Abstract
Importance
Community-based interventions can reduce neonatal mortality when health systems are weak. Population coverage of target groups may be an important determinant of their effect on behavior and mortality. A women’s group trial at coverage of 1 group per 1414 population in rural Bangladesh showed no effect on neonatal mortality, despite a similar intervention having a significant effect on neonatal and maternal death in comparable settings.
Objective
To assess the effect of a participatory women’s group intervention with higher population coverage on neonatal mortality in Bangladesh.
Design
A cluster randomized controlled trial in 9 intervention and 9 control clusters.
Setting
Rural Bangladesh.
Participants
Women permanently residing in 18 unions in 3 districts and accounting for 19 301 births during the final 24 months of the intervention.
Interventions
Women’s groups at a coverage of 1 per 309 population that proceed through a participatory learning and action cycle in which they prioritize issues that affected maternal and neonatal health and design and implement strategies to address these issues.
Main Outcomes and Measures
Neonatal mortality rate.
Results
Analysis included 19 301 births during the final 24 months of the intervention. More than one-third of newly pregnant women joined the groups. The neonatal mortality rate was significantly lower in the intervention arm (21.3 neonatal deaths per 1000 live births vs 30.1 per 1000 in control areas), a reduction in neonatal mortality of 38% (risk ratio, 0.62 [95% CI, 0.43-0.89]) when adjusted for socioeconomic factors. The cost-effectiveness was US $220 to $393 per year of life lost averted. Cause-specific mortality rates suggest reduced deaths due to infections and those associated with prematurity/low birth weight. Improvements were seen in hygienic home delivery practices, newborn thermal care, and breastfeeding practices.
Conclusions and Relevance
Women’s group community mobilization, delivered at adequate population coverage, is a highly cost-effective approach to improve newborn survival and health behavior indicators in rural Bangladesh.
Trial Registration
isrctn.org Identifier: ISRCTN01805825
Community-based interventions have the potential to reduce neonatal mortality, as demonstrated by cluster randomized trials of participatory women’s groups in rural Nepal1 and India2 that showed a 30% to 45% reduction in neonatal mortality. A similar women’s group trial in Bangladesh,3 however, showed no impact on neonatal mortality. A possible explanation for the absence of effect was the lower population coverage of women’s groups (approximately 1 group per 1414 population) compared with the studies in India (1 per 468) and Nepal (1 per 756). In particular, the coverage of pregnant women, a key target group for the intervention, was only 3% in Bangladesh compared with 55% in the final study year in India.
Given that participatory women’s groups are low-cost and potentially highly effective and scalable,4 we wished to assess the effect of the intervention at higher population coverage in Bangladesh. We hypothesized that the intervention delivered at higher coverage could reduce neonatal mortality by at least 30% and improve home care practices and health care use for pregnant women and newborns and their mothers.
Methods
Intervention and Study Design
We used a cluster randomized controlled trial to evaluate the effect of the participatory learning and action cycle with women’s groups when delivered at higher population coverage in 18 “unions” in 3 districts (Bogra, Molavibazar, and Faridpur) of rural Bangladesh. Unions are the lowest administrative unit in Bangladesh, representing a geographically adjacent collection of villages. To fully assess the effect of scale-up of the women’s groups, the same intervention and control unions were used as for the earlier 2005-2007 trial.3 Randomization for that trial is described elsewhere.3,5 It involved the purposeful selection of the 3 districts on the basis of having active Diabetic Association of Bangladesh offices and some-what representing the social and geographical diversity of Bangladesh.5 Per district, 2 upazillas (subdistricts) and, per upazilla, 3 unions were purposefully sampled on the basis of perceived limited access to perinatal health care and feasible accessibility from Diabetic Association of Bangladesh district headquarters. The 6 unions (clusters) in each district (stratum) were randomly allocated to either intervention or control by blindly pulling pieces of paper, each representing 1 union, from a bottle. The allocation sequence had been decided on before drawing the papers.
In intervention areas, 648 new groups were formed by newly recruited facilitators in addition to the 162 women’s groups set up in the intervention areas as part of the 2005-2007 trial. The 162 “old” groups continued to meet monthly from late 2004 until the end of June 2011, during which their focus expanded beyond maternal and newborn health to include the health of children younger than 5 years of age and women’s health. From January 2009, the 648 “new” groups proceeded through a cycle of monthly meetings on maternal and newborn health, giving a coverage of 1 per 386 population. The combined 810 women’s groups constituted a coverage of 1 group per 309 population. The nature of the intervention means that allocation was not masked. All unions, irrespective of allocation arm, received health system–strengthening initiatives from the Diabetic Association of Bangladesh–Perinatal Care Project. These included the provision of basic medical equipment, traditional birth attendant training in essential newborn care, physician training, and establishing links between communities and health services.
