPA. Due to the high mass of construct and high Gd loading, significantly reduced concentrations of mPEG-g-PAA-(DTTA-Gd) were required to achieve MRI contrast comparable to clinical applications.
T1 relaxivity can also be increased by reducing the molecular tumbling (increasing the correlation time) of the contrast agent by attaching low molecular contrast agent groups to a massive macromolecular carrier (Grogna et al.)12. To this end, a macromolecular platform was developed by a reversible addition fragmentation chain transfer (RAFT) polymerization followed by the esterification with 4-pentynoic acid and conjugation with Gd-DOTA by CuAAC using CuI as a catalyst and stirring at room temperature overnight. Based on the relaxometry measurements, novel macrocontrast agents demonstrated 250% increase in T1 relaxivity per Gd, compared to free Gd-DOTA. The relaxivity can be further enhanced using a shorter linker connecting Gd-DOTA complexes with the macromolecular platform.13
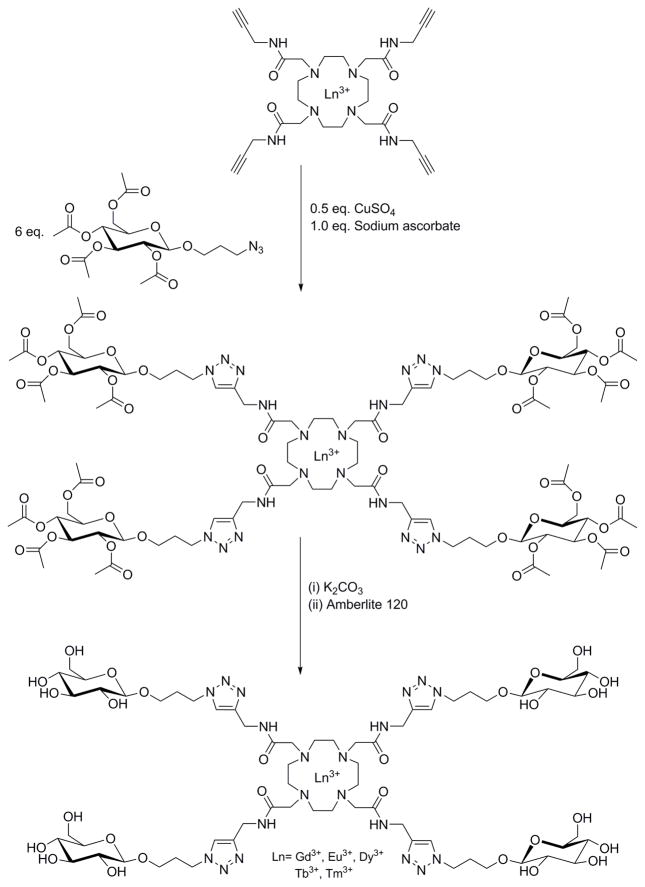
CuAAC click chemistry has been used for tetra-functionalization of DOTA-based lanthanide contrast agents with glucosides for the paramagnetic chemical exchange saturation transfer (ParaCEST) MR imaging (Scheme 2).14 Similar to this study, Suchy et al. described a development of a synthetic methodology for Eu3+ complexes and its analogs utilizing the CuAAC and used them for ParaCEST MRI.15 This study has extensively described the use of click chemistry and synthetic handling of intermediates and chemistry of their azido and alkyne derivatives in these syntheses.15
Scheme 2.
