Abstract
Objective
To determine if mediastinal lymph node dissection (MLND) improves survival compared to mediastinal lymph node sampling (MLNS) in patients undergoing resection for N0 or non-hilar N1, T1 or T2 non-small cell lung cancer (NSCLC).
Methods
Patients with NSCLC underwent sampling of 2R, 4R, 7 and 10R for right sided tumors, and 5, 6, 7 and 10L for left sided tumors. If all were negative for malignancy, patients were randomized to no further lymph node sampling (MLNS) or complete MLND.
Results
Of 1,111 patients randomized, 1,023 (498 MLNS, 525 MLND) were eligible/evaluable. There were no significant differences between the two groups in terms of demographics, ECOG status, histology, location of the cancer, type or extent of resection, or pathological stage. Occult N2 disease was found in 21 patients in the MLND group. At median follow-up of 6.5 years, 435 (43%) patients have died; (MLNS: 217 (44%);MLND:218 (42%)). The median survival for MLNS is8.1 years, and 8.5 years for MLND (p=0.25). The 5-year disease free survival rate was 69% (95% CI: 64%-74%) in the MLNS group versus 68%(95% CI: 64%-73%) years in the MLND group (p=0.92). There was no difference for local (p=0.52), regional (p=0.10), or distant (p=0.76) recurrence between the two groups.
Conclusions
If systematic, thorough presection sampling of the mediastinal and hilar lymph nodes is negative, MLND does not improve survival in patients with early stage NSCLC but these results are not generalizable to patients staged radiographically or those with higher stage tumors.
Keywords: Lung Cancer, lymph nodes, mediastinum, staging, randomized controlled trial
Introduction
Lung cancer is the leading cause of cancer deaths1. Non-small cell lung cancer (NSCLC) may be curable by surgical resection; however, even tumors which appear localized by imaging may have lymph node metastases. Lymph node assessment is important for accurate staging of NSCLC. However the extent of lymph node removal required and the impact of mediastinal node removal on survival is controversial. Unfortunately, in a pattern of care study, only 57.3% of patients had any mediastinal nodes removed at the time of pulmonary resection.2
Studies addressing the survival benefit of MLND have been inconclusive with only one out of three previous randomized trials reporting a survival advantage.3 Proponentsargue that MLND by removing occult N2 disease, would lower recurrence and increase survival. However, two-thirds of patients with N2 disease develop distant metastases as their first site of recurrence4.
The American College of Surgery Oncology Group (ACOSOG) Z0030 study was a randomized, multi-institutional, prospective trial of MLND versus MLNS during pulmonary resection for patients with early stage NSCLC. The aim of this study was to address the question of whether survival was improved by MLND as compared to MLNS in early stage NSCLC and compare recurrence patterns.
Methods
The protocol was approved by a central Institutional Review Board (IRB) and the IRB at each participating institution. All patients provided written informed consent before trial enrollment.
Participating surgeons were required to read a detailed description of the technique of mediastinal lymph node dissection and watch an instructional video. All operative notes and pathology reports were reviewed by the principal investigators (MSA or GED) for completeness of the mediastinal dissection. Specifically, it was ascertained that additional lymph nodes were removed during MLND as per protocol. Lymph nodes were named according to the American Thoracic Society (ATS) lymph node stations5. Eligibility requirements and methods have been published previously.6 Patients with proven NSCLC underwent a rigorous mediastinal and hilar lymph node sampling as per protocol prior to randomization. For tumors in the right lung lymph node stations 2R, 4R, 7, and 10R were sampled. For tumors in the left lung, stations 5, 6, 7, and 10L were sampled. Any suspicious lymph nodes were also biopsied. The surgeon had the option of sampling by mediastinoscopy (2R/L, 4R/L and 7), thoracotomy or Video Assisted Thoracic Surgery (VATS). Station 10 nodes were sampled at thoracotomy or VATS. If all sampled lymph nodes showed no evidence of cancer on frozen section examination, patients were randomized intra operatively via telephone by the central coordinating center to either lymph node sampling only (MLNS) with no further lymph node removal or to complete mediastinal lymph node dissection (MLND).