Monitoring and Evaluation
The trial ran from January 1, 2009, to June 30, 2011. The period from January to December 2008 was designated the baseline period, and the period from January to June 2009 was designated the scale-up period, leaving 24 months between July 2009 and June 2011 for impact evaluation. The study participants were women whose childbirth or death was recorded in the study areas. A prospective surveillance system recorded all deaths during pregnancy and for up to 6 weeks postpartum and all births taking place in the study areas and their outcomes.6 Each of approximately 500 key informants, all traditional birth attendants, received an honorarium for identifying births and pregnancy-related and newborn deaths in the study areas. Each traditional birth attendant was visited fortnightly by monitors who collected details of all registered events. The monitor subsequently verified all events through household visits and further asked household members if they were aware of any other births or deaths in their community to ensure complete capture of these vital events.
Between 6 and 52 weeks after the event, the monitors revisited households to gather data on background characteristics, home care practices, and health care use. In the event of a stillbirth or a neonatal- or pregnancy-related death, data on the signs, symptoms, and circumstances of death were collected using structured verbal autopsy questions administered to the mother or other close caregiver.
Data were checked for quality and completeness in the district offices, and errors were referred back to the field. Between 10% and 20% of questions in 10% to 20% of the completed interviews were randomly selected for verification in the field by revisiting respondents to check the accuracy and completeness of data collected. Verified data were sent to Dhaka, Bangladesh, for data entry into a Microsoft Access database where further quality assurance took place. Process evaluation data were collected from monthly women’s group attendance registers and from a 2009 cross-sectional survey of women’s group members’ socioeconomics and basic demographics.4,7
Statistical Analysis
The control areas in Moulavibazar included tea-garden estates, which differ from the majority of non–tea-garden Bangladesh in terms of socioeconomics and history. The majority of tea-garden estate residents work on the tea estates and are descendants of workers originating mostly from Orissa and Bihar, in India, who were brought to the area by the former British rulers. The relative socioeconomic disadvantage of tea-garden estates has been described previously.8,9 Given these underlying differences and as specified a priori in the study protocol, analyses were performed with and without the tea-garden residents.
Participants were assigned to the cluster in which their birth or death was registered, and the intention-to-treat analysis only included those who permanently resided in the study areas, as determined by the registration process. Analysis of secondary outcomes only included women who were successfully interviewed.
The trial’s primary end point was the neonatal mortality rate in the final 24 months of the 30-month intervention implementation. All analyses were specified a priori in the trial protocol5 and were based on cluster-level summaries using regression techniques that took the stratified and clustered study design into account, using Stata version 12.1 (StataCorp LP). Process evaluation data from registers and implementation documents were used to estimate intervention coverage (groups per population and proportion of pregnant women attending groups) and “dosage” received by women’s group members (average proportion of meetings attended) as described elsewhere.4
To account for potential confounding and to facilitate comparisons with the previous trial, adjustments for maternal age, maternal education, and household assets were made using a 2-stage analysis. At the first stage, a regression model was fitted that incorporated the strata (as a fixed effect) and all co-variates of interest (but not the intervention effect). Fitted values from the model were used to compute a residual for each cluster, and these residuals then replaced the cluster-specific observations in the analysis, following the methods detailed by Hayes and Moulton (2009).10
Verbal autopsy data for neonatal deaths were analyzed using InterVA4 software, which uses Bayesian reasoning to identify likely causes of death and has been widely applied.11–14 Aggregated cause-specific mortality fractions were used to derive cause-specific mortality rates by dividing the total number of deaths from each cause category by the total number of live births. Crude cause-specific rate ratios were derived using the Stata incidence rate ratio calculator.
We did not expect the intervention to have any adverse effects, and therefore we had no stopping rules. Interim analysis of the first 18 months of data was performed in June 2011 and presented to a data safety monitoring board, and, again, interim analysis of the first 24 months of data was performed in March 2012. The board, as well as the implementation and in-country monitoring and evaluation teams, were blind to the allocation arm on both occasions. The board recommended completion of the full trial period and a final analysis of data from July 2009 to June 2011, with and without the inclusion of tea-garden residents.
Cost-effectiveness
A cost-effectiveness analysis was conducted from a provider perspective using methods consistent with similar trials of community participatory interventions.1,2,15 A description of methods is provided (eAppendix in the Supplement).
Ethics
Community consent was acquired for intervention implementation and monitoring activities. Individual informed consent was obtained before each interview. The trial was approved by the University College London research ethics committee (identification no. 1488/001) and the ethical review committee of the Diabetic Association of Bangladesh.
Results
Process
A total of 648 new women's groups were added to the pre–scale-up 162 groups. All new groups completed their first meeting in January 2009, and 198 groups had completed at least 20 meetings by June 2011. The remaining 450 groups completed at least 20 meetings by September 2011, with various reasons being given for delays in holding meetings, including religious holidays, harvest commitments, and flooding.
Total population coverage was 1 group per 309 population and 386 for the new groups, an approximately 4- to 5-fold increase in coverage relative to pre–scale-up levels. There was 1 women's group per 57 ever-married women of reproductive age, compared with 1 per 283 prior to scale-up, and 23% of the 45 330 ever-married women living in the intervention areas and of reproductive age were women's group members. Approximately 36.8% of women who gave birth and were interviewed between July 2009 and June 2011 reported attending women's groups compared with 3% prior to scale-up. Average attendance at each women's group meeting in the newly formed groups was 21 women per group (range, 19-24 women). Process evaluation data indicated a mean age of participants of 31 years, and 95% of group members were 15 to 49 years of age. Approximately 3% of attendees were men. Attendees also included traditional birth attendants, health service providers, teachers, and community leaders. The intensity or “dose” of the exposure to the intervention among women's group members was estimated at 70%, meaning that, on average, women's group members were exposed to 14 of 20 meetings.
