Abstract
The survival of insect larvae often depends on the mother’s choice of oviposition substrate, and thus, this choice is an essential part of an insect species’ ecology. Especially species with narrow substrate preferences may suffer from changes in substrate availability triggered by, for example, climate change. Recent climate warming is affecting species directly (e.g., physiology) but also indirectly (e.g., biological interactions) leading to mismatching phenologies and distributions. However, the preferred oviposition substrate is still unknown for many drosophilid species, especially for those at higher elevations. In this study, we investigated the oviposition-substrate preference of the montane-alpine fly Drosophila nigrosparsa in rearing and multiple-choice experiments using natural substrates in the laboratory. Insect emergence from field-collected substrates was tested. More than 650 insects were reared from natural substrates, among them 152 drosophilids but no individual of D. nigrosparsa. In the multiple-choice experiments, D. nigrosparsa preferred ovipositing on mushrooms (> 93% of eggs); additionally, a few eggs were laid on berries but none on other substrates such as cow faeces, rotten plant material, and soil. The flies laid 24 times more eggs per day when mushrooms were included in the substrates than when they were excluded. We infer that D. nigrosparsa is a mushroom breeder with some variation in oviposition choice. The flies favoured some mushrooms over others, but they were not specialised on a single fungal taxon. Although it is unclear if and how climate change will affect D. nigrosparsa, our results indicate that this species will not be threatened by oviposition-substrate limitations in the near future because of the broad altitudinal distribution of the mushrooms considered here, even if the flies will have to shift upwards to withstand increasing temperatures.
Introduction
Ovipositing on the preferred substrate can increase the likelihood of insect offspring’s survival [1], and especially poorly agile insect larvae strongly depend on the mother’s substrate selection. According to the major hypothesis on the evolution of oviposition choice, there should be strong selective pressure on oviposition substrate to enable optimum larval performance [2]. However, females often do not prefer the substrate optimising larval performance but rather their own performance [3,4]. This highlights that other factors than offspring survival may play a role in oviposition choice, such as host chemistry, predictability, genetics, and presence or absence of predators [5,6].
The fly family Drosophilidae is among the best studied animal taxa, and several drosophilid species are well characterised in, for example, physiology, genetics, life-cycle, developmental biology, and ecology [7,8]. The preferred oviposition substrate, however, is known for only a few drosophilids [9–11], and in-depth knowledge especially for species from higher elevations is lacking. Shorrocks [11] defined four categories of breeding substrates for European drosophilids, namely, decaying plant material, fermenting fruits, fungi, and sap fluxes of trees. Although many drosophilid species can use several feeding substrates as adults, the oviposition substrate preference is often narrower and not the same as the food preference [9,12]. Some species are highly specialised in their oviposition preference, such as Drosophila pachea [13]; others are more generalist breeders and are able to use various substrates for successful larval development, such as D. subobscura [14]. Furthermore, oviposition choice can vary within species [9,15].
One major factor of recent climate change is increasing temperature; worst-case scenarios predict increments of up to 4.8°C until the end of the 21st century [16]. Species are affected directly, by physiological changes, and indirectly, by effects on interacting species [17]. Distribution ranges and synchrony of interacting partners can become mismatched so that the overlapping area and period are reduced, respectively [18,19]. Models predict increasing mismatches, spatially and temporally [20], which can result in the extinction of interacting species [21]. Insect species with narrow substrate preferences for egg laying are especially vulnerable [17]. Hence, knowledge of the preferred oviposition substrate is a crucial factor when predicting a species’ future under changed environmental conditions such as under climate warming.
Drosophila (Drosophila) nigrosparsa Strobl, 1898 is distributed in European mountain regions, where it is most abundant at the timber line [22]. Currently, we are establishing D. nigrosparsa as a new study system for climate change research (Austrian Science Fund, project number P 23949, https://pf.fwf.ac.at/project_pdfs/pdf_abstracts/p23949e.pdf). This species was chosen because the genus Drosophila contains some of the best studied animals [7], and among drosophilids, only D. nigrosparsa is both confined to high altitudes and culturable in the laboratory. In this project, we are performing laboratory selection experiments to investigate the ability of this fly species to adapt to increasing temperatures in the alpine ecosystem. The species’ transcriptome has been sequenced [23], and various life history traits and physiological limits of D. nigrosparsa have been characterised under laboratory conditions (P. Krapf, M.-C. Kinzner, M. Nindl, C. Heussler, A. A. Hoffmann, W. Arthofer, B. C. Schlick-Steiner, F. M. Steiner, unpubl.). However, there is currently no information available concerning the oviposition preference of D. nigrosparsa. This can hamper progress in climate change research—even if the fly is able to adapt to higher temperatures, an interacting partner might not be. To illuminate this: Assume that D. nigrosparsa is specialised to oviposit on species X, both having a similar altitudinal distribution at the timberline in the Alps. Then, if temperatures in the Alps rise strongly until the end of the 21st century, as predicted [24], D. nigrosparsa might not be able to adapt to the higher temperatures, but species X might be. In this situation, D. nigrosparsa would migrate to higher altitudes, but species X would keep its recent distribution, as observed for other biotic observations (e.g., [19,20]), leading to an increasing distribution mismatch. Once the two species’ distributions do not overlap any more, D. nigrosparsa would lose its biological partner, and, due to the fly’s specialised oviposition biology, at least some of its populations might vanish. This is just one theoretically possible future scenario, but it stresses the importance for climate change research to gain information about biological interactions in addition to selection experiments in the laboratory.