Synthesis of a tetraglucoside functionalized DOTAM via the acetyl-protected intermediate by Huisgen copper-catalyzed alkyne-azide cycloaddition (CuAAC). Adapted from Milne et al.14
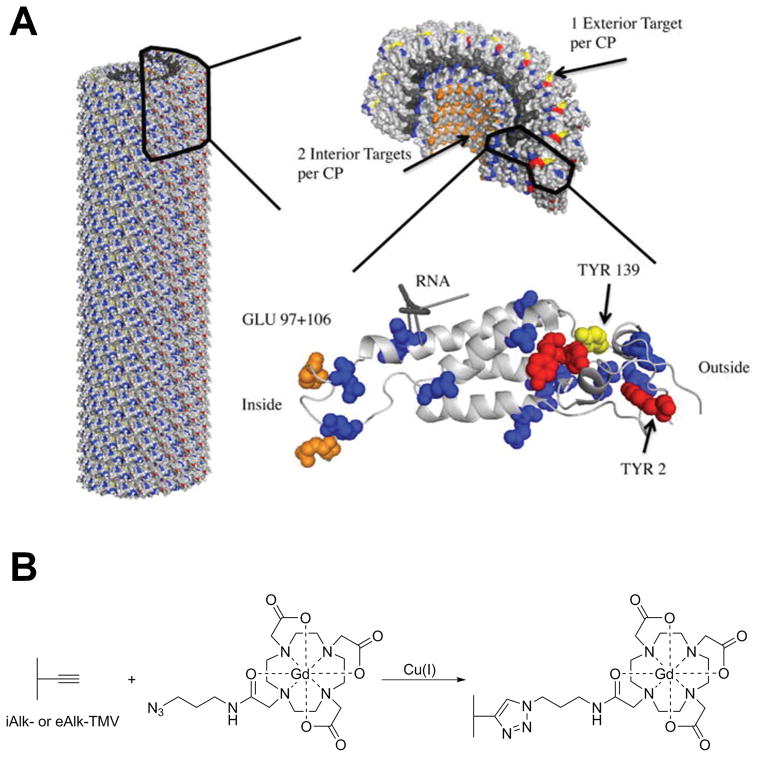
Bruckman et al., also used reduced tumbling rates of Gd-DOTA complexes conjugated to supramolecular carriers by covalent linkers to enhance ionic T1 relaxivity in MRI.16 They have proven this phenomenon by conjugating azido-Gd-DOTA with a plant viral particle, tobacco mosaic virus (TMV). This study formed the foundation of using TMV based-nanoconstructs as contrast agents for MRI (Figure 2). A similar study has been performed by Douglas and coworkers, who synthesized a branched oligomer conjugated to multiple Gd-DTPA complexes encapsulated in P22 “wiffleball” viral capsids.17 This structure has produced particles with the highest relaxivities among viral-capsid based systems, and has significantly improved in ionic relaxivity compared to free Gd-DTPA. For improved encapsulation of Gd-DTPA complexes inside viral particles they have used flexible trialkyne molecules conjugated with the viral particle. CuAAC conjugation resulted in the formation of a rigid linker with Gd(III)-DTPA, which results in increased contrast.
Figure 2.
Tobacco mosaic virus rods and spheres as supramolecular high-relaxivity MRI contrast agents (A). A PyMol image of tobacco mosaic virus highlighting the interior glutamic acids, GLU97 and GLU106 (orange), exterior tyrosine, TYR139 (yellow), for bioconjugation. Additional glutamic and aspartic acid residues (blue) and tyrosine residues (red) are highlighted for reference. (B). Schematic illustration of the CuAAC reaction to label TMV particles with Gd-DOTA. Adapted from Bruckman et al.13
Wu et al. used click chemistry conjugation of multivalent manganese complexes with amphiphilic dextran micelles to develop an imaging probe that has much higher T1 relaxivity (13.3 Mn mmol−1 s−1) than free Mn(II) complexes (4.8 Mn mmol−1 s−1).18 After the click reaction conjugation, a rigid triazole linker is formed connecting the rigid six-member ring of glucose with Mn(II) complexes. Such linkers are important to hinder the local rotation of Mn(II) complexes. The amphiphilic dextran micelles are a stationary platform that has a longer rotational correlation time (τR′). Based on the Bloembergen-Solomon-Morgan theory, the T1 relaxation rate at typically used magnetic fields, can be enhanced by increasing τR′, which is depending on the structure of the composites and the linker. Combination of multiple contrast generating Mn(ii) complexes with a prolong τR′ significantly enhanced relaxivity of the complexes.
Wu and co-workers have suggested that the improved T1 relaxivity of the paramagnetic nano-composites is due to the rigidity of nano-micelle surface and the triazole linker used to conjugate Mn(II) complex.18 In addition, the triazole linker formed by the azide-alkyne cycloaddition has been found strong, stable, and biocompatible for designing molecular probes.
Germeroth et al. utilized the advantage of rigid triazole linkers for designing and synthesis of their biotinidase-resistant biotin-Gd-DOTA contrast agents.19 They have synthesized contrast agents conjugated with biotin via a triazole linkers generated by azide-alkyne cycloaddition. These rigid conjugates are excellent isosteres for similar biotin-amide contrast agents.