MLND was performed according to protocol as previously described. For tumors on the right, all lymph tissue was removed from an area bounded by the takeoff of the right upper lobe bronchus, the innominate artery, the superior vena cava and the trachea (stations 2R and 4R). Lymph nodes in the prevascular area, adjacent to the superior vena cava, and retrotracheal nodes were removed. Complete MLND for tumors on the left involved removing all lymph tissue between the phrenic and vagusnerves extending down to the left main stem bronchus (stations 5 and 6). At the completion of the dissection the aortopulmonary window was free of lymph tissue and the recurrent nerve was preserved. Regardless of the side of the tumor, complete subcarinal lymph node dissection was performed removing all lymph tissue caudal to the carina and both left and right mainstem bronchi (station 7). Lymph nodes in the inferior pulmonary ligament and adjacent to the caudal half of the esophagus were also removed (stations 8 and 9). When the dissection was complete, mainstem bronchi, posterior pericardium, and the esophagus were free of all lymph tissue. In both arms, all lobar and interlobar lymph nodes were resected during the lung resection.
Statistical methods
The target accrual for this study was 1037 with the final analysis to occur after 459 deaths. This was determined based on the assumption that following surgery there would be four pathologic stage groups: pT1N0, pT2N0, pN1, and pN2/pIIIA with expected proportions of 40%, 30%, 15%, and 15%, respectively. For calculating the sample size, it was assumed that the 5-year survival rates in the sampling arm for the four groups would be 75%, 60%, 40% and 30%, respectively. However, observed proportions of the four pathologic stage groups in the sampling arm of thestudy--pT1N0, pT2N0, pN1, and pN2/pIIIA--were 41%, 41%, 13%, and 4%, respectively. In addition, the observed 5-year survival rates in the sampling arm for the four groups was 74%, 59%, 44% and 27%, respectively. This translated to a weighted yearly death hazard rate of 0.0998 (assuming survival time is exponentially distributed). An 8% higher five-year survival in Arm 2 was considered clinically important. Under exponential distribution assumptions this translated to a death hazard rate that is 25%less, or a hazard ratio of 0.75 (0.0751/0.0998). In calculating the sample size for survival, the following specifications were made: One-sided significance level of 0.05; statistical power of 0.90; uniform patient accrual; a five-year accrual period, and a follow-up period of five years.
Patient and surgical characteristics were compared between treatment arms using the chi-square test for categorical variables, Kruskal-Wallis test for continuous variables, and the Wilcoxon test for ordinal variables. Cumulative time to event (survival, recurrence) probabilities was estimated using the Kaplan-Meier method. The log rank test was used to compare survival and recurrence by treatment group. For the recurrence analysis deaths were censored. An additional comparison of local, regional, and distant recurrence was performed using a cumulative incidence approach. This methodology was used to account for competing risks when determining recurrence rates as only first sites of recurrence were recorded. Death was considered a competing event. One sided statistical tests were used for the primary endpoint of overall survival. In all cases p-values <0.05 were considered statistically significant.
Results
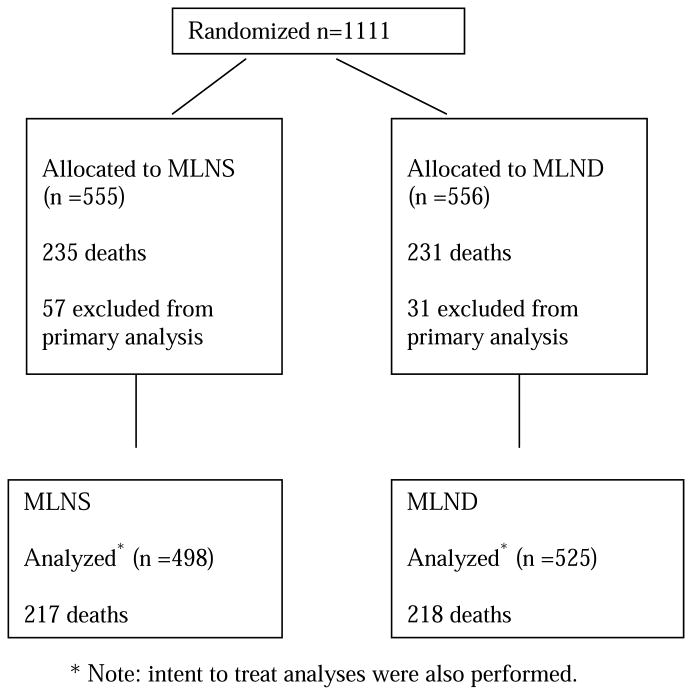
From June 1999 to February 2004, 1,111 patients were randomized by 102 different surgeons from 63 institutions. All participating surgeons were general thoracic surgeons and diplomats of the American Board of Thoracic Surgery (ABTS) or equivalent. After randomization, retrospective review found 155 patients (13.9%) to be ineligible (see figure 1). In 67 of these patients the reason for ineligibility was minor (e.g. timing violation) and these patients were included in this analysis. The remaining 88 patients (57 in the LNS group and 31 in the LND group) were excluded for major violations including incorrect clinical stage in 29 patients, inadequate lymph node sampling in 14, benign disease in 6, insufficient documentation in 5 and other reasons in 34 patients. All analyses were performed on the 1023 (498 MLNS and 525 MLND) eligible subjects and additional intent-to-treat analyses were performed on all randomized subjects. Details of the demographic profile have been published previously (Table 1).6 The median age was 68 years with a range of 23 to 89 with a slight male predominance (n=529; 52%), 955 (93%) were Caucasian with 46 (5%) black and other racial backgrounds in 22 (2%). Most patients were Eastern Cooperative Oncology Group (ECOG) performance score zero (n=688; 67%) or one (n=307; 30%). There were no clinically significant differences in demographic profile or ECOG status between the study arms.
