Abstract
Current commercial tensile testing systems use spring-loaded or other compression-based grips to clamp materials in place posing a problem for very soft or delicate materials that cannot withstand this mechanical clamping force. In order to perform uniaxial tensile tests on soft tissues or materials, we have created a novel vacuum-assisted anchor (VAA).
Fibrin gels were subjected to uniaxial extension, and the testing data was used to determine material mechanical properties.
Utilizing the VAA, we achieved successful tensile breaks of soft fibrin gels while finding statistically significant differences between the mechanical properties of gels fabricated at two different fibrinogen concentrations.
Keywords: tissue grip, tensile testing, failure, soft materials, fibrin, elastic modulus
1 Introduction
Tensile testing systems are commonly used to impart mechanical load to materials in order to experimentally evaluate mechanical properties including stiffness and tensile strength. Current commercial tensile testing systems rely on spring-loaded or other compression-based grips to clamp tissues in place to avoid slipping during uniaxial extension. For robust and mature tissues or materials, the clamping force is typically not a problem. However, when attempting to clamp soft materials (which we define as tissue or materials with a tangent modulus ~15 kPA), the clamping force can cause catastrophic damage to the sample. This also poses a problem when studying the mechanobiology of soft materials when loading is desired. For instance, cell-seeded fibrin gels are used to create tissue engineered constructs, such as vascular grafts(1–3) and heart valve leaflets(4, 5), and as three dimensional (3D) in-vitro culture systems(6–8). Unless these gels are polymerized around a grippable post(9) or integrated into a stronger material(10), they cannot be secured in conventional uniaxial tensile loading systems until significant remodeling has occurred. Attempts to mechanically test or load purely gel-based tissue constructs have been limited to ring tests on sections of tubular cell-seeded constructs(11–13). While ring tests represent a valuable tool for determining mechanical properties, the method has inherent deficiencies including non-constant strain rates, compression at the pulling posts, and convoluting edge effects(14). Using either ring tests or custom molds restricts the geometry and possibly the material of samples that can be used for testing. Performing uniaxial tensile tests mitigates the problems surrounding ring tests and will allow researchers to gather meaningful tensile data that might reveal the role of the mechanical environment during the initiation of tissue remodeling in these experimental systems.
Due to the limitations of current uniaxial tensile testing methods, we sought to design an alternative gripping mechanism for soft materials. Drawing inspiration from micro-aspiration techniques that are used for membrane mechanics studies(15), we designed a vacuum-based anchorage system to grip a soft material subjected to uniaxial tensile loading. Here we present our solution: a novel vacuum-assisted anchor (VAA). Our design was validated by using the VAA to grip and mechanically test two soft materials (fibrin gels) that were previously unable to withstand uniaxial tensile testing using conventional methods.
2 Materials and Methods
2.1 Design and functional principles of the VAA
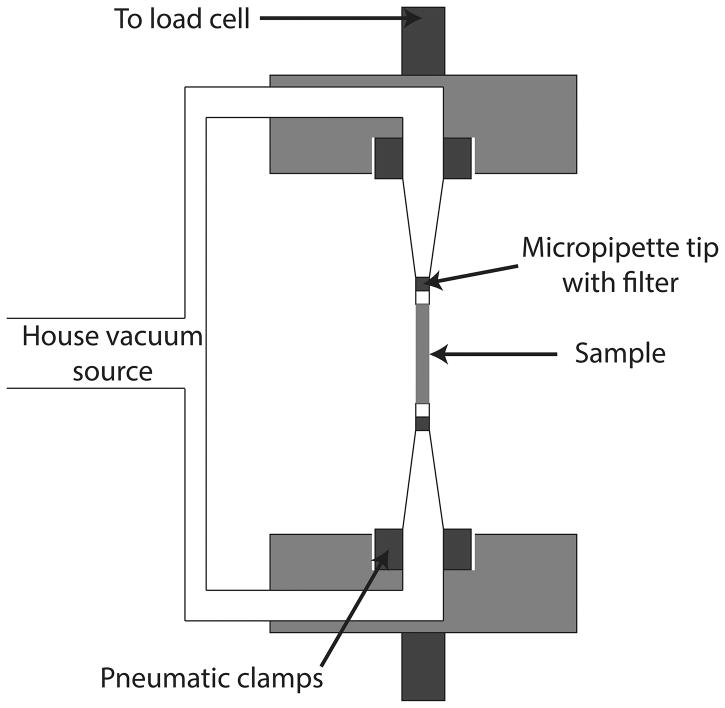
The VAA design is shown in Figure 1. The prototype uses a typical house vacuum source (−45 kPa gauge pressure) to secure a sample against an in-line filter via a 90 degree plastic elbow. The elbow (1/4″ outer diameter) fits inside the pneumatic clamps of a commercial tensile testing system and can therefore be moved up and down. The open end of the elbow is connected to a truncated, aerosol-resistant micropipette tip, allowing the sample to be secured without being pulled into the vacuum line.