To improve sensitivity of detection to practically acceptable levels, specific probes for molecular MRI of protein expression should provide high-load of Gd(III) per single protein binding event. To increase the Gd(III) payload Vistain and co-workers have used gold nanoparticles and linked them to 24 polydeoxythymidine (dT) oligonucleotides per particle and conjugated up to six Gd-DOTA complexes per oligonucleotide by CuAAC click chemistry.20 The stability of the Gd(III) complexes with triazole moieties has been studied by Mishra and co-workers.21 They have synthesized a ligand, DO3A-bis(triazole-triazaspirodecanone), complexed it with Gd(III) (Figure 3) and determined the protonation and dissociation constants with endogenous cations Cu2+ and Zn2+. This study was important because the DO3A ligand changes the chelation from heptadentate to octadentate when the pH of the media is changing from acidic to basic. Mishra and co-workers have found three protonation constants corresponding to three carboxylic groups in the complex. Importantly, the whole structure with triazole moieties was stable in the entire range of pH (pH 2-12) same as for Gd-DOTA and Gd-DTPA complexes without transmetallation with endogeneous Cu2+ and Zn2+.
Figure 3.
Triazole linked Gd(III)-DO3A-BT-bistriazaspirodecanone as a potential MRI contrast agent. (A). Structure of Gd(III)-DO3A-BT-bistriazaspirodecanone. (B). T1-weighted MR images in Balb/c mice with (a) Dotarem and (b) Gd–DO3A-bis(triazole-triazaspirodecanone) (0.1 mL, 1.25 mM). Adapted from Varshney et al.21
Goswami et al. have demonstrated that the effectiveness of Gd-based contrast agents in MRI can be enhanced by increasing the steric constraint of the Gd centers and lowering the water exchange rates.22 Hence, they have developed an octahedral closo-B122− that can support 12 Gd(III) complexes. Due to the sterically constraint structure and requirement of a rigid linker, Gd(III) complexes are conjugated via azide-alkyne click chemistry. In in vivo experiments in severe combined immunodeficient (SCID) mice, bearing human PC-3 prostate cancer xenografts, these closomer complexes showed strongly enhanced MR signals at 30 min and 1 h post-injection, compared to Omniscan, which was rapidly cleared and disappeared after 1 h. Wang and co-workers have developed a small molecular Gd complex, Gd(DODAS) which is similar to other Gd-based contrast agents used for MR imaging of myelination.23 This complex is water soluble and does not permeate across the blood-brain barrier (BBB). MR imaging in Sprague-Dawley rats with stereotactic injection of Gd(DODAS) showed a dramatic shortening of T1 which was localized in brain areas corresponded to the corpus callosum.
Design and synthesis of small molecular multimodal imaging probes are challenging due to the complexity of the synthetic procedure. For in vivo multimodality imaging applications, contrast agents should combine high sensitivity and specificity with high spatial and temporal resolutions. Meade and co-workers have developed an MR and optical imaging probe with Gd(III) chelates and IR-783 dye that can be used for collecting both molecular and anatomical information in vivo.24 As they have described in Vistain et al. the synthesis of this macromolecule included copper catalyzed azide-alkyne cycloaddition and produced a rigid triazole linker connecting Gd(III) complexes. The novel probe was used for MR imaging of a xenograft MCF7 breast tumor model in athymic nude mice. Images have shown a significant enhancement of the bladder but not the tumor. On the other hand, optical imaging confirmed the accumulation of the contrast agent in the tumor, raising a question about the MR sensitivity of the complex. To improve MRI sensitivity, this group has also developed an optical/MR imaging multimodal and multimeric contrast agent with three Gd(III) groups per fluorophore utilizing the CuAAC click chemistry.25 This hydrophilic, cell permeable agent has high relaxivity at both low (1.4T) and high (7T) and provides excellent cell labeling capabilities and image contrast at 7T.