Figure 1. Consort Diagram.
Table 1. Patient and surgical characteristics of the Patients in Each Arm of the ACOSOG Z0030 Study.
| Lymph Node Sampling | Lymph Node Dissection | ||
|---|---|---|---|
| Arm (n=498) | Arm (n=525) | P* | |
| Male | 257 (52%) | 272 (52%) | 0.95 |
| Median Age (range) | 68 (23 – 89) | 67 (37 – 87) | 0.026 |
| ECOG Performance | |||
| Score | |||
| 0 | 344 (69%) | 344 (66%) | 0.34 |
| 1 | 139 (28%) | 168 (32%) | |
| 2 | 15 (3%) | 13 (3%) | |
| Tumor location ** | |||
| RUL | 193 (39%) | 194 (37%) | 0.55 |
| RML | 36 (7%) | 29 (6%) | 0.26 |
| RLL | 88 (18%) | 101 (19%) | 0.52 |
| LUL | 129 (26%) | 144 (27%) | 0.58 |
| LLL | 58 (12%) | 64 (12%) | 0.79 |
| Histology | 0.53 | ||
| Squamous Cell | 132 (27%) | 141 (27%) | |
| Adenocarcinoma | 195 (39%) | 228 (44%) | |
| Large Cell | 27 (5%) | 22 (4%) | |
| Bronchoalveolar | 36 (7%) | 32 (6%) | |
| Other NSCLC | 106 (21%) | 99 (19%) | |
| Type of resection† | 0.66 | ||
| Segmentectomy | 36 (7%) | 34 (7%) | |
| Lobectomy | 379 (76%) | 385 (74%) | |
| Bilobectomy | 18 (4%) | 25 (5%) | |
| Pneumonectomy | 18 (4%) | 24 (5%) | |
| Combination | 45 (9%) | 55 (11%) | |
| Extent of resection | 0.36 | ||
| R0 | 488 (98%) | 512 (98%) | |
| R1 | 10 (2%) | 9 (2%) | |
| R2 | 0 (0%) | 2 (0.4%) | |
| Stage | 0.34 | ||
| IA | 211 (43%) | 212 (41%) | |
| IB | 205 (41%) | 213 (41%) | |
| IIA | 13 (3%) | 24 (5%) | |
| IIB | 56 (11%) | 41 (8%) | |
| IIIA | 4 (1%) | 22 (4%) | |
| IIIB | 8 (2%) | 11 (2%) |
Chi-square test, Kruskal-Wallis test, or Wilcoxon test as appropriate
Counts do not sum to 100% since some patients had disease that involved multiple lobes
The type of resection could not be determined in 4 patients
Approximately 61% of tumors were located in the upper lobes and adenocarcinoma was the predominant histology (42%), see Table 1. Mediastinoscopy was performed in 303 patients overall, MLNS:162 (33%) patients versus MLND: 141 (27%) (p=0.05). As per protocol, anatomic pulmonary resections were performed in all patients:lobectomy in 764 (75%) patients, segmentectomy in 70 (7%), pneumonectomy in 42 (4%), and bilobectomy in 43 (4%). Combinations were performed in 100 patients (10%). Resection was complete (R0) in 1000 patients (98%), incomplete (R1) in 19 (2%), and grossly incomplete (R2) in 2 (0.2%). There were no statistical difference in type of resection, operative approach (thoracotomy versus VATS) or completeness of resection between the lymph node dissection (MLND) and the lymph node sampling (MLNS) group6. See table 1.
Only 67 patients (7%) were resected via VATS. There was no difference in the number of lymph nodes removed by VATS as compared to open resections (median nodes removed: 15 vs 19, p=0.17). However, more nodes were harvested via lobectomy (median = 18) compared to segmentectomy (median = 14), p= 0.006.
