Abstract
Cancer stem-like cells (CSC) are thought to drive brain cancer but their cellular and molecular origins remain uncertain. Here we report the successful generation of induced CSC (iCSC) from primary human astrocytes through the expression of defined genetic factors. Combined transduction of four factors - myc, Oct-4, p53DD, and ras - induced efficient transformation of primary human astrocytes into malignant cells with powerful tumor-initiating capabilities. Notably, transplantation of 100 tranduced cells into nude mice was sufficient for tumor formation. The cells showed unlimited self-renewal ability with robust telomerase activities. In addition, they expressed typical glioma stem-like cell markers such as CD133, CD15 and CD90. Moreover, these cells could form spheres in culture and differentiate into neuron-like, astrocyte-like and oligodendrocyte-like cells. Lastly, they also displayed resistance to the widely used brain cancer drug temozolomide. These iCSC could provide important tools for studies of glioma biology and therapeutics development.
Keywords: brain cancer stem cells, oncogene-induced reprogramming, primary astrocytes
Introduction
Cancer stem cells or tumor initiating cells are tumor cells with characteristics of normal stem cells and strong ability to form tumors in mice(1–3). They have been shown to play key roles in carcinogenesis (4,5), metastasis(6,7), and tumor response to treatment(5,8,9). Cancer stem cells have been isolated from a wide variety of cancer types. Examples of those include: leukemia(1), glioma(3), and breast cancer(2), etc. However, there are still many confusions and controversies concerning cancer stem cells(10,11). For example, in many instances there is no consensus on the most appropriate molecular markers to identify cancer stem cells, in contrast to normal tissue stem cells or embryonic stem cells (11). Despite tremendous growth in cancer stem cell research in recent years, the molecular mechanisms involved in the development and maintenance of cancer stem cells are poorly understood.
We are particularly interested in brain cancer stem cells because they are one of the first to be isolated and they have been implicated tumor therapeutic responses (5,8,9). An important question in brain cancer is whether differentiated brain cells such as astrocytes can de-differentiate and become cancer stem cells. One approach to answer this question is to carry out oncogene-mediated in vitro transformation of astrocytes and determine if brain cancer stem cell-like cells could be obtained. Previously, successful in vitro transformation of primary human astrocytes has been achieved by serial transduction of oncogenes such as SV40T(t) antigen, HPV E6, E7 genes, H-ras, and hTERT genes(12,13). The transformed cells can form colonies in soft agar and are tumorigenic in nude mice. However, they were significantly less tumorigenic when compared with patient-derived human brain cancer stem cells in their tumorigenic abilities and there was no evidence that the transformed cells possess cancer stem cell properties.
In the present study, we attempted to derive cancer stem cells from cultured primary human astrocytes, which are the cells of origin for several types of brain tumors including glioblastoma multiforme, a devastating brain malignancy with very poor prognosis. Drawing lessons from the success in making induced pluripotent stem cells from differentiated human cells, we were able to generate brain cancer stem cell-like cells from primary human astrocytes with only 4 defined genetic factors,.
Materials and Methods
Tissue culture conditions
Normal human primary astrocyte cells purchased from Lonza (Cat# CC-2565, Walkersville, MD) in 2012 were used in the experiments. Cells arrived as passage 3 and were cultured in AGM BulletKit astrocyte growth medium (Lonza Cat# CC-3186). U87MG cells (ATCC, Manassas, VA) was obtained in 2012 from the Tissue Culture Facility of the Duke Cancer Institute, which validated the cell lines by use of microsatellite analysis. U87MG and the transformed astrocytes were cultured in DMEM medium plus 10% FBS. In some experiments, the transformed cells were also culture in neurosphere growth medium. A patient-derived glioma stem cell line ALPS1459, which was derived from a patient at Duke University Medical Center, was obtained in 2012. It was cultured in neural stem cell growth medium: DMEM/F12 supplemented with non–essential amino acid, glutamine, B-27 supplement without vitamin A, 0.2% heparin, 20 ng/ml EGF and 25ng/ml b-FGF.
Soft agar assay
Soft agar assay were carried out according to established protocols (14). Growth of cells in soft agar was determined by plating 0.5–5×104 cells in triplicate in 0.3% Noble agar in 6 well tissue culture plates. Three weeks after plating, soft agar plates were stained with 0.05% crystal violet. Colonies were then photographed and counted with the aid of a microscope.