In the following, we addressed three questions: (i) What natural substrate is preferred by D. nigrosparsa for oviposition; (ii) is D. nigrosparsa a generalist or a specialist concerning oviposition substrate; and (iii) is D. nigrosparsa opportunistic when the preferred substrate is not available, changing oviposition to non-preferred substrates?
Materials and Methods
Origin and maintenance of experimental flies
In summer 2010, Drosophila nigrosparsa was collected using fermented-banana baits at Kaserstattalm (Tyrol, Austria, 11.29°E 47.13°N) at 2000 m above sea level. No specific permissions were required because none of the species used in this study is endangered or protected. Flies were kept in the laboratory as mass bred population using grape agar medium (30 g agar, 1000 mL deionised water, 334 mL grape juice, 3.4 g methyl-4-hydroxybenzoate, and 34 g sucrose; modified from Sullivan et al. [25]) and malt medium (10 g agar, 1000 mL deionised water, 15 g dried yeast, 100 g malt, 3 g methyl-4-hydroxybenzoate, 3.6 mL propionic acid, and 50 g semolina; modified from Lakovaara [26]) at 19°C and ca. 60% relative humidity in a 16L:8D photoperiod. After about 100 days, a pair of flies was isolated for oviposition on grape agar to initiate a strongly inbred laboratory population. After establishment, laboratory populations were maintained at a population size of approximately 200 for four years.
Experiment 1—Rearing experiment
In August 2012, 239 substrates (S1 Table) were collected at three natural habitats of D. nigrosparsa at 2000 m above sea level, at Arztal (Tyrol, Austria, 11.50°E 47.18°N), Kaserstattalm, and Pfitscherjoch (South Tyrol, Italy, 11.68°E 46.98°N), and brought to an environmental chamber (DWM, Weiss Technik, Germany; modified) at the University of Innsbruck. Temperature and relative humidity in the chamber were 19°C and 50–70%, respectively, in a 16L:8D photoperiod. The surface of substrates was disinfected using a mixture of 1000 mL deionised water, 3 g methyl-4-hydroxybenzoate, and 3.6 mL propionic acid (pH 8.9). Substrates were placed individually in 500 mL plastic cups containing ca. 40 mL malt medium. Emerged adults were collected weekly and stored in 96% ethanol at -20°C. After three months, the chamber was cooled down to 1–4°C to simulate winter. Five weeks later, the chamber was warmed up again to 19°C, and substrates were controlled for emerged adults for another two months. Drosophila species were identified morphologically according to Bächli & Burla [22].
Experiment 2—General oviposition preference
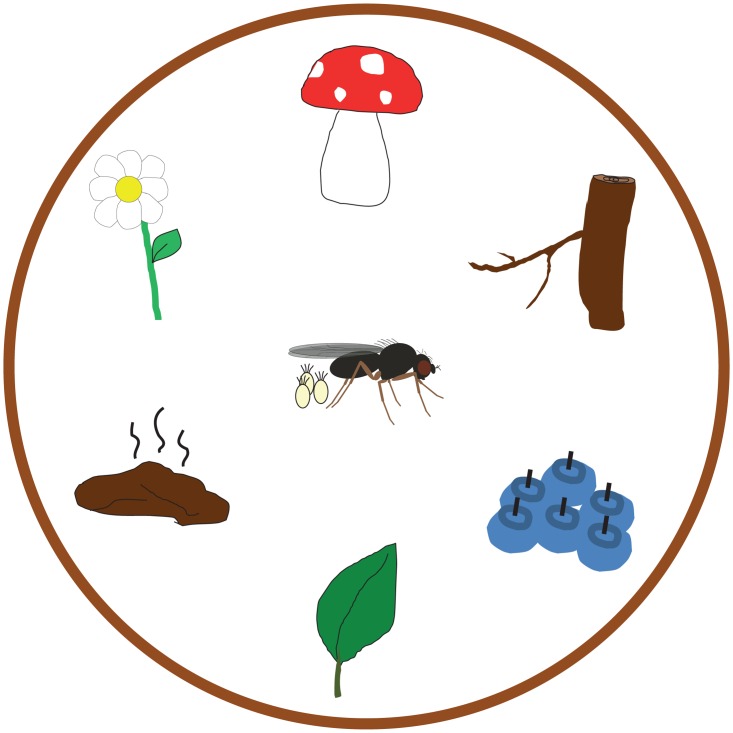
In September 2014, 24 substrates were collected at Kaserstattalm (S2 Table) and brought to the environmental chamber used also in Experiment 1. Temperature, relative humidity, and photoperiod in the chamber were equal to Experiment 1. Substrates were placed individually in 90-mm-diameter Petri dishes, which were randomly positioned along the inner side of a circular untransparent plastic cage of 530 mm diameter with a transparent observation window at the top (Fig 1). For water supply, a Petri dish containing ca. 40 mL 3.0% plain agar was placed in the centre of the cage. Seventy females and 40 males of mixed age of the inbred laboratory population were briefly CO2 anaesthetised for sexing and released on the plain agar equidistantly from the 24 potential oviposition substrates. After 54 hours, the number of eggs laid on each substrate was counted under the microscope (Nikon SMZ-10A, Nikon Corporation, Japan, 7.5 − 49x magnification). Three replicate cages were run in parallel. To control for potentially overlooked eggs after recording the number of eggs, substrates on which no eggs had been detected were placed individually in 500 mL plastic cups containing 8 mL malt medium enabling the development of eggs to adulthood. Emerged adults were collected weekly for 14 weeks.
Fig 1. Schematic illustration of the multiple-choice oviposition Experiments 2–4.
The brown circle indicates the cage. Size relations do not represent the real situation.