A total of 385 community meetings, organized by the groups after their 14th women’s group meeting, were held from March to August 2010, with approximately 43 000 community participants. Approximately one-third of participants at community meetings were men.
Recruitment
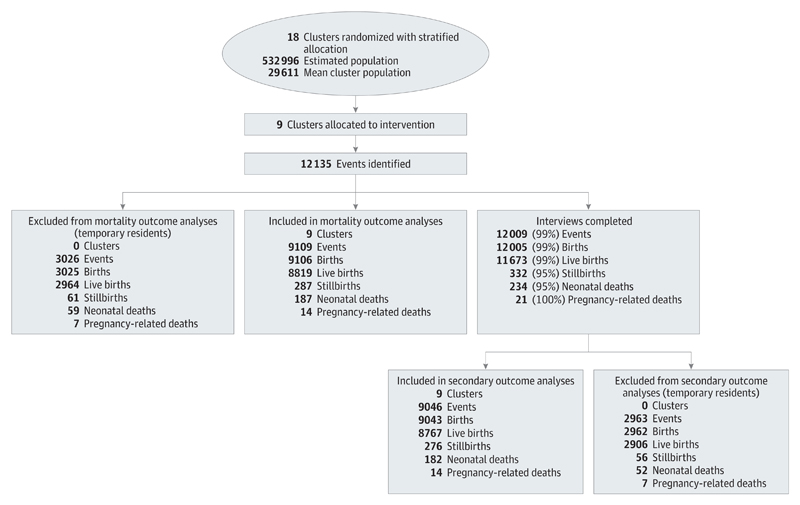
Figure 1 and Figure 2 show the trial profile during the final 24 study months for the intervention and control arms, respectively. Including tea-garden residents, the estimated population size was 532 996 people, in 117 914 households, representing 99 998 women of reproductive age. A total of 25 594 events were registered during the final 24 months, for which 25 321 interviews were completed (99%) with a median follow-up time of 67 days following birth (interquartile range, 52-100 days). The interview completion rates were the same for both arms of the trial. The interview rates were slightly lower for neonatal deaths and stillbirths, and the follow-up times were slightly higher (median, 94 days; interquartile range, 58-174 days), largely owing to difficulties in tracking these families for an interview after the event.
Figure 1. Trial Profile for the Intervention Arm.
The numbers in parentheses show the proportion of all events for which an interview was successfully completed. All numbers are from the final 24-month evaluation period.
Figure 2. Trial Profile for the Control Arm.
The numbers in parentheses show the proportion of all events for which an interview was successfully completed. All numbers are from the final 24-month evaluation period.
Baseline Comparisons
Table 1 shows cluster-level summaries of the baseline characteristics of the study population, including cluster size. Sociodemographic indicators and primary and secondary outcome indictors were similar in both arms, irrespective of the inclusion of tea-garden residents. Baseline comparisons did not significantly change with the inclusion of tea-garden residents, except that more women with a primary education were in the intervention clusters than in the control clusters and that fewer Hindus were in the intervention clusters (10.4%) than in the control clusters (17.6%).
Table 1. Cluster-Level Mean Baseline Summaries of Socioeconomic, Mortality, and Secondary Outcome Indicators for Intervention and Control Areasa.
