Abstract
An in vitro skin diffusion study of pure forskolin (1) versus a 1-containing Plectranthus barbatus root extract (P. barbatus extract) in hairless guinea pig skin and human skin in a flow-through diffusion cell system was conducted and is being reported for the first time. Both topical agents were formulated in a solution of 70% ethanol and 30% propylene glycol (v/v). The results showed that forskolin can be delivered through the stratum corneum and that the flux of this compound was enhanced when 1 was delivered as a constituent of the P. barbatus extract as compared to an equivalent amount in pure form. These results suggest that the P. barbatus extract used contains permeation enhancement activity from other compound(s) contained in the crude root extract. It is possible that P. barbatus root extract may be used as an economical source of 1 to perform topical chemical manipulation of pigmentation in high-risk populations.
Fair-skinned people are much more likely to be diagnosed with melanoma and other skin cancers when compared with dark-skinned individuals (up to 500 times). This is because fairness of skin correlates with diminished epidermal expression of UV-protective eumelanin, which is caused in part by defective signaling in melanocytes by the melanocortin-1 receptor (MC1R), a Gs-coupled melanocytic surface receptor that promotes cyclic adenosine monophosphate (cAMP) accumulation in the cytoplasm when bound by its cognate ligand, α-melanocyte stimulating hormone (α-MSH).1–5 Individuals with loss-of-function MC1R polymorphisms exhibit a defective tanning response and therefore may receive dangerous DNA damage when exposed to sun for extended periods of time.5 The incidence of melanoma, the most lethal skin disease induced by UV irradiation, has been increasing over the last several decades. In the United States, the current risk of developing melanoma is 2.4% for men and 1.6% for women. In 2008, 62 480 newly diagnosed melanomas were expected with 8420 individuals predicted to die of melanoma.6 Eumelanin and pheomelanin are two basic types of pigment in the skin. Eumelanin is the brown-black pigment polymer preferentially expressed in people with the darkest complexion and is highly protective against UV radiation. Pheomelanin, on the other hand, is a more soluble, red-yellow compound that is the major pigment found in fair-skinned individuals and is much less able to absorb UV radiation in the skin.7 A person with dark pigmentation is protected from immediate effects of UV radiation (sunburn) as well as chronic UV effects (skin degenerative changes, keratinocyte malignancies, and melanomas) due to the strong ability of UV absorption by eumelanin, which blocks UV penetration into deeper layers of the skin.7,8
The roots of Plectranthus barbatus Andrews (Lamiaceae) (previously known as Coleus forskohlii) have been used for centuries in traditional Ayurvedic medicine.9 Forskolin (1), a cell-permeable diterpenoid that stimulates adenyl cyclase to promote cAMP accumulation, is a major component isolated from P. barbatus extract.10–12 The activity of 1 as a direct activator of adenylyl cyclase and enhancer of cytoplasmic cAMP levels has been known for more than two decades and been applied as an aid for weight loss, glaucoma, prostatism, hypertension, and other physiologic responses.13–15 However, the potential use of forskolin in sunless tanning products has been revealed only recently.5 Thus, when forskolin (both when present in a crude extract of P. barbatus and chemically pure 1) was applied topically to a murine model with humanized skin and a mutant MC1R, significant melanization was observed.5 This study implied that 1 is a potential candidate for clinical application in sunless tanning products as well as a topical small-molecule manipulation of pigmentation that would protect against UV-induced cutaneous DNA damage and tumorigenesis.5 It has been proposed that chemically induced pigmentation by forskolin bypassed the defective cAMP signaling associated with mutant MC1R and induced production of protective eumelanin.5 To chemically trigger epidermal pigmentation, topically applied drugs need to penetrate through the stratum corneum to reach melanocytes, cells located in the lower part of the skin’s epidermis. As a potential candidate for topical administration in sunless tanning products and UV protection, it is essential to study the permeation of 1 in human skin. However, no human skin permeation studies of forskolin have been reported in the literature to date. The current study focuses on the topical delivery of pure 1 and P. barbatus extract on guinea pig and human skin to determine the skin disposition and rates of transport of 1. An in vitro skin diffusion study of pure forskolin (1) versus P. barbatus extract in hairless guinea pig skin and human skin in a flow-through diffusion cell system was conducted. Both pure 1 and P. barbatus extract were formulated in 70% ethanol and 30% propylene glycol (v/v). The hairless guinea pig was used because it is the best small animal model for comparison to humans for skin permeation studies.16,17
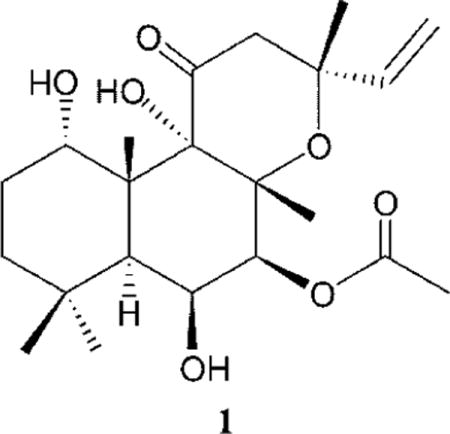
The purposes of this study were to investigate if forskolin can be delivered across the stratum corneum at a significant rate and to determine if there is a difference between human skin flux values using pure forskolin versus a P. barbatus extract containing 1.