After pre-randomization sampling, a median of 18 additional nodes were removed in those patients randomized to MLND (range 1-72 for right sided tumors, 4-69 for left sided tumors) and 516 patients (99%) had at least 6 nodes removed from 3 mediastinal node stations. With MLND at least one additional mediastinal lymph node was removed from each station with a range of median number of nodes removed of 1-4.
The pathological stage is shown in Table 1. There were 45 (4.4%) patients who had pathological stage IIIA or IIIB; 12 in the MLNS arm and 33 in the MLND arm. Positive mediastinal lymph nodes (N2) were discovered in 21 patients who had negative sampling and were randomized to MLND (4%, 95% CI: 2.5%-6.1%). There were 303 patients who underwent mediastinoscopy for lymph node sampling prior to randomization. Despite a negative mediastinoscopy, after MLND 8 patients (2.6%) were found to be N2 on final pathology whereas of the 718 patients who had lymph node sampling at thoracotomy or VATS prior to randomization, 13 (1.8%) were N2 on final pathology. The reason for designation of stage IIIA/B in the 12 patients on the MLNS arm and 12 (non N2) in the MLND arm included: another cancer in the same lobe, tumor < 2 cm from carina, involvement of phrenic nerve and tracheal involvement.
In the group who had initial sampling by mediastinoscopy there were 56 patients (19%) who were found to be N1 positive on final pathology as compared to 71 patients (10%) who had sampling by thoracotomy or VATS. There was no difference in survival between patients who had their initial nodal assessment by mediastinoscopy and those assessed by thoracotomy or VATS.
At a median follow-up of 6.5 years, (range: 0 to10. 1 years), 466 (42%) of the 1111 patients have died; 235 (42%) in the MLNS arm and 231 (42%) in the MLND arm. Of the 1023 eligible patients there were 217 (44%) deaths in the MLNS arm and 218 (42%) in the MLND arm. The median survival was 8.1 years (95% CI: 7.0-9.0) in the MLNS group versus 8.5 years (95% CI: 7.4-NA) in the MLND group (p=0.25; figure 2, table 2). Similar results were observed when the analysis was performed on all 1111 randomized subjects (median survival in the MLNS group was 8.1 years (95% CI: 7.0-9.0) versus 8.4 years (95% CI: 7.3-NA) in the MLND group; p=0.34). There were 285 recurrences reported including 54 local; 74 regional; and 225 distant in the eligible subjects. Of these 138 (24 local, 43 regional, and 111 distant) occurred in the MLNS arm and 148 (30 local, 31 regional, and 114 distant) occurred in the MLND arm. The 5-year disease free survival (DFS) rate was 69% (95% CI: 64%-74%) in the MLNS arm and 68% (95% CI: 64%-73%) in the MLND arm (p=0.92, table 2). Similar results were obtained on all randomized subjects; 5 year DFS was 68% (95% CI: 64%-73%) in the MLNS group and 67% (95% CI: 62%-71%) in the MLND group (p=0.89). An additional analysis was performed separately for T1 (p=0.83) and T2 (p=0.63) tumors and no differences were observed in DFS between treatment arms, see table 3. There was no difference between local (p=0.52), regional recurrence (p=0.10) or distant recurrence (p=0.76) between the two treatment arms. Similar results were observed for all randomized subjects (p=0.54, p=0.24, and p=0.77 for local, regional, and distant recurrence, respectively).
Figure 2. Overall Survival.
Table 2. Overall and disease free survival estimates on all eligible patients.
| Time | MLNS (N=498) Estimate (95% CI) |
MNLD (n=525) Estimate (95% CI) |
|---|---|---|
| Overall survival | ||
| 2 year | 85.1 (82.0, 88.3) | 83.0 (79.8, 86.4) |
| 4 year | 67.8 (63.7, 72.2) | 70.5 (66.5, 74.6) |
| 6 year | 58.1 (53.7, 62.9) | 61.4 (57.1, 66.0) |
| 8 year | 50.9 (45.9, 56.5) | 52.4 (47.6, 57.7) |
| Disease free survival | ||
| 2 year | 79.5 (75.7, 83.4) | 80.5 (76.9, 84.3) |
| 4 year | 70.6 (66.2, 75.2) | 71.7 (67.4, 76.2) |
| 6 year | 65.2 (60.4, 70.4) | 66.3 (61.8, 71.3) |
| 8 year | 61.1 (55.4, 67.3) | 59.4 (53.8, 65.6) |
Table 3. Disease free survival on T1/T2 eligible patients.