Experiment 3—Generalist vs. specialist
In September 2014, additional samples of blueberries (fruits of Vaccinium myrtillus), bog bilberries (fruits of V. uliginosum), and fruiting bodies of seven different fungi (all species detectable at that time) were collected at Kaserstattalm (S3 Table) and brought to the environmental chamber used also in Experiments 1 & 2. Substrates were placed individually in 55-mm-diameter Petri dishes containing ca. 10 mL 0.4% plain agar to prevent desiccation. As mushrooms were determined morphologically after the experiment [27] using photographs, one dish inadvertently contained two different species (Lycoperdon sp. & Bovista sp.). Dishes were randomly positioned along the inner side of a circular untransparent plastic cage of 270 mm diameter with a transparent observation window at the top (Fig 1). A Petri dish containing ca. 10 mL 3.0% plain agar was added as negative control. For water supply, a 90-mm-diameter Petri dish containing ca. 40 mL 3.0% plain agar was placed in the centre of the cage. Fifty females and 30 males of mixed age of the inbred laboratory population were briefly CO2 anaesthetised for sexing and released on the plain agar. The experimental procedure was equal to that in Experiment 2, but the time for egg laying was extended to 64 hours.
Experiment 4—Opportunistic ovipositing
In September 2014, nine not-favoured substrates from Experiment 2 (S4 Table) were recollected at Kaserstattalm based on a subjective choice of those substrates on which, according to the main oviposition types [11] and to personal observations, D. nigrosparsa might possibly lay eggs in the absence of mushrooms. The experimental design was the same as in Experiment 3.
Lacking knowledge about oviposition behaviour and clutch sizes, there was no justifiable specific probability model for the egg numbers. Therefore, we took a pragmatic approach and Box-Cox transformed (after adding 1 to handle zeroes) the egg counts to meet the criteria for an analysis of variance (ANOVA). Substrates without any eggs over all replicates having zero variance were excluded from analysis. The transformed data were analysed using ANOVA with Bonferroni-corrected post-hoc tests to compare substrates in R version 3.1.1 [28]. For homogenous groups shown in graphs, a p-level of 0.05 was applied.
Molecular analysis
DNA of four drosophilids eclosed from Experiment 1 and of 39 D. nigrosparsa adults eclosed from Experiments 2–4 was extracted using the GenElute Mammalian Genomic DNA miniprep kit (Sigma, USA). PCR amplification of a mitochondrial COI gene stretch for species identification of drosophilids eclosed in Experiment 1 was carried out in a 10 μL reaction volume with 1.0 μL template DNA, 1× Rotor-Gene Probe PCR Master Mix (Qiagen, Germany), and 0.2 μM forward and reverse primers (UEA5 and UEA10, respectively; [29]). PCRs were performed at 94°C for 2 min followed by 35 cycles at 94°C for 30 s, 50°C for 45 s, 72°C for 2 min and a final extension at 72°C for 10 min.
We were aware that by counting eggs under the microscope, some eggs could have been overlooked (e.g., because of deep insertion in substrates), and some eggs (and/or larvae, pupae) could have been already on substrates before bringing them to the laboratory. Thus, the population of origin (wildtype or inbred laboratory population) of adults emerged from substrates on which no eggs were found in Experiments 2–4 (S2–S5 Tables) were identified using molecular markers. Therefore, flies were genotyped using five polymorphic microsatellite loci, namely, DN37, DN40, DN41, DN48, and DN49 [30]. PCR for genotyping was performed in a 5 μL reaction volume with 0.5 μL template DNA, 1× reaction buffer (Bioline, UK), 0.2 μM fluorescent-labelled M13 primer, 0.02 μM M13 tailed locus specific forward primer, 0.2 μM untailed specific reverse primer, and 0.25 U MyTaq polymerase (Bioline). For loci DN37, DN41, DN48, and DN49, cycling conditions were 94°C for 2 min followed by 35 cycles at 94°C for 30 s, 55°C for 45 s, 72°C for 1 min and a final extension at 72°C for 10 min. For locus DN40, cycling conditions were equal but annealing temperature was 48°C. Fragment analysis was performed on an ABI 3130 genetic analyzer (Applied Biosystems, USA). Traces were visualised and scored manually using PeakScanner Software v2.0 (Applied Biosystems). GeneClass v2.0 [31] was used for the classification of the population of origin (wildtype or inbred laboratory population) by using a two-step approach: First, the software was trained with microsatellite data from the wildtype and the inbred laboratory population already available [30] using the Bayesian method of Rannala & Mountain [32]. Second, as more than ten generations of laboratory evolution occurred between the inbred laboratory population characterised in 2013 [30] and the flies used in this study, all eclosed individuals from this study unambiguously assigned to the inbred laboratory population in the first step were used to train the software again. Individuals not assigned unambiguously to the inbred laboratory population in the first step were classified again using the new training set.
Results
Experiment 1—Rearing experiment
A total of 614 adult Diptera from 14 families were reared from 60 different substrates (S6 Table) whereby most individuals emerged from cow and sheep faeces and from mushrooms. The only drosophilid species was Drosophila transversa (GenBank accession numbers KU934279–KU934282), with all 152 individuals eclosing from mushrooms. In addition to dipterans, 11 Coleoptera, 20 Hemiptera, 2 Hymenoptera, and 41 Thysanoptera emerged (S6 Table).