Neurosphere formation
Transformed cells were cultured at low density 50 (1–2 cells per mm2) on uncoated plates in neurosphere growth medium (DMEM/F12 supplemented with non–essential amino acid, Glutamine, B-27 supplement without vitamin A, 0.2% heparin, 20 ng/ml EGF and 25ng/ml b-FGF). Cells were cultured up to 10 days during which time they were monitored for neurosphere formation. To generate secondary neurospheres, primary neurospheres harvested dissociated with 0.05% trypsin/EDTA and replated under identical neurosphere growth conditions for an additional 10 days.
Tumor growth in vivo
For tumor xenograft experiments, athymic nude mice (6–8 weeks old, Jackson Laboratory, Bar Harbor, Maine) were used. For subcutaneous tumor growth, 100 of the transformed cells grown in neurosphere medium were injected subcutaneously into mouse hind leg. Intracranial tumor cell injection was carried out using a published protocol(15) in commercially obtained nude mice. Briefly, transformed astrocytes were labeled with an EGFP and Luciferease fusion reporter introduced through lentivirus infection. About 500 cells in 3 μl of DMEM/F12 medium was injected into the brains of mice (4–5 weeks old of immunodeficient athymic nude mice, Jackson lab). Tumor growth was monitored for intracranial bioluminescence by use of an IVIS Kinetic imager (PerkinElmer, Waltham, Massachusetts) available through the Optical Molecular Imaging and Analysis Core of Duke Cancer Institute. Tumor samples from mouse brain were fixed in 10% formalin. The samples were embedded with paraffin and sections were made for immunohistochemical or H&E (hematoxylin and eosin) analysis. All animal procedures described above have been reviewed and approved by the Institutional Animal Use and Care Committee at Duke University Medical Center.
Additional information on methods used in this study is provided in the supplementary information section.
Results
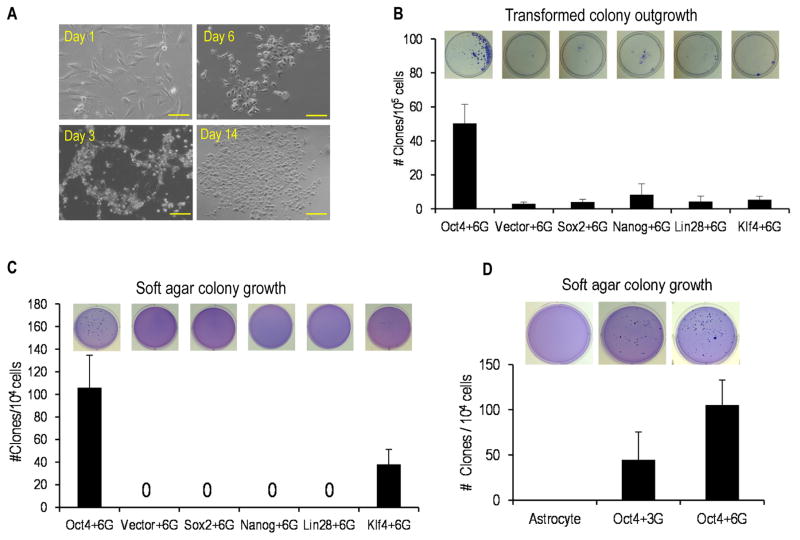
We decided to include factors involved in making induced pluripotent stem cells (iPSCs)(16) in our transformation protocol because we reasoned that there should be a significant amount of epigenetic reprogramming if successful transformation of differentiated astrocytes into cancer stem cells were to happen. We therefore tested iPSC inducing factors Oct4, Nanog, Klf4, Sox2, Lin28 in addition to established oncogenic factors H-Ras(G12V), myc(T58A), p53DD (a dominant negative form of p53), cyclin D1, CDK4(RC24), and hTERT. The latter factors have been shown to induce oncogenic transformation in primary human mammary epithelial and muscle cells independent of oncogenes(17). All factors were cloned into recombinant lentiviral vectors. The vectors were then used to infect primary human astrocytes following a scheme depicted in supplementary Fig. S1A. We evaluated each of the five iPS factors in combination with a cocktail of the six oncogenic factors for their ability to transform the astrocytes. Transduced astrocytes showed a generally similar pattern of behavior: initial cell death, survival of a small fraction of cells, and finally growth and expansion from the surviving cells (Fig. 1A). Transduced cells that survived and proliferated showed a very different morphology when compared with parental astrocytes (Fig. 1A). After 2–3 weeks, colonies are visible in the Petri dish after staining with crystal violet, indicating cellular survival and clonal expansion (Fig. 1B). It is interesting to note that the timing of the emergence of the transformed astrocyte colonies was very similar to