| Time | Disease Free Survival on T1/T2 Eligible patients | |||
|---|---|---|---|---|
| T1 MLNS Estimate (95% CI) (%) |
T2 MLNS Estimate (95% CI) (%) |
T1 MNLD Estimate (95% CI) (%) |
T2 MNLD Estimate (95% CI) (%) |
|
| 2 year | 87.2 (82.5, 92.1) | 75.1 (69.5, 81.2) | 91.0 (87.1, 95.1) | 71.7 (66.0, 77.9) |
| 4 year | 79.4 (73.7, 85.6) | 64.7 (58.3, 71.7) | 82.1 (76.6, 87.9) | 62.9 (56.7, 69.8) |
| 6 year | 75.0 (68.5, 82.1) | 58.1 (51.2, 65.8) | 73.7 (67.1, 81.0) | 59.0 (52.6, 66.3) |
| 8 year | 69.7 (61.5, 79.0) | 54.8 (47.0, 63.7) | 66.3 (58.0, 75.8) | 53.0 (45.5, 61.7) |
Discussion
In this prospective randomized controlled clinical trial we found no difference in long-term survival between MLND and MLNS during pulmonary resection for patients with T1 or T2, N0 or non-hilar N1 NSCLC. We also did not see a difference in the recurrence rates or in the pattern of recurrence between MLND and MLNS groups. Previous reports from this trial have shown no increase in morbidity or mortality with the addition of a mediastinal lymph node dissection6.
After Evarts A. Graham's first successful pneumonectomy, surgical resection for lung cancer became popular in the United States. Although Davies in 1912 reported an anatomic lobectomy for lung cancer pneumonectomy remained the mainstay of therapy until it was challenged by Johnson8 and subsequently in a 1962 report by Shimkin9 lobectomy was shown to be acceptable therapy. Although the extent of resection was reduced, removal of all mediastinal lymph tissue was still considered necessary extrapolating from other solid tumors such as breast or gastric cancer, where removal of all the draining lymph nodes was considered the standard of care. In 1951, Cahan from Memorial Sloan Kettering Cancer Center reporting on complete mediastinal lymphadenectomy found “that some patients experienced long-term survival when the positive regional lymph nodes also were removed”10. In a subsequent review, the authors commented that complete mediastinal lymph node dissection led to “more favorable long-term survival”11. Thus, complete MLND has been considered the standard of care for lung cancer resection at most academic centers.
This “standard” has not been followed by the majority of surgeons. In a review of surgical care in the United States in 2001, Little found that only 57.8% of patients who had surgery as their initial treatment of lung cancer had any mediastinal lymph nodes sampled or removed. In community hospitals the rate of any surgical lymph node assessment was even lower at only 48.1%.2
MLND improves staging accuracy by increasing lymph node harvest improving identification of occult N2 disease.12-14 However, whether survival is improved by MLND is controversial. In a subgroup analysis of patients with stage II or IIIA NSCLC entered into Intergroup trial 0115 of adjuvant chemoradiotherapy vs. radiotherapy following resection, Keller15 reported improved long-term survival in patients with right upper lobe tumors who had MLND with a median survival of 57.5 months versus MLNS with a median survival of 29.2 months. The choice of MLND versus MLNS was not randomized and was based on surgeon preference. In contrast to the Z0030 study, Keller's analysis included only patients who had positive N1 or N2 nodes. Interestingly, there was no difference in the recurrence rates between the two cohorts.
A randomized trial comparing MLND and MLNS in 169 patients with stage I, II or IIIA NSCLC reported by Izbicki16 found no significant difference in survival after a median follow-up of 47.5 months. In the MLND group, lymph nodes were removed from stations 12, 11, 10, 7, 4 and 5 in all patients whereas in the sampling group mediastinal nodes were removed only if they appeared suspicious. At a median followup of 47.5 months, there was no difference in overall or disease free survival except in patients with N1 or single station N2 in whom both overall and disease free survival was improved. Although their sampling methodology was a bit different than ours, they also found only a small number of patients (5.5%) had unsuspected mediastinal lymph node involvement after MLND.
Using a protocol similar to that used in the Izbicki study in patients with clinical stage I, small (<2cm) T1 NSCLC (87% nonsquamous cancers), Sugi found no difference in survival between the MLND and MLNS groups (five year survival: 81% and 84% respectively). Unsuspected N2 disease was identified in 12% and 14% of MLND and MLNS groups.14
However, a larger randomized trial of 532 patients with clinical stage I, II or IIIA NSCLC reported by Wu3 comparing MLND to MLNS reported significantly improved survival with MLND. The median survival in the MLND group was 43 months and only 32 months in the MLNS group (p=0.0001). By multivariate analysis, number of lymph node metastasis, type of nodal dissection (MLND vs MLNS), tumor size and pathological stage were all significant prognostic factors.