| Variable | Intervention | Control |
|
|---|---|---|---|
| Without TGRs | With TGRs | ||
| Cluster size, No. | |||
| Households | 6092 | 6238 | 7010 |
| Population | 27 817 | 27 645 | 31 405 |
| Age, mean (SD), y | 24.7 (1.1) | 24.7 (0.9) | 24.6 (0.7) |
| Age, % | |||
| <20 y | 16.6 | 16.0 | 15.9 |
| 20-29 y | 62.7 | 64.9 | 65.3 |
| 30-39 y | 19.5 | 18.2 | 18.0 |
| ≥40 y | 1.1 | 0.9 | 0.9 |
| Age at first pregnancy, mean (SD), y | 18.3 (0.8) | 18.7 (0.8) | 18.6 (0.7) |
| Gravidity, mean (SD), No. | 2.7 (0.4) | 2.5 (0.3) | 2.5 (0.3) |
| Religion, % | |||
| Islam | 89.4 | 89.4 | 82.3 |
| Hindu | 10.4 | 10.5 | 17.6 |
| Other | 0.1 | 0 | 0 |
| Education, % | |||
| Never went to school | 25.2 | 22.5 | 26.1 |
| Primary education | 36.0 | 31.5 | 30.3 |
| Secondary or above | 38.8 | 46.0 | 43.7 |
| Literacy, % | |||
| Illiterate | 29.8 | 27.2 | 31.0 |
| Reads with difficulty | 21.2 | 20.1 | 20.1 |
| Reads with ease | 49.0 | 52.7 | 48.8 |
| Household assets, % | |||
| None | 36.5 | 32.6 | 34.8 |
| 1 | 20.9 | 20.5 | 19.5 |
| 2 | 14.5 | 12.8 | 12.6 |
| ≥3 | 28.1 | 34.1 | 33.1 |
| Counts of births and deaths | |||
| Mean No. of births per cluster | 570 | 569 | 634 |
| No. of births | 5128 | 5120 | 5706 |
| No. of live births | 4965 | 4930 | 5485 |
| No. of stillbirths | 163 | 190 | 221 |
| No. of early neonatal deaths | 149 | 129 | 145 |
| No. of late neonatal deaths | 41 | 45 | 59 |
| No. of neonatal deaths | 190 | 174 | 204 |
| No. of perinatal deaths | 312 | 319 | 366 |
| No. of pregnancy-related deaths | 12 | 20 | 22 |
| Mortality indicators, clusterwise mean rates | |||
| Neonatal mortality rate per 1000 live births | 38.5 | 34.8 | 37.4 |
| Stillbirth rate per 1000 births | 31.5 | 36.7 | 37.9 |
| Early neonatal mortality rate per 1000 live births | 30.0 | 25.7 | 26.3 |
| Late neonatal mortality rate per 1000 live births | 8.5 | 9.1 | 11.2 |
| Perinatal mortality rate per 1000 births | 60.5 | 61.5 | 63.2 |
| Pregnancy-related mortality ratio per 100 000 live births | 141.5 | 247.6 | 233.5 |
| Secondary outcome indicators, clusterwise mean proportions | |||
| Facility deliveries | 18.6 | 20.7 | 19.6 |
| Birth attendant home delivery practices | |||
| Washed hands with soapb | 81.3 | 79.8 | 80.1 |
| Used safe delivery kitb | 12.0 | 13.2 | 11.9 |
| Used plastic sheetb | 49.7 | 47.9 | 47.4 |
| Used boiled or new instrumentb,c | 98.8 | 99.0 | 99.1 |
| Boiled threadb,c | 54.1 | 49.4 | 51.1 |
| Left cord undressed or dressed with antisepticb,c | 37.0 | 37.5 | 35.3 |
| Thermal care of newborns | |||
| Infant wiped and wrapped within 10 min of deliveryb,c | 33.5 | 23.2 | 25.9 |
| Infant not bathed in first 24 hb,c | 81.7 | 69.2 | 70.6 |
| Infant placed on mother’s skin within 30 min of deliveryb,c | 35.1 | 35.9 | 37.2 |
| Early infant feeding practices | |||
| Infant put to breast within 1 hc | 64.7 | 60.6 | 60.5 |
| Exclusive breastfeeding for at least 6 wkc | 64.8 | 64.2 | 65.5 |
| Health service utilization | |||
| Antepartum | |||
| ≥4 Antenatal care checkups by formal provider | 11.0 | 13.5 | 12.0 |
| Intrapartum | |||
| Birth attended by a formal providerd | 20.0 | 23.2 | 21.7 |
| Postpartum | |||
| Mother received checkup in first 6 wk by formal providere | 14.0 | 16.5 | 15.7 |
| Infant received checkup in first 6 wk by formal providere | 13.1 | 14.3 | 13.1 |
Abbreviation: TGRs, tea-garden residents.
We have a maximum of 0.3% missing data on background information, a maximum of 13% missing data on home delivery practices, and a maximum of 0.8% missing data on other secondary outcome measures.
Nonfacility deliveries only.
Live births only.
Excludes mothers who died during pregnancy.
Only includes mothers alive at 6 weeks.
Impact Evaluation
The intention-to-treat analysis included 17 940 births over the final 24 study months, a mean of 997 births per cluster (19 315 births, 1073 per cluster when including tea-garden residents) and a coefficient of variation for neonatal mortality of 0.2. Analysis of home care practices and health care use was based on successfully completed interviews relating to 17 817 events (99%) (19 148 events [99%] when including tea-garden residents). The rate of completion of interviews was equally high in the intervention and control arms.
Table 2 presents the counts of births and deaths during the trial period, and Table 3 presents the mortality rates and rate ratios during the trial period. The crude neonatal mortality rate was 29% lower in intervention arm than in the control arm, increasing to 38% when adjusted for maternal age, maternal education, and household assets. The inclusion of tea-garden residents reduced the crude mortality rate to 35%, although adjustment for background factors reduced this effect and made the effect on the overall neonatal morality rate (but not the early neonatal morality rate) statistically nonsignificant. The risk ratios indicate that most of the survival gain was in the early neonatal period. Substantial reductions in stillbirth mortality in control areas were not observed in intervention areas, where the stillbirth mortality rate did not change over the trial period.