the induction of iPS cells. After the transformed cells grew to sufficient numbers, we evaluated their ability for anchorage-independent growth in soft agar, which is a well-recognized characteristic of tumorigenic cells. Our results show that cells transduced with Oct4 or Klf4 in combination with the six-gene oncogenic cocktail (6G in short) could grow in soft agar and form large colonies after three weeks (Figure 1C). Cells derived from other gene transductions did not form any soft agar colonies. In further experiments, we show that cellular transformation and growth in soft agar could be achieved using only four factors: Oct4, Myc(T58A), H-ras(G12V), and p53DD (OMRP or Oct4+3G) (Fig. 1D). These four factors could not be reduced any further. Among OMRP-transduced cells, about 0.45% (~45 out of 10,000 seeded) of the cells could form soft agar colonies. In contrast in the Oct4+6G transformed cells, about 105 colonies formed out of 10,000 cells seeded (Fig. 1D). Supplementary Fig. S1B shows the expression of the various exogenous factors in Oct4+6G and Oct+3G transformed astrocytes. Please see Supplementary Table S1 for information on the antibodies used in this study.
Figure 1. Rapid induction of transformation in primary human astrocytes.
A). Changes in astrocyte cellular morphology during the course of induced transformation. Shown are examples from Oct+6G transformed group. The scale bars represent 200 μm.
B). Colony outgrowth in astrocytes transformed with a variety of gene combinations, n=3.
C). Soft agar colony growth from cells transduced with a variety of gene combinations, n=3.
D). Soft agar colony growth from astrocytes transduced with either the Oct4+3G or the Oct4+6G combinations, n=3.
In B–D, the error bars represent standard error of the mean (SEM).
Microarray analysis indicated that both the 7-gene and 4-gene transformed cells shared similar gene expression profiles (supplementary Fig. S2A, ArrayExpress accession#E-MTAB-4771). The profiles from both cells are also similar to that of ALPS1459, a patient-derived glioma stem cell line. Less but still significant similarities also exist between the transformed cells and U87G, a well-established glioma multiforme cell line. Principal component analysis (PCA) confirmed the similarities between the transformed cells and with patient-derived glioma stem cells (supplementary Fig. S2B). An analysis of genes also showed the major biological pathways related to differentially expressed genes in transformed vs parental astrocytes (>2 fold difference) (supplementary Fig. S2C).
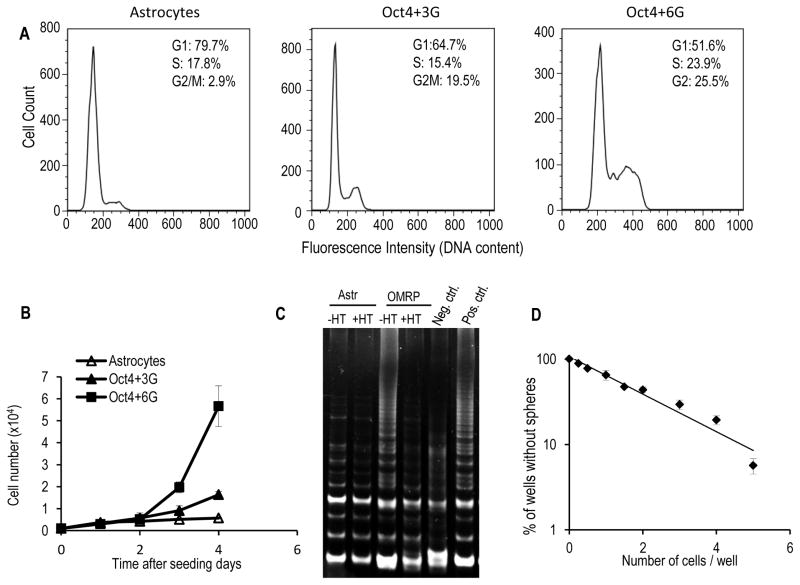
We also conducted flow cytometry analysis of the cell cycle distribution of the transformed and parental astrocytes. Compared with the parental astrocytes, the transformed cells have very different cell cycle distribution profiles (Figure 2A). In the transformed cells (Oct4+3G or Oct4+6G), the fractions of cells in G1 are much less than those in the control cells. Oct4+3G transformed had similar S phase cells as the controls while Oct+6G transformed cells had significantly increased S phase cells. In both Oct4+3G and Oct4+6G transformed astrocytes, the fraction of cells in the S+G2/M phases were significantly higher. A careful examination indicate that in Oct4+6G transduced astrocytes, many cells were had >4N DNA content. Chromosome number analysis indicate that more than 60 percent of transformed cells had chromosome aneuploidy with most possessing more than 46 chromosome, indicating wide-spread chromosomal instability (supplementary Fig. S3A &B).