In contrast to the ACOSOG Z0030 trial, the patients in the trial reported by Wu were only staged clinically prior to randomization. As a result, 48% of the patients in the MLND had pathological stage IIIA disease versus 28% in the MLNS arm. Furthermore, in the MLNS arm, mediastinal nodes were sampled only if suspicious based on size greater than 1 cm, or hardness. Unlike the ACOSOG Z0030 trial, systematic sampling of the mediastinal nodes was not performed.
A meta-analysis of these 3 trials reported a survival advantage for stages I, II and IIIA and a fixed-effects model reported by the same group reported MLND reduced the risk of death for early stage disease.17-18
The ACOSOG Z0030 protocol required systematic sampling of mediastinal nodes either by mediastinoscopy, thoracotomy or VATS. Additionally, any suspicious nodes were also sampled. Only after all required node stations were proven to be negative for metastatic disease was the patient eligible for randomization to either no further lymph node removal (MLNS) or to formal complete lymph node dissection (MLND). This process allowed us to eliminate many patients who may have had “occult” positive mediastinal lymph nodes and explains the low (4%) incidence of unsuspected N2 disease found in patents who underwent complete mediastinal lymphadenectomy. In this regard the Z0030 trial differs from previous randomized trials in that all patients truly had early stage disease.
Lymph node sampling by mediastinoscopy, VATS or thoracotomy were equally efficacious in accomplishing systematic lymph node sampling. EBUS-TBNA- or EUS-FNA were not evaluated in this trial.
Clinical staging with CT and PET are not equivalent to surgical staging, thus in the absence of surgical staging of the mediastinal lymph nodes as was performed in the Z0030 trial, a MLND is essential for both accurate staging and improved survival as suggested by Wu's study3. In patients who are surgically staged as in our trial, MLND identifies truly occult N2 disease and thereby provides such patients with the opportunity to receive adjuvant chemotherapy which has now been shown to improve survival. At the time this study was performed, adjuvant chemotherapy was not the standard of care. This may have contributed to the lack of survival benefit in the MLND arm.
Determination of the amount of lymph node tissue removed in both arms of the trial was based on number or fragments of nodes rather than weight and this has some inherent inaccuracies. This is a limitation of the study and potentially may have contributed to the lack of difference between the two arms of the trial.
This study was conducted by many surgeons, all ABTS (or equivalent) certified and in many institutions (both community and academic), hence the results are generalizable and are not limited to a few specialized surgeons or centers. The breadth of surgical participation also introduced a potential problem of standardization. However, since each operative and pathology note was reviewed by one of the principle investigators (MSA or GED) the variation was minimized, since patients with inadequate MLND or overly aggressive sampling were deemed ineligible.
Conclusions
MLND does not improve longterm survival in patients with early stage (T1or T2, N0 or non-hilar N1) NSCLC who have pathologically negative mediastinal and hilar nodes after rigorous systematic preresection lymph node sampling. In such patients MLND also does not affect the rate of local or regional recurrence. Our results do not apply to patients with T3 or T4 tumors or those with known hilar or N2 disease as these were not included in our study. Staging by PET-CT or CT alone is not equivalent to the invasive staging performed in this study and surgeons cannot use this study to justify excluding invasive mediastinal staging from their evaluation of patients with early stage NSCLC.
MLND provides patients with the most accurate staging and the opportunity for adjuvant therapy if occult metastatic disease is present. Since current preoperative staging cannot definitively identify patients with mediastinal lymph node involvement and patients with known hilar or mediastinal disease (N2) or those with T3 or T4 tumors may benefit from MLND as the pretest probability of N2 disease is higher, we still recommend that all patients with resectable NSCLC undergo MLND as MLND does not increased mortality or morbidity.
Acknowledgments
This study was coordinated by the American College of Surgeons Oncology Group and is supported by funding from the U.S. National Cancer Institute to the American College of Surgeons Oncology Group, grant U10 CA 76001.
The authors wish to express their appreciation to the late Dr. Robert J. Ginsberg for his valuable leadership in designing this trial.