Figure 1. VAA Design Concept.
Schematic of the VAA design is shown. A house vacuum source is directed to the testing sample and clamped by pneumatic grips.
Our prototype was designed to be used within a specific commercial uniaxial tensile testing system (Instron, #5543a, Norwood, MA) although vacuum supply line sizes can also be altered to fit inside a wide variety of commercial testing systems. All components of the prototype VAA are readily available in most laboratory settings.
2.2 Fibrin gels as testing materials
Fibrin gel was used as a sample material to demonstrate proof-of-concept of the VAA for tensile testing. Two densities of fibrin gels were fabricated by mixing bovine fibrinogen type I at different concentrations (5 mg/mL and 10 mg/mL, Sigma-Aldrich, St. Louis MO) with bovine thrombin (1 NIHU/mL, Sigma-Aldrich, St. Louis, MO) within the troughs of Flexcell™ Tissue-Train™ plates (Flexcell Int., Hillsborough, NC). Gels were allowed to polymerize for at least 2 hours in incubator conditions (37 °C, 5% CO2).
Additional gels were fabricated using the same concentrations of fibrinogen and thrombin mentioned above to verify the VAA. These gels were formed between two strips of polyvinyl alcohol (PVA) sponge material that served as anchorage points for mechanical testing.
2.3 Mechanical testing
The VAA was clamped into an Instron uniaxial tensile testing system (Instron, #5543a, Norwood, MA,) and fibrin gels were gently placed next to the open ends of the VAA while the vacuum was turned on. Samples were stretched to initial, unloaded lengths by moving the crosshead until force readings were present. A constant crosshead speed of 0.1 mm/sec was used to pull on the samples, and the applied load and resulting displacement (d) were recorded continuously using the Instron-packaged software (Bluehill, Version 2, Instron, Norwood, MA). The axial component of the First Piola Kirchhoff Stress (the P11 component of the full First Piola Kirchhoff Stress Tensor, P) was calculated using the force measurement (f) from a 25 N load cell (MDB-25, Transducer Techniques, Temecula, CA) and the initial measured area (Ao, diameter measured from pictures of stress free samples held in VAA), as follows:
| (1) |
The fibrin gels that were formed between the PVA-sponge strips were tested in the same manner as described above. The PVA-sponge served as the anchor point for clamping.
2.4 Material assumptions and mechanical properties
We assumed that all fibrin gels displayed transverse isotropy for a rod-like material. A Poisson’s Ratio (ν) of 0.25 was assumed which was consistent with previously reported values for fibrin(12). The deformation gradient tensor, F, is described as:
| (2) |
The axial stretch (λa) is calculated from the initial length measurement (L0) and the displacement (d) measured during testing as follows:
| (3) |
The transverse stretch (λt) was calculated from the exact relation for finite stretch values as follows(16):
| (4) |
The Cauchy stress is defined as:
| (5) |
where the Jacobian, J, is defined as the determinant of F
| (6) |
For a uniaxial loaded, we will consider the axial component of the Cauchy stress (σ11):
| (7) |
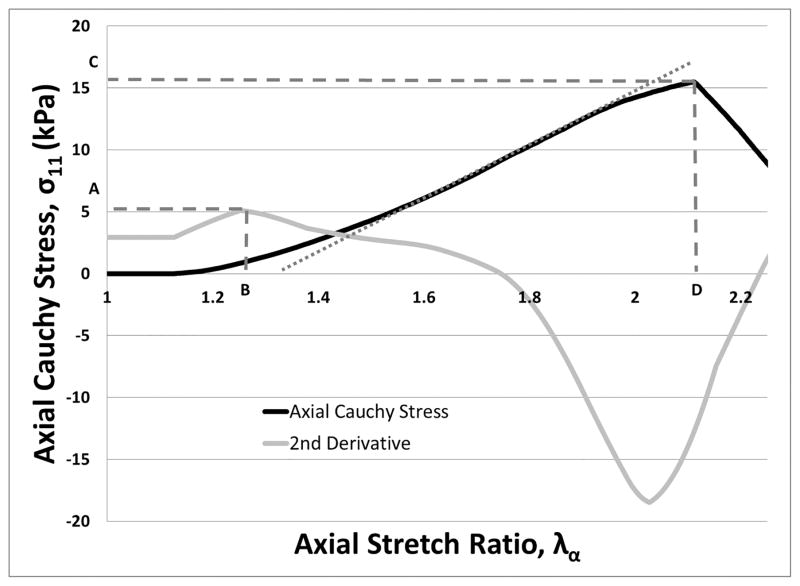