Experiment 2—General oviposition preference
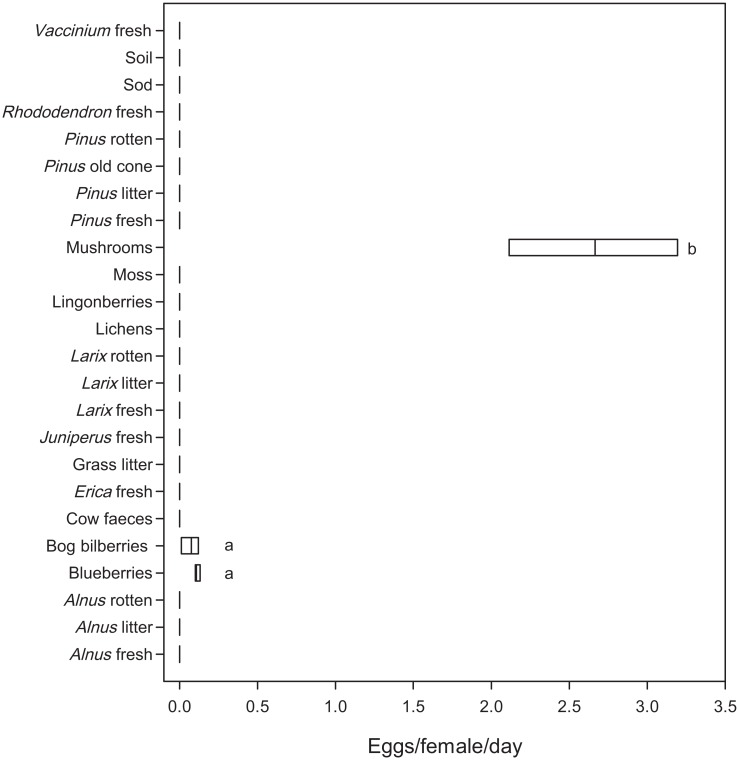
Females of D. nigrosparsa oviposited on only three of the 24 offered substrates (Fig 2, S2 Table), consistently across all replicates. A total of 1343 eggs (2.842 eggs/female/day) were laid across all replicates. Most eggs were laid on the mixed mushroom sample, on average 2.658 ± 0.540 eggs/female/day (93.5 ± 19.0% of all eggs in Experiment 2, mean ± standard deviation). Only a few eggs were laid on blueberries and bog bilberries, on average 0.114 ± 0.017 (4.0 ± 0.6%) and 0.070 ± 0.054 eggs/female/day (2.5 ± 1.9%), respectively. The number of eggs differed significantly among substrates (Fig 2, Table 1). Blueberries and bog bilberries were not different in the pairwise comparison, but both differed significantly from mushrooms (S7 Table). After placing the substrates on malt medium, adults emerged from the three substrates on which eggs had been detected but from no other substrate (S2 Table).
Fig 2. Number of eggs/female/day laid on various substrates from Experiment 2—General oviposition preference.
The same lower case letters indicate homogenous significance groups (p = 0.05). For details about substrate nomenclature, see S1 Table.
Table 1. Results of the analysis of variance for Experiments 2–4.
| Factor | Df | SS | MS | F-value | p-value | |
|---|---|---|---|---|---|---|
| Experiment 2 | substrate | 2 | 748.67 | 374.33 | 109.63 | 1.89 E-5 |
| error | 6 | 20.49 | 3.41 | |||
| Experiment 3 | substrate | 8 | 335.11 | 41.89 | 13.00 | 4.75 E-6 |
| error | 18 | 57.98 | 3.22 | |||
| Experiment 4 | substrate | 2 | 1.37 | 0.69 | 0.89 | 0.46 |
| error | 6 | 4.61 | 0.77 |
Df, degrees of freedom. SS, sum of squares. MS, mean square.
Experiment 3—Generalist vs. specialist
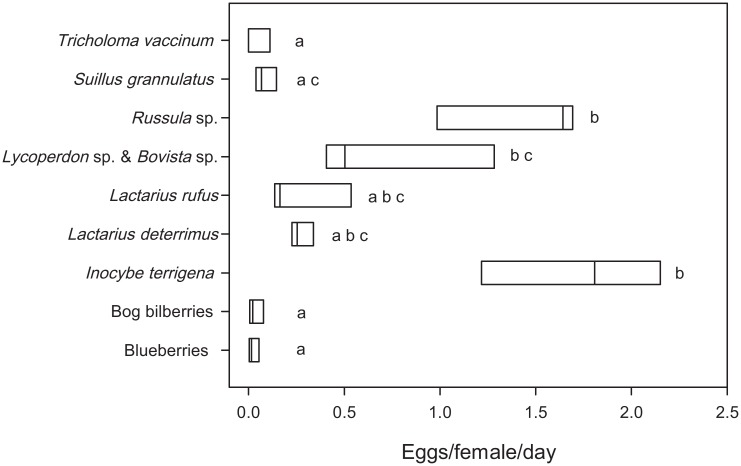
Eggs were laid on all substrates at least in one replicate (Fig 3, S3 Table). In total, 1873 eggs (4.683 eggs/female/day) were counted across all replicates. The most attractive substrate was the mushroom Inocybe terrigena (36.3 ± 13.4% of all eggs in Experiment 3, mean ± standard deviation), followed by Russula sp. (29.3 ± 11.3%) and Lycoperdon sp. & Bovista sp. (17.3 ± 13.7%). The least attractive were blueberries (0.6 ± 0.8%), bog bilberries (0.9 ± 1.1%), and the mushroom Tricholoma vaccinum (1.1 ± 1.9%). The number of eggs differed significantly among substrates (Fig 3, Table 1); three homogenous substrate groups were found in post-hoc tests (S7 Table). In the sample Lycoperdon sp. & Bovista sp., only one egg was laid on Bovista sp. (replicate B); all other eggs were found on Lycoperdon sp. Adults emerged from two of four substrates (blueberries, bog bilberries, S3 Table) where zero eggs were counted; all had inbred laboratory population genotypes (S5 and S8 Tables).