Table 2. Numbers of Births and Deaths During the Trial Period (July 2009 to June 2011).
| No. of Births or Deaths |
|||
|---|---|---|---|
| Control |
|||
| Type of Birth or Death | Intervention | Without TGRs | With TGRs |
| Mean births per cluster | 1012 | 982 | 1134 |
| Births | 9106 | 8834 | 10 204 |
| Live births | 8819 | 8602 | 9896 |
| Stillbirths | 287 | 232 | 308 |
| Early neonatal deaths | 148 | 210 | 244 |
| Late neonatal deaths | 39 | 61 | 69 |
| Neonatal deaths | 187 | 271 | 313 |
| Perinatal deaths | 435 | 442 | 552 |
| Pregnancy-related deaths | 14 | 23 | 28 |
Abbreviation: TGRs, tea-garden residents.
Table 3. Mortality Rates and Ratios for the Trial Period (July 2009–June 2011).
| Clusterwise Mean Rates |
Risk Ratio (95% CI) |
||||||
|---|---|---|---|---|---|---|---|
| Control |
Without TGRs |
With TGRs |
|||||
| Mortality Outcome | Intervention | Without TGRs | With TGRs | Unadjusteda | Adjustedb | Unadjusteda | Adjustedb |
| Neonatal mortality rate per 1000 live births | 21.3 | 30.1 | 31.8 | 0.71 (0.52-0.96) | 0.62 (0.43-0.89) | 0.65 (0.50-0.84) | 0.71 (0.50-1.03) |
| Stillbirth rate per 1000 births | 31.6 | 26 | 29.8 | 1.22 (1.02-1.45) | 1.07 (0.75-1.53) | 1.09 (0.92-1.28) | 1.20 (0.90-1.60) |
| Early neonatal mortality rate per 1000 live births | 16.8 | 23.5 | 25.2 | 0.70 (0.47-1.04) | 0.61 (0.38-0.99) | 0.64 (0.44-0.92) | 0.61 (0.38-0.99) |
| Late neonatal mortality rate per 1000 live births | 4.5 | 6.6 | 6.6 | 0.69 (0.45-1.08) | 0.60 (0.41-0.89) | 0.68 (0.45-1.04) | 0.75 (0.51-1.11) |
| Perinatal mortality rate per 1000 births | 47.9 | 48.9 | 54.3 | 0.99 (0.82-1.19) | 0.87 (0.62-1.22) | 0.89 (0.75-1.05) | 0.98 (0.72-1.33) |
| Pregnancy-related mortality ratio per 100 000 live births | 153.4 | 276.1 | 254.3 | 0.73 (0.39-1.40) | 0.74 (0.34-1.64) | 0.77 (0.39-1.49) | 1.02 (0.50-2.10) |
Abbreviation: TGRs, tea-garden residents.
Adjusted for clustering and stratification only.
Adjusted for clustering and stratification, as well as for maternal age group, maternal education, and household assets.
Gains were observed in hygienic home delivery practices, thermal care of the newborn, and feeding practices (Table 4). Quantitative gains in health service utilization were statistically significant only when including tea-garden residents (eTable in Supplement). Adjustment for socioeconomic indicators had little quantitative effect on the estimated intervention effect on secondary outcomes.
Table 4. Secondary Outcome Indicators During the Trial Period (July 2009 to June 2011) in Intervention and Non–Tea-Garden Control Areasa.
| Secondary Outcome | Clusterwise Mean Proportion |
Odds Ratio (95% CI) |
||
|---|---|---|---|---|
| Intervention | Control | Crude | Adjusted | |
| Facility deliveries | 26.8 | 27.7 | 0.99 (0.79-1.25) | 1.05 (0.88-1.25) |
| Home delivery practices of birth attendant | ||||
| Washed hands with soapb | 91.3 | 83.8 | 1.09 (1.01-1.18) | 1.18 (1.02-1.35) |
| Used safe delivery kitb | 29.1 | 15.5 | 2.24 (1.29-3.88) | 2.26 (1.31-3.89) |
| Used plastic sheetb | 72.5 | 62.1 | 1.18 (1.05-1.33) | 1.19 (1.06-1.34) |
| Used boiled or new instrumentb,c | 99.5 | 98.9 | 1.01 (1.00-1.01) | 1.02 (1.00-1.04) |
| Boiled threadb,c | 66.8 | 56.2 | 1.18 (0.98-1.43) | 1.22 (1.02-1.47) |
| Left cord undressed or dressed with antisepticb,c | 36.9 | 25.4 | 1.57 (1.01-2.45) | 1.58 (1.01-2.48) |
| Thermal care of newborns, home deliveries | ||||
| Infant wiped and wrapped within 10 min of deliveryb,c | 36.0 | 33.3 | 1.11 (0.67-1.86) | 1.11 (0.66-1.85) |
| Infant not bathed in first 24 hb,c | 90.0 | 70.1 | 1.33 (1.04-1.71) | 1.33 (1.04-1.71) |
| Infant placed on mother’s skin within 30 min of deliveryb,c | 80.7 | 51.7 | 1.46 (0.85-2.49) | 1.46 (0.86-2.47) |
| Early infant feeding practices, all live births | ||||
| Infant put to breast within 1 hc | 78.8 | 67.3 | 1.17 (1.06-1.29) | 1.16 (1.05-1.28) |
| Exclusive breastfeeding for at least 6 wkc | 77.9 | 74.4 | 1.05 (0.99-1.11) | 1.05 (1.00-1.11) |
| Health service utilization | ||||
| Antepartum | ||||
| ≥4 Antenatal care checkups by formal provider | 18.9 | 14.8 | 1.26 (0.90-1.77) | 1.37 (0.99-1.88) |
| Intrapartum | ||||
| Birth attended by formal providerd | 28.4 | 30.8 | 0.95 (0.76-1.19) | 1.00 (0.84-1.20) |
| Postpartum | ||||
| Mother received checkup in first 6 wk by formal providere | 22.5 | 23.1 | 0.98 (0.69-1.38) | 1.04 (0.78-1.39) |
| Infant received checkup in first 6 wk by formal providerc | 20.4 | 23.6 | 0.84 (0.49-1.46) | 0.89 (0.53-1.50) |
We have a maximum 13% of missing data on home delivery practices and a maximum 3% of missing data on other secondary outcome measures.