Copper-free azide-alkyne click reaction
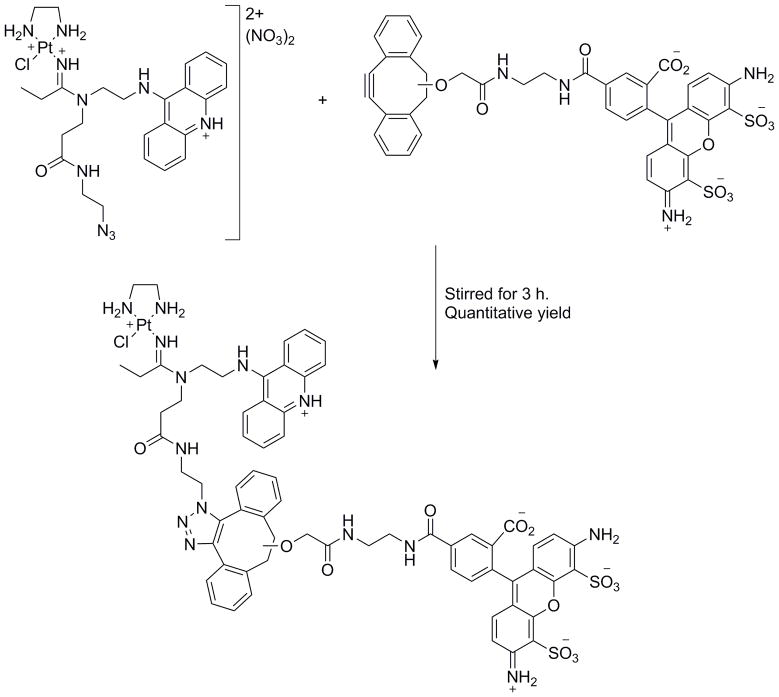
The CuAAC click chemistry has been used widely for the preparation of contrast agents and nanostructures on the bench; however, it cannot be used in vitro or in vivo due to the Cu toxicity and the requirement of elevated temperature. Nwe and Brechbiel have described the importance of the complete removal of catalytic copper traces and use of alternative click chemistry methodologies, especially for in vivo application.26 To avoid these toxicological issues, Bertozzi and co-workers (Scheme 3) and Bierbach and co-workers (Figure 4) developed strain-promoted copper-free azide-alkyne click reactions and specifically used this method for live cell fluorescence imaging. 27,28
Scheme 3.
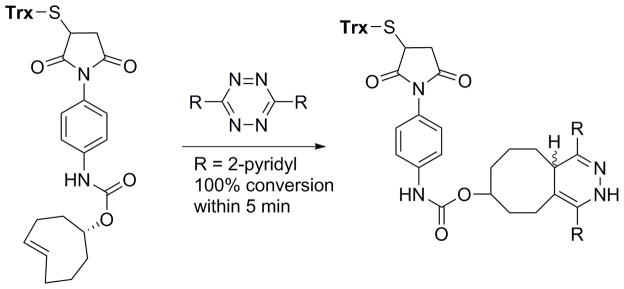
Structures and copper-free click reaction of the probe used in Qiao et al. study.28
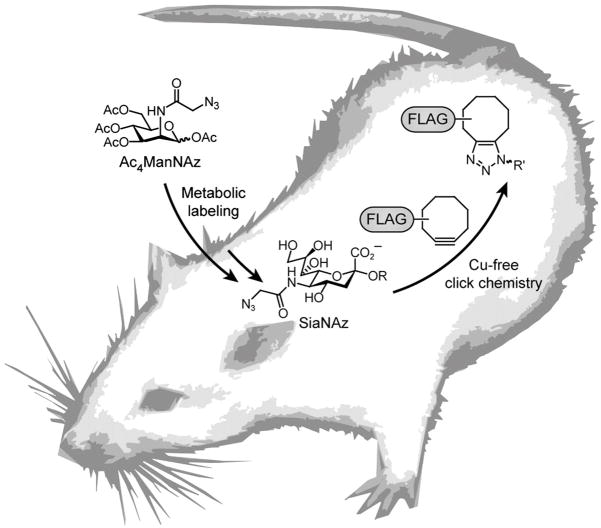
Figure 4.
Cu-free click chemistry in mice as described in Chang et al.27 Mice were injected with Ac4ManNAz daily for 1 wk to allow for metabolic labeling of glycans with SiaNAz. The mice were then injected with a cyclooctyne-FLAG conjugate for in vivo covalent labeling of azido glycans.
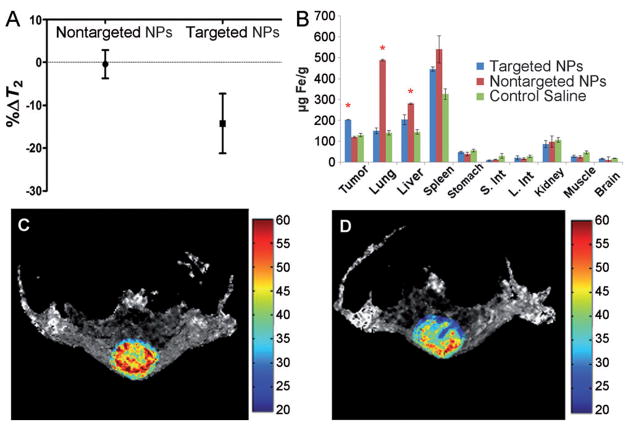
Long and co-workers utilized advantage of these reactions in their syntheses of CXCR4-targeted and matrix metalloproteinase (MMP)-responsive iron oxide nanoparticles (IONPs) for enhanced MRI.29 In their study, two types of IONPs-based, CXCR4 receptor targeted nanoparticles were conjugated with either azide or alkyne functional groups. Both IONPs were conjugated with protease-specific peptide sequence, which was cleavable by MMP enzymes in MMP2/6 overexpressing tumors. A mixture of two IONPs was injected intratumorally into subcutaneous U87.CD4.CXCR4 brain cell xenografts in BALB/c nude mice. Upon cleavage of the protease-specific peptide groups, azide and alkyne functional groups were exposed leading to the strain-promoted [3+2] cycloaddition under copper free condition and self-assembly of IONP clusters at the tumor site. After self-assembling the T2 relaxivity of the aggregates was significantly increased, enhancing the contrast in T2-weighted MR images (Figure 5).