Fig 3. Number of eggs/female/day laid on various substrates from Experiment 3—Generalist vs. specialist.
The same lower case letters indicate homogenous significance groups (p = 0.05).
Experiment 4—Opportunistic ovipositing
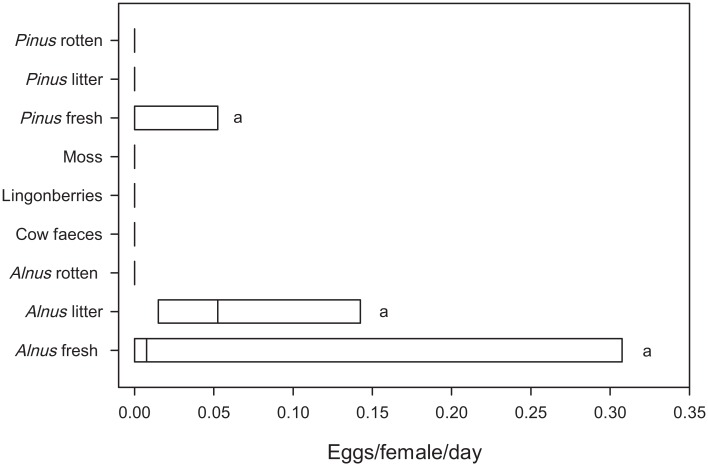
A total of 77 eggs (0.193 eggs/female/day) were laid across all replicates (Fig 4, S4 Table), whereof 70 (90.9%) were found on fresh Alnus or Alnus litter. The remaining seven eggs (9.1%) were laid on fresh Pinus (replicate B). The number of eggs did not differ significantly among substrates in the ANOVA (Fig 4, Table 1) and in the post hoc tests (S7 Table). In addition, adults emerged from five of 21 substrates (Alnus fresh, cow faeces, lingonberries, Pinus fresh, and Pinus rotten, S4 Table) where no eggs had been detected. All had inbred laboratory population genotypes (S5 and S8 Tables).
Fig 4. Number of eggs/female/day laid on various substrates from Experiment 4—Opportunistic ovipositing.
The same lower case letters indicate homogenous significance groups (p = 0.05). For details about substrate nomenclature, see S1 Table.
Discussion
What natural substrate is preferred by D. nigrosparsa for oviposition?
Information on the oviposition preference is scarce for many insects, but oviposition substrate selection is an essential part of a species’ ecology [12]. We reveal here that the mountain fly Drosophila nigrosparsa prefers ovipositing on fungal fruiting bodies (Fig 2, S2 Table). Including this fly, 15 drosophilid species are now known to oviposit on fungi in Europe, of which 14 are Drosophila and one Scaptomyza [33]. Burla & Bächli [34] and Burla et al. [14] reared 11 Drosophila species from mushrooms in Switzerland lacking, apart from D. nigrosparsa, also D. transversa and D. subobscura, which both oviposit on fungi in England [35]. The latter two species are common at the natural habitat at Kaserstattalm, and thus, competition among them and D. nigrosparsa is possible by sharing the same resource, a topic now open for future research.
The rearing experiment in this study (Experiment 1) yielded 614 Diptera, among them 152 Drosophilidae (Table 1) but no Drosophila nigrosparsa. The only drosophilid species found was D. transversa, which was also reared from fungi in England [35] but was missing in a study in Switzerland, probably because of low elevation and latitude [14]. However, after knowing the mushroom preference of D. nigrosparsa, we would have expected at least a small rearing success from fungi. Probably, our failure reflects the low frequency of D. nigrosparsa in nature [22] in combination with the “aggregation model of species coexistence” [36,37], which assumes that females gregariously oviposit on resource patches, so that some patches are strongly occupied and a large number of patches remain uninhabited. By chance, we may not have collected occupied patches in our experiment. Possibly, not all substrates indeed relevant for D. nigrosparsa were considered here due to experimental limitations and seasonal variation in substrate availability. As we have searched intensively for any oviposition substrate mentioned in the literature, however, we are positive that at least various types of each substrate category according to Shorrocks [11] were included. For a final confirmation of our assumptions concerning D. nigrosparsa, we suggest additional field experiments and long-term studies focusing on changing distributions of both the flies and the mushrooms as well as on possible adaptations to rising temperature and other environmental factors.
Is D. nigrosparsa a generalist or a specialist concerning oviposition substrate?
Species specialised on a single food or habitat source are predicted to be more threatened by changing environments than generalists [17]. Thus, information on the degree of specialisation helps making more reliable predictions of a species’ future survival. Even though D. nigrosparsa prefers mushrooms for oviposition (Fig 2), and some mushrooms were more attractive than others (Fig 3), the fly seems not to be specialised on a single fungal species or genus. Moreover, we think that the variation among replicates in Experiment 3 corroborates this conclusion. This finding is in accordance with those for most other drosophilid species of the fungus guild, which was explained by the unpredictability of many fungal fruiting bodies as the fruiting mainly depends on temperature and rainfall [38]. However, at least some fruiting bodies of various mushroom species can be found during the whole vegetation period also in high altitudes, although species richness decreases with increasing elevation [39]. Correlated with increasing temperature, the number of fungus species as well as the number of fungal fruiting bodies increased over the past decades [40]. Moreover, most fungi were observed to prolong their fruiting period in spring and autumn [41]. Hence, mushrooms will most probably not be a limiting resource in the future, even at higher altitudes. Currently, we are performing laboratory selection experiments to assess the evolutionary potential of D. nigrosparsa to adapt to increased temperature. Although we do not know if and how D. nigrosparsa will be affected by climate change, it seems unlikely that the oviposition substrate will be a limiting resource for D. nigrosparsa (and other fungus breeders) in the near future, even not under scenarios of the flies migrating vertically to habitats with more adequate temperatures. However, on the longer run, when temperatures will have risen even further, additional factors such as soil formation in high altitudes [42] may play an important role in the limitation of fungi and fungus-associated species.