In this study, the diffusion of forskolin was quantified by LC-MS. Representative profiles of the in vitro permeation study of human skin and guinea pig skin treated with pure 1 and P. barbatus extract are shown in Figure 1. Permeation data obtained with pure 1 and P. barbatus extract are shown in Table 1. The guinea pig skin flux value for pure 1 was 3.4 ± 1.7 nmol/cm2/h with a lag time of 2.8 ± 1.8 h. Unexpectedly, it was found that topical treatment with an equimolar concentration of 1 in P. barbatus extract was significantly different, with flux and lag time values of 18.5 ± 2.3 nmol/cm2/h and 5.5 ± 2.9 h, respectively. Similar differences were observed in human skin; flux values for forskolin from pure 1 and P. barbatus extract were 0.18 ± 0.04 and 3.6 ± 1.0 nmol/cm2/h, with lag times of 1.5 ± 0.6 and 4.8 ± 3.0 h, respectively. As expected in human skin, a tissue more resistant to drug transport, flux values for forskolin (both from pure 1 and from P. barbatus extract) were lower [19-fold for pure 1 (p < 0.001) and 5-fold for P. barbatus extract (p < 0.001)] than the corresponding values in guinea pig skin. The results of these permeation studies showed clearly that 1 (both from the P. barbatus extract and chemically pure 1) can be delivered transdermally, although at different permeation rates through the skin from pure 1 as compared to P. barbatus extract. The flux of forskolin is much greater in human (p < 0.001) and guinea pig skin (p < 0.001) from the P. barbatus extract than the pure compound 1 when used at equivalent active concentrations and in identical vehicles. This might suggest that skin permeation of 1 is enhanced by compound(s) other than forskolin in the crude root extract. Solutions of 10 μg/mL pure 1 in CH3CN and 10 μg/mL P. barbatus extract in CH3CN were scanned with UV wavelength 200–300 nm with a photodiode array detector. Chromatograms are shown in Figure S1, Supporting Information. Compared with pure 1, several new peaks were found after 12 min in the chromatogram of P. barbatus extract, which might be from the potential enhancers of the natural product. It is also possible that the constituents of the root extract modified the solubility and percent saturation of 1 so that the flux was higher from the extract because it had a higher thermodynamic activity than the pure compound in solution. A higher flux of forskolin could result in an enhanced pigmentation effect in clinical applications, and 1 as part of a P. barbatus root extract is much less expensive than pure 1. Thus, P. barbatus root extract might be useful as an economical source of 1 for topical administration for UV protection and/or sunless tanning products. Many chemicals have enhanced skin permeation of drugs via different mechanisms, such as altering skin barrier lipids or changing partitioning of the drug.18–20 Additional work needs to be completed to identify the potential permeation enhancer(s) in P. barbatus extract and determine whether skin permeation rates of 1 are enhanced through barrier disruption and/or changing physicochemical properties of the drug in the matrix, such as stratum corneum/vehicle partition coefficient changes or solubility changes in the vehicle.
Figure 1.