Figure 5.
CXCR4-targeted and MMP-responsive iron oxide nanoparticles for enhanced MRI. (A) Results of the T2 maps analysis from a series of spin echo images acquired with different TEs. Only the region of interest comprising the tumor was considered for the analyses. (B) Iron concentrations in the different organs measured 48 h after the injection of targeted NPs. The results are expressed as the mean of three independent experiments ± the standard deviation. * Statistically different with p<0.05, S. Int - small intestine, L. Int - large intestine. Representative tumor T2 maps are shown from a series of spin echo images acquired with different TEs before (C) and 4 h after (D) the intravenous injection of targeted NPs. The color bar scale is in milliseconds. Adapted from Gallo et al.29
Trans-cyclooctene and tetrazine click chemistry
With increasing interest in click chemistry for in vitro and in vivo studies, Fox and co-workers have introduced a trans-cyclooctene - tetrazine ligation, which can be used in living systems without any catalysts.30 This ligation is approximately >10,000 times faster, than the known CuAAC or azide-alkyne copper-free click chemistries (Scheme 4).
Scheme 4.
Bioorthogonal reaction that proceeds with unusually fast reaction rates without need for catalysis: the cycloaddition of s-tetrazine and trans-cyclooctene derivatives. The reactions tolerate a broad range of functionality and proceed in high yield in organic solvents, water, cell media, or cell lysate. The rate of the ligation between trans-cyclooctene and 3,6-di-(2-pyridyl)-s-tetrazine is very rapid (k2 2000 M−1 s−1). This fast reactivity enables protein modification at low concentration. Adapted from Blackman et al.30
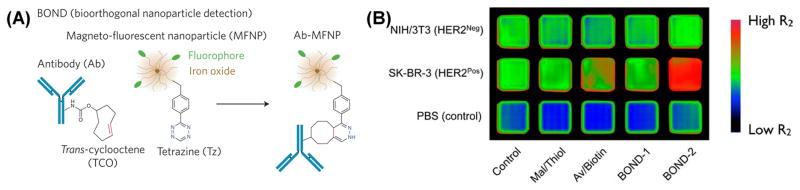
However, Fox and co-workers have not used this chemistry for MR imaging. The Weissleder group reported the use of trans-cyclooctene - tetrazine click chemistry in a prelabeling strategy that amplifies nanoparticle binding on the targeted cell surface enhancing the sensitivity of cell detection in MRI.31 In this strategy, pretargeting antibodies and IONPs were functionalized with trans-cyclooctene (TCO) and tetrazine (Tt), respectively (Figure 6A). Cancer cells were first prelabeled with TCO conjugated anti-HER2 antibodies. Then the immunolabelled cells were treated with tetrazine-IONPs. Two-components underwent catalyst-free click reactions and accumulated in the targeted cell, allowing magnetic detection using a preclinical MRI system (Figure 6B).
Figure 6.