We defined the experimentally determined elastic limit as the stretch ratio where the second derivative of the axial component of Cauchy stress (σ11) with respect to λa is a maxima(17) (see Figure 2.) The “yield stress” was defined as the value of σ11 at the experimentally determined elastic limit; i.e. the “yield stretch.” The tangent modulus is defined as the slope of the linear portion of the σ11 vs. λa curve. The ultimate tensile strength (UTS) is defined as the maximum value of the σ11 vs. λa curve, and the ultimate stretch is the value of λa where the UTS is defined. The ultimate properties were only calculated for samples that had a confirmed tensile failure. Any samples that demonstrated slipping behavior were used to find only the tangent modulus of the sample and not UTS.
Figure 2. Features of Stress-Strain Behavior Captured by the VAA.
The black curve shows σ11 vs. λa from a sample test using 5 mg/mL fibrinogen. The gray curve is a scaled form of the second derivative of the σ11 with respect to λa. Points A and B indicate the yield stress and yield stretch, respectively. The ultimate tensile strength (UTS) and ultimate stretch are noted by points C and D, respectively. The linear portion of the black stress-strain curve is estimated by a dotted gray line. The slope of this line is the tangent modulus.
2.5 Statistics
Student t-tests were used to compare mechanical properties (yield stretch, yield stress, tangent modulus, ultimate stretch, ultimate tensile strength) between material groups. Statistical significance was assigned to p-values < 0.05. Data given as mean ± SD.
3 Results
3.1 Device performance
The VAA was able to successfully grip soft, delicate fibrin gels subjected to uniaxial tensile testing leading to tensile failures (confirmed with frames from slow-motion video in Figure 3.) By using vacuum, the VAA does not damage the sample at the gripped location and creates a highly tunable method of delivering anchoring force to soft materials. The use of the VAA yielded true tensile breaks in 47% of all tests (compared to a 0% success rate we experienced in our lab using conventional gripping methods). A total of 59% of samples provided useful yielding data (i.e. stress-strain data through stretch ratios up to the yield stretch, see Table 1).
Figure 3. Visual Confirmation of a Tensile Break.
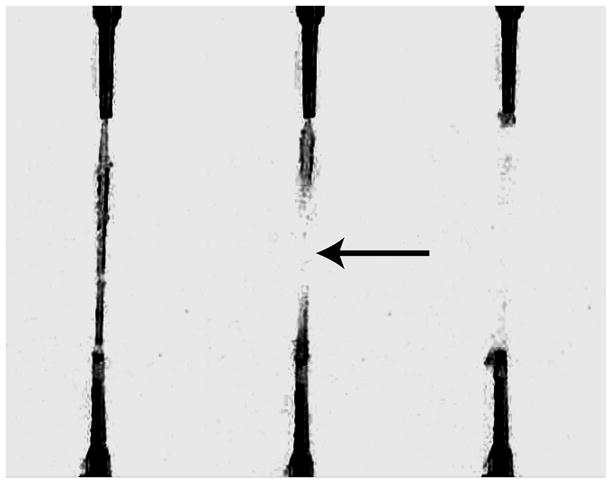
Three frames (captured at 30 frames per second) from a video of uniaxial tensile test are shown. The left frame shows the gel just before its tensile failure. The middle frame confirms a tensile break in the center of the fibrin gel (arrow) with the two halves of the fibrin gel recoiling back to their respective VAA grips (right frame).
Table 1.
Breakdown of Useful Sample Numbers by Group
| Group | Total Samples | Useful Yield Data | Useful Ultimate Data |
|---|---|---|---|
| Low | n=29 | n=20 (69%) | n=18 (62%) |
| High | n=29 | n=14 (48%) | n=9 (31%) |
| Total | n=58 | n=34 (59%) | n=27 (47%) |
Successful tensile tests illustrate the gripping efficiency of the VAA. By utilizing the VAA during uniaxial tensile loading, we were able to collect meaningful tensile and ultimate data from samples in both groups with much greater efficiency over conventional gripping mechanisms.