American College of Surgeons Oncology Group Z0030 Trial - Abramson Cancer Center of the University of Pennsylvania; Shrager, Joseph B.; Allegheny Cancer Center Network, Keenan, Robert J., Landreneau, Rodney J.; Beth Israel Deaconess Medical Center Boston MA, LoCicero, Joseph, Thurer, Robert Lee; Beth Israel Medical Center New York NY, Keller, Steven M.; Bethesda North Hospital, Buckley, Donald C.; Cedars-Sinai Medical Center, McKenna, Robert J.; Central Baptist Hospital; Creighton University Medical Center, Scott, Walter J. Veterans Affairs Medical Center, Mitchell, John D.;: Edward Hospital, Bleck, Phyllis C.;: Englewood Hospital and Medical Center: Fox Chase Cancer Center, Goldberg, Melvyn, Scott, Walter J.; Good Samaritan Hospital (Cincinnati), Smith, J. Michael; Holmes Regional Medical Center, Greene, Michael A., Malias, Mark A.; Huntington Memorial Hospital, Cohen, Robbin G., Jameson Hospital, Landreneau, Rodney J.; Jewish Hospital, Bowling, Roy G.; Lankenau Medical Research Center, Carp, Ned Z.; Latter Day Saints Hospital, Collins, Michael P.; Lenoir Memorial Hospital, Whitlark, Joseph D.; London Health Sciences Centre (Univ of Western Ontario Med Ctr), Inculet, Richard I., Malthaner, Richard A.; Loyola University Medical Center, Vigneswaran, Wickii T.; M.D. Anderson Cancer Center (Univ of Texas), Putnam, Joe B., Rice, David Christopher, Roth, Jack A., Vaporciyan, Ara A.; Mary Imogene Bassett Hospital, Ryan, M. Bernadette; Massachusetts General Hospital, Gaissert, Henning A.; Mayo Clinic (Rochester), Allen, Mark S., Deschamps, Claude, Miller, Daniel L., Nichols, Francis C.; Medical Center of Central Georgia; Medical Center of Southwest Louisiana, Lirtzman, Mitchell D.; Medical College of Virginia Hospital (Virginia Commonwealth Univ), Cohen, Neri M.; Memorial Medical Center (Southern Illinois Univ School of Medicine), Hazelrigg, Stephen R.; Memorial Sloan-Kettering Cancer Center, Bains, Manjit S., Rusch, Valerie W. Robert J. Downey, Robert J. Ginsberg; Mobile Infirmary Medical Center, Bradley Scott, Walker, Gaylord T.; New York Hospital - Cornell University Medical Center, Altorki, Nasser K., Port, Jeffrey L.; Omaha Veterans Administration Medical Center, Scott, Walter J.; Peter MacCallum Cancer Institute, Wright, Gavin M.; Providence Portland Medical Center, Douville, Emery Charles, Handy, John R., Ott, Gary Yee, Tsen, Andrew C.; Rhode Island Hospital, Gaissert, Henning A.; Roswell Park Cancer Institute, Anderson, Timothy M., Demmy, Todd L., Nwogu, Chukwumere E.; Saint Clair Hospital, Landreneau, Rodney J., Saint John's Hospital, Hazelrigg, Stephen R.; Saint Luke's Hospital of Duluth, Streitz, John M.; Saint Thomas Hospital (Nashville), Nesbitt, Jonathan C.; Saint Vincent Hospital (Green Bay), Coleman, Edward J.; Saint Vincent's Hospital, Melbourne, Wright, Gavin M; Stanford University (Hospital), Whyte, Richard I.; Stony Brook University Hospital; Thomas Jefferson University Hospital, Pechet, Taine T.; Toronto General Hospital, Darling, Gail E., Johnston, Michael R., Keshavjee, Shafique, Pierre, Andrew F., Waddell, Thomas K.; Trinity Cancer Care Center (Minot), Rothberg, Martin L.; University of California San Francisco Medical Center (Long-Moffitt), Jablons, David M.; University of California, Irvine, Milliken, Jeffrey C.; University of Chicago (Hospitals), Ferguson, Mark K.; University of Cincinnati Medical Center, Howington, John A., Reed, Michael F.; University of Miami, Katariya, Kushagra, Thurer, Richard J.; University of Missouri - Ellis Fischel, Demmy, Todd L.; University of Pittsburgh - Presbyterian Hospital, Buenaventura, Percival O., Keenan, Robert J., Landreneau, Rodney J., Luketich, James D.; University of Pittsburgh (Shadyside Hospital), Christie, Neil A., Landreneau, Rodney J., Luketich, James D.; University of Pittsburgh Medical Center-St. Margaret Hospital, Landreneau, Rodney J.; University of Rochester (Medical Center), Feins, Richard H., Johnstone, David W., Watson, Thomas J.; University of South Alabama, LoCicero, Joseph; University of Virginia (Medical Center), Daniel, Thomas M., Jones, David R.; University of Washington Medical Center, Vallieres, Eric, Wood, Douglas E.; University of Wisconsin, Weigel, Tracey L.; Upstate Medical University (SUNY-Upstate Syracuse), Dexter, Elizabeth U., Kohman, Leslie J.; VA