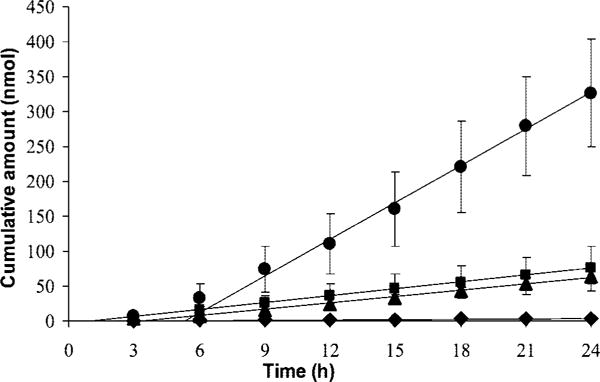
Representative permeation profile of human skin and guinea pig skin treated with pure forskolin (1) and P. barbatus extract. Pure 1-treated human skin (◆) [n = 7], P. barbatus extract-treated human skin (▲) [n = 3], pure 1-treated guinea pig skin (■) [n = 4], and P. barbatus extract-treated guinea pig skin (●) [n = 4].
Table 1.
Permeation Data from Pure Forskolin (1)-Treated Human Skin (1, n = 7), P. barbatus Extract-Treated Human Skin (n = 3), Pure 1-Treated Guinea Pig Skin (1, n = 4), and P. barbatus Extract-Treated Guinea Pig Skin (n = 4)
| treatment | 24 h skin concentration (μmol/g)a | 24 h cumulative amount (nmol) | flux (nmol/cm2/h)a | flux enhancement | lag time (h) |
|---|---|---|---|---|---|
| pure 1-treated human skin | 20.0 ± 10.6b | 3.8 ± 1.0 | 0.18 ± 0.04c,d | 1.5 ± 0.6 | |
| P. barbatus extract-treated human skin | 1.7 ± 1.7b | 63.5 ± 10.1 | 3.6 ± 1.0c,f | 20.0 | 4.8 ± 3.0 |
| pure 1-treated guinea pig skin | 5.8 ± 6.4 | 75.6 ± 31.8 | 3.4 ± 1.7d,e | 2.8 ± 1.8 | |
| P. barbatus extract-treated guinea pig skin | 2.5 ± 2.9 | 326.1 ± 76.9 | 18.5 ± 2.3e,f | 5.4 | 5.5 ± 2.9 |
Student’s t-test was performed on data of 24 h skin concentration and flux of pure 1 vs P. barbatus extract. This test was also performed on data of the same drug treatment on guinea pig skin vs human skin. p-values were calculated between two numbers with the same letter. Levels of significance with different p-values are shown below. All p-values shown are less than 0.001 except for the human skin content of 1 vs P. barbatus extract (p < 0.05).
p < 0.05.
p < 0.001.
Drug disposition concentrations in skin treated with a pure forskolin and a P. barbatus extract containing 1 were different. In human skin, when treated with pure 1 and P. barbatus extract, skin contents of 1 were found to be 20.0 ± 10.6 and 1.7 ± 1.7 μmol/g, respectively (p < 0.05). This significant difference suggests different skin permeation mechanisms through human skin for pure 1 and the forskolin from P. barbatus extract, which supports the notion that other compound(s) in the P. barbatus extract enhance transdermal delivery.
Experimental Section
General Experimental Procedures
Pure forskolin (1), absolute ethanol, and propylene glycol were purchased from Sigma-Aldrich (St. Louis, MO). A crude extract of P. barbatus root (Lot # C40360) was obtained from ATZ Natural (Edgewater, NJ). The certificate of analysis of this substance is shown in Table S1, Supporting Information. Acetonitrile (HPLC grade) and 70% ethanol were purchased from Fisher Scientific (Fairlawn, NJ). Water was purified with a NANOpure Diamond ultrapure water system (Barnstead International; Dubuque, IA).
Formulation Preparation
Pure forskolin (1) and a P. barbatus extract containing 1 were used as two separate sources of this compound. All topical agents used in the skin permeation experiment were prepared as a w/v solution in a standard dermatologic vehicle of 70% ethanol and 30% propylene glycol. The formulation of pure 1 was prepared by mixing this substance with the above dermatologic vehicle to make a solution that contained 64.8 mg/mL. The formulation of P. barbatus extract was made by mixing 40 g of the powder of crude extract of P. barbatus root with 100 mL of the above vehicle for 1 h at room temperature on a stir plate with constant agitation. The solution was centrifuged (10 min, room temperature, 2000g), and the soluble portion (supernatant) was collected and filtered (0.45 μm cellulose acetate filter). The P. barbatus extract was stored at room temperature with no discernible loss of activity over time. The same batch of P. barbatus extract was used to conduct the skin permeation experiments. The concentration of 1 in the extract solution was assayed by LC-MS as 64.8 mg/mL.