Is D. nigrosparsa opportunistic when the preferred substrate is not available, changing oviposition to non-preferred substrates?
Mismatches among interacting species are predicted to increase with ongoing climate change [20]. Thus, it might become advantageous, or even unavoidable, to oviposit opportunistically on others than the preferred substrate in the future. However, in our study, D. nigrosparsa reduced oviposition by 96% when the preferred substrate was not available: 0.193 eggs/female/day were laid on non-favoured substrate (Fig 4) versus 4.683 eggs/female/day on favoured substrates (Fig 3). Although there was some variation among replicates, this does not invalidate the conclusion of reduced oviposition behaviour—these results have to be seen relative to the oviposition behaviour in experiments with mushrooms present (Experiment 2 & 3), in which the flies laid up to 24 times more eggs. Even the maximum number of eggs laid in Experiment 4 was more than four times lower than the mean number of eggs on mushrooms in Experiment 2. Moreover, even on the laboratory substrate they had become accustomed to over four years (grape agar with malt and yeast, see Materials and Methods for details), flies from the same lines and generation laid fewer eggs than on mushrooms (2.760 eggs/50 females/day, data not shown). After knowing this strong preference for mushrooms, an optimisation of the laboratory media would potentially increase the flies’ reproductive success for further culturing. Although the flies laid a few eggs on blueberries and bog bilberries (Figs 2 & 3), fruits are unlikely to be the major oviposition substrate and likely are an alternative for egg laying just because of the late and short fruiting period of berries at high altitudes. In contrast, mushrooms are available during the whole vegetation period. The oviposition on sap fluxes and decaying plant material, which are also possible oviposition substrates of drosophilids in Europe [11], was avoided by D. nigrosparsa in our experiments, except when the favoured substrates were not offered (Fig 4). We conclude that D. nigrosparsa generally does not perform opportunistic ovipositing, but that there is some variation in oviposition choice.
In a few of our samples of Experiments 3 & 4, adults emerged where no eggs had been observed visually (S3–S5 Tables). Thus, egg-counting seems not to be an error-free method but a simple proxy for oviposition preferences. Probably, eggs had been overlooked because of deep insertion in the substrate, undetectable by examining the substrates’ surface, and possibly, those substrates had contained eggs already laid by wildtype females in nature. It would have been an important finding if eclosed adults had been of the wildtype genotype as this would have proven the substrate under question as oviposition substrate in nature. However, all eclosed adults had inbred laboratory population genotypes lacking wildtype-specific alleles (S5 Table). Thus, we infer that these eggs were laid by inbred laboratory population females and had not been introduced from the field. We also note that the lacking proof of the concerned substrates as oviposition substrates in nature does not bear on our laboratory-based inferences, given the low density of D. nigrosparsa in the field [22].
When putting a group of flies together (70 females in Experiment 2 and 50 females in Experiment 3 & 4), the oviposition behaviour of single females might not be independent from the behaviour of other individuals. Preliminary experiments with D. nigrosparsa showed that when individualising pairs of flies, oviposition ceased nearly completely. This observation is in line with gregarious oviposition behaviour assumed in nature [43]. Thus, performing the experiments with single individuals was not possible.
In future experiments, a higher number of replicates would be preferable to increase statistical power. Anyway, four reasons limit the number of feasible replicates: First, in the Alps, some of the relevant substrates are available for just a short period, such as blueberries and lingonberries (August to September, depending on the weather during the vegetation period). Second, D. nigrosparsa has a low oviposition rate and a low egg-to-adult survival rate (4 eggs/female/day and 20%, respectively, data not shown), at least in the laboratory. Thus, the number of adult flies available is limited, and for the presented experiments, we have used as many flies as possible at that time. Third, D. nigrosparsa has a relatively long minimum egg-to-adult developmental time (60 days, data not shown) compared with, for example, D. melanogaster (9 days [8]). This is an additional limiting factor for the number of flies available and for the synchronisation with various substrates. Fourth, climate chambers are limited in size. For true replicates, it is necessary to have all replicates in parallel in the same chamber; otherwise, the results could be influenced by different conditions in different chambers or in the same chamber at different points in time. Here, we placed as many replicates in parallel as possible in the available climate chamber. We do not believe that increasing the number of replicates would have changed the conclusions of our study, in that the major results were congruent among replicates. Anyway, validating our findings by larger-scale investigations will be desirable.
Conclusion and Outlook
We showed that Drosophila nigrosparsa prefers ovipositing on fungal fruit bodies. This fly species seems to have preferences among mushrooms for oviposition, but is not specialised on a single fungal taxon. Opportunistic ovipositing on not-favoured substrates, that is, others than mushrooms, is generally not performed, but not impossible, highlighting some variation in oviposition choice. Due to the broad altitudinal distribution of the fungi considered here and D. nigrosparsa’s generalist ovipositing among mushrooms, we assume that the oviposition substrate will not limit D. nigrosparsa in the near future, even if the species will have to migrate vertically when temperatures are rising.
By being mostly descriptive, studies on basic life-history traits are currently not in vogue [44,45]. Nevertheless, information on life-history traits is highly important for a deeper understanding of a species’ ecology and evolution [46–49] and is available only for a few organisms [50]. We suggest—in addition to studies on the molecular baseline, not instead of them—focussing research on basic life-history traits to gain a more complete picture of a species’ biology.