Bioorthogonal chemistry amplifies nanoparticle binding and enhances the sensitivity of cell detection. (A) Schematic showing the conjugation chemistry between antibody and nanoparticle. (B) MRI of the different nanoparticle targeting strategies in cells. Cancer (SK-BR-3 breast cancer) and normal (NIH/3T3 fibroblast) cells were labeled using the different targeting strategies and imaged by a magnetic resonance scanner (4.7 T; Bruker Pharmascan). Consistent with fluorescence and diagnostic magnetic resonance measurements, BOND-2 yielded the most pronounced T2 changes due to higher nanoparticle binding. Prior to imaging, labeled cells (106) were lysed and embedded in agar. Adapted from Haun et al.31
Future Directions
Click chemistry is a rapidly growing field and search for faster, quantitative and chemoselective click reactions for diagnostic imaging is still continuing. While copper-free click chemistry can be used for in situ ligation in living systems under physiological conditions, these experiments are still challenging. As described in the previous section, Weissleder and co-workers have successfully applied a click reaction-based prelabeling strategy for MRI in cells in vitro. Generally, targeting strategies, which based on IONPs, are challenging to apply in vivo due to the biological barriers for the delivery of nanomaterials and potential toxicity issue. To reduce the molecular size and to improve in vivo delivery, a strategy to replace IONP with Gd is required. Potential toxicity issues of Gd in kidney and more recently brain and bones have to be carefully examined.32,33 To implement this concept, the following important prerequisites should be considered: a) choosing of rigid and biocompatible carrier structure to enhance relaxivity, b) increasing pay-load of the contrast agent per delivery component without interfering specific affinity and/or click reactions, and c) signal amplification mechanism by accumulation of the contrast agent at the target site. To this end, a promising development has been reported by Artemov and co-workers introducing two-component, two step pretargeting delivery driven by in situ click chemistry.
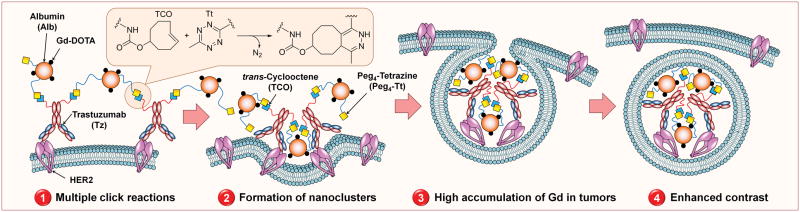
As described in Hapuarachchige et al. in 2014, a therapeutic monoclonal antibody (mAb), trastuzumab, functionalized with azide groups was used to prelabeled HER2(+) cancer cells followed by the delivery of drug-loaded albumin functionalized with dibenzylcyclooctyne (DIBO).34 Two components undergo multiple click reactions and crosslink on the targeted cell surface making nanoclusters. These naoclusters are readily internalized and delivered drugs into the cytoplasm. In Hapuarachchige et al. in 2016, the same strategy was applied in vivo in HER2(+) BT-474 human breast cancer mouse models using trans-cyclooctene and tetrazine click chemistry.35 In both cases, the delivery components were cross-linked on the target cell surface, which significantly enhanced internalization. This system can be applied for the delivery of MRI contrast agents (Figure 7) since, a) albumin is a biocompatible rigid platform to load Gd-based contrast agents with appropriate pharmacokinetics and delivery profile in vivo36–39, b) high loading capacity of albumin allows for conjugation of multiple Gd groups to increase sensitivity; and c) in situ generation of nanoclusters enhanced the internalization of target-specific nanoclusters at the tumor site efficiently amplifies the signal and increases MRI contrast.40
Figure 7.
Envision of using two-component, two-step pretargeting delivery system in MR imaging of tumors with enhanced contrast driven by in situ click chemistry.
In summary, novel click chemistry technology has already found widespread use for synthesis of complex multifunctional MRI probes by rapid, clean, and mild reactions with good yield and purity of the product. In addition, the recently developed copper-free click ligation can find increased application for in vivo MRI by assembling macromolecular complexes with significantly increased MR relaxivity in situ, while preserving good pharmacokinetics and delivery properties of substrates with relatively small molecular size.
Footnotes
The authors have no potential conflicts of interest and no sources of funding to disclose.