Human Skin Preparation
Human skin harvested during abdominal reduction surgery was used for the diffusion study. Skin sections were obtained by using a Padgett dermatome (Integra LifeSciences Corporation; Plainsboro, NJ) set to 250 μm; these sections were stored at −20 °C. The human tissue use was approved by the University of Kentucky Institutional Review Board.
Guinea Pig Skin Preparation
Guinea pig skin was harvested after pentobarbital overdose and used for the diffusion study. Full thickness skin was stored at −20 °C. Prior to the diffusion study, skin sections were obtained using a Padgett dermatome set to 250 μm. Guinea pig tissue use was approved by the University of Kentucky Institutional Animal Care and Use Committee (IACUC).
In Vitro Permeation Studies
PermeGear flow-through (In-line, Hellertown, PA) diffusion cells were kept at 32 °C with a circulating water bath. Data were collected using skin from a single donor with three or four cells for pure 1 and the P. barbatus extract. The receiver solution was 60:40 nanopure water–ethanol, and the pump was set at a flow rate of 0.8 mL/h. A solution of 70:30 absolute ethanol–propylene glycol was applied to the skin, and each cell was charged with 0.175 mL of the drug solution. Both P. barbatus extract and pure 1 solutions contained 64.8 mg/mL forskolin. Samples were collected in 3 h increments for 24 h. All samples were stored at 4 °C, until analyzed by liquid chromatography/mass spectroscopy (LC-MS). The cumulative quantity of forskolin collected in the receiver compartment from pure 1 and P. barbatus extract was plotted as a function of time (Figure 1). The flux value for a given experiment was obtained from the slope (steady-state portion) of the cumulative amount of drug permeated versus time plot.
Drug disposition in skin samples was measured at the end of the 24 h experiment. The skin tissue was rinsed with nanopure water (3 times) for a total of 30 s and wiped off with an alcohol pad. The entire piece of skin was blotted dry with a paper towel. To remove drug formulation adhering to the surface, the skin was tape stripped twice using Scotch book tape (3M; St. Paul, MN). Skin was rinsed an additional time with Nanopure water and blotted dry again. The area of skin in contact with drug was excised, minced with a scalpel, and placed in a preweighed vial. Drug was extracted from skin by equilibrating with 10 mL of CH3CN and shaking overnight at ambient temperature. Samples were analyzed by LC-MS to determine 1 content in μmol of drug per gram of wet tissue weight (Table 1).
Quantitative Analysis
The LC-MS system was used to analyze samples.21 It consisted of a Waters Alliance 2695 pump and autosampler, a Waters Micromass ZQ detector, a Waters 996 photodiode array detector, and MassLynx software (Milford, MA). A Symmetry C18 (2.1 × 150 mm, 5 μm) column with a Sentry Symmetry C18 guard column (2.1 × 10 mm, 3.5 μm) was used with the LC-MS system. Volume injected onto the column was 20 μL. The flow rate was set at 0.25 mL/min. The MS detection was conducted using electrospray ionization (ESI) in positive mode. Selected-ion monitoring (SIM) was conducted for 1 at m/z 411.1 (dwell time 0.30 s) and m/z 428.2 (dwell time 0.30 s). The peak at m/z 428.2 was used for quantification because of the peak shape and lack of background interference. The capillary voltage was set at 3.0 kV and cone voltage set at 25 V, where the source block temperature and desolvation temperature were 120 and 250 °C, respectively. Nitrogen gas was used, and desolvation and cone gas flows were set at 450 and 50 L/h, respectively. The gradient elution mobile phase initially consisted of 45% A:55% B. A was pure CH3CN and B was Nanopure water with 5% CH3CN (v/v). The initial 45% mobile phase A was increased linearly to 62% over 10 min, maintained for 1 min, and then decreased linearly to 45% in 1 min and maintained for 8 min. Diffusion samples were directly injected onto the column. CH3CN solutions used to extract drug from the skins were diluted with additional CH3CN and injected onto the column. The retention time for 1 was 8.0–8.2 min. The total run time for each sample was 20 min. The detection limit of 1 was 20 ng/mL.
Data Evaluation and Statistical Analysis
The in vitro permeation data were calculated to assess permeation activity of forskolin on human skin and guinea pig skin treated with pure 1 and P. barbatus extract. Results are expressed as mean ± standard deviation from n = 3–7 replicates. Values of 1 flux and skin content treated with P. barbatus extract were compared to those treated with pure 1 on the same type of skin; flux data from the same drug solutions on different skin types were also compared. Differences between the above values were evaluated by the Student’s t-test for unpaired data using SigmaStat (Systat Software Inc., Richmond, CA), with p ≤ 0.05 taken to be significant.