Supporting Information
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Significant comparisons (p = 0.05) are marked in red. For details about substrate nomenclature, see S1 Table.
(XLS)
Wildtype and inbred laboratory population strains (KG0L0 & ILP) were used as references for the classification of emerged adults.
(XLSX)
Acknowledgments
To B. Frajman and P. Schönswetter for plant identification, to G. Degasperi for Coleoptera identification, to J. Schied for Coleoptera and Hymenoptera identification, to T. Friess for Heteroptera identification. Further, to C. Folterbauer for help in the laboratory, to P. Krapf, I. S. and J. S. Schlick-Steiner, and H. C. Wagner for help in the field, and to T. Kremser and E. K. Mayr for help in the field and in the laboratory. To P. Kirschner, E. M. C. Skoulakis, M. Knaden, and one anonymous reviewer for helpful comments on earlier versions of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the University of Innsbruck and the Austrian Science Fund (FWF, http://www.fwf.ac.at/) under grant P23949 awarded to FMS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Thompson JN. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol Exp Appl. 1988;47: 3–14. [Google Scholar]
- 2.Jaenike J. On optimal oviposition behavior in phytophagous insects. Theor Popul Biol. 1978;14: 350–356. [DOI] [PubMed] [Google Scholar]
- 3.Thompson JN, Pellmyr O. Evolution of oviposition behavior and host preference in Lepidoptera. Annu Rev Entomol. 1991;36: 65–89. [Google Scholar]
- 4.Mayhew PJ. Herbivore host choice and optimal bad motherhood. Trends Ecol Evol. 2001;16: 165–167. [DOI] [PubMed] [Google Scholar]
- 5.Jaenike J. Host specialization in phytophagous insects. Annu Rev Ecol Syst. 1990;21: 243–273. [Google Scholar]
- 6.Markow TA, O’Grady PM. Reproductive ecology of Drosophila. Funct Ecol. 2008;22: 747–759. [Google Scholar]
- 7.Hoffmann AA, Sørensen JG, Loeschcke V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J Therm Biol. 2003;28: 175–216. [Google Scholar]
- 8.Ashburner M, Golic KG, Hawley RS, editors. Drosophila—A Laboratory Handbook. 2nd ed Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2005. [Google Scholar]
- 9.Carson HL. The ecology of Drosophila breeding sites. Hawaii: University of Hawaii, Harold L. Lyon Arboretum; 1971. [Google Scholar]
- 10.Kimura MT, Toda M, Beppu K, Watabe H. Breeding sites of drosophilid flies in and near Sapporo, northern Japan, with supplementary notes on adult feeding habits. Jap J Entomol. 1977;45: 571–582. [Google Scholar]
- 11.Shorrocks B. The breeding sites of temperate woodland Drosophila. Genetics and biology of Drosophila. 1982;3: 385–428. [Google Scholar]
- 12.Carson HL, Stalker HD. Natural breeding sites for some wild species of Drosophila in the eastern United States. Ecology. 1951;32: 317–330. [Google Scholar]
- 13.Heed WB, Kircher HW. Unique sterol in the ecology and nutrition of Drosophila pachea. Science. 1965;149: 758–761. [DOI] [PubMed] [Google Scholar]
- 14.Burla H, Bächli G, Huber H. Drosophila reared from the stinkhorn, Phallus impudicus, near Zurich, Switzerland. Z zool Syst Evolut-forsch. 1991;29: 97–107. [Google Scholar]
- 15.Begon M. The relationships of Drosophila obscura Fallén and D. subobscura Collin to naturally-occurring fruits. Oecologia. 1975;20: 255–277. [DOI] [PubMed] [Google Scholar]
- 16.IPCC. Climate Change 2014: Synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. Geneva, Switzerland: IPCC; 2014.
- 17.Cornelissen T. Climate change and its effects on terrestrial insects and herbivory patterns. Neotrop Entomol. 2011;40: 155–163. [DOI] [PubMed] [Google Scholar]
- 18.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006;37: 637–669. [Google Scholar]
- 19.Merrill RM, Gutiérrez D, Lewis OT, Gutiérrez J, Díez SB, Wilson RJ. Combined effects of climate and biotic interactions on the elevational range of a phytophagous insect. J Anim Ecol. 2008;77: 145–155. 10.1111/j.1365-2656.2007.01303.x [DOI] [PubMed] [Google Scholar]
- 20.Schweiger O, Heikkinen RK, Harpke A, Hickler T, Klotz S, Kudrna O, et al. Increasing range mismatching of interacting species under global change is related to their ecological characteristics. Glob Ecol Biogeogr. 2012;21: 88–99. [Google Scholar]
- 21.Memmott J, Craze PG, Waser NM, Price MV. Global warming and the disruption of plant—pollinator interactions. Ecol Lett. 2007;10: 710–717. 10.1111/j.1461-0248.2007.01061.x [DOI] [PubMed] [Google Scholar]
- 22.Bächli G, Burla H. Diptera, Drosophilidae. Zürich: Schweizerische Entomologische Gesellschaft; 1985. [Google Scholar]
- 23.Genomic Resources Development Consortium, Arthofer W, Banbury BL, Carneiro M, Cicconardi F, Duda TF, et al. Genomic Resources Notes Accepted 1 August 2014–30 September 2014. Mol Ecol Resour. 2015;15: 228–229. 10.1111/1755-0998.12340 [DOI] [PubMed] [Google Scholar]
