Kay discusses the recent characterization of the different modes of retinal ganglion cell migration and their cell biological basis.
Abstract
Newborn neuron radial migration is a key force shaping the nervous system. In this issue, Icha et al. (2016. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201604095) use zebrafish retinal ganglion cells as a model to investigate the cell biological basis of radial migration and the consequences for retinal histogenesis when migration is impaired.
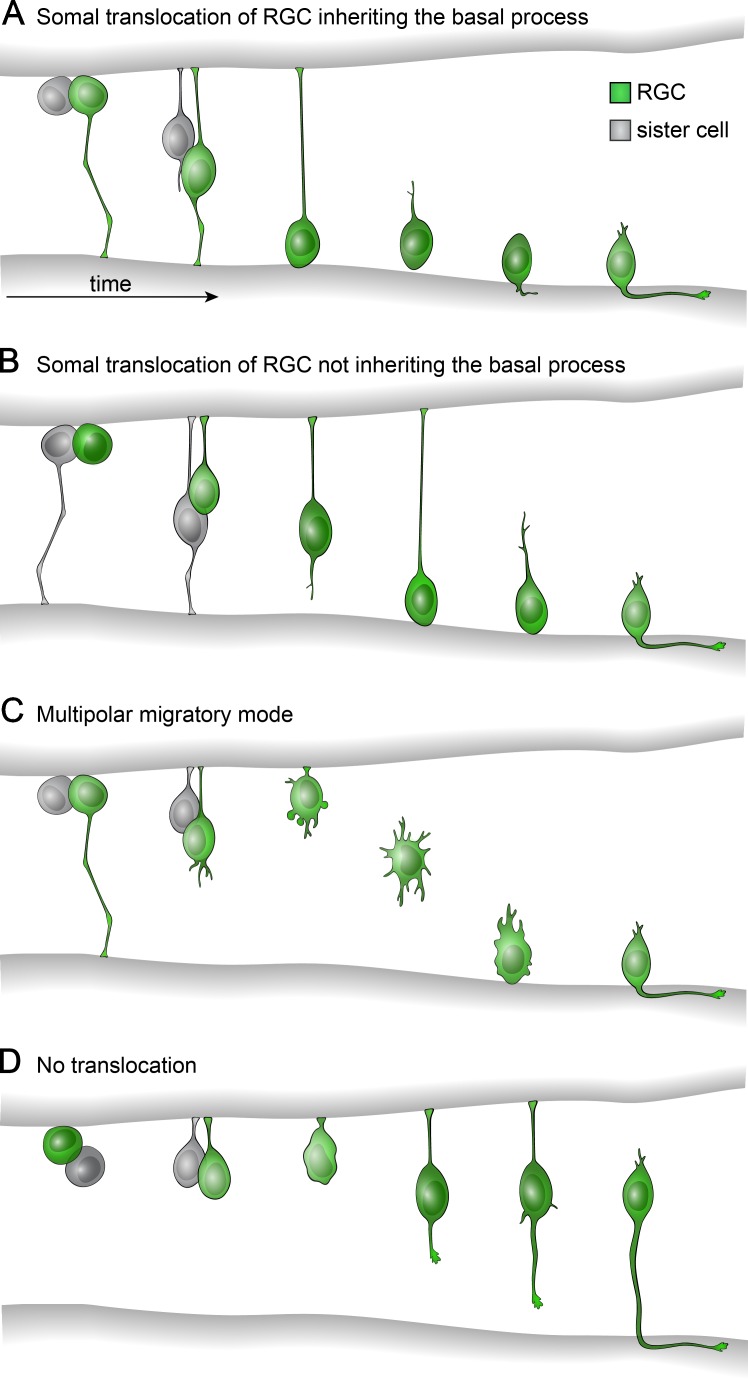
Neurons of the vertebrate nervous system are usually born in a different site than where they will ultimately reside. Migration of newborn neurons is therefore a critical step in nervous system development. The most common form of cell transit is known as radial migration. Upon exiting the cell cycle, immature neurons depart the germinal zone, which lines the ventricles at the inner (apical) surface of the neural tube, and migrate radially into the overlying neuropil. In many regions of the nervous system (for instance, the cerebral cortex and retina) each cell type settles at a specific radial location, giving rise to a laminar structure in which neurons are arranged according to their type and function. Radial migration therefore serves not only to deliver neurons to the appropriate layer but also, through successive waves of neurogenesis and migration, to generate the laminar structure itself. Because radial migration has such a central role in building the nervous system, there has been great interest in understanding how neurons accomplish their journey. Over 40 years ago, it was discovered that newborn neurons can migrate along the radially oriented stalks of neural progenitor cells, also known as radial glia (Rakic, 1971). This is the best-known mode of radial migration, and thanks to many studies in cerebral cortex and cerebellum, we know a great deal about the cell biological mechanisms involved (Solecki, 2012; Kawauchi, 2015). However, there are other ways for neurons to move radially (Ramon y Cajal, 1972; Hinds and Hinds, 1974, 1978; Nadarajah et al., 2001; Tabata and Nakajima, 2003). Some neurons use what is known as somal translocation: they extend long apical and basal protrusions, termed processes, and then shift their nucleus within this structure to bring about cell movement. Others use a multipolar migration mode, with many short dynamic arbors that extend in all directions as the cell crawls toward its final position (Fig. 1, A-C, republished from Icha et al., 2016). Although somal translocation and multipolar migration are less famous than glial-guided migration, they may be more common. Some regions of the nervous system, like the retina, use glial-guided migration only rarely, if ever (Wong and Godinho, 2003). Moreover, cortical neurons that begin in contact with a progenitor often switch to one of the other modes during their migration (Noctor et al., 2004). Despite their importance, the mechanisms underlying translocation and multipolar migration are poorly understood. In this issue, Icha et al. use in vivo live imaging of larval zebrafish retina to investigate the cell biological mechanisms of somal translocation. They uncover specific functions of the apical and basal processes, helping to clarify how the unusual morphology of translocating cells facilitates their migration.
Figure 1.