Nonfacility deliveries only.
Live births only.
Excludes mothers who died during pregnancy.
Only includes mothers alive at 6 weeks.
Cause of Death
Verbal autopsies for all deaths followed up with interview show that the major causes of neonatal mortality were infections, perinatal asphyxia, and complications associated with prematurity and low birth weight (Table 5). Notably, infection-specific mortality rates decreased in the intervention areas during the trial period but remained relatively stable in the control areas, and preterm/small-baby mortality rates decreased more in the intervention areas than in the control areas.
Table 5. Population Cause-Specific Mortality Rates per 1000 Live Births by Intervention Arm and Study Period and Crude Rate Ratios During the Trial Period (July 2009 to June 2011).
| Mortality Rate per 1000 Live Births |
||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (2008) |
Trial Period (July 2009–June 2011) |
|||||||
| Control | Control | Crude Rate Ratio (95% CI) | ||||||
| Cause | Intervention | Without TGRs | With TGRs | Intervention | Without TGRs | With TGRs | Without TGRs | With TGRs |
| Infectiona | 17.2 | 13 | 14.4 | 11.1 | 14.1 | 14.4 | 0.78 (0.59-1.03) | 0.77 (0.59-1.00) |
| Asphyxia (ICD code 10.02) | 8.5 | 8.1 | 7.6 | 4.2 | 4.9 | 4.8 | 0.86 (0.54-1.37) | 0.86 (0.55-1.35) |
| Prematurity (ICD code 10.01) | 5.1 | 4.1 | 4.5 | 1.7 | 3 | 3.2 | 0.58 (0.29-1.15) | 0.52 (0.26-1.00) |
| Otherb | 1.9 | 2.3 | 2.7 | 2.2 | 2.4 | 2.6 | 0.93 (0.47-1.83) | 0.82 (0.43-1.54) |
| Indeterminate cause | 3.5 | 4.3 | 4.1 | 1.6 | 5.8 | 5.1 | 0.29 (0.14-0.51) | 0.31 (0.16-0.56) |
Abbreviations: ICD, International Classification of Diseases; TGRs, tea-garden residents.
Includes determinate causes of diarrheal disease (ICD code 1.04), meningitis (ICD code 1.07), neonatal pneumonia (ICD code 10.03), and neonatal sepsis (ICD code 10.04).
Includes determinate causes of congenital malformations (ICD code 10.06) and other and unspecified neonatal causes (ICD code 10.99).
Cost-effectiveness
The prospective cost-effectiveness was US $11 974 ($10 053 with tea-garden residents) per neonatal death averted and $393 ($330 with tea-garden residents) per year of life lost averted. The estimated cost-effectiveness of going straight to scale is $7975 ($6695 with tea-garden residents) per neonatal death averted and $261 ($220 with tea-garden residents) per year of life lost averted.
Discussion
Our trial showed that participatory women’s groups with high population coverage in Bangladesh strongly and statistically significantly reduced neonatal mortality. A 4- to 5-fold increase in population coverage of women’s groups was achieved, and more than one-third of pregnant women attended the groups. Intervention coverage at this scale, combined with active engagement with this target group of women, led to reductions in neonatal mortality of up to 38%. Gains in home delivery practices, essential newborn care, and feeding practices were also observed in the intervention clusters compared with the control clusters, which may explain part of the observed reduction in neonatal mortality. The evidence of the effect on health service utilization was less strong, with statistical significance only being reached when intervention areas were compared with control areas that included tea-garden residents, who are typically more disadvantaged and thus less likely to access services. The cost-effectiveness of $220 to $393 per year of life lost averted is less than the Bangladesh gross domestic product per capita of $775 (in 2011), which indicates that the intervention is very cost-effective.16
This trial follows an earlier trial of women’s groups,3 delivered at lower coverage in the same areas, that showed no effect on neonatal mortality (risk ratio, 0.93 [95% CI, 0.80-1.09]). For pragmatic and practical reasons and to test the effect of scale-up and assess whether coverage is an important determinant of the success of the intervention, we used the same clusters and allocation arms as in the earlier trial.3 A difference-in-difference analysis, which accounted for the non-statistical difference in baseline neonatal mortality rates that favored the control areas, had a negligible effect on the estimated effect of the intervention (data not shown).