Medical Center –Cincinnati, Howington, John A., Reed, Michael F.; ValleyHospita, Lee, Youngick, Tsoukas, Elias N.; Veterans Administration Center, Seattle (Puget Sound), Vallieres, Eric; Veterans Administration Medical Center-Minneapolis, Kelly, Rosemary F.; Washington University (Barnes Jewish Hospital), Battafarano, Richard J., Cooper, Joel D., Meyers, Bryan F., Patterson, G. Alexander; West Virginia University (Mary Babb Randolph Cancer Center), Graeber, Geoffrey M.; Western Pennsylvania Hospital, Keenan, Robert J., Landreneau, Rodney J.; Westmoreland Hospital, Keenan, Robert J., Landreneau, Rodney J.; William Beaumont Hospital (Royal Oak), Chmielewski, Gary W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altekruse SF, Kosary CL, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2007. National Cancer Institute; Bethesda, MD: 2010. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg. 2005;80:2051–2056. doi: 10.1016/j.athoracsur.2005.06.071. discussion 2056. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer. 2002;36:1–6. doi: 10.1016/s0169-5002(01)00445-7. [DOI] [PubMed] [Google Scholar]
- 4.Caglar HB, Baldini EH, Othus M, Rabin MS, Bueno R, Sugarbaker DJ, Mentzer SJ, Janne PA, Johnson BE, Allen AM. Outcomes of patients with stage III nonsmall cell lung cancer treated with chemotherapy and radiation with and without surgery. Cancer. 2009;115:4156–66. doi: 10.1002/cncr.24492. [DOI] [PubMed] [Google Scholar]
- 5.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–1723. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 6.Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006;81:1013–1019. doi: 10.1016/j.athoracsur.2005.06.066. discussion 1019-1020. [DOI] [PubMed] [Google Scholar]
- 7.Darling GE, Allen MS, Decker PA, et al. Number of Lymph nodes harvested from a mediastinal lymphadenectomy: Results of the randomized, prospective ACOSOG ZOO30 trial. Accepted for publication CHEST. 2010 doi: 10.1378/chest.10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson J, Kirby CK, Blackmore WS. Should we insist on radical pneumonectomy as routine procedure in the treatment of carcinoma of the lung? Journal of Thoracic Surgery. 1958;36:309–315. [PubMed] [Google Scholar]
- 9.Shimkin MB, Connelly RR, Marcus SC. Pneumonectomy and lobectomy in bronchogenic carcinoma: A comparison of end results of the Overholt and Ochsner clinic. Journal of Thoracic and Cardiovascular Surgery. 1962;44:503–519. [PubMed] [Google Scholar]
- 10.Cahan WG, Watson WL, Pool JL. Radial pneumonectomy Journal of Thoracic Surgery. 1951;22:449–473. [PubMed] [Google Scholar]
- 11.Martini N. Mediastinal lymph node dissection for lung cancer. The Memorial experience. Chest Surg Clin N Am. 1995;5:189–203. [PubMed] [Google Scholar]
- 12.Doddali C, Aragon A, Barlesi F, Chetaille B, Robitail S. Does the extent of lymph node dissection influence outcome in patients with stage I non-small cell lung cancer? European Journal of Cardiothoracic Surgery. 2005;27:680–685. doi: 10.1016/j.ejcts.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 13.Gajra A, Newman N, Gamble GP, et al. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol. 2003;21:1029–1034. doi: 10.1200/JCO.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Sugi K, Nawata K, Fujita N, et al. Systematic lymph node dissection for clinically diagnosed peripheral non-small-cell lung cancer less than 2 cm in diameter. World J Surg. 1998;22:290–294. doi: 10.1007/s002689900384. discussion 294-295. [DOI] [PubMed] [Google Scholar]
- 15.Keller SM, Adak S, Wagner H, et al. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group Ann Thorac Surg. 2000;70:358–365. doi: 10.1016/s0003-4975(00)01673-8. [DOI] [PubMed] [Google Scholar]
- 16.Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg. 1998;227:138–144. doi: 10.1097/00000658-199801000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright G, Manser RL, Byrnes G, et al. Surgery for non-small cell lung cancer: systematic review and meta-analysis of randomised controlled trials. Thorax. 2006;61:597–603. doi: 10.1136/thx.2005.051995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manser R, Wright GDH. Surgery for early stage non-small cell lung cancer. Cochrane Library. 2006;2:1–39. doi: 10.1002/14651858.CD004699.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]