3.2 Device validation and verification
The VAA was validated by determining the mechanical properties of two groups of soft materials that were previously unable to be evaluated using conventional methods for uniaxial tensile testing. A summary of the elastic mechanical properties of the fibrin gels made with two different concentrations of fibrinogen (low - 5 mg/ml, or high - 10 mg/ml) is provided in Table 2. The lower concentration group had a higher yield stress (2.74±1.62 vs. 1.53±0.74 kPa; p=0.014) and tangent modulus (12.94±4.85 vs. 9.64±3.77 kPa; p=0.033). A summary of the ultimate mechanical properties is provided in Table 3. The lower concentration group also had a higher ultimate tensile strength (UTS; 8.18±3.97 vs. 4.12±1.55 kPa; p=0.001) and ultimate stretch (2.00±0.23 vs. 1.76±0.25; p=0.031).
Table 2.
Fibrin Gel Elastic Mechanical Properties
| Group | Yield Stretch | Yield Stress (kPa) | Tangent Modulus (kPa) |
|---|---|---|---|
| Low (n=20) | 1.20 ± 0.11 | 2.74 ± 1.62 | 12.94 ± 4.85 |
| High (n=14) | 1.24 ± 0.12 | 1.53 ± 0.74 | 9.64 ± 3.77 |
| p-value | 0.322 | 0.014 * | 0.033 * |
Significant differences (*) in the elastic mechanical properties of the fibrin gel groups are revealed during tensile tests using the VAA. Yield stretch, yield stress, and tangent modulus values are reported as mean ± SD from each fibrin gel group. The low concentration group had higher yield stress and tangent modulus values.
Table 3.
Fibrin Gel Ultimate Mechanical Properties
| Group | Ultimate Stretch | UTS (kPa) |
|---|---|---|
| Low (n=18) | 2.00 ± 0.23 | 8.18 ± 3.97 |
| High (n=9) | 1.76 ± 0.25 | 4.12 ± 1.55 |
| p-value | 0.031 * | 0.001 * |
Significant differences (*) in the ultimate mechanical properties of the fibrin gel groups are revealed during tensile tests using the VAA. Ultimate stretch and ultimate tensile strength (UTS) are reported as mean ± SD from each fibrin gel group. The low concentration group had higher ultimate stretch and UTS values.
The lower concentration VAA gels had a similar yield stress and tangent modulus when compared to the verification gels [yield stress: (2.74±1.62 vs. 3.95±1.88 kPa, respectively; p=0.20), tangent modulus: (12.94±4.85 vs. 12.1±2.97 kPa, respectively; p=0.72)]. The higher concentration VAA gels also had a similar yield stress and tangent modulus when compared to the verification gels [yield stress: (1.53±0.74 vs. 1.97±0.58 kPa; p=0.29), tangent modulus: (9.64±3.77 vs. 10.40±1.69 kPa; p=0.69)].
4 Discussion
In this study, we developed a device for uniaxial tensile loading of soft materials such as gels. Previous attempts to do so using conventional mechanical grips consistently resulted in failures at the gripped location. We designed our vacuum-based gripping mechanism to provide a means to adequately grip the sample during tensile loading – even to true tensile failures – while avoiding the edge effects associated with ring tests. Our novel grip described here could be used to evaluate the uniaxial mechanical properties of a wide array of soft, gelatinous-like tissues and materials, both biological and non-biological. Further, it could be used to impart uniaxial loading on materials, such as cell-seeded gels for mechanobiology studies.
Analysis of our mechanical tests revealed differences in the mechanical properties between two groups of soft fibrin gel strips, which could not be achieved using other conventional testing systems. Our results are similar to a previously published study by Cummings et al.(11), which performed ring tests of tubular fibrin constructs, and calculated a tangential modulus of 19 kPa for gels created with 4 mg/mL fibrinogen and 0.4 NIHU/mL thrombin. We calculated a mean tangent modulus of ~13 kPa for our low concentration group, which was a similar fibrinogen concentration (5 mg/mL) to the Cummings et al. study. The VAA was also verified through our own testing using alternative means.