Figure 2. Robust self-renewal and sphere-forming abilities of the transformed astrocytes.
A). Flow cytometry analysis of the cell cycle distribution profiles of control (primary astrocytes), Oct4+3G− and Oct4+6G− transformed astrocytes.
B). Growth curve of control human astrocytes, Oct4+3G− and Oct4+6G-transformed astrocyte cells.
C). TRAP assay for telomerase activities of primary astrocytes and OMRP (4G)-transduced astrocytes. HT: heat treatment. Positive and negative controls were provided by manufacturer.
D). Results of limited dilution assay for the sphere-forming ability of OMRP-transduced cells.
The error bars in B & D represent standard error of the mean.
Our analysis also showed that compared with the parental astrocytes, both the 4-factor (Oct4+3G) and the 7-factor (Oct4+6G) transformed cells showed significantly faster growth rate (Figure 2B). The doubling times for transformed cells are 16.7 hours and 25.1 hours for Oct+3G− or Oct4+6G-transformed cells. In contrast, it is longer than 43.6 hours for the parental astrocytes. Both Oct4+6G− and OMRP-transduced cells showed unlimited growth potential, exhibiting robust proliferative ability even after 12 months of continuous cell culture (data not shown). In comparison, the parental human primary astrocytes could only undergo 4–5 population doublings before losing their ability to proliferate. Indeed, TRAP (telomeric repeat amplification protocol) assay showed that OMRP-transformed cells had strong telomerase activities, which indicated activation of the endogenous telomerase (hTERT) genes (Figure 2C). Further evidence of hTERT gene activation was demonstrated when semi-quantitative RT-PCR was carried out to examine hTERT mRNA transcripts in parental and Oct4+3G or Oct4+6G-transformed astrocytes. Our data shown that hTERT transcript increased from barely any expression to a robust level comparable to established cancer lines (Supplementary Fig. S3C). The activation of the hTERT gene indicated that OMRP-transduced cells gained immortality after OMRP gene transduction. Activation of the endogenous hTERT gene in the 4-factor transformed astrocytes is reminiscent of iPS cell induction procedures where cells gained immortality through endogenous rather than exogenous hTERT gene activation. In comparison, most previously published oncogenic protocols called for the use of an exogenous copy of the hTERT gene(18,19).
OMRP-transformed astrocytes showed clear sphere forming ability in stem cell media, an important characteristic of cancer stem cells. Furthermore, when the spheres were disaggregated into single cells and seeded into 96-well plates, the cells showed strong ability to form spheres again, with most individual cells able to form spheres. When cells grown as spheres in 96-well plates were taken out and plated as individual cells in wells of new 96-well plates, they formed secondary spheres at a frequency similar to parental cells. Cells from the secondary spheres, in turn, formed tertiary spheres also at similar frequencies when plated again in 96-well dishes, indicating undiminished ability to form spheres in serially diluted cultures. Furthermore, limited dilution assays(20,21) (Fig. 2D) indicated that the average number of OMRP-transformed cells it took to form a sphere was around one in 2.1, a number that compared very favorably to published frequency of cancer stem cells isolated from patients derived tumor tissues(3,22).