Radial migration modes used by RGCs. RGCs (green) are born at the apical side of the retina after a progenitor division that also gives rise to a sister cell (gray). The RGC may transit basally in several different ways. (A) Most commonly, the RGC inherits the progenitor cell’s basal process and moves by somal translocation. (B) In ∼20% of cases, the sister cell inherits the basal process, forcing the RGC to use a slower version of somal translocation as it regrows its basal process. (C) Multipolar migration mode, rare in wild-type RGCs but commonly seen after cytoskeletal disruptions that affect basal process attachment. The RGC detaches its apical process to initiate this mode. (D) RGCs that lack a basal process and are prevented from releasing their apical process do not migrate efficiently, causing them to differentiate at ectopic localizations. Figure republished from Icha et al. (2016).
The model cell type used in this study is the retinal ganglion cell (RGC), which extends apical and basal processes that attach the cell to each surface of the retinal neuroepithelium (Ramon y Cajal, 1972; Hinds and Hinds, 1974). RGCs then translocate to occupy the ganglion cell layer, the most basal layer of this highly stratified tissue (Fig. 1). A key technical advance is the use of light-sheet microscopy, which Icha et al. (2016) find produces less phototoxicity than other time-lapse imaging methods. This permits long recordings that encompass the entire RGC migration period, from the last cell division before cell cycle exit until the newborn neuron arrives in the ganglion cell layer. Icha et al. (2016) use this method to probe the role of the apical and basal processes in the radial movement of RGCs. They first show that attachment of the basal process to the basement membrane of the retinal neuroepithelium is important for efficient translocation. In wild-type retina, most RGCs were observed to inherit an attached basal process from the progenitor that produced it. However, some did not inherit the progenitor’s basal process and needed to grow one during migration (Fig. 1 B). The authors found that cells inheriting the basal process moved faster than those that did not, suggesting that basal process attachment promotes translocation. To test this, the authors interfered with attachment to the basement membrane in a variety of ways. Their most powerful trick was to express cytoskeletal regulators in a sparse fashion, using a RGC-specific promoter, so that the cell being imaged is perturbed but the surrounding tissue is not. The researchers focused on two manipulations that prevent basal process attachment: overexpression of the microtubule-destabilizing protein Stathmin-1 and overexpression of constitutively active WASP, which perturbs Arp 2/3 function and thereby disrupts actin organization. The precise mechanisms by which these molecules influence the basal process still need to be worked out, but it appears that microtubule manipulation may affect the stability of the basal process, whereas actin manipulation might interfere with focal adhesions, preventing cell–matrix interactions. Both molecules caused the basal process to disappear, whereas the apical process remained intact, at least initially. In both cases, RGCs migrated much less efficiently; indeed, some never reached the ganglion cell layer and differentiated in ectopic retinal regions. Thus, the basal process plays a key role in paving the way for somal translocation.