The VAA will allow for uniaxial tensile testing of soft materials that were previously limited to other mechanical testing methods such as compressive testing and atomic force microscopy (AFM) to determine mechanical properties. For example, Kirchmajer et al.(18) have developed a degradable genipin cross-linked gelatin hydrogel to be used as a scaffold for tissue engineering purposes. The mechanical characterization of this soft material was limited to compression and rheological studies - sufficient for researchers looking to use this hydrogel scaffold for creating tissue engineered cartilage or other compressive load bearing tissues. However, the same hydrogel might also be useful for tissues bearing tensile loads (e.g. tendon, blood vessels, skin). Evaluation of soft material tensile properties with a device such as the VAA will be a critical step towards engineering new tensile load bearing tissue equivalents(19–22).
AFM is a technique often used to investigate the mechanical properties of individual cells, fibers, or other materials that may be too delicate to undergo conventional uniaxial tensile testing where compressive grips are employed. Equipment cost considerations and availability can make using AFM impractical; however the desire to know tensile properties of cells, their substrates, and the effects of the substrate’s mechanical properties has left researchers with no other options for very soft materials. For example, Solon et al.(23) used AFM to examine the functional effect of polyacrylamide gel stiffness on cultured fibroblasts, revealing important insights into how substrate stiffness can affect cell stiffness and f-actin organization. The VAA provides an alternative tool for performing uniaxial tensile tests on soft materials like polyacrylamide gels that is more affordable and readily available than AFM.
The VAA is inherently limited by the strength of the vacuum source, the normal area of the sample in contact with the device, and the integrity of the seal between the sample and the device. A quality seal will ultimately lead to higher success rates during mechanical testing. This is demonstrated in the differences in the success rates between the high and low groups. The higher concentration fibrinogen group will have more dense fibers (24) and less water in the sample. As the sample is pulled, water will be squeezed out, thus eventually compromising the quality of the seal. This phenomenon would partially explain the differences in success rates between the two testing groups. The theoretical maximum gripping force is limited to the product of atmospheric pressure and the normal area of the sample in contact with the VAA. A practical limit to maximum gripping force is dependent upon the strength of vacuum source (house source or commonly available vacuum pumps). In order to extend this technology to hydrated samples, improvements in the quality and repeatability of the seal would need to be made.
Our mechanical analysis has limitations inherent to the material fabrication and assumptions made regarding our test materials (fibrin gels). When fabricating our gels, we only varied the fibrinogen concentration. The amount of thrombin used was held constant. This meant that the ratio of fibrinogen to thrombin in each sample was also different and may have introduced convoluting effects such as changing relative fiber diameters, lengths and branchpoint densities (24). Additionally, we assumed a Poisson’s Ratio of 0.25 for both groups although the actual value is likely to change when changing the concentration of fibrinogen in the sample groups. Lastly, we assumed a uniform strain across the length of the sample though this may not be the case. Future studies will determine any convoluting effects of non-constant fibrinogen to thrombin ratios by testing additional samples where this ratio is kept constant. Future studies will also focus on measuring transverse stretch in order to calculate an experimental Poisson’s Ratio and determining the axial strain distribution through video analysis and ink spattering of the samples. These analyses will also help determine if the VAA affects the strain conditions near the seal. These considerations were beyond the scope of the current study, which was simply to perform a proof-of-concept of our novel VAA gripping system to perform tensile testing on soft materials.
4.1 Conclusions
Our VAA design was validated through the successful mechanical test of two soft materials (fibrin gels) that were previously unable to withstand uniaxial tensile testing using conventional methods. This anchorage method will allow new studies into the uniaxial mechanics and/or mechanobiology of soft materials.
Acknowledgments
We would like to acknowledge funding support by the National Institutes of Health (Cardiovascular Bioengineering Training Program T32 HL076124 and Biomechanics in Regenerative Medicine T32 EB000392 to K.J.B.).