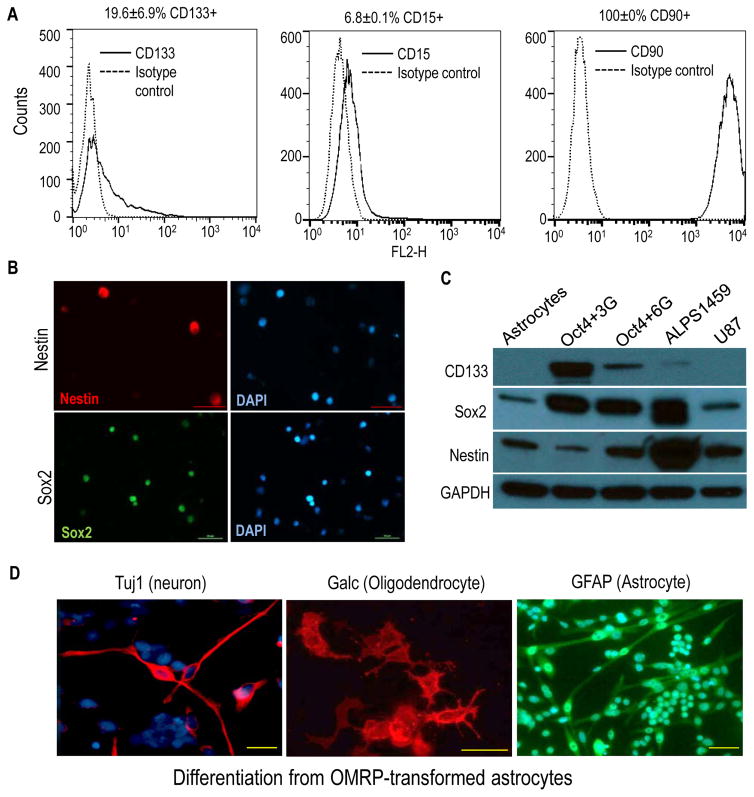
We next examined the OMRP-transduced cells for expression of various cancer stem cell markers established by previous studies. Flow cytometry analyses showed that CD133, one of the most commonly used glioma stem cell markers(3), was expressed by OMRP-transformed astrocytes at robust levels (Figure 3A, left panel). In addition, CD15, an established neural stem cell and brain cancer stem cell marker(23), was also detected by flow cytometry in the cells (Fig. 3A, mid panel). Furthermore, CD90, another well-recognized glioma stem cell marker(24), was expressed at very high levels in OMRP-transformed astrocytes (Fig. 3A, right panel). In addition, the transformed cells also showed positive staining of Sox2 and nestin expression (Fig. 3B). Western blot analysis confirmed the presence of CD133, Sox2, and nestin in both Oct4+3G− and Oct4+6G− transduced cells, similar to patient-derived glioma stem cells (Fig. 3C).
Figure 3. OMRP-transduced cells exhibit characteristics of cancer stem cells.
A). Flow cytometry analysis of cell surface expression of cancer stem cell markers CD133 (left panel), CD15 (mid panel), and CD90 (right panel) in OMRP-transformed cells.
B). Immunofluorescence staining of Nestin and Sox2 in OMRP-transduced cells. Scale bars represent 50 μm.
C). Western blot analysis of stem cell markers CD133, Sox2, and Nestin in control astrocytes, Oct4+3G gene- and Oct4+7G-gene transformed astrocytes, ALPS1459 (a patient-derived glioma stem cell line), and U87MG, an established glioma cell line.
D). Differentiation of OMRP-transformed cells in neuronal, oligodendrocyte, and astrocyte differentiation media. Immunofluorescence staining of characteristic markers (Tuj1, Galc, and GFAP) were done for each of the cell types, respectively. Scale bars represent 50 μm.
In subsequent experiments, we show that when spheres of OMRP-transduced cells cultured in stem cell medium were disaggregated and placed into neuron-specific media, they could differentiate into cells with neuron-like morphology that stained positive for Tuj1, a neuron-specific marker (Fig. 3D, left panel). We also show that sphere-derived cells could be differentiated into oligodendrocyte-like cells that stained positive for galatcocerebroside (GalC) (Fig. 3D, mid panel), or astrocyte-like cells that stained positive for glial fibrillary acidic protein (GFAP) (Fig. 3D, right panel). Therefore, the OMRP-transformed astrocyte cells grown in stem cell media can form spheres and behave like brain tumor stem cells, which can differentiate into different cell types in a manner similar to neural stem cells(22).