Of the 810 women’s groups evaluated, 162 were existing groups, which had been meeting from late 2004 onward, and 648 were new groups, which started in January 2009. We did not intend to separate out the effects of the old and new groups. Thus, any difference in outcomes between trial arms could, in theory, be due to either increased population coverage of groups from 1 group per 1414 population to 1 group per 309 population, as hypothesized, or a residual effect of the 162 original groups that have all continued to meet. Given that the previous trial of 162 groups showed no effect on primary and most secondary outcomes and that the 162 original groups have subsequently focused largely on the postneonatal period, we expect that the observed effect on primary and secondary outcomes in the present trial is mainly due to increased coverage of new women’s groups addressing maternal and neonatal health issues.
We expect that the prospective key-informant surveillance system used in this study captures most, if not all, births and deaths in our study areas. Exceptions may be deaths in early pregnancy, in which a woman’s pregnancy status at the time of death may remain unknown. The reported pregnancy-related mortality rate may therefore be underestimated, although the proportion of pregnancy-related deaths in early pregnancy is generally small, so the absolute number of missed events is also likely to be small. A further potential limitation of the system is that classification of deaths as stillbirths or neonatal deaths depends on lay reporting and could, in theory, be misclassified. The monitoring and evaluation questionnaire, which had an extremely high response rate of 99% in both arms, can correct for this to a certain extent in that it gathers information on signs of life after delivery.
Verbal autopsy results suggest that the intervention reduced deaths due to infections and prematurity or low birth weight. This plausibly correlates with improvements in hygienic delivery and essential newborn care practices and is supported by the observation that most of the survival gains were in the first week of life, when most deaths due to prematurity/low birth weight and approximately half of the deaths due to infections are likely to occur.17
Our findings add to the growing body of evidence on the effectiveness of women’s groups in low-income settings. Trials from rural South Asia provide strong evidence of the effects of women’s groups on neonatal mortality rates, with reductions ranging from 30% in Nepal to 45% in India,1,2 although, as demonstrated in the work from Bangladesh, coverage matters. An evaluation of the intervention in Mumbai, India, showed no effect on mortality outcomes, with More et al18 suggesting that transient populations, low-baseline mortality rates, poor social cohesion, and a dynamic environment hindered the collective action and resulted in the diffusion of messages in urban slum areas. Issues of coverage and the participation of women of reproductive age were also highlighted18 that reinforced the findings of the present study that population coverage and the appropriate targeting of the intervention are important factors for success. Furthermore, it is reasonable to expect that population-level improvements in neonatal mortality require interventions that address both the supply side and the demand side of health care.
In conclusion, population coverage of women’s groups and the extent to which they reach target groups are important determinants of their success. Even at high coverage, the intervention is highly cost-effective and could improve maternal and neonatal health in Bangladesh and other rural settings.
Acknowledgments
Funding/Support: The implementation and evaluation of the women’s groups was funded by a Big Lottery Fund International Strategic Grant. This study was supported with funds from a Wellcome Trust Strategic Award (085417ma /Z/08/Z).
Role of the Sponsor: The sponsors did not participate in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Fottrell had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Azad, Kuddus, Younes, Nahar, Skordis-Worrall, Prost, Costello, Houweling.
Acquisition of data: Fottrell, Azad, Shaha, Aumon, Beard, Skordis-Worrall, Costello, Houweling.
Analysis and interpretation of data: Fottrell, Azad, Kuddus, Younes, Shaha, Nahar, Hossen, Hossain, Pulkki-Brannstrom, Skordis-Worrall, Prost, Costello, Houweling.
Drafting of the manuscript: Fottrell, Aumon, Hossen, Pulkki-Brannstrom, Skordis-Worrall, Houweling.
Critical revision of the manuscript for important intellectual content: Fottrell, Azad, Kuddus, Younes, Shaha, Beard, Hossain, Pulkki-Brannstrom, Skordis-Worrall, Prost, Costello, Houweling.
Statistical analysis: Fottrell, Hossen, and Skordis-Worrall.
Obtained funding: Azad Costello.
Administrative, technical, and material support: Fottrell, Azad, Kuddus, Younes, Nahar, Aumon, Beard, Hossain, Prost, Costello, Houweling.
Study supervision: Fottrell, Azad, Shaha, Skordis-Worrall, Costello, Houweling.
Additional Contributions: We thank the many women and their families who took part in this study. We thank the members of the technical advisory committee (Meg Braddock, Andrew Copas, David Osrin, Sarwar Ali, Ramesh Sharan, Azad Khan, Snehil Kumar, and Nazmun Nahar) and the data safety monitoring board (Sam Richmond, Shams El Arifeen, and Mohammed Shahidullah). We also thank Ros Davies and colleagues at Women and Children First UK, who contributed to the implementation of the women’s group interventions in Bangladesh. We also thank the Diabetic Association of Bangladesh and its director, Azad Khan, for support given throughout this project. Particular thanks and dedication to Dr Sam Richmond, chair of the data safety monitoring board, who passed away shortly after the trial ended. His technical advice, guidance, and friendship were invaluable
Conflict of Interest Disclosures: None reported.