References
- 1.Swartz DD, Russell JA, Andreadis ST. Engineering of fibrin-based functional and implantable small-diameter blood vessels. American Journal of Physiology-Heart and Circulatory Physiology. 2005;288(3):H1451–H60. doi: 10.1152/ajpheart.00479.2004. [DOI] [PubMed] [Google Scholar]
- 2.Syedain ZH, Meier LA, Bjork JW, Lee A, Tranquillo RT. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials. 2011;32(3):714–22. doi: 10.1016/j.biomaterials.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syedain ZH, Weinberg JS, Tranquillo RT. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proceedings of the National Academy of Sciences. 2008;105(18):6537–42. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flanagan TC, Cornelissen C, Koch S, Tschoeke B, Sachweh JS, Schmitz-Rode T, et al. The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials. 2007;28(23):3388–97. doi: 10.1016/j.biomaterials.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Robinson PS, Johnson SL, Evans MC, Barocas VH, Tranquillo RT. Functional tissue-engineered valves from cell-remodeled fibrin with commissural alignment of cell-produced collagen. Tissue Engineering Part A. 2008;14(1):83–95. doi: 10.1089/ten.a.2007.0148. [DOI] [PubMed] [Google Scholar]
- 6.Garvin J, Qi J, Maloney M, Banes AJ. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Engineering. 2003;9(5):967–79. doi: 10.1089/107632703322495619. [DOI] [PubMed] [Google Scholar]
- 7.Nieponice A, Maul TM, Cumer JM, Soletti L, Vorp DA. Mechanical stimulation induces morphological and phenotypic changes in bone marrow-derived progenitor cells within a three-dimensional fibrin matrix. Journal of Biomedical Materials Research Part A. 2007;81A(3):523–30. doi: 10.1002/jbm.a.31041. [DOI] [PubMed] [Google Scholar]
- 8.Weinbaum JS, Schmidt JB, Tranquillo RT. Combating Adaptation to Cyclic Stretching by Prolonging Activation of Extracellular Signal-Regulated Kinase. Cellular and Molecular Bioengineering. 2013:1–8. doi: 10.1007/s12195-013-0289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sander E, Barocas V, Tranquillo R. Initial fiber alignment pattern alters extracellular matrix synthesis in fibroblast-populated fibrin gel cruciforms and correlates with predicted tension. Annals of biomedical engineering. 2011;39(2):714–29. doi: 10.1007/s10439-010-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lake SP, Barocas VH. Mechanical and structural contribution of non-fibrillar matrix in uniaxial tension: a collagen-agarose co-gel model. Annals of biomedical engineering. 2011;39(7):1891–903. doi: 10.1007/s10439-011-0298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings CL, Gawlitta D, Nerem RM, Stegemann JP. Properties of engineered vascular constructs made from collagen, fibrin, and collagen–fibrin mixtures. Biomaterials. 2004;25(17):3699–706. doi: 10.1016/j.biomaterials.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 12.Rowe SL, Lee S, Stegemann JP. Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomaterialia. 2007;3(1):59–67. doi: 10.1016/j.actbio.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowe SL, Stegemann JP. Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios. Biomacromolecules. 2006;7(11):2942–8. doi: 10.1021/bm0602233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis JR. Tensile testing: ASM international. 2004. [Google Scholar]
- 15.Kwok R, Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophysical Journal. 1981;35(3):637–52. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivlin RS, Saunders D. Large elastic deformations of isotropic materials. VII. Experiments on the deformation of rubber. Philosophical Transactions of the Royal Society of London Series A, Mathematical and Physical Sciences. 1951;243(865):251–88. [Google Scholar]
- 17.Christensen RM. Observations on the definition of yield stress. Acta Mechanica. 2008;196(3–4):239–44. [Google Scholar]
- 18.Kirchmajer DM, Watson CA, Ranson M. Gelapin, a degradable genipin cross-linked gelatin hydrogel. RSC Advances. 2013;3(4):1073–81. [Google Scholar]
- 19.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly (ethylene glycol) hydrogels. Journal of Biomedical Materials Research. 2002;59(1):63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 20.Cloyd JM, Malhotra NR, Weng L, Chen W, Mauck RL, Elliott DM. Material properties in unconfined compression of human nucleus pulposus, injectable hyaluronic acid-based hydrogels and tissue engineering scaffolds. European Spine Journal. 2007;16(11):1892–8. doi: 10.1007/s00586-007-0443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao P-HG, Valhmu WB, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. Journal of biomechanical engineering. 2000;122(3):252–60. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 22.Smeds KA, Grinstaff MW. Photocrosslinkable polysaccharides for in situ hydrogel formation. Journal of biomedical materials research. 2001;54(1):115–21. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 23.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast Adaptation and Stiffness Matching to Soft Elastic Substrates. Biophysical Journal. 2007;93(12):4453–61. doi: 10.1529/biophysj.106.101386. doi: http://dx.doi.org/10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophysical Journal. 1999;77(5):2813–26. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]