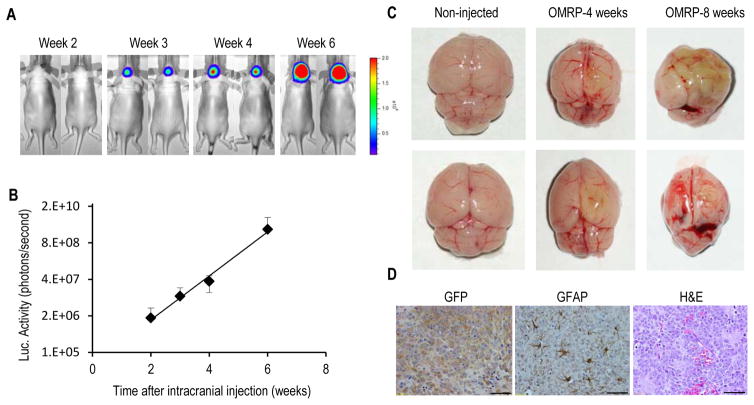
Thus OMRP-transformed astrocytes behaved similarly to patient-derived glioma stem cells in several aspects as described above. However, the “gold standard” of human cancer stem cells is the ability to form tumors in immuno-deficient mice. We therefore evaluated the capacity of OMRP-transformed cells to grow in nude mice, which is a more stringent host than either the NOD/SCID or the NOD/SCID/gamma mice that many previous studies used when testing human cancer stem cells. Injection of only 100 OMRP-transformed astrocytes subcutaneously into nude mice gave rise to tumor growth in 8 out of 10 injected mice (supplementary Fig. S4A) during 8 weeks of observation, indicating powerful tumor initiating ability of the cells. Because primary tumors do not grow outside of the CNS in human patients, we examined the tumor-forming abilities of the OMRP-transformed cells to form orthotopic tumors by injecting them intracranially into nude mice. Intracranial injections of about 500 of firefly luciferase-EGFP labeled OMRP-transduced astrocytes into nude mice led to robust tumor growth in 5 of 5 mice when measured through bioluminescence imaging (Fig. 4A). Based on luciferase signals, it is estimated that during the course of 4 weeks (from week 2 to week 6) tumor cellular numbers increased exponentially almost 1,000 fold (Fig. 4B), with a doubling time of 64.6 hrs. These numbers demonstrated the extraordinary potent tumor forming abilities of the OMRP-transduced cells orthotopically. When mice with the intracranial tumors were sacrificed, their brains showed signs of invasive tumor growth (Fig. 4C). In addition, immunohistological staining showed diffuse tumor growth in large areas of the brain (as shown by GFP staining, Fig. 4D left panel). They also show infiltration of astrocytes (as revealed by GFAP staining) in the tumors (supplementary Fig. 4D, mid panel), which has been associated with invasive of brain tumors(25). H&E staining shows areas of high cellularity and vascularity mixed with necrotic areas (Fig. 4D, right panel). Further immunofluorescence staining indicates expression of the stem cell marker CD133 (supplementary Fig. S4B). Expression of the original exogenous factors was confirmed by immunofluorescence (Oct4, supplementary Fig. S4C) and immunohistochemistry (Myc, p53, and Ras, supplementary Fig. S4D–F) staining.
Figure 4. Tumor initiation in vivo from OMRP-transduced astrocytes.
A). Tumor formation in nude mice from 500 firefly luciferase-labeled, OMRP-transformed astrocytes injected intracranially. Bioluminescence imaging was used to follow tumor growth non-invasively at different time points.
B). Exponential increase in intracranial bioluminescence signals from the OMRP-transformed cells during week 2–6. The error bars represent standard error of the mean (SEM), n=5.
C). Brains of non-injected mice (left panels) and those injected with OMRP-transformed cells (middle and right panels). Notice with smaller, deformed shapes of the brains and abnormal tissue growth with greenish tint (from EGFP-labeled tumor cells) in the latter group.
D). Immunohistochemical (left and middle panels) and H&E (right panel) staining of brain sections from the tumor-bearing mice. Scale bars represent 50 μm.