- 24.Nogués-Bravo D, Araújo MB, Errea MP, Martínez-Rica JP. Exposure of global mountain systems to climate warming during the 21st Century. Globspekulativ Environ Change. 2007;17: 420–428. [Google Scholar]
- 25.Sullivan W, Ashburner M, Hawley RS. Drosophila protocols. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 26.Lakovaara S. Malt as a culture medium for Drosophila species. Drosoph Inf Serv. 1969; 128. [Google Scholar]
- 27.Knudsen H, Vesterholt J, editors. Funga nordica: agaricoid, boletoid and cyphelloid genera. Copenhagen: Nordsvamp; 2008. [Google Scholar]
- 28.R: A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2014. Available: http://www.R-project.org/ [Google Scholar]
- 29.Lunt DH, Zhang D-X, Szymura JM, Hewltt OM. The insect cytochrome oxidase I gene: evolutionary patterns and conserved primers for phylogenetic studies. Insect Mol Biol. 1996;5: 153–165. [DOI] [PubMed] [Google Scholar]
- 30.Molecular Ecology Resources Primer Development Consortium, Agostini C, Albaladejo RG, Aparicio A, Arthofer W, Berrebi P, et al. Permanent genetic resources added to Molecular Ecology Resources database 1 April 2013–31 May 2013. Mol Ecol Resour. 2013;13: 966–968. 10.1111/1755-0998.12140 [DOI] [PubMed] [Google Scholar]
- 31.Piry S, Alapetite A, Cornuet J-M, Paetkau D, Baudouin L, Estoup A. GENECLASS2: A software for genetic assignment and first-generation migrant detection. J Hered. 2004;95: 536–539. 10.1093/jhered/esh074 [DOI] [PubMed] [Google Scholar]
- 32.Rannala B, Mountain JL. Detecting immigration by using multilocus genotypes. Proc Natl Acad Sci USA. 1997;94: 9197–9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Law GR, Parslow R, Davis AJ, Jenkinson LS. Drosophilidae (Diptera) exploiting decaying herbage and fungi in a Scottish wetland habitat. The Entomologist. 1997; [Google Scholar]
- 34.Burla H, Bächli G. Beitrag zur Kenntnis der schweizerischen Dipteren, insbesondere Drosophila-Arten, die sich in Fruchtkörpern von Hutpilzen entwickeln Vierteljahrsschrift der Naturforschenden Gesellschaft in Zürich. 1968; 311–336. [Google Scholar]
- 35.Shorrocks B. An ecological classification of European Drosophila species. Oecologia. 1977;26: 335–345. [DOI] [PubMed] [Google Scholar]
- 36.Shorrocks B, Rosewell J, Edwards K, Atkinson W. Interspecific competition is not a major organizing force in many insect communities. Nature. 1984;310: 310–312. [Google Scholar]
- 37.Hoffmeister TS, Rohlfs M. Aggregative egg distributions may promote species co-existence—but why do they exist? Evol Ecol Res. 2001; 37–50. [Google Scholar]
- 38.Grimaldi D, Jaenike J. Competition in natural populations of mycophagous Drosophila. Ecology. 1984;65: 1113–1120. [Google Scholar]
- 39.Kernaghan G, Harper KA. Community structure of ectomycorrhizal fungi across an alpine/subalpine ecotone. Ecography. 2001;24: 181–188. [Google Scholar]
- 40.Büntgen U, Peter M, Kauserud H, Egli S. Unraveling environmental drivers of a recent increase in Swiss fungi fruiting. Glob Change Biol. 2013;19: 2785–2794. [DOI] [PubMed] [Google Scholar]
- 41.Boddy L, Büntgen U, Egli S, Gange AC, Heegaard E, Kirk PM, et al. Climate variation effects on fungal fruiting. Fungal Ecol. 2014;10: 20–33. [Google Scholar]
- 42.Körner C. Alpine plant life: functional plant ecology of high mountain ecosystems. 2. Berlin, Heidelberg: Springer-Verlag; 2003. [Google Scholar]
- 43.Shorrocks B, Atkinson WD, Charlesworth P. Competition on a divided and ephemeral resource. Journal of Animal Ecology. 1979;48: 899–908. [Google Scholar]
- 44.Mitchell JC, Pague CA. Filling gaps in life-history data: clutch size for 21 species of North American anurans. Herpetol Conserv Biol. 2014;3: 495–501. [Google Scholar]
- 45.Tschinkel WR. Back to basics: sociometry and sociogenesis of ant societies (Hymenoptera: Formicidae). Myrmecol News. 2011;14: 49–54. [Google Scholar]
- 46.Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Philos Trans R Soc Lond B Biol Sci. 2012;367: 1665–1679. 10.1098/rstb.2012.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steiner FM, Crozier RH, Schlick-Steiner BC. Colony structure In: Lach L, Parr CL, Abbott KL, editors. Ant ecology. Oxford: Oxford University Press; 2009. pp. 177–193. [Google Scholar]
- 48.Markow TA. The secret lives of Drosophila flies. eLife. 2015;4: e06793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LoPresti EF, Karban R, Robinson M, Grof-Tisza P, Wetzel W. The natural history supplement: furthering natural history amongst ecologists and evolutionary biologists. Bull Ecol Soc Am. 2016;97: 305–310. [Google Scholar]
- 50.Hoffmann AA, Sgrò CM. Climate change and evolutionary adaptation. Nature. 2011;470: 479–485. 10.1038/nature09670 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Significant comparisons (p = 0.05) are marked in red. For details about substrate nomenclature, see S1 Table.
(XLS)
Wildtype and inbred laboratory population strains (KG0L0 & ILP) were used as references for the classification of emerged adults.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.