References
- 1.Kolb HC, Finn MG, Sharpless KB. Click chemistry: Diverse chemical function from a few good reactions. Angew Chem Int Edit. 2001;40:2004-+. doi: 10.1002/1521-3773(20010601)40:11<2004::Aid-Anie2004>3.0.Co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Moses JE, Moorhouse AD. The growing applications of click chemistry. Chem Soc Rev. 2007;36:1249–1262. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]
- 3.Hein CD, Liu XM, Wang D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharm Res-Dordr. 2008;25:2216–2230. doi: 10.1007/s11095-008-9616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thirumurugan P, Matosiuk D, Jozwiak K. Click Chemistry for Drug Development and Diverse Chemical-Biology Applications. Chem Rev. 2013;113:4905–4979. doi: 10.1021/cr200409f. [DOI] [PubMed] [Google Scholar]
- 5.Kantheti S, Narayan R, Raju KVSN. Click Chemistry Engineered Hyperbranched Polyurethane-Urea for Functional Coating Applications. Ind Eng Chem Res. 2014;53:8357–8365. doi: 10.1021/ie500627x. [DOI] [Google Scholar]
- 6.Yang M, et al. Biocompatible click chemistry enabled compartment-specific pH measurement inside E. coli. Nat Commun. 2014;5:4981. doi: 10.1038/ncomms5981. ncomms5981 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Habnouni S, et al. MRI-Visible Poly(epsilon-caprolactone) with Controlled Contrast Agent Ratios for Enhanced Visualization in Temporary Imaging Applications. Biomacromolecules. 2013;14:3626–3634. doi: 10.1021/bm400978a. [DOI] [PubMed] [Google Scholar]
- 8.Trivedi ER, et al. Synthesis and characterization of a porphyrazine-Gd(III) MRI contrast agent and in vivo imaging of a breast cancer xenograft model. Contrast Media Mol Imaging. 2014;9:313–322. doi: 10.1002/cmmi.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang WL, et al. Fabrication of Polymer-Gadolinium (III) Complex Nanomicelle from Poly(ethylene glycol)-Polysuccinimide Conjugate and Diethylenetriaminetetraacetic Acid-Gadolinium as Magnetic Resonance Imaging Contrast Agents. J Appl Polym Sci. 2011;120:2596–2605. doi: 10.1002/app.33464. [DOI] [Google Scholar]
- 10.Hubbell JA, Langer R. Translating materials design to the clinic. Nat Mater. 2013;12:963–966. doi: 10.1038/nmat3788. nmat3788 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Tan MQ, Wu XM, Jeong EK, Chen QJ, Lu ZR. Peptide-Targeted Nanoglobular Gd-DOTA Monoamide Conjugates for Magnetic Resonance Cancer Molecular Imaging. Biomacromolecules. 2010;11:754–761. doi: 10.1021/bm901352v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grogna M, et al. Stealth macromolecular platforms for the design of MRI blood pool contrast agents. Polym Chem-Uk. 2011;2:2316–2327. doi: 10.1039/c1py00198a. [DOI] [Google Scholar]
- 13.Bruckman MA, Yu X, Steinmetz NF. Engineering Gd-loaded nanoparticles to enhance MRI sensitivity via T(1) shortening. Nanotechnology. 2013;24:462001. doi: 10.1088/0957-4484/24/46/462001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milne M, Chicas K, Li A, Bartha R, Hudson RH. ParaCEST MRI contrast agents capable of derivatization via “click” chemistry. Org Biomol Chem. 2012;10:287–292. doi: 10.1039/c1ob06162c. [DOI] [PubMed] [Google Scholar]
- 15.Suchy M, et al. Mono- and Tetraalkyne Modified Ligands and Their Eu3+ Complexes - Utilizing “Click” Chemistry to Expand the Scope of Conjugation Chemistry. Eur J Org Chem. 2011:6532–6543. doi: 10.1002/ejoc.201100945. [DOI] [Google Scholar]
- 16.Bruckman MA, et al. Tobacco mosaic virus rods and spheres as supramolecular high-relaxivity MRI contrast agents. J Mater Chem B Mater Biol Med. 2013;1:1482–1490. doi: 10.1039/C3TB00461A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qazi S, et al. P22 Viral Capsids as Nanocomposite High-Relaxivity MRI Contrast Agents. Mol Pharmaceut. 2013;10:11–17. doi: 10.1021/mp300208g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu CQ, et al. Multivalent manganese complex decorated amphiphilic dextran micelles as sensitive MRI probes. J Mater Chem B. 2015;3:1470–1473. doi: 10.1039/c4tb02036g. [DOI] [PubMed] [Google Scholar]
- 19.Germeroth AI, et al. Triazole biotin: a tight-binding biotinidase-resistant conjugate. Org Biomol Chem. 2013;11:7700–7704. doi: 10.1039/c3ob41837e. [DOI] [PubMed] [Google Scholar]
- 20.Vistain LF, Rotz MW, Rathore R, Preslar AT, Meade TJ. Targeted delivery of gold nanoparticle contrast agents for reporting gene detection by magnetic resonance imaging. Chem Commun (Camb) 2016;52:160–163. doi: 10.1039/c5cc06565h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varshney R, Sethi SK, Rangaswamy S, Tiwari AK, Milton MD, Kumaran S, Mishra AK. Design, synthesis and relaxation studies of triazole linked gadolinium(III)–DO3A-BT-bistriazaspirodecanone as a potential MRI contrast agent. New Journal of Chemistry. 2016 doi: 10.1039/C5NJ03220B. [DOI] [Google Scholar]