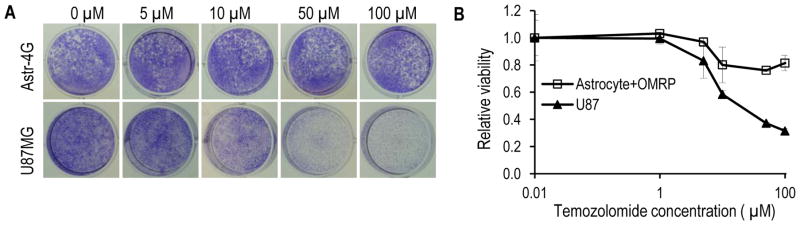
Another reported characteristic of cancer stem cells is their elevated resistance to chemotherapy. To determine if the OMRP-transformed cells exhibit similar properties, we measured the resistance of the cells to temozolomide, an alkylating agent used commonly for advanced stage brain cancer patients. OMRP-transduced cells are significantly more resistant to temozolomide than U87MG (Fig. 5A), with IC50 at least more than 10x higher in the former (Fig. 5B).
Fig. 5.
Drug resistance of OMRP-transduced astrocytes.
A). Crystal violet staining of OMRP-transformed astrocytes and U87MG cells after treatment with various concentrations of temozolomide.
B). Quantitative estimate of the relative survival of OMRP-transduced astrocytes and U87MG cells after temozolomide treatment. The error bars represent standard deviation (n=3).
Discussion
Thus we have successfully generated cancer stem cell-like cells from primary human astrocytes with a relatively simple cocktail of defined genetic factors: Oct4, Myc, Ras, and p53DD. Most remarkably, the cells possess strong tumor initiating abilities and express various molecular markers of glioma stem cells, which distinguishes them from previously successful transformation of human astrocytes(12,13). The critical importance of Oct4 implicates a key role for epigenetic reprogramming in facilitating the transformation process.
Our study should contribute to the cells of origin studies in brain tumor. Mouse genetic studies conducted so far has indicated that glioma can arise from neural stem cells (NSCs)(26,27) and oligodendrocyte progenitor cells (OPCs)(28). Attempts to transform mature astrocytes was less successful(29). However, a more recent study indicated that lentivirus mediated transduction of shNF1-shp53 or H-RasV12-shp53 could cause dedifferentiation and transformation of mature astrocytes in mice (30). Our results therefore provide evidence that mature human astrocytes could be dedifferentiated and transformed into glioma-like cells, similar to mouse cells.
Of the four factors used for carry out oncogenic transformation of astrocytes, Oct4 and myc have been shown to be over-expressed in brain tumors(31) while p53(32) has been shown to be frequently mutated. Therefore all three are quite relevant for human glioma development. On the other hand, H-Ras, one of the four factors used in our transformation of primary astrocytes, is not frequently mutated in brain cancers. Despite the fact that Ras has been shown to play some important roles in glioma (33), we believe our model could be made more relevant by replacing Ras with other more relevant oncogenic factors such as EGFR or EGFRvIII(34).
We propose to name the transformed cells induced cancer stem cells (iCSCs) or induced tumor-initiating cells (iTICs). We believe it should be possible to establish iCSCs from other cell lineages using similar procedures. The iCSC or iTICs should provide genetically tractable models to study cancer stem cell biology or develop novel cancer therapeutics.
Supplementary Material
Acknowledgments
Grant Support: This study was supported in part by grants CA155270, and ES024015 from the National Institutes of Health (to C-Y Li). In addition, it is also supported in part by the Duke Skin Disease Research Center (AR066527).
The authors thank Drs. James Thomson, Shinya Yamanaka, and Christopher Counter for making their plasmids available through Addgene. We would like to thank the Duke Microarray Share Resource for their technical support, data management and generation of the microarray data reported in this manuscript. We would also like to thank the Optical Imaging Resource at the Duke Cancer Institute.
Footnotes
Conflict of interest statement: The authors declare no financial interest in the work described in this study.