What about the role of the apical process? Icha et al. (2016) observed a large amount of stable, acetylated microtubules in the apical process, which they suggest might prevent the translocating nucleus from moving in the wrong direction. In support of this idea, live imaging of RGCs subjected to microtubule depolymerization showed the soma moving back and forth, instead of progressing basally. A second role of the apical process may be to control the mode of migration. When the basal process was experimentally ablated, RGC migration was initially stymied but the authors noticed that, later in the imaging session, most RGCs detached their apical process and resumed migrating in a multipolar mode (Fig. 1 C). Upon doing so, their migration speed substantially increased, although they did not reach the speed achieved by translocating RGCs. Nevertheless, multipolar migration was sufficient to deliver most RGCs to their appropriate layer. This strategy for overcoming the lack of a basal process was occasionally even observed in wild-type RGCs, suggesting that it is within the behavioral repertoire of normal cells.
To ask if apical process removal is required for the switch to multipolar migration, Icha et al. (2016) searched for a cytoskeletal manipulation that would stabilize the apical process and prevent detachment. They found that overexpression of membrane-targeted atypical protein kinase C-ζ (aPKC), which increases cortical actin on the apical side of polarized cells, eliminated the basal process and stabilized the apical one. Most aPKC-expressing RGCs failed to use either translocation or multipolar migration modes, instead remaining anchored to the apical surface (Fig. 1 D). This caused a deficit in colonization of the ganglion cell layer, much more severe than in Stathmin-1 overexpressing cells. This finding suggests that signals impinging on the regulation of cortical actin are important for determining whether a neuron detaches from the apical epithelium and migrates in multipolar mode. It will be interesting to define how aPKC controls stability of the apical process and to identify upstream signals that regulate actin to determine if the neuron holds on or lets go. Such upstream signals might differ between RGCs and other retinal neurons, such as amacrine cells, which normally use multipolar migration and therefore do not have an apical attachment (Hinds and Hinds, 1978; Chow et al., 2015).
Having identified a way to block both the preferred and the backup modes of RGC migration, Icha et al. (2016) found themselves in a position to ask how radial migration of a single cell type contributes to the formation of retinal layers. They discovered that when RGCs expressed aPKC and failed to reach their normal layer, they nucleated striking laminar defects in their vicinity that affected all other retinal neuron types. This result highlights the importance of radial migration in the histogenesis of laminar nervous structures. When one cell type fails to get to the right place, the cues those cells express will be mislocalized, leading to cascading effects on cells that arrive later. An exciting future direction will be to identify the RGC-derived signals that influence lamination of their neighbors.
The work of Icha et al. (2016) provides important new insight into the cellular requirements for somal translocation. We now know a lot more about the roles of the apical and basal processes, and the significance of their attachment, in promoting movement. This work also demonstrates that radial migration is a robust phenomenon that cells can accomplish in multiple ways, likely because of the severe consequences for tissue histogenesis when it fails. Finally, this paper provides some first steps toward understanding the molecular mechanisms of translocation. Future studies are needed to delve more deeply into these molecular mechanisms. For instance, the cytoskeletal differences between apical and basal processes are still in need of further clarification, as are the mechanisms through which the manipulations used by Icha et al. (2016) alter the cytoskeleton to differentially affect each process. Given that the two processes are clearly different, how do they become distinct? Recently, the planar cell polarity molecule Fat3 and the downstream Ena/VASP actin regulators were found to be necessary for efficient multipolar migration in amacrine cells (Krol et al., 2016). Perhaps this signaling pathway also contributes to the polarization of the newborn RGC, giving the apical and basal process distinct properties.