- 22.Goswami LN, et al. Discrete Nanomolecular Polyhedral Borane Scaffold Supporting Multiple Gadolinium(III) Complexes as a High Performance MRI Contrast Agent. Inorg Chem. 2013;52:1694–1700. doi: 10.1021/ic3017613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frullano L, Zhu J, Miller RH, Wang Y. Synthesis and characterization of a novel gadolinium-based contrast agent for magnetic resonance imaging of myelination. J Med Chem. 2013;56:1629–1640. doi: 10.1021/jm301435z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison VS, Carney CE, MacRenaris KW, Waters EA, Meade TJ. Multimeric Near IR-MR Contrast Agent for Multimodal In Vivo Imaging. J Am Chem Soc. 2015;137:9108–9116. doi: 10.1021/jacs.5b04509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison VSR, Carney CE, Macrenaris KW, Meade TJ. A multimeric MR-optical contrast agent for multimodal imaging. Chem Commun. 2014;50:11469–11471. doi: 10.1039/c4cc05651e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nwe K, Brechbiel MW. Growing Applications of “Click Chemistry” for Bioconjugation in Contemporary Biomedical Research. Cancer Biother Radio. 2009;24:289–302. doi: 10.1089/cbr.2008.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang PV, et al. Copper-free click chemistry in living animals. P Natl Acad Sci USA. 2010;107:1821–1826. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiao X, Ding S, Liu F, Kucera GL, Bierbach U. Investigating the cellular fate of a DNA-targeted platinum-based anticancer agent by orthogonal double-click chemistry. J Biol Inorg Chem. 2014;19:415–426. doi: 10.1007/s00775-013-1086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallo J, et al. CXCR4-targeted and MMP-responsive iron oxide nanoparticles for enhanced magnetic resonance imaging. Angew Chem Int Ed Engl. 2014;53:9550–9554. doi: 10.1002/anie.201405442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackman ML, Royzen M, Fox JM. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J Am Chem Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haun JB, Devaraj NK, Hilderbrand SA, Lee H, Weissleder R. Bioorthogonal chemistry amplifies nanoparticle binding and enhances the sensitivity of cell detection. Nat Nanotechnol. 2010;5:660–665. doi: 10.1038/Nnano.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murata N, et al. Macrocyclic and Other Non-Group 1 Gadolinium Contrast Agents Deposit Low Levels of Gadolinium in Brain and Bone Tissue: Preliminary Results From 9 Patients With Normal Renal Function. Invest Radiol. 2016;51:447–453. doi: 10.1097/RLI.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 33.Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals. 2016;29:365–376. doi: 10.1007/s10534-016-9931-7. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hapuarachchige S, Zhu WL, Kato Y, Artemov D. Bioorthogonal, two-component delivery systems based on antibody and drug-loaded nanocarriers for enhanced internalization of nanotherapeutics. Biomaterials. 2014;35:2346–2354. doi: 10.1016/j.biomaterials.2013.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hapuarachchige S, Kato Y, Artemov D. Bioorthogonal two-component drug delivery in HER2(+) breast cancer mouse models. Sci Rep. 2016;6:24298. doi: 10.1038/srep24298. srep24298 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhujwalla ZM, Artemov D, Natarajan K, Ackerstaff E, Solaiyappan M. Vascular differences detected by MRI for metastatic versus nonmetastatic breast and prostate cancer xenografts. Neoplasia. 2001;3:143–153. doi: 10.1038/sj/neo/7900129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gimi B, et al. Molecular Imaging of Cancer: Applications of Magnetic Resonance Methods. Proc IEEE Inst Electr Electron Eng. 2005;93:784–799. doi: 10.1109/JPROC.2005.844266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu W, Kato Y, Artemov D. Water exchange-minimizing DCE-MRI protocol to detect changes in tumor vascular parameters: effect of bevacizumab/paclitaxel combination therapy. MAGMA. 2014;27:161–170. doi: 10.1007/s10334-013-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nwe K, Milenic D, Bryant LH, Regino CA, Brechbiel MW. Preparation, characterization and in vivo assessment of Gd-albumin and Gd-dendrimer conjugates as intravascular contrast-enhancing agents for MRI. J Inorg Biochem. 2011;105:722–727. doi: 10.1016/j.jinorgbio.2011.01.017. S0162-0134(11)00028-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu WL, Okollie B, Arlemov D. Controlled internalization of Her-2/neu receptors by cross-linking for targeted delivery. Cancer Biology & Therapy. 2007;6:1960–1966. doi: 10.4161/cbt.6.12.4979. [DOI] [PubMed] [Google Scholar]