References
- 1.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 4.Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–30. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–6. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603–15. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 9.Kreso A, van Galen P, Pedley NM, Lima-Fernandes E, Frelin C, Davis T, et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med. 2013 doi: 10.1038/nm.3418. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–43. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 11.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rich JN, Guo C, McLendon RE, Bigner DD, Wang XF, Counter CM. A genetically tractable model of human glioma formation. Cancer Res. 2001;61:3556–60. [PubMed] [Google Scholar]
- 13.Sonoda Y, Ozawa T, Hirose Y, Aldape KD, McMahon M, Berger MS, et al. Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res. 2001;61:4956–60. [PubMed] [Google Scholar]
- 14.Cifone MA, Fidler IJ. Correlation of patterns of anchorage-independent growth with in vivo behavior of cells from a murine fibrosarcoma. Proc Natl Acad Sci U S A. 1980;77:1039–43. doi: 10.1073/pnas.77.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozawa T, James CD. Establishing intracranial brain tumor xenografts with subsequent analysis of tumor growth and response to therapy using bioluminescence imaging. J Vis Exp. 2010 doi: 10.3791/1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Kendall SD, Linardic CM, Adam SJ, Counter CM. A network of genetic events sufficient to convert normal human cells to a tumorigenic state. Cancer Res. 2005;65:9824–8. doi: 10.1158/0008-5472.CAN-05-1543. [DOI] [PubMed] [Google Scholar]
- 18.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–8. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 19.Boehm JS, Hession MT, Bulmer SE, Hahn WC. Transformation of human and murine fibroblasts without viral oncoproteins. Mol Cell Biol. 2005;25:6464–74. doi: 10.1128/MCB.25.15.6464-6474.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellows CG, Aubin JE. Determination of numbers of osteoprogenitors present in isolated fetal rat calvaria cells in vitro. Dev Biol. 1989;133:8–13. doi: 10.1016/0012-1606(89)90291-1. [DOI] [PubMed] [Google Scholar]
- 21.Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–88. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- 22.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 23.Read TA, Fogarty MP, Markant SL, McLendon RE, Wei Z, Ellison DW, et al. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell. 2009;15:135–47. doi: 10.1016/j.ccr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parry PV, Engh JA. CD90 is identified as a marker for cancer stem cells in high-grade gliomas using tissue microarrays. Neurosurgery. 2012;70:N23–4. doi: 10.1227/01.neu.0000413227.80467.92. [DOI] [PubMed] [Google Scholar]
- 25.Le DM, Besson A, Fogg DK, Choi KS, Waisman DM, Goodyer CG, et al. Exploitation of astrocytes by glioma cells to facilitate invasiveness: a mechanism involving matrix metalloproteinase-2 and the urokinase-type plasminogen activator-plasmin cascade. J Neurosci. 2003;23:4034–43. doi: 10.1523/JNEUROSCI.23-10-04034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon CH, Zhao D, Chen J, Alcantara S, Li Y, Burns DK, et al. Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 2008;68:3286–94. doi: 10.1158/0008-5472.CAN-07-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C, Sage JC, Miller MR, Verhaak RG, Hippenmeyer S, Vogel H, et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146:209–21. doi: 10.1016/j.cell.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacques TS, Swales A, Brzozowski MJ, Henriquez NV, Linehan JM, Mirzadeh Z, et al. Combinations of genetic mutations in the adult neural stem cell compartment determine brain tumour phenotypes. EMBO J. 2010;29:222–35. doi: 10.1038/emboj.2009.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedmann-Morvinski D, Bushong EA, Ke E, Soda Y, Marumoto T, Singer O, et al. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science. 2012;338:1080–4. doi: 10.1126/science.1226929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frankel RH, Bayona W, Koslow M, Newcomb EW. p53 mutations in human malignant gliomas: comparison of loss of heterozygosity with mutation frequency. Cancer Res. 1992;52:1427–33. [PubMed] [Google Scholar]
- 33.Feldkamp MM, Lala P, Lau N, Roncari L, Guha A. Expression of activated epidermal growth factor receptors, Ras-guanosine triphosphate, and mitogen-activated protein kinase in human glioblastoma multiforme specimens. Neurosurgery. 1999;45:1442–53. doi: 10.1097/00006123-199912000-00034. [DOI] [PubMed] [Google Scholar]
- 34.Fan QW, Cheng CK, Gustafson WC, Charron E, Zipper P, Wong RA, et al. EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell. 2013;24:438–49. doi: 10.1016/j.ccr.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.