Overall, this study provides new details that are important for understanding not only the development of the retina but also the cerebral cortex and indeed any brain region where translocation occurs. This work also has implications for understanding the pathogenesis of several congenital human diseases in which radial migration is impaired (Manzini and Walsh, 2011). One of the most interesting remaining questions is how the soma moves during translocation. Clearly, the presence of a basal process is critical. But is the basal process important because it provides a permissive cytoskeletal substrate for translocation? Or is it perhaps because elements that push or pull the nucleus anchor within the basal process? More broadly, what are the forces that propel the nucleus specifically in the basal direction? In glia-guided migration, the centrosome is positioned on the leading side and drags the nucleus using a microtubule “cage” (Solecki et al., 2004), but Icha et al. (2016) found that the centrosome is in the apical process of migrating RGCs on the trailing side. Thus, even though the mechanism by which the nucleus translocates is still unclear, we know enough to conclude that somal translocation differs significantly from other radial migration modes. Once we understand more about the different cytoskeletal configurations that distinguish the different migration modes, it will be particularly fascinating to learn how these are reconfigured when migrating neurons switch modes, something that happens quite frequently in both retina and cortex (Noctor et al., 2004; Chow et al., 2015). Of course, we can expect that answering these questions will take time. With its powerful combination of genetics and live imaging, the zebrafish retina appears poised as a powerful model to give us at least a few of those answers.
Acknowledgments
The author declares no competing financial interests.
References
- Chow R.W., Almeida A.D., Randlett O., Norden C., and Harris W.A.. 2015. Inhibitory neuron migration and IPL formation in the developing zebrafish retina. Development. 142:2665–2677. 10.1242/dev.122473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds J.W., and Hinds P.L.. 1974. Early ganglion cell differentiation in the mouse retina: An electron microscopic analysis utilizing serial sections. Dev. Biol. 37:381–416. 10.1016/0012-1606(74)90156-0 [DOI] [PubMed] [Google Scholar]
- Hinds J.W., and Hinds P.L.. 1978. Early development of amacrine cells in the mouse retina: An electron microscopic, serial section analysis. J. Comp. Neurol. 179:277–300. 10.1002/cne.901790204 [DOI] [PubMed] [Google Scholar]
- Icha J., Kunath C., Rocha-Martins M., and Norden C.. 2016. Independent modes of ganglion cell translocation ensure correct lamination of the zebrafish retina. J. Cell Biol. 10.1083/jcb.201604095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi T. 2015. Cellullar insights into cerebral cortical development: Focusing on the locomotion mode of neuronal migration. Front. Cell. Neurosci. 9:394 10.3389/fncel.2015.00394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A., Henle S.J., and Goodrich L.V.. 2016. Fat3 and Ena/VASP proteins influence the emergence of asymmetric cell morphology in the developing retina. Development. 143:2172–2182. 10.1242/dev.133678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini M.C., and Walsh C.A.. 2011. What disorders of cortical development tell us about the cortex: One plus one does not always make two. Curr. Opin. Genet. Dev. 21:333–339. 10.1016/j.gde.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah B., Brunstrom J.E., Grutzendler J., Wong R.O., and Pearlman A.L.. 2001. Two modes of radial migration in early development of the cerebral cortex. Nat. Neurosci. 4:143–150. 10.1038/83967 [DOI] [PubMed] [Google Scholar]
- Noctor S.C., Martínez-Cerdeño V., Ivic L., and Kriegstein A.R.. 2004. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7:136–144. 10.1038/nn1172 [DOI] [PubMed] [Google Scholar]
- Rakic P. 1971. Guidance of neurons migrating to the fetal monkey neocortex. Brain Res. 33:471–476. 10.1016/0006-8993(71)90119-3 [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. 1972. The Structure of the Retina. Thorpe S.A., and Glickstein M., editors. Charles Thomas, Springfield, IL. 196 pp. [Google Scholar]
- Solecki D.J. 2012. Sticky situations: Recent advances in control of cell adhesion during neuronal migration. Curr. Opin. Neurobiol. 22:791–798. 10.1016/j.conb.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecki D.J., Model L., Gaetz J., Kapoor T.M., and Hatten M.E.. 2004. Par6α signaling controls glial-guided neuronal migration. Nat. Neurosci. 7:1195–1203. 10.1038/nn1332 [DOI] [PubMed] [Google Scholar]
- Tabata H., and Nakajima K.. 2003. Multipolar migration: The third mode of radial neuronal migration in the developing cerebral cortex. J. Neurosci. 23:9996–10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R.O., and Godinho L.. 2003. Development of the vertebrate retina. In The Visual Neurosciences. Chalupa L.M., and Werner J.S., editors. MIT Press, Cambridge, MA: 77–93. [Google Scholar]