References
- 1.Manandhar DS, Osrin D, Shrestha BP, et al. Members of the MIRA Makwanpur trial team. Effect of a participatory intervention with women’s groups on birth outcomes in Nepal: cluster-randomised controlled trial. Lancet. 2004;364(9438):970–979. doi: 10.1016/S0140-6736(04)17021-9. [DOI] [PubMed] [Google Scholar]
- 2.Tripathy P, Nair N, Barnett S, et al. Effect of a participatory intervention with women’s groups on birth outcomes and maternal depression in Jharkhand and Orissa, India: a cluster-randomised controlled trial. Lancet. 2010;375(9721):1182–1192. doi: 10.1016/S0140-6736(09)62042-0. [DOI] [PubMed] [Google Scholar]
- 3.Azad K, Barnett S, Banerjee B, et al. Effect of scaling up women’s groups on birth outcomes in three rural districts in Bangladesh: a cluster-randomised controlled trial. Lancet. 2010;375(9721):1193–1202. doi: 10.1016/S0140-6736(10)60142-0. [DOI] [PubMed] [Google Scholar]
- 4.Nahar T, Azad K, Aumon BH, et al. Scaling up community mobilisation through women’s groups for maternal and neonatal health: experiences from rural Bangladesh. BMC Pregnancy Childbirth. doi: 10.1186/1471-2393-12-5. [published online January 24, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houweling TAJ, Azad K, Younes L, et al. PCP study team. The effect of participatory women's groups on birth outcomes in Bangladesh: does coverage matter? study protocol for a randomized controlled trial. Trials. doi: 10.1186/1745-6215-12-208. [published online September 26, 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnett S, Nair N, Tripathy P, Borghi J, Rath S, Costello A. A prospective key informant surveillance system to measure maternal mortality—findings from indigenous populations in Jharkhand and Orissa, India. BMC Pregnancy Childbirth. doi: 10.1186/1471-2393-8-6. [published online February 28, 2008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younes L, Houweling TA, Azad K, Costello A, Fottrell E. Estimating coverage of a women’s group intervention among a population of pregnant women in rural Bangladesh. BMC Pregnancy Childbirth. doi: 10.1186/1471-2393-12-60. [published online June 29, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdhury M, Hasan G, Karim M. A study on existing WATSAN condition in two tea gardens in Maulvibazar. J Environ Sci Nat Resources. 2011;4(2):13–18. doi: 10.3329/jesnr.v4i2.10125. [DOI] [Google Scholar]
- 9.Ahmed M, Begum A, Chowdhury MA. Social constraints before sanitation improvement in tea gardens of Sylhet, Bangladesh. Environ Monit Assess. 2010;164(1-4):263–271. doi: 10.1007/s10661-009-0890-0. [DOI] [PubMed] [Google Scholar]
- 10.Hayes RJ, Moulton LH. Cluster Randomised Trials. New York, NY: Taylor & Francis; 2009. [Google Scholar]
- 11.Lemma H, Byass P, Desta A, et al. Deploying artemether-lumefantrine with rapid testing in Ethiopian communities: impact on malaria morbidity, mortality and healthcare resources. Trop Med Int Health. 2010;15(2):241–250. doi: 10.1111/j.1365-3156.2009.02447.x. [DOI] [PubMed] [Google Scholar]
- 12.Ramroth H, Lorenz E, Rankin JC, et al. Cause of death distribution with InterVA and physician coding in a rural area of Burkina Faso. Trop Med Int Health. 2012;17(7):904–913. doi: 10.1111/j.1365-3156.2012.02998.x. [DOI] [PubMed] [Google Scholar]
- 13.Byass P, Chandramohan D, Clark SJ, et al. Strengthening standardized interpretation of verbal autopsy data: the new InterVA-4 tool. Global Health Action. 2012;5:1–8. doi: 10.3402/gha.v5i0.19281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO) Verbal autopsy standards: The 2012 WHO verbal autopsy instruments: Release Candidate 1. Geneva, Switzerland: WHO; 2012. [Google Scholar]
- 15.Lewycka S, Mwansambo C, Rosato M, et al. Effect of women’s groups and volunteer peer counsellors on mortality, morbidity and health behaviours among mothers and children in rural Malawi: the MaiMwana cluster randomised controlled trial. Lancet. doi: 10.1016/S0140-6736(12)61959-X. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization (WHO) Macroeconomics and Health: Investing in Health for Economic Development. Geneva, Switzerland: WHO; 2001. Commission on Macroeconomics and Health. [Google Scholar]
- 17.Baqui AH, Darmstadt GL, Williams EK, et al. Rates, timing and causes of neonatal deaths in rural India: implications for neonatal health programmes. Bull World Health Organ. 2006;84(9):706–713. doi: 10.2471/blt.05.026443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.More NS, Bapat U, Das S, et al. Community mobilization in Mumbai slums to improve perinatal care and outcomes: a cluster randomized controlled trial. PLoS Med. 2012;9(7):e1001257. doi: 10.1371/journal.pmed.1001257. [DOI] [PMC free article] [PubMed] [Google Scholar]