Supplementary Material
Acknowledgments
A.L.S. acknowledges funding by the American Cancer Society (RSG-00-027-04-CDD).
Footnotes
Supporting Information Available: Certificate of analysis of Plectranthus barbatus extract and chromatograms of 10 μg/mL CH3CN solution of pure forskolin (1) and 10 μg/mL CH3CN solution of the P. barbatus extract containing 1 scanned with a photodiode array detector with UV wavelength 200–300 nm. This material is provided free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Valverde P, Healy E, Sikkink S, Haldane F, Thody AJ, Carothers A, Jackson IJ, Rees JL. Hum Mol Genet. 1996;5:1663–1666. doi: 10.1093/hmg/5.10.1663. [DOI] [PubMed] [Google Scholar]
- 2.Gruijl RF. Eur J Cancer. 1999;35:2003–2009. doi: 10.1016/s0959-8049(99)00283-x. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Malek Z, Scott MC, Suzuki I, Tada A, Im S, Lamoreux L, Ito S, Barsh G, Hearing VJ. Pigment Cell Res. 2000;13:156–162. doi: 10.1034/j.1600-0749.13.s8.28.x. [DOI] [PubMed] [Google Scholar]
- 4.Sturm RA, Duffy DL, Box NF, Chen W, Smit DJ, Brown DL, Stow JL, Leonard JH, Martin NG. Pigment Cell Res. 2003;16:266–272. doi: 10.1034/j.1600-0749.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 5.D’Orazio JA, Nobuhisa T, Cui R, Arya M, Spry M, Wakamatsu K, Igras V, Kunisada T, Granter SR, Nishimura EK, Ito S, Fisher DE. Nature. 2006;443:340–344. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 7.Schaffer JV, Bolognia JL. Arch Dermatol. 2001;137:1477–1485. doi: 10.1001/archderm.137.11.1477. [DOI] [PubMed] [Google Scholar]
- 8.Rees JL. Am J Hum Genet. 2004;75:739–751. doi: 10.1086/425285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ammon HT, Müller A. Planta Med. 1985;51:473–477. doi: 10.1055/s-2007-969566. [DOI] [PubMed] [Google Scholar]
- 10.Seamon KB, Padgett W, Daly JW. Proc Natl Acad Sci USA. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly JW, Padgett W, Seamon KB. J Neurochem. 1982;38:532–544. doi: 10.1111/j.1471-4159.1982.tb08660.x. [DOI] [PubMed] [Google Scholar]
- 12.Seamon K, Daly JW, Metzger H, De Souza NJ, Reden J. J Med Chem. 1983;26:436–439. doi: 10.1021/jm00357a021. [DOI] [PubMed] [Google Scholar]
- 13.Greenway FL, Bray GA, Heber D. Obes Res. 1995;3:561S–568S. doi: 10.1002/j.1550-8528.1995.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 14.Fleischner AM. 9,928,714. US Patent. 2003
- 15.Godard MP, Johnson BA, Richmond SR. Obesity. 2005;13:1335–1343. doi: 10.1038/oby.2005.162. [DOI] [PubMed] [Google Scholar]
- 16.Hood HL, Kraeling MEK, Robl MG, Bronaugh RL. Food Chem Toxicol. 1999;37:1105–1111. doi: 10.1016/s0278-6915(99)00100-3. [DOI] [PubMed] [Google Scholar]
- 17.Wilhelm K-P, Surber C, Maibach HI. J InVestig Dermatol. 1991;97:927–932. doi: 10.1111/1523-1747.ep12491710. [DOI] [PubMed] [Google Scholar]
- 18.Barry BW. J Controlled Release. 1987;6:85–97. [Google Scholar]
- 19.Barrie C, Finnin TMM. J Pharm Sci. 1999;88:955–958. doi: 10.1021/js990154g. [DOI] [PubMed] [Google Scholar]
- 20.Asbill CS, Michniak BB. Pharm Sci Technol Today. 2000;3:36–41. doi: 10.1016/s1461-5347(99)00225-4. [DOI] [PubMed] [Google Scholar]
- 21.Schaneberg BT, Khan IA. J AOAC Int. 2003